Introduction
One of the most important objectives of gene therapy
is the development of safe and efficient systems for gene transfer
in eukaryotic cells. There are 2 strategies to provide target genes
for gene transfer: viral-based and non-viral-based systems
(1). Although viral-based systems
have shown high transfection efficiency in vivo, they have
serious disadvantages, such as immunogenicity and inflammatory
response (2). Non-viral gene
delivery strategies are usually based on plasmid DNA (pDNA)
carrying the gene of interest. pDNA is an attractive platform for
gene delivery since it is a non-viral, non-integrating vector that
is safe, inexpensive, stable and easily manipulated (3). The popularity of pDNA continues to
increase (18.3% of trials compared to 18% in 2007 and 14% in 2004),
and it is the most popular non-viral system used in clinical trials
(4). Conventional plasmid vectors
include a bacterial backbone and a transcription unit. However,
these sequences may cause undesirable effects such as the
production of antibodies against bacterial proteins expressed from
cryptic upstream eukaryotic expression signals, changes in
eukaryotic gene expression caused by the antibiotic resistance
marker, and immune responses to CpG sequences (5,6).
Compared to conventional plasmids, minicircle (MC)
DNAs devoid of plasmid bacterial sequences are superior as
non-viral DNA vector for multiple reasons: i) relative safety due
to the reduced numbers of inflammatory unmethylated CpG motifs; ii)
more efficient transgene expression due to its reduced size; and
iii) more robust and persistent transgene expression (7–9).
Previous studies have demonstrated that the use of MCs may offer a
promising avenue for safe and efficacious non-viral-based gene
therapies (6,10–12).
The goal of cancer treatment is to selectively
eliminate malignant cells while leaving normal tissues intact
(13). Therefore, targeted
strategies need to be implemented for future therapies to ensure
efficient activity at the site of patient primary tumors or
metastases without causing intolerable side-effects. Nasopharyngeal
carcinoma (NPC) is prevalent in South China, North Africa and among
Alaskan Eskimos. A unique feature of NPC is that nearly 100% of
anaplastic or poorly differentiated NPCs contain Epstein-Barr virus
(EBV) genomes and express EBV proteins (14), which are expressed exclusively in
the malignant tissues but not in the surrounding normal tissues.
This difference provides an exploitable opportunity for
tumor-specific targeting. Initial genetic dissections of EBV
identified one viral protein, Epstein-Barr nuclear antigen 1
(EBNA1), and one region of the viral genome, termed latent origin
of plasmid replication (oriP), as being necessary and sufficient
for replication of the viral plasmid. Previous studies have
determined that EBNA1 is essential for regulating the transcription
of the transforming genes of EBV (15–17).
Additionally, EBNA-1, the only viral protein required for the
replication of EBV in latently-infected cells, is found in all
EBV-associated malignancies (18).
The oriP is composed of two separable cis elements, the family of
repeats (FR) and dyad symmetry element (DS) (19). The FR element consists of 20 tandem
30-bp repeats and acts as a transcriptional enhancer for
heterologous promoters when it is bound by EBNA1. Based on these
features, the oriP-CMV promoter has been exploited for targeted
gene therapy in EBV-positive NPC (20).
Due to these factors, we have developed a novel MC
targeted therapy system in which transgene expression is under the
transcriptional regulation of the oriP-CMV promoter (hereinafter
referred to as oriP promoter). The binding of EBNA1 to the FR
domain in oriP region activates the transcription of downstream
genes. Selective expression of the therapeutic gene is successfully
achieved in vitro and in vivo, indicating the
feasibility of MC-oriP as a safe and highly effective gene therapy
system for the treatment of NPC (21).
However, the major obstacle to widespread use of MCs
is their time-consuming, labor-intensive production. In previous MC
production schemes, the MC producer plasmid p2ΦC31 contained a
transgene expression cassette flanked with attB and attP, a set of
inducible enzyme genes (a gene encoding homing endonuclease I-SceI
and two copies of the gene encoding ΦC31 integrase) and an I-SceI
recognition site (9). The attB and
attP sites are the bacterial and phage attachment sites of ΦC31
integrase, and the ΦC31 and I-SceI genes are regulated by the
l-arabinose-inducible araCBAD system. MC DNA is generated by
recombination between the attB and attP sites, and I-SceI initiates
the destruction of the pDNA backbone circle by cutting through the
engineered I-SceI site. Although the yields from this protocol were
~1 mg of MC DNA from 1 liter of overnight culture, the preparations
still contained ~3–15% of the input MC producer plasmid plus the
plasmid backbone circle as contaminants. In addition to CsCl
equilibrium gradient centrifugation to remove these unwanted DNAs,
the production procedure is four labor-intensive days longer than
routine plasmid production protocols (22).
Therefore, Chen et al presented a new system
including the bacterial strain ZYCY10P3S2T plus the MC producer
plasmid pMC.BESPX that allows simple, rapid and inexpensive pro
duction of a high-quality form of MCs. It enabled three
improvements over the previous system (9). First, the procedure was simplified
considerably and, compared to a routine plasmid preparation,
required only an additional temperature change and 5-h incubation
after addition of l-arabinose. Second, the yield of MC was 3.4–4.8
mg/1,000 ml of overnight culture, making it ~3–5-fold higher than
the previous MC producing system. In addition, compared with the
previous MC production protocol, there were 10-fold fewer
contaminating pDNAs, ranging from 0.4% to 1.5% of the input MC
preparation. On a molar scale, the yield of MC was 20–70% higher
than the MC producer plasmid. Third, the cost of MC production was
similar to that of a standard plasmid. These production
improvements, along with their superior expression profiles, make
it feasible for MC DNA vectors to be used in place of pDNAs in
mammalian expression studies (22).
In the present study, we developed a new targeted MC
producing system by introducing targeted promoter oriP into the MC
producer plasmid pMC.BESPX. Then, we demonstrated that this system
could produce high-quality form of targeted MCs and the targeted MC
containing oriP promoter could selectively express gene in
vitro. To our knowledge, this is the first report on the new MC
producer plasmid pMC. BESPX used in targeted gene expression. This
system (pMC. BESPX-oriP) provides new targeted therapeutic vector
for EBV-positive NPC.
Materials and methods
Construction of recombinant parent
plasmids
Plasmid pMC. BESPX (4,084 bp) and bacterial strain
ZYCY10P3S2T were gifts from Dr Zhiying Chen (Stanford University,
Stanford, CA, USA) (22). Plasmid
pSP72-oriP-luci-polyA was constructed by our laboratory (21). pcDNA3.1 (5,428 bp) and the E.
coli strains top 10 were purchased from Invitrogen.
pGL3-control was purchased from Promega. pEGFP-C2 (4.7 kb) was
obtained from Clontech.
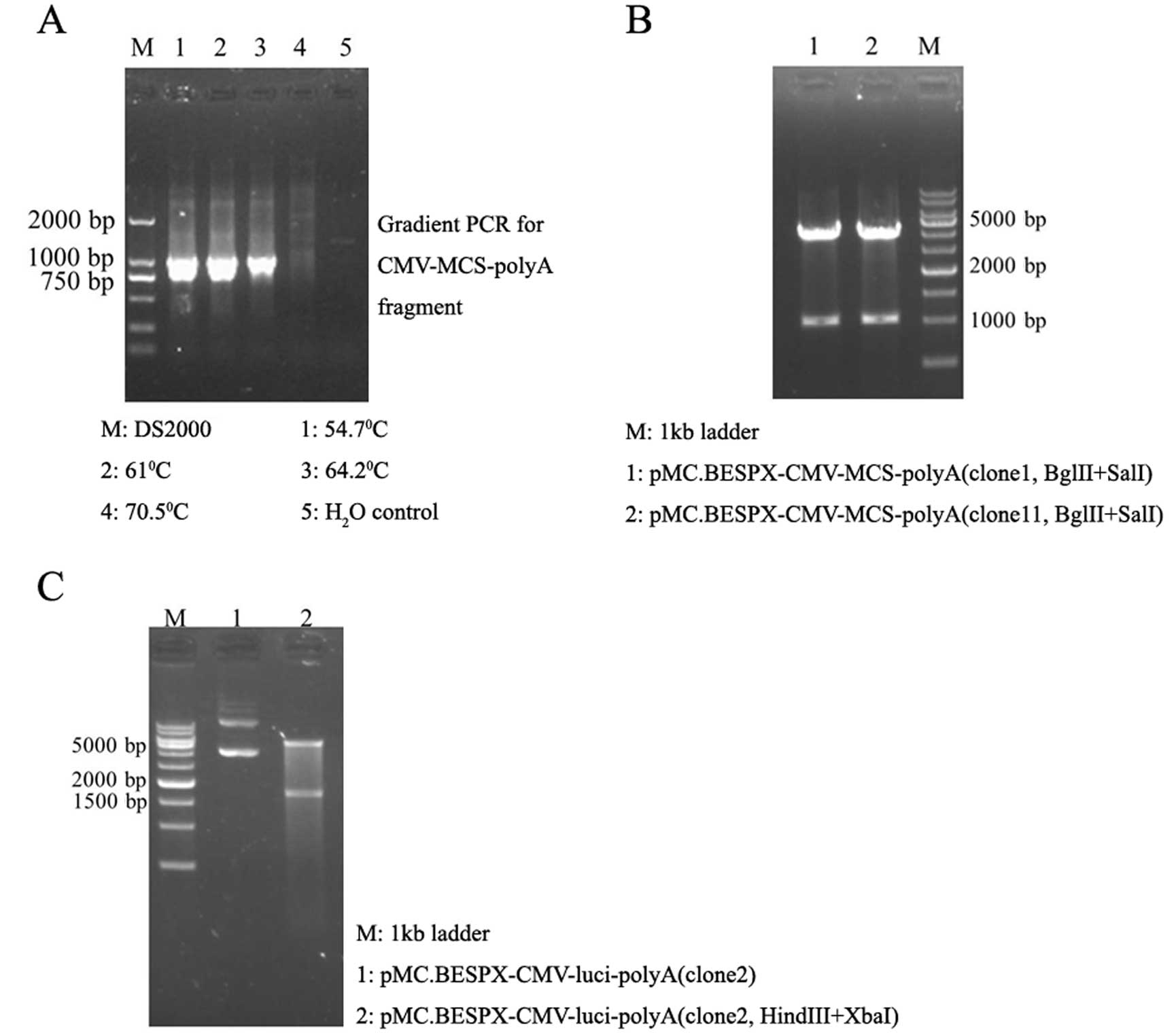
The 1-kb CMV-multiple cloning sites (MCS)-polyA
fragment was amplified by PCR from pcDNA3.1 plasmid (Fig. 1A) and subcloned into the pMC.BESPX
plasmid to create intermediate plasmid pMC.BESPX-CMV-MCS-polyA (5.1
kb, Fig. 1B). Then, parent plasmid
pMC.BESPX-CMV-luci (6.7 kb, Fig.
1C) was constructed by replacing MCS of intermediate plasmid
with luciferase gene (1.7 kb) obtained from plasmid
pGL3-control (digested by HindIII and XbaI).
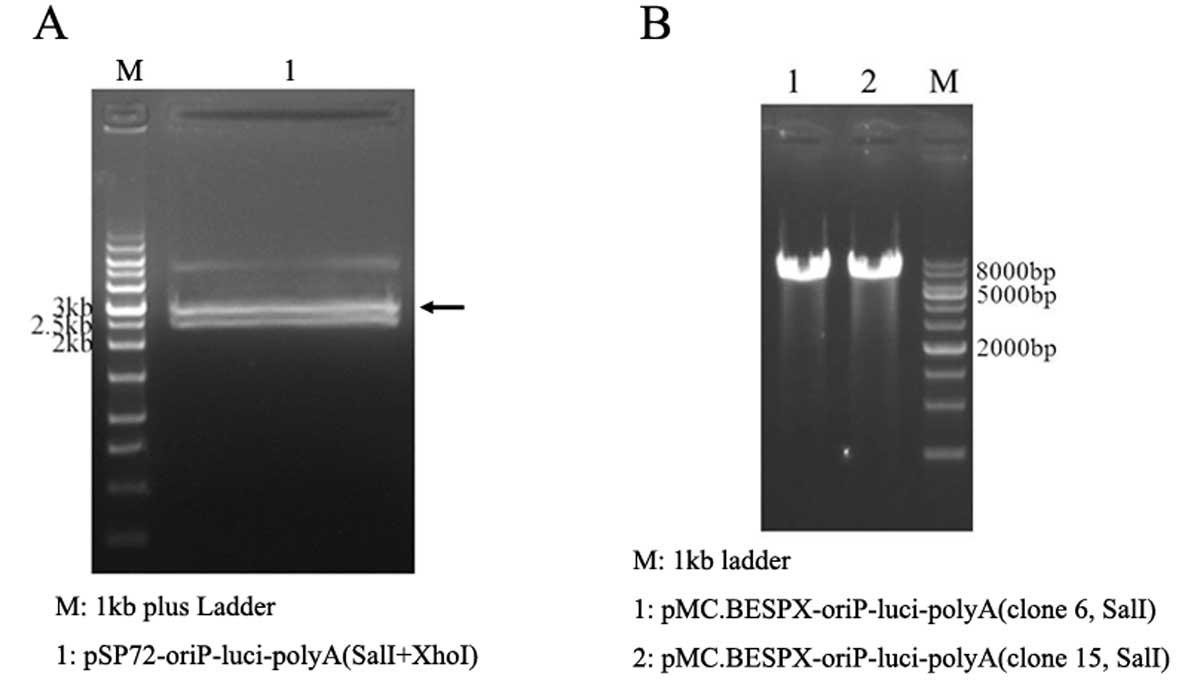
Parent plasmid pMC.BESPX-oriP-luci (6.9 kb) was
constructed by inserting the 2.8-kb
SalI-oriP-luciferase-polyA-XhoI fragment from the
intermediate plasmid pSP72-oriP-luci-polyA (Fig. 2A) into the SalI sites of pMC.
BESPX; SalI and XhoI are isocaudamers (Fig. 2B).
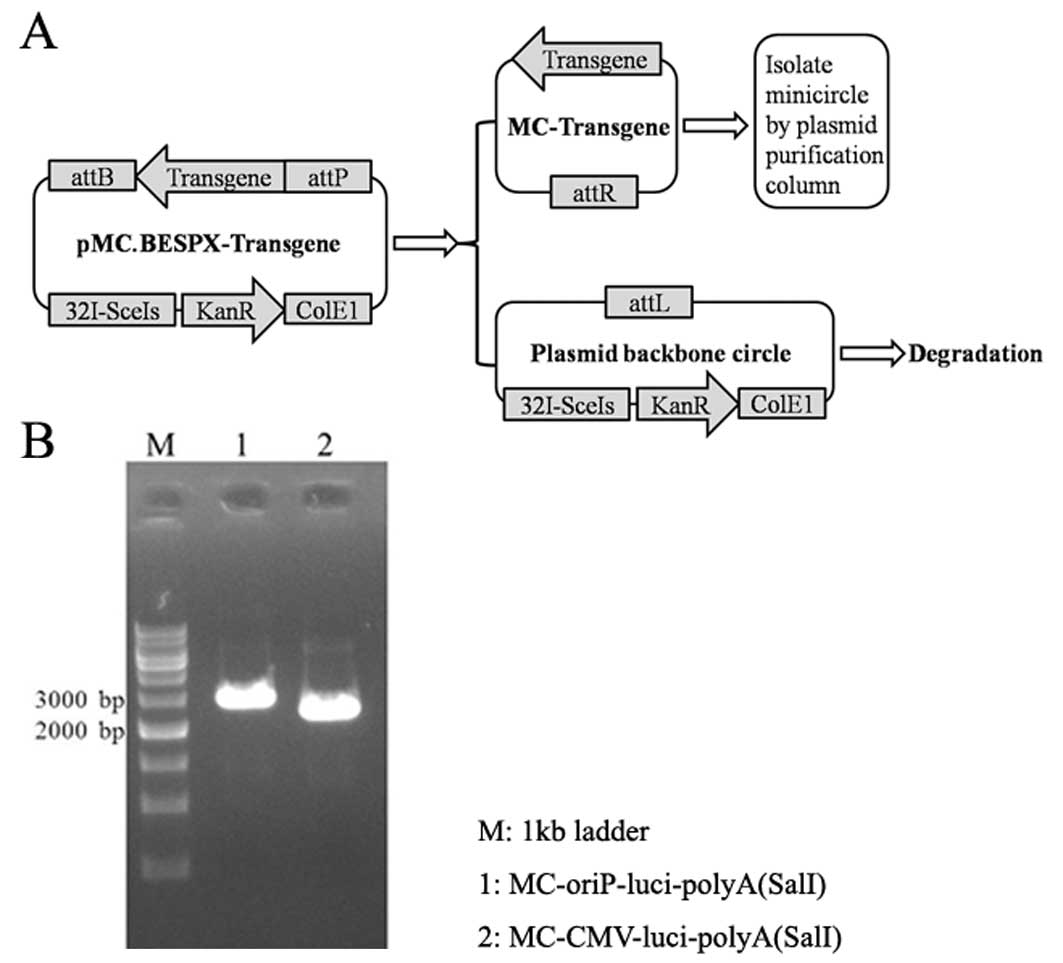
Production and purification of MCs
MC-luciferase (2.8-kb
MC-oriP-luciand2.6-kbcontrolMC-CMV-luci)wereproduced according to
the methods described by Chen et al (22). Our present MC producing system, the
strain ZYCY10P3S2T plus the MC producer plasmid pMC.BESPX-oriP-luci
and pMC.BESPX-CMV-luci, allowed for a greatly simplified MC
production protocol (Fig. 3A). On
the morning of day 1, we inoculated cells from one transformed
colony in 2 ml of TB (pH 7.0) with Kan (50 μg/ml) and incubated at
37°C with shaking at 250 rpm. Later that day, we amplified the
bacteria by combining 25 μl of culture to every 100 ml TB
containing Kan (50 μg/ml) and continued incubation overnight. At
the end of the culture period, the OD600 was 4–5 with a pH of ~6.5.
On day 2, we prepared an MC induction mix comprising 100 ml fresh
LB, 4 ml 1N sodium hydroxide and 0.1 ml 20% L-arabinose and
combined it with a 100 ml overnight culture, and incubated the
culture at 32°C with shaking at 250 rpm for an additional 5 h. We
used Qiagen plasmid purification kits to isolate MC from bacterial
lysates following the manufacturer’s protocol (22).
Cells and culture conditions
The cell lines used in the present study were 293
(human embryonic kidney cell line, EBV-negative), NP69
(immortalized human nasopharyngeal epithelial cell line,
EBV-negative), 5–8F (highly metastasized NPC cell line,
EBV-negative), and C666-1 (undifferentiated and the only available
EBV-positive NPC cell line) (23–25).
5–8F and C666-1 cells were maintained in RPMI-1640 containing 100
U/ml penicillin, 100 mg/ml streptomycin and 10% fetal bovine serum
(Gibco, Paisley, Scotland) at 37°C in a 5% CO2
humidified atmosphere. NP69 cells were maintained in
Keratinocyte-SFM (cat. 10724; Gibco, Invitrogen), and the
experiments were conducted when the cells were in an exponential
growth phase. C666-1 was a gift from Dr Saiwah Tsao (University of
Hong Kong, Hong Kong, China). NP69 and 5–8F were kindly provided by
Professor Musheng Zeng (State Key Laboratory of Oncology in South
China, Cancer Center, Sun Yat-sen University, Guangzhou, China).
The 293 cell line was maintained by our laboratory (21).
Quantitative assay of luciferase
expression mediated by targeted or control promoter
To evaluate luciferase gene expression from
MC-oriP-luci or MC-CMV-luci in EBV-negative and -positive cells,
luciferase activity was measured using the Dual-Luciferase reporter
assay system (Promega). Cells were seeded in 24-well culture plates
the day before transfection. After one doubling, cells were
cotransfected with different luciferase expressing plasmid and
pGL4.73 (Promega) simultaneously at a ratio of 50:1. Cell lysates
were analyzed for luciferase activity using the Dual-Luciferase
reporter assay system and a Luminometer (Centro LB-960; Berthold
Technologies) according to the manufacturers’ protocols (21).
Fluorescence detection of egfp expression
mediated by parent plasmid or MC plasmid
To further validate that MC mediated more efficient
transgene expression compared with routine plasmid, we also
constructed MC producer plasmid pMC.BESPX-CMV-EGFP, and isolated MC
plasmid MC-CMV-EGFP. Transfections were conducted according to the
manufacturer’s instructions (Invitrogen). Twenty-four hours after
transfection, fluorescence images of transfected 5–8F and C666-1
cells were captured using the Olympus IX71 motorized inverted
microscope using FITC filter at ×10 magnification (26).
Statistical analysis
All results were evaluated using Student’s t-test
with SPSS 17.0 software (SSPS, Inc., Chicago, IL, USA). p<0.05
was considered to indicate a statistically significant difference.
Representative results from three independent experiments are
shown, and the data are presented as means ± SD.
Results
Analysis of molecular weight and purity
of MCs by gel electrophoresis
MC DNA produced by using designated protocols was
purified using Qiagen plasmid purification kits and analyzed by gel
electrophoresis (Fig. 3B). Prior to
electrophoresis, DNAs were digested with restriction enzyme
SalI. As shown in Fig. 3,
the size of MCs was close to their expression cassette
(oriP-luciferase-polyA and CMV-luciferase-polyA). Notably, there
was no contaminating pDNA by macroscopic observation. This is
consistent with a previous report (22).
Selective expression of luciferase in
EBV-positive C666-1 cells mediated by oriP-vectors
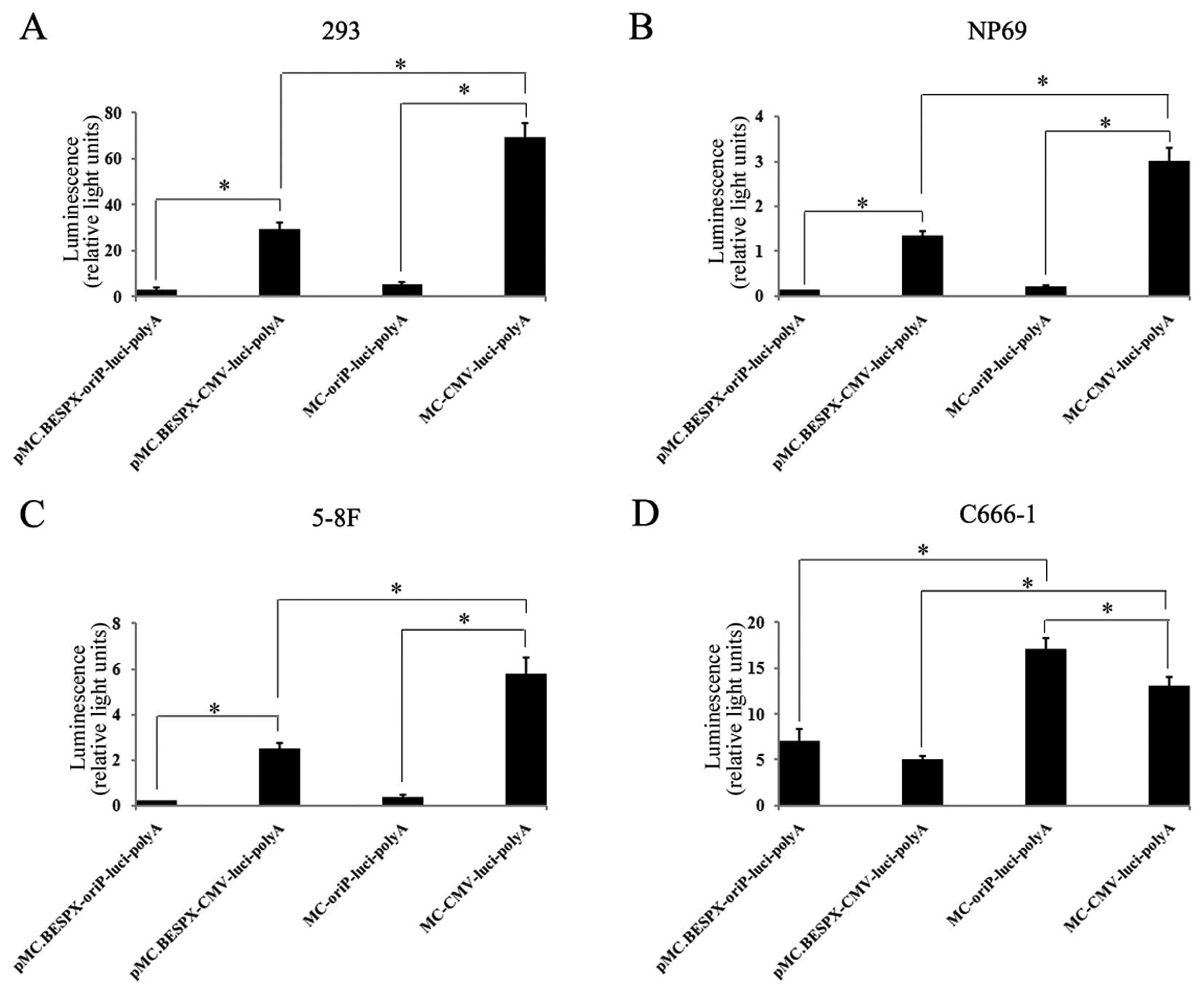
To determine the transgene expression provided by
the novel EBNA1-regulated MC vectors in EBV-negative cells (293,
NP69 and 5–8F cells), and the only available EBV-positive NPC cell
line (C666-1), the MC-luci was compared with its parent plasmid
pMC.BESPX-luci. All these plasmids contained a luciferase
expression cassette driven by an oriP promoter or cytomegalovirus
promoter (Fig. 4). Luciferase
activity was assessed 48 h (72 h for C666-1 due to its low growth
rate) after transfection using the Dual-Luciferase reporter assay
system. Luciferase activities were 10–20-fold lower when driven by
oriP promoter than by CMV promoter in EBV-negative cells
(p<0.001; Fig. 4). In contrast,
luciferase activity was higher when driven by oriP promoter than by
CMV promoter in the EBV-positive C666-1 cells (p<0.05; Fig. 4). Occasionally, low levels of
luciferase activity were detected in some EBV-negative cells when
driven by the oriP promoter, which suggested the basal expression
induced by the minimal CMV IE promoter included in oriP promoter.
This observation is consistent with a previous study (20). The expression levels of the MC
groups were significantly higher than those of the corresponding
parent groups, demonstrating that the MC is more efficient in
mediating transgene expression in vitro (p<0.01; Fig. 4).
Enhanced expression of MC-encoded egfp
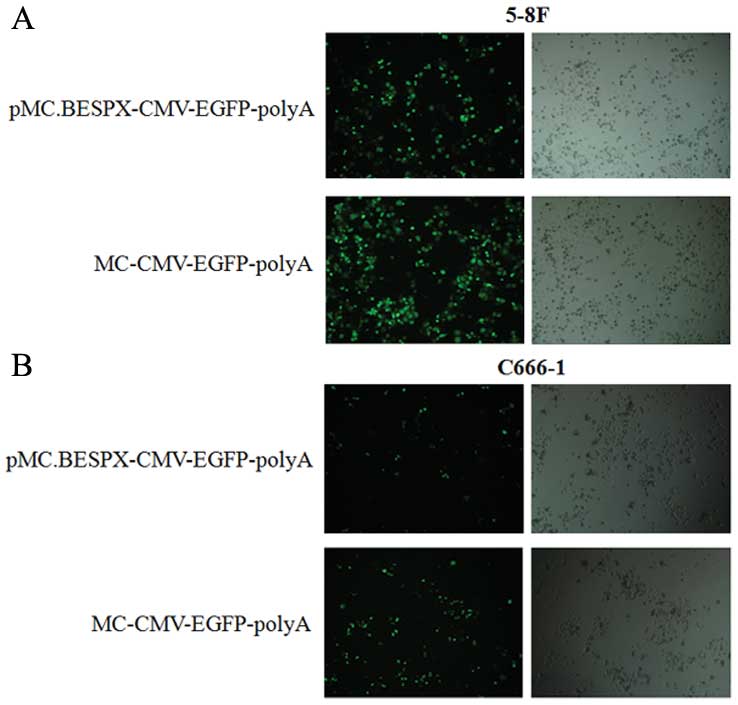
gene compared with parent plasmid in vitro
The transfection efficiency of parent plasmid and MC
were compared in 5–8F (Fig. 5A) and
C666-1 (Fig. 5B) cells using
Lipofectamine 2000 (Invitrogen). Twenty-four hours after
transfection, there was a significant increase in GFP expression in
the MC-transfected cell population compared with the parent
plasmid-transfected cell population (Fig. 5).
Discussion
Our data indicate the novel minicircle (MC)
producing system pMC.BESPX-oriP could produce high-quality form of
MC-oriP. Targeted and increased gene expression is successfully
achieved in MC-oriP-transfected EBV-positive cells. More efficient
transgene expression is reconfirmed in MC-EGFP compared with
pMC.BESPX-EGFP. The novelty of the present study lies in the
introduction of oriP promoter into the new MC producing system
pMC.BESPX and application of this system in gene expression in
EBV-positive nasopharyn-geal carcinoma (NPC).
Gene therapy is an emerging field in medical and
pharmaceutical sciences due to its potential to treat chronic
diseases such as cancer, viral infections, myocardial infarctions
and genetic disorders. Application of gene therapy is limited due
to lack of suitable methods for proper introduction of genes into
cells and, therefore, this is an area of interest for most
researchers. To achieve successful gene therapy, development of
proper gene delivery systems may be one of the most important
factors. Several non-viral and viral gene transfer methods have
been developed. Although the viral agents have a high transferring
efficiency, they are difficult to handle due to their toxicity. To
overcome the safety problems of the viral counterpart, several
non-viral in vitro and in vivo gene delivery systems
have been developed (27). Plasmid
DNA (pDNA) is the most popular non-viral system used in clinical
trials (4).
Standard pDNA has two components: the bacterial
backbone required for plasmid propagation in bacteria and the
transcription cassette for expression in mammalian cells. An MC
episomal DNA vector is a circular expression cassette devoid of the
bacterial pDNA backbone. pDNA size is inversely related to
transfection efficiency and correspondingly, the transfection
efficiency and expression levels of MC are higher than pDNA levels
(12). MCs have been used for years
in preclinical gene transfer research due to their 10–1,000-fold
enhancement compared with regular plasmids in long-term transgene
expression in quiescent tissues in vivo and in vitro
(22). Owing to this advantage,
Huang et al created MC carrying HIF-1α (MC-HIF-1α)
therapeutic gene for treatment of myocardial infarction. They also
demonstrated that MC could significantly improve transfection
efficiency, duration of transgene expression and cardiac
contractility (6). Jia et al
used a single MC vector to generate transgene-free induced
pluripotent stem cells (iPSCs) from adult human adipose stem cells
(10). Gao et al developed a
magnetic resonance imaging (MRI) visible nanoparticle to monitor MC
DNA gene delivery and explore the potential of gene therapy in
vivo (28). Dietz et al
also demonstrated that MC DNA is superior to pDNA in eliciting
antigen-specific CD8+ T-cell responses (12).
In our previous study, we generated a safe and
highly effective gene therapy system for NPC by using the MC
producer plasmid p2ΦC31, which was provided by Dr Zhiying Chen
(Stanford University) (21). The MC
preparations contained ~3–15% of the input MC producer plasmid plus
the plasmid backbone circle as contaminants. A new system pMC.BESPX
and the bacterial strain ZYCY10P3S2T that allow simple, rapid and
inexpensive production of a high-quality form of MCs has been
presented (22).
In the present study, we constructed a targeted MC
producing system by using the novel parent plasmid pMC.BESPX and
the bacterial strain ZYCY10P3S2T. We isolated MCs without
macroscopic plasmid contamination (Fig.
3B). Our results verified the superiority of pMC.BESPX in MC
preparation, and is consistent with other reports (12,22).
Then, we detected the targeted gene expression of pMC.BESPX-oriP
and MC-oriP in EBV-negative and EBV-positive cells. Both in parent
plasmid and in MC group, oriP promoter could mediate selective gene
expression compared to CMV promoter (Fig. 4). This indicates that MC-oriP which
is produced by the new producing system constructed in the present
study, could also mediate targeted gene expression. It is similar
to our previous study (21).
Finally, we verified that MC was more efficient in gene expression
compared with parent plasmid (Fig.
5). Our results suggest that pMC.BESPX-oriP could carry various
therapeutic gene and produce high-quality MC which can be used in
targeted gene therapy for EBV-positive NPC.
In conclusion, we showed for the first time the
application of targeted promoter oriP in the novel MC producing
plasmid pMC.BESPX, and demonstrated the selective expression of
pMC.BESPX-oriP, as well as its inducing product MC-oriP. We
constructed a targeted expression vector which could carry
diversified therapeutic gene for EBV-positive NPC. The present
study provides a new approach toward MC-based therapies.
Acknowledgements
The authors thank Dr Zhiying Chen (Stanford
University, Stanford, CA, USA) for his generous gift of plasmid
pMC. BESPX and bacterial strain ZYCY10P3S2T. This study was
supported by grants from the Open Project Program of State Key
Laboratory of Oncology in Southern China (HN2012-06), the PhD
Programs Foundation of Guangdong Medical College (XB1228), and the
National Natural Science Foundation of China (no. 81201736).
Abbreviations:
|
MC
|
minicircle
|
|
NPC
|
nasopharyngeal carcinoma
|
|
EGFP
|
enhanced green fluorescence
protein
|
|
EBV
|
Epstein-Barr virus
|
|
EBNA1
|
Epstein-Barr nuclear antigen 1
|
|
oriP
|
origin of plasmid replication
|
References
|
1
|
Lyon AR, Sato M, Hajjar RJ, Samulski RJ
and Harding SE: Gene therapy: targeting the myocardium. Heart.
94:89–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marshall E: Gene therapy death prompts
review of adenovirus vector. Science. 286:2244–2245. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faurez F, Dory D, Le Moigne V, Gravier R
and Jestin A: Biosafety of DNA vaccines: new generation of DNA
vectors and current knowledge on the fate of plasmids after
injection. Vaccine. 28:3888–3895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ginn SL, Alexander IE, Edelstein ML, Abedi
MR and Wixon J: Gene therapy clinical trials worldwide to 2012 - an
update. J Gene Med. 15:65–77. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jechlinger W: Optimization and delivery of
plasmid DNA for vaccination. Expert Rev Vaccines. 5:803–825. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang M, Chen Z, Hu S, et al: Novel
minicircle vector for gene therapy in murine myocardial infarction.
Circulation. 120(Suppl 11): S230–S237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen ZY, He CY, Ehrhardt A and Kay MA:
Minicircle DNA vectors devoid of bacterial DNA result in persistent
and high-level transgene expression in vivo. Mol Ther. 8:495–500.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen ZY, He CY, Meuse L and Kay MA:
Silencing of episomal transgene expression by plasmid bacterial DNA
elements in vivo. Gene Ther. 11:856–864. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen ZY, He CY and Kay MA: Improved
production and purification of minicircle DNA vector free of
plasmid bacterial sequences and capable of persistent transgene
expression in vivo. Hum Gene Ther. 16:126–131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia F, Wilson KD, Sun N, et al: A nonviral
minicircle vector for deriving human iPS cells. Nat Methods.
7:197–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang C, Gao S, Jiang W, et al: Targeted
minicircle DNA delivery using folate-poly(ethylene
glycol)-polyethylenimine as non-viral carrier. Biomaterials.
31:6075–6086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dietz WM, Skinner NE, Hamilton SE, et al:
Minicircle DNA is superior to plasmid DNA in eliciting
antigen-specific CD8+ T-cell responses. Mol Ther.
21:1526–1535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hine CM, Seluanov A and Gorbunova V: Use
of the Rad51 promoter for targeted anti-cancer therapy. Proc Natl
Acad Sci USA. 105:20810–20815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen JI: Epstein-Barr virus infection. N
Engl J Med. 343:481–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bochkarev A, Barwell JA, Pfuetzner RA,
Bochkareva E, Frappier L and Edwards AM: Crystal structure of the
DNA-binding domain of the Epstein-Barr virus origin-binding
protein, EBNA1, bound to DNA. Cell. 84:791–800. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niedobitek G, Agathanggelou A and Nicholls
JM: Epstein-Barr virus infection and the pathogenesis of
nasopharyngeal carcinoma: viral gene expression, tumour cell
phenotype, and the role of the lymphoid stroma. Semin Cancer Biol.
7:165–174. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altmann M, Pich D, Ruiss R, Wang J, Sugden
B and Hammerschmidt W: Transcriptional activation by EBV nuclear
antigen 1 is essential for the expression of EBV’s transforming
genes. Proc Natl Acad Sci USA. 103:14188–14193. 2006.
|
|
18
|
Leight ER and Sugden B: EBNA-1: a protein
pivotal to latent infection by Epstein-Barr virus. Rev Med Virol.
10:83–100. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lindner SE and Sugden B: The plasmid
replicon of Epstein-Barr virus: mechanistic insights into
efficient, licensed, extrachromosomal replication in human cells.
Plasmid. 58:1–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li JH, Chia M, Shi W, et al:
Tumor-targeted gene therapy for nasopharyngeal carcinoma. Cancer
Res. 62:171–178. 2002.PubMed/NCBI
|
|
21
|
Zuo Y, Wu J, Xu Z, et al:
Minicircle-oriP-IFNγ: a novel targeted gene therapeutic system for
EBV positive human nasopharyngeal carcinoma. PLoS One.
6:e194072011.
|
|
22
|
Kay MA, He CY and Chen ZY: A robust system
for production of minicircle DNA vectors. Nat Biotechnol.
28:1287–1289. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teng ZP, Ooka T, Huang DP and Zeng Y:
Detection of Epstein-Barr virus DNA in well and poorly
differentiated nasopharyngeal carcinoma cell lines. Virus Genes.
13:53–60. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Ding Y, Xie W, et al: An imageable
metastatic treatment model of nasopharyngeal carcinoma. Clin Cancer
Res. 13:3960–3967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheung ST, Huang DP, Hui AB, et al:
Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring
Epstein-Barr virus. Int J Cancer. 83:121–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong Y, Aied A, Li J, Wang Q, Hu X and
Wang W: An in vitro approach for production of non-scar minicircle
DNA vectors. J Biotechnol. 166:84–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manjila SB, Baby JN, Bijin EN, Constantine
I, Pramod K and Valsalakumari J: Novel gene delivery systems. Int J
Pharm Investig. 3:1–7. 2013. View Article : Google Scholar
|
|
28
|
Gao L, Xie L, Long X, et al: Efficacy of
MRI visible iron oxide nanoparticles in delivering minicircle DNA
into liver via intra-biliary infusion. Biomaterials. 34:3688–3696.
2013. View Article : Google Scholar : PubMed/NCBI
|