Introduction
Hepatocellular carcinoma (HCC) ranks as the most
common type of primary liver cancer with poor survival rates, and
accounts for 90% of all liver cancers (1,2). Its
incidence is currently increasing around the world, particularly in
Asia and sub-Saharan Africa, with as many as 120 cases per 100,000
(3,4). The high proliferation and invasion
characteristics of HCC make it the leading cause of cancer-related
mortality worldwide. Although considerable advances in the
treatment of HCC have been made, its intrinsic resistance to many
clinical therapies, such as chemotherapy and radio-therapy, have
led to ineffective therapeutic options, and the 5-year survival
rate of patients is only ~10% (5).
Therefore, it is necessary to understand the molecular mechanism
underlying HCC development and progression.
Syntenin, also known as melanoma differentiation
associated gene-9 (mda-9), is an evolutionarily conserved sytosolic
protein, that was initially identified as a molecule linking
syndecan-mediated signaling to the cytoskeleton. It is now
recognized as a significant member of the expanding family of
scaffolding proteins with highly potent and diverse biological
activities, including protein trafficking, cell adhesion,
regeneration and signal transduction (6–8).
Syntenin comprises four separate structural domains; the presence
of two tandem PDZ domains (PDZ1 and PDZ2) is the notable feature,
reflecting its indispensable role in assembling cell signaling
processes. The function of syntenin has attracted increasing
attention regarding its critical roles in cancer growth, invasion
and metastasis (9–11). Extensive studies have demonstrated
that syntenin is upregulated in several types of cancer, including
breast cancer, melanoma and bladder cancer (10,12,13).
Overexpression of syntenin in breast cancer cells promotes cell
migration, invasion and tumor growth, as well as poor patient
survival, indicating a significant role in breast cancer
progression (10). Blocking
syntenin expression inhibits melanoma cell migration, growth,
invasion and metastasis by suppressing the p38 MAPK and NF-κB
pathway (12). Furthermore, a novel
role of syntenin in regulating urothelial cell carcinoma (UCC) cell
proliferation by epidermal growth factor receptor (EGFR) signaling
has been observed, suggesting it is a promising target for
developing detection, monitoring and therapeutic strategies for
managing UCC (14). Although
numerous studies on syntenin function in cancer have been
performed, little is known regarding its roles and underlying
molecular mechanism in the development of HCC.
In the present study, we investigated the expression
of syntenin in HCC cell lines and explored its role in HCC cell
proliferation and invasion. Furthermore, the underlying molecular
mechanisms were also examined.
Materials and methods
Antibodies and reagents
SB203580 (p38 MAPK inhibitor) was obtained from
Sigma (St. Louis, MO, USA). Mouse anti-p38 and phospho-p38
antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA,
USA). Antibodies specific for syntenin (sc-100336) were purchased
from Santa Cruz Biotechnology. Mouse anti-Ki67 monoclonal
antibodies were from Sigma. The corresponding horseradish
peroxidase-conjugated secondary antibodies were obtained from
Calbiochem (La Jolla, CA, USA).
Cell culture
The human liver cancer cell lines HepG2, MHCC97-L,
MHCC97-H and HCCLM3 were obtained from the American Type Culture
Collection (ATCC; Rockville, MD, USA). Cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal
bovine serum, 100 μg/ml streptomycin and penicillin. Immortalized
normal liver epithelial cells THLE3 were also purchased from ATCC
and were maintained in bronchial epithelial growth medium,
supplemented with 5 ng/ml epithelial growth factor, 70 ng/ml
phosphoethanolamine and 10% fetal bovine serum. All cells were
cultured in a humidified atmosphere at 37°C with 5%
CO2.
Real-time PCR
Total RNA was isolated from human hepatoma cells
HepG2 with the RNAiso Plus kit (Roche Diagnostics, Mannheim,
Germany), and was then reverse-transcribed to synthesize first
strand cDNA using the cDNA Synthesis kit (Fermentas). Then, ~4 μl
cDNA was subjected to real-time PCR with specific primers for
syntenin (sense, 5′-GAA TCC TGC AAA AAT GTC TC-3′ and antisense,
5′-GCC ATG GTG CCG TGA ATT TTA-3′); EGFR (sense, 5′-TCG GGG AGC AGC
GAT GCG AC-3′ and antisense, 5′-TCA TGT GAT AAT TCA GCT C-3′); and
MMP-2 (sense, 5′-CAG GGA GCG CTA CGA TGG A-3′ and antisense, 5′-GAG
CCA GGG CCA GCT CAG C-3′). Each reaction consisted of 10 μl
SYBR® Premix Ex Taq™ II, 10 μmol/l specific
primers, 4 μl of DNA and H2O to a final volume of 20 μl.
The reaction conditions were introduced according to the
instructions of SYBR Premix Ex Taq™ II kit (Takara Bio Inc.,
Otsu, Japan). For normalization, β-actin mRNA was used. All samples
were performed in triplicate, and results were calculated according
to the 2−ΔΔCt method.
Construction of syntenin expression
vectors and stable transfection
To obtain the syntenin expression vectors, the
syntenin cDNA was digested with BamHI and XhoI
restriction enzymes, and was then ligated into the BamHI and
XhoI cloning site of the pcDNA3.1(+) vector (Invitrogen) to
induce syntenin expression. All sequences were confirmed by DNA
sequencing. Transfection was performed using 8 μl of Lipofectamine
2000 (Invitrogen) and 15 μg of pcDNA3.1-syntenin or pcDNA3.1 empty
vector in HCCLM3 cells. Forty-eight hours after transfection, 400
μg/ml of G418 was used to select the stable transfectants. The
empty vector was packaged as a negative control. A limiting
dilution was introduced to produce several stable
syntenin-expressing clones.
RNA interference against EGFR
To knock down EGFR expression in HCCLM3 cells, the
specific siRNA fragments of EGFR and control siRNA were designed as
previously described (15). The
siRNA strands were synthesized by Shanghai Sangon Co., Ltd.
(Shanghai, China). For transfection, HCCLM3 cells were seeded in
24-well microplates to reach 40–50% confluence. Then, cells were
transfected with 2 μg/ml EGFR siRNA and 1 ml Lipofectamine™
RNAi-MAX using the GeneSilencer® siRNA Transfection
Reagent (GeneTherapy System, San Diego, CA, USA). Approximately 24
h later, the transfection efficiency was analyzed by western
blotting as described above. Cell viability was evaluated by
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay.
Proliferation assays
Cell proliferation was assessed by MTT assay.
Briefly, cells were seeded into 96-well culture plates at a density
of 1×105 cells/well. After incubation for 24 h, the
culture medium was replaced with fresh medium containing 500 μg/ml
MTT (Sigma) for an additional 5 h at 37°C and 5% CO2.
Then, the supernatant was replaced with 200 μl isopropanol to
dissolve formazan production and the absorbance was measured at 570
nm on a microplate reader (Bio-Rad, Hercules, CA, USA).
Colony formation assay
HCCLM3 cells transfected with syntenin were plated
in 0.35% agar mixed culture medium in 12-well plates. After 12
days, cells were washed with phosphate-buffered saline (PBS) three
times, and were then fixed with methanol. Cell colony formation in
soft agar was analyzed by counting the number of colonies under a
stereoscopic microscope in triplicate of a 12-well plate. The
results are expressed as percentage control of colonies.
Invasion assay
The ability of cells to invade through
Matrigel-coated filters was measured by a modified Boyden chamber
(BD Biosciences, Bedford, MA, USA). Briefly,
syntenin-over-expressed HCCLM3 cells were pre-treated with
anti-MMP-2 antibody for 4 h, and were then seeded at a density of
3.0×104 cells in the upper compartment. DMEM medium
containing 10% fetal bovine serum was added to the lower
compartment. After incubation for 48 h at 37°C, cells were stained
with hematoxylin and eosin (Sigma), and counted in six randomly
selected high-powered fields in the center of each well. Each
experiment was repeated three times.
Western blotting
Following rinses with PBS three times, cells were
homogenized and lysed with RIPA lysis buffer (100 mM NaCl, 50 mM
Tris-HCl pH 7.5, 1% Triton X-100, 1 mM EDTA, 10 mM
b-glycerophosphate, 2 mM sodium vanadate and protease inhibitor) to
extract the total protein, and then a BCA assay (Pierce, Rockford,
IL, USA) was introduced to quantify the protein concentrations.
Following electrophoresis by SDS-PAGE, the obtained protein was
transferred onto a polyvinylidene difluoride (PVDF) membrane in a
semi-dry transblot apparatus. To perform the western blot assay,
non-specific binding was blocked by incubating with 5% non-fat milk
in TBST buffer at room temperature at 4°C overnight. Then,
immune-detection of syntenin, EGFR, Ki67, MMP-2, p-p38 MAPK and p38
MAPK was performed using specific antibodies against them for 1 h
at 37°C, followed by incubation with HRP-conjugated secondary
antibodies for 1 h. To visualize the bound antibodies, the LumiGLO
reagent (KPL, Gaithersburg, MD, USA) was used. The protein
expression levels were normalized by β-actin.
Statistical analysis
The Student’s t-test was used to evaluate
statistically significant differences between two groups in all
relevant experiments. All numerical results were analyzed by SPSS
11.0 and are presented as means ± SD. A P-value <0.05 was
considered to indicate a statistically significant result.
Results
Expression of syntenin is increased in
HCC cell lines
Syntenin has been confirmed to be upregulated in
several cancers and is critical for the development of cancer
(9,13). However, research regarding syntenin
in HCC remains to be performed. To address this, we analyzed the
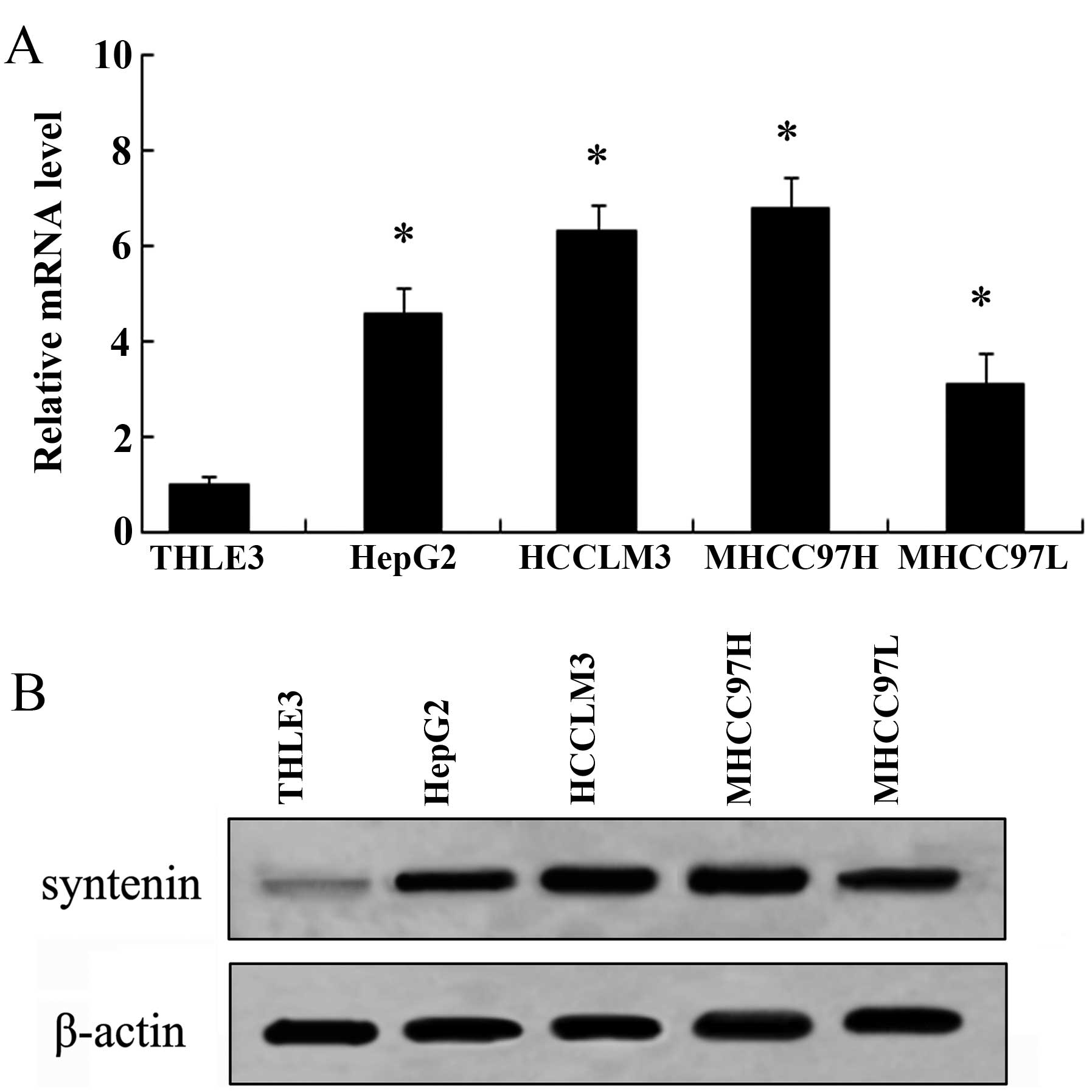
expression of syntenin in various HCC cell lines. Compared with
normal liver epithelial cells THLE3, an apparent increase in
syntenin mRNA levels was observed in all four HCC cell lines
including HepG2, MHCC97-L, MHCC97-H and HCCLM3 by RT-PCR analysis
(Fig. 1A). Furthermore, western
blot analysis demonstrated that the expression levels of syntenin
were also markedly upregulated in these four HCC cell lines,
compared with THLE3 cells (Fig.
1B). Moreover, the mRNA and protein levels of syntenin in the
high metastatic potential cell line MHCC97-H were higher than in
the low-invasive cell line MHCC97-L, indicating a potential
correlation between syntenin expression and the metastatic ability
of HCC cell lines. Collectively, these results corroborated that
syntenin is upregulated in HCC cells, particularly in higher
metastatic cells.
Overexpression of syntenin enhances
HCCLM3 cell proliferation in vitro
To clarify the role of syntenin in the development
and progression of HCC, we assessed the effect of syntenin
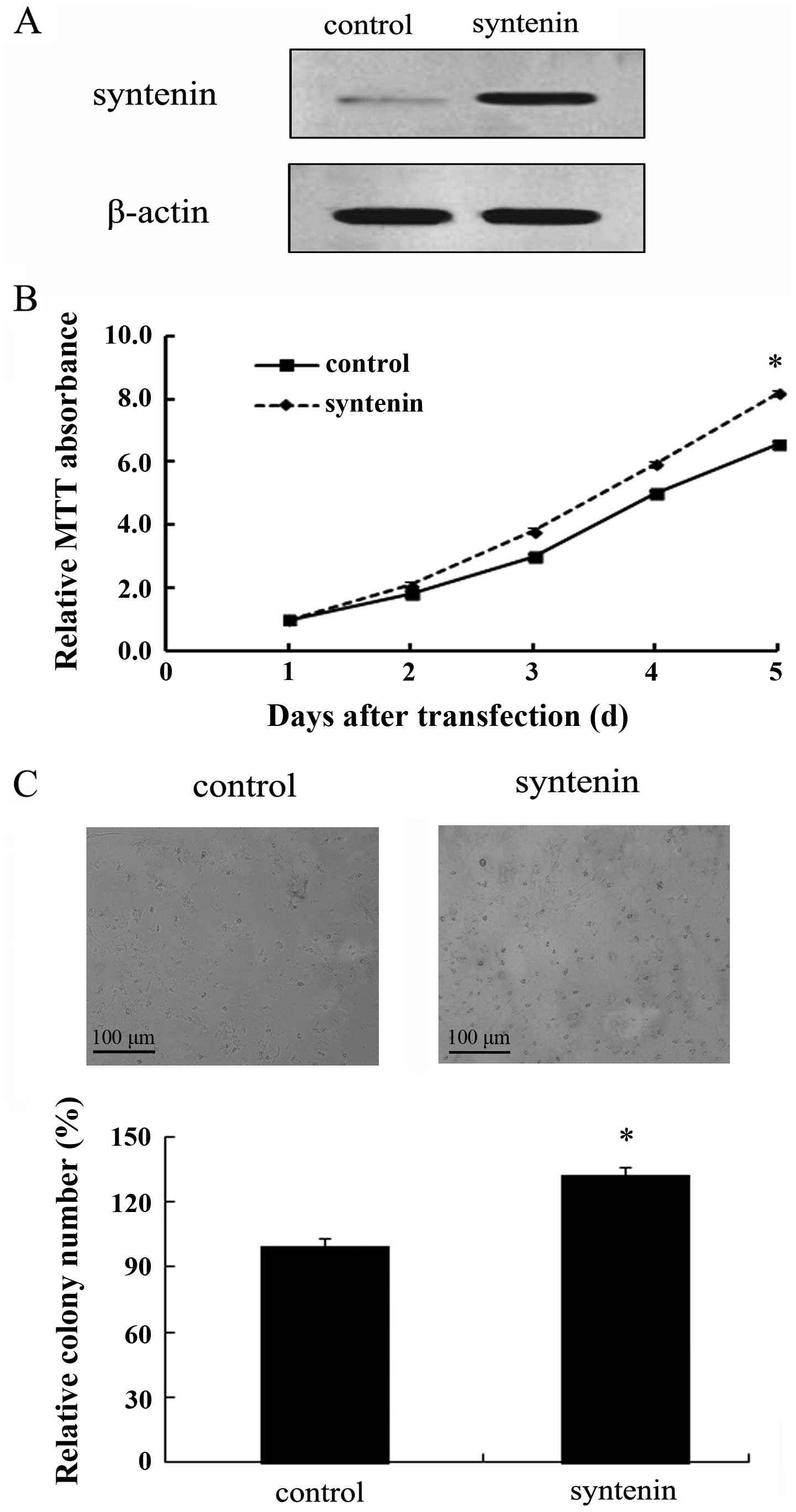
overexpression on HCCLM3 cell growth. To quantify the direct
contribution of syntenin in hepatoma, the recombinant
pcDNA3.1-syntenin was transfected into HCCLM3 cells, and then a
stable syntenin overexpression clone termed HCCLM3-150 cell line
was obtained. As shown in Fig. 2A,
a striking upregulation of syntenin protein was detected in the
HCCLM3-150 cell line, compared with the vector control clone. Based
on the stable expression of syntenin in HCCLM3-150 cells, we
evaluated syntenin function on cell growth by MTT and colony
formation assays. As shown in Fig.
2B, an apparent increase in cell proliferation was observed as
the gradually increased transfection times in
syntenin-overexpressed group, compared with the control group.
Furthermore, syntenin overexpression displayed more colonies in
contrast to controls (Fig. 2C).
Therefore, these results showed that syntenin transfection strongly
promotes cell proliferation.
Syntenin enhances hepatoma cell
proliferation in an EGFR-dependent manner
It is generally believed that EGFR is overexpressed
in several cancers, including colorectal cancer, HCC and urothelial
carcinoma, and can activate a cascade of multiple signaling
pathways that facilitate the tumor growth process (16–18).
The fact that syntenin can promote hepatoma cell proliferation was
demonstrated above. However, the precise molecular mechanism of
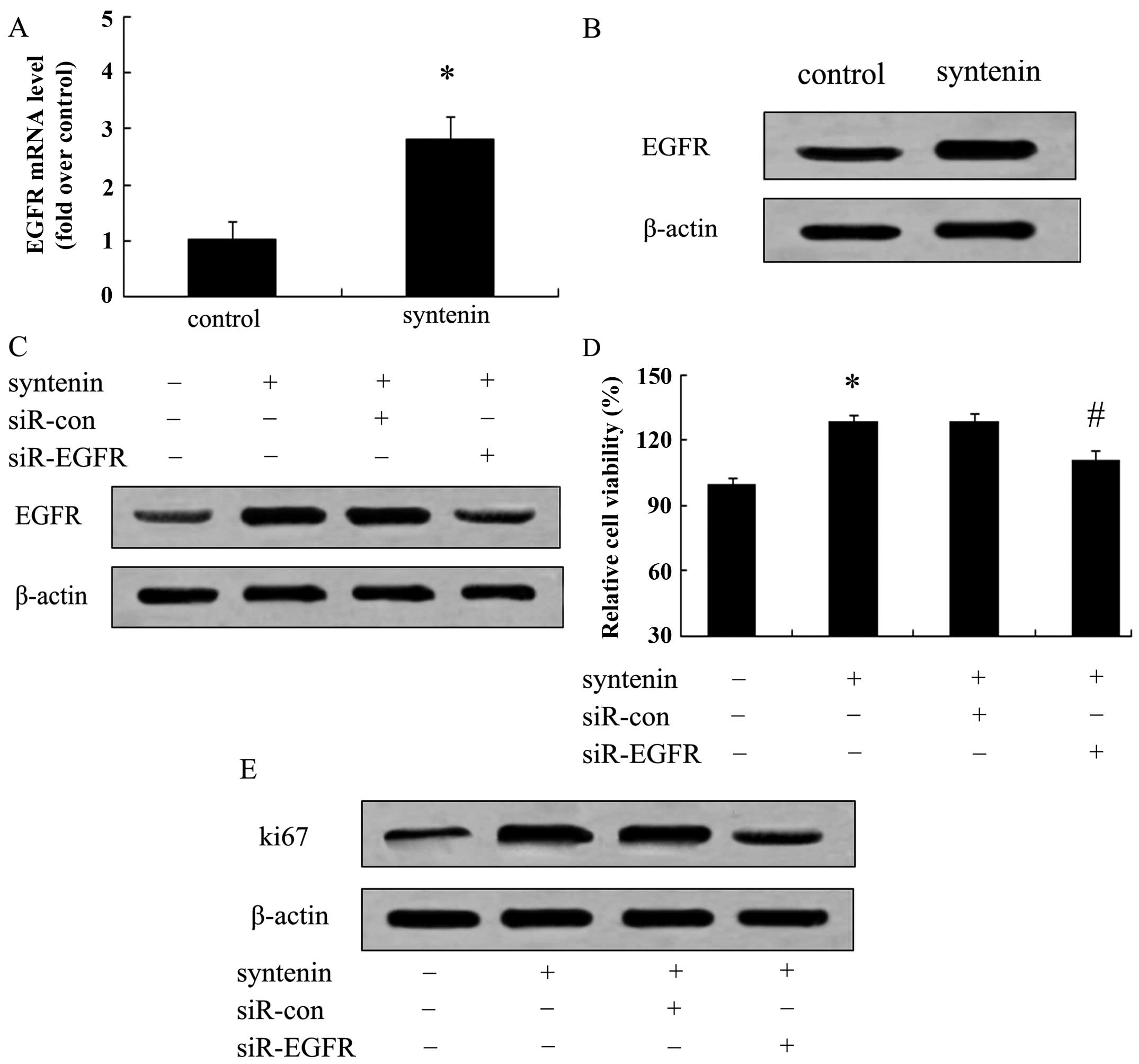
this action remains unclear. Hence, we attempted to link EGFR to
syntenin-induced cell proliferation mechanistically. When cells
were stably transfected with syntenin, the mRNA levels of EGFR were
2.7-fold over controls, indicating that elevated syntenin
expression strongly upregulated EGFR mRNA levels (Fig. 3A). Moreover, a similar upregulation
of EGFR protein levels was also ascertained by western blot assay
(Fig. 3B). Further mechanistic
analysis corroborated that EGFR silencing significantly reduced
cell viability induced by syntenin overexpression (Fig. 3C). Consistently, the expression
levels of proliferation marker Ki67 were markedly attenuated when
blocking EGFR expression in HCCLM3-150 cells (Fig. 3D). Taken together, our data
demonstrated that syntenin overexpression promotes hepatoma cell
proliferation predominantly through the EGFR pathway.
Elevated syntenin expression stimulates
hepatoma cell invasion by MMP-2
To further assess the function of syntenin in
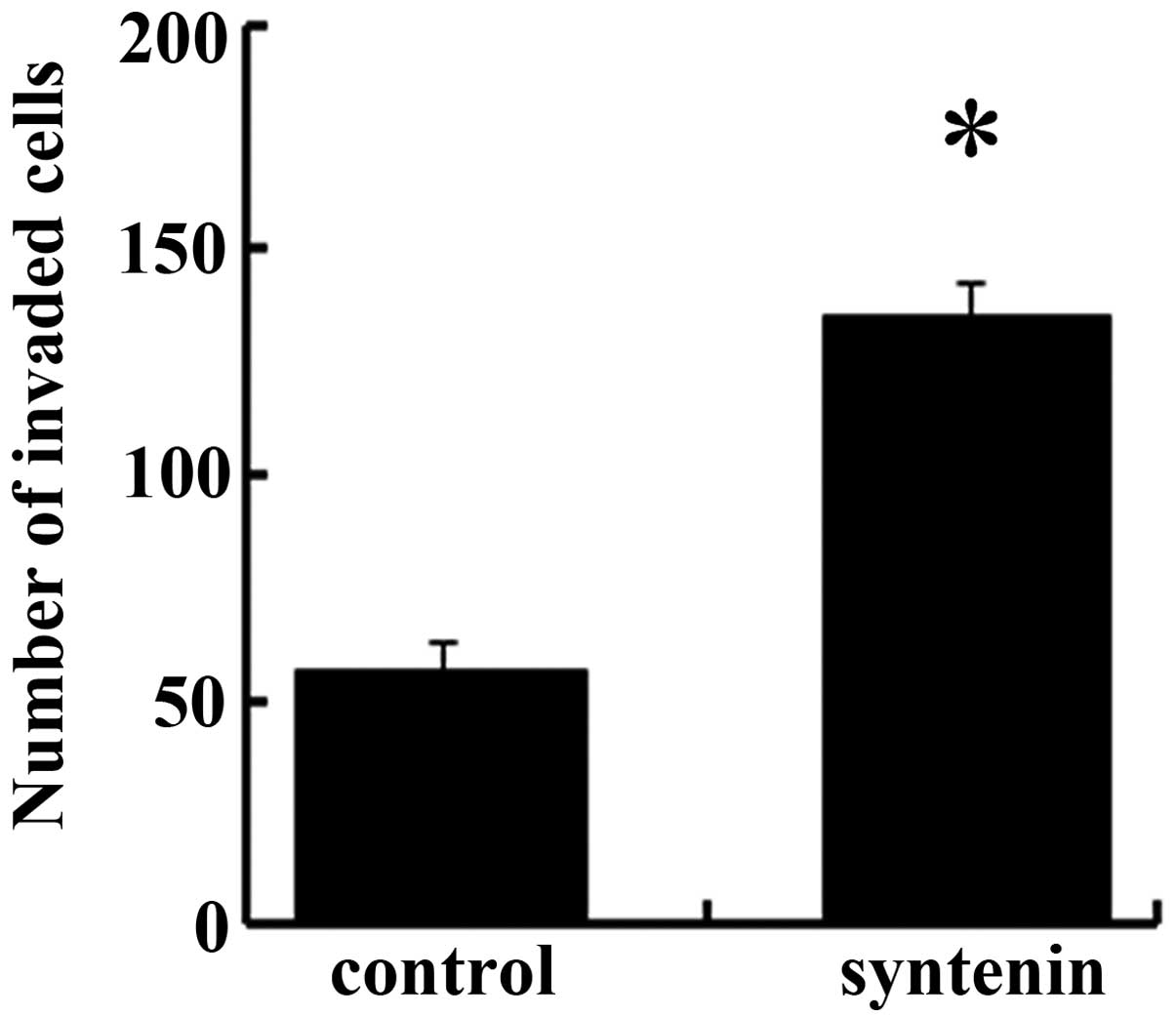
hepatoma cells, we detected cell invasion ability in HCCLM-150,
which stably overexpressed syntenin. As shown in Fig. 4, the forced syntenin expression
notably upregulated the number of invaded cells from 56 to 135
compared to the control groups, indicating it is a positive
regulator for HCC cell invasion. It is known that MMP-2 can degrade
extracellular matrix to facilitate tumor cell invasion (19). Thus, we linked the MMP-2 to
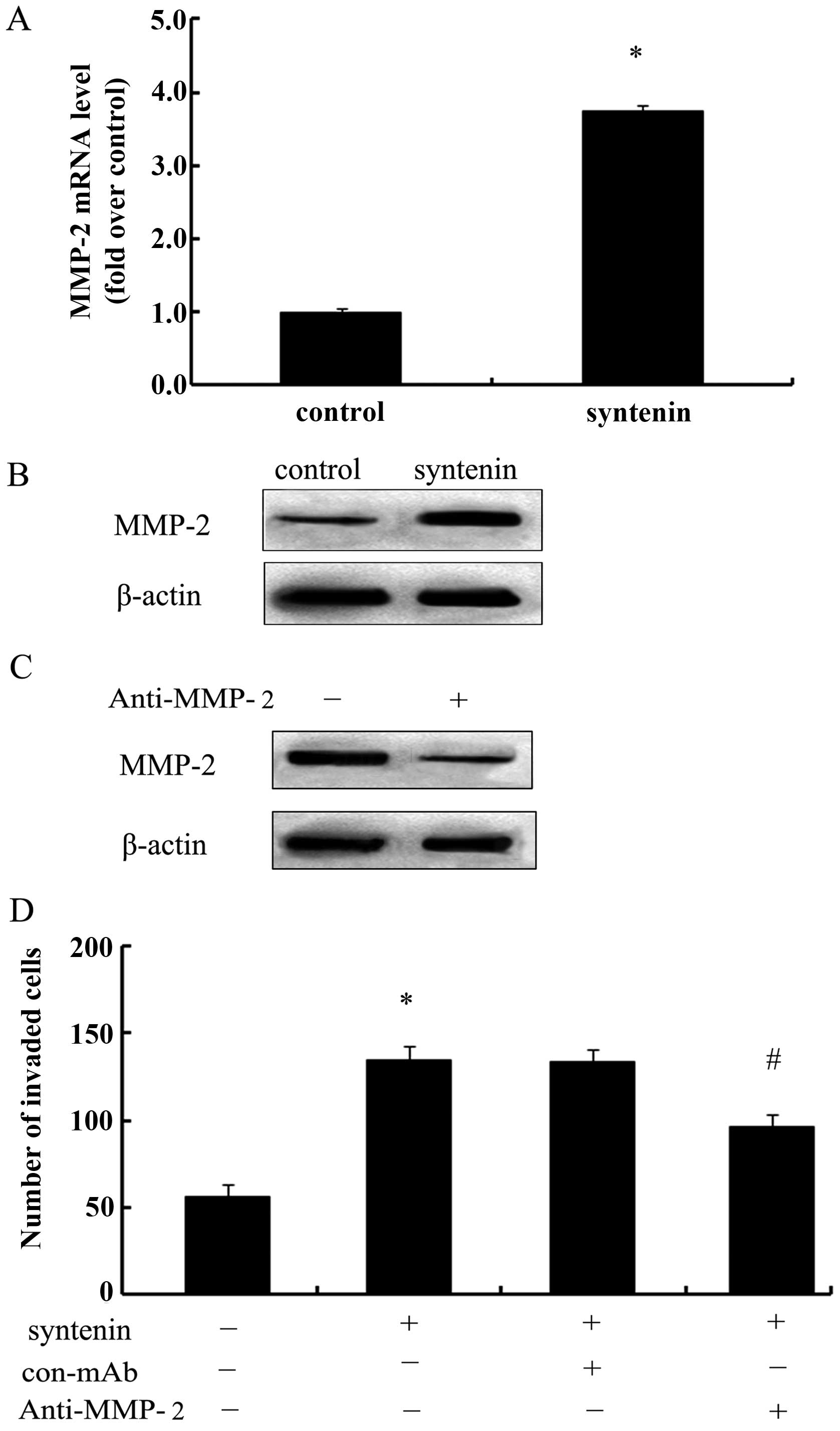
syntenin-induced cell invasion. Following transfection of syntenin
in HCCLM3 cells, the expression levels of MMP-2 mRNA were clearly
increased and were 3.75-fold times higher than those of the
controls (Fig. 5A). Consistent with
the above observation, syntenin overexpression significantly
enhanced the expression of MMP-2 protein (Fig. 5B). To further analyze the underlying
mechanism involved in syntenin-induced cell invasion, we silenced
the expression levels of MMP-2 with its specific antibody and a
notable downregulation of MMP-2 levels was observed in HCCLM3-150
cells (Fig. 5C). Also, MMP-2
silencing clearly abrogated syntenin-induced cell invasion number
(Fig. 5D). Hence, these data
confirmed that syntenin enhances cell invasion ability in an
MMP-2-dependent manner.
Syntenin-induced MMP-2 expression is
regulated by the p38 MAPK pathway
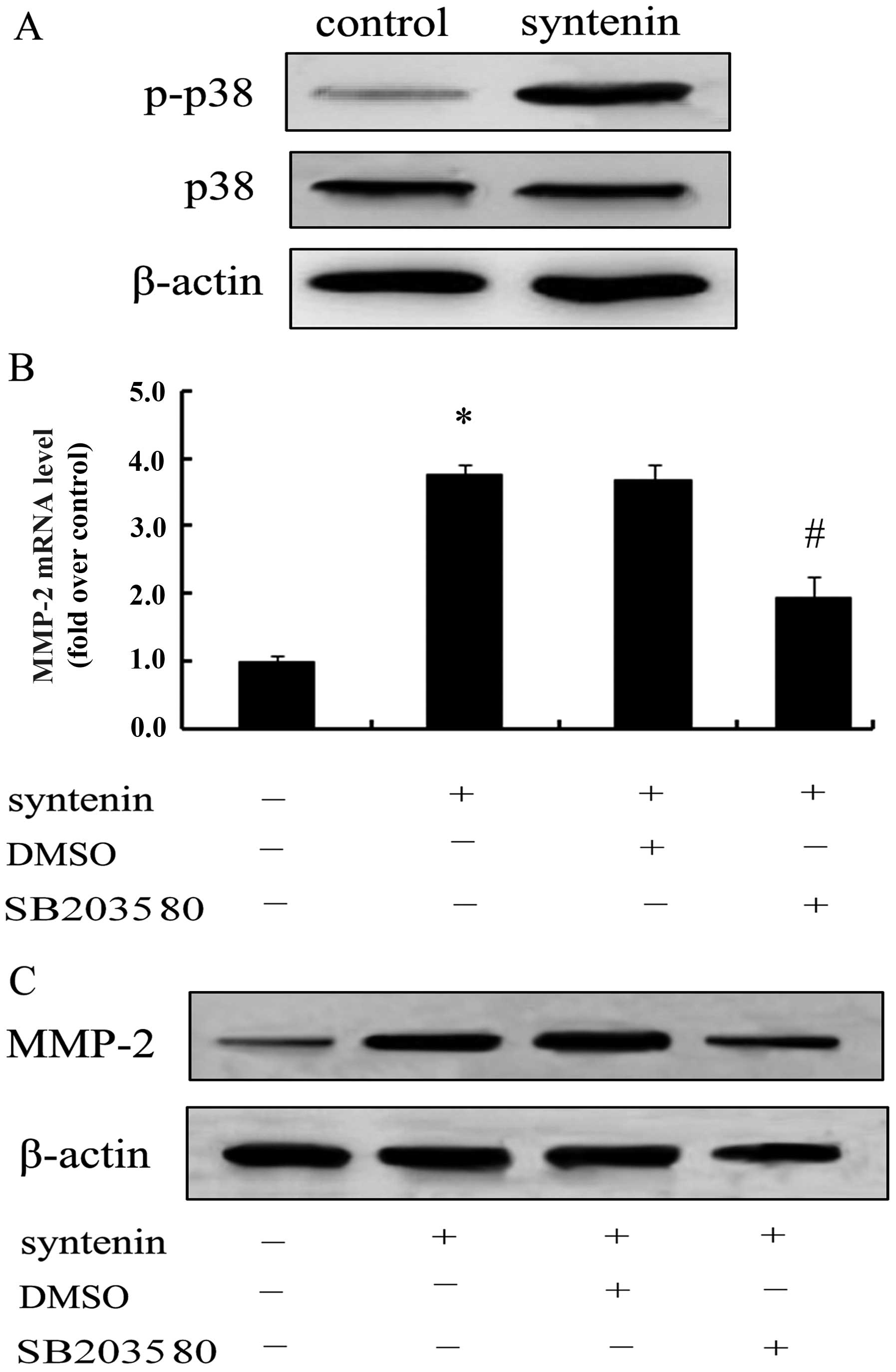
To further clarify the mechanism associated with
syntenin-triggered hepatoma cell invasion through MMP-2, we
evaluated the activation of p38 MAPK. Only slight phosphor-p38 MAPK
was observed in the control group; however, when cells
overexpressed syntenin, the expression of p-p38 MAPK was
significantly increased, suggesting that syntenin overexpression
induced the activation of the p38 MAPK pathway (Fig. 6A). Notably, the specific inhibitor
of p38 MAPK, SB203580, strikingly blocked syntenin-induced MMP-2
mRNA levels (Fig. 6B), concomitant
with the reduction of MMP-2 protein levels (Fig. 6C). These results revealed that
syntenin promotes cell invasion via MMP-2 expression through the
activation of the p38 MAPK pathway.
Discussion
Hepatocellular carcinoma (HCC) is the sixth most
common malignancy, resulting in more than 560,000 deaths per year,
and it is the third leading cause of tumor-related mortality around
the world (1,2,20). The
present study demonstrated that syntenin was markedly upregulated
in several HCC cell lines compared with its expression in normal
liver epithelial THLE3 cells. Moreover, syntenin upregulation
promoted HCCLM3 cell proliferation by epidermal growth factor
receptor (EGFR) signaling. Notably, syntenin overexpression
enhanced hepatoma cell invasion through the activation of the p38
MAPK pathway. These findings suggest that syntenin may play an
important role in the development and progression of hepatoma.
Syntenin is also known as mda-9 and was originally
cloned from human melanoma cells. As a PDZ domain-containing
molecule, syntenin can exert multiple roles and regulate diverse
and central physiologic processes (8,9). The
function of syntenin in cancer has attracted increasing attention
for its important roles in the development of various cancers,
including melanoma, breast and bladder cancer (10,12,13).
It has been confirmed that syntenin is highly expressed in several
cancer cells and tissues, and it is involved in the progression of
cancer, including cancer cell growth, invasion and metastasis
(9,21,22).
However, its function in the development of hepatoma remains
unclear. In the present study, we firstly confirmed a similar high
expression of syntenin mRNA and protein levels in HCC cells.
Moreover, cell proliferation ability and clone formation ability
were strongly elevated after stable expression of syntenin in
HCCLM3 cells, indicating a critical role of syntenin in hepatoma
cell growth. However, the underlying mechanism remains
undefined.
EGFR belongs to the ErbB proto-oncogene receptors
family (ErbB tyrosine kinase receptors), and its cognate ligand has
been considered as a common component of various cancer types to
induce tumor growth (23,24). EGFR overexpression has been
confirmed in multiple carcinomas including breast cancer,
urothelial cell carcinoma and hepatoma (10,16).
It is believed that EGFR can trigger a cascade of multiple
signaling pathways to regulate cancer cell proliferation,
maintenance, invasion, metastasis, survival and prognosis (16,17,25,26).
Furthermore, EGFR elicits a vital role in MDA-9/syntenin-induced
urothelial carcinoma cell proliferation (14). Accordingly, to further corroborate
the molecular mechanism underlying syntenin-induced HCC cell
proliferation, we analyzed the expression levels of EGFR. When
syntenin was stably transfected into HCCLM cells, the expression
levels of EGFR mRNA and protein were notably upregulated. More
importantly, blocking its expression with specific siRNA markedly
attenuated syntenin-triggered cell proliferation as well as the
expression of proliferation marker Ki67, suggesting a pivotal
function of EGFR in syntenin-regulated hepatoma cell
proliferation.
The high invasiveness is critical for tumor
progression and is known to be a vital contributor to morbidity and
mortality in cancer (27). The fact
that a higher expression of syntenin was observed in the high
metastatic potential cell line MHCC97-H, compared with the
low-invasive cell line MHCC97-L, indicated a potential role in HCC
cell invasion and metastasis. To better understand the roles of
syntenin in the development of hepatoma, we further assessed the
effect of syntenin overexpression on cell invasion. As expected,
syntenin upregulation significantly enhanced hepatoma cell
invasion. Matrix metal-loproteinases (MMPs) are known to possess an
indispensable role during the degradation of extracellular matrix
(ECM), a crucial step in tumor invasion and metastasis (28,29).
Among them, MMP-2 has exhibited an important role in hepatoma cell
adhesion and invasion (30,31). To clarify the underlying mechanism
involved in syntenin-induced cell invasion, we well linked MMP-2
together. In the present study, syntenin over-expression enhanced
the expression levels of MMP-2 mRNA and protein. When silencing its
expression with anti-MMP-2 antibody, syntenin-triggered hepatoma
cell invasion ability was clearly abrogated, indicating that
syntenin may enhance cell invasion by MMP-2 expression.
P38 MAPK pathways can trigger a cascade of
responses, from cell growth and proliferation to motility and
invasion, which drive tumor development and progression (32,33).
To further elucidate the exact molecular mechanism involved in
syntenin-induced cancer cell invasion via MMP-2 expression, the
phosphor-p38 MAPK was explored. After overexpression of syntenin,
the expression levels of p-p38 MAPK were significantly enhanced,
indicating a pivotal effect of syntenin on the activation of the
p38 MAPK pathway. Following pretreatment with SB203580,
syntenin-induced MMP-2 expression levels were significantly
reduced. Accordingly, we can conclude that syntenin may promote the
invasiveness of hepatoma cells by MMP-2 expression through the p38
MAPK pathway.
In conclusion, we confirmed the upregulation of
syntenin in hepatoma cells. In the present study, syntenin
overexpression enhanced hepatoma cell proliferation and invasion by
EGFR pathway and p38 MAPK signaling, which ultimately benefit
hepatoma development and progression. These findings provide
insight into how syntenin accelerates the pathogenesis of hepatoma.
Therefore, syntenin may prove to be a promising therapeutic agent
against hepatoma. Further studies will focus on its function and
regulation mechanism on hepatoma progression in vivo.
Furthermore, its PDZ domain function in the development of hepatoma
will also be investigated in a forthcoming study.
Acknowledgements
Financial support was provided by the National
Natural Science Foundation of China (NSFC) (no. 81101873).
References
|
1
|
Jelic S and Sotiropoulos GC; ESMO
Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21:v59–v64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGlynn KA and London WT: International
liver cancer incidence trends - letter. Cancer Epidemiol Biomarkers
Prev. 21:384–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: a global and regional perspective. Oncologist. 15:5–13.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarkar D, Boukerche H, Su Z-Z and Fisher
PB: mda-9/syntenin: recent insights into a novel cell
signaling and metastasis-associated gene. Pharmacol Ther.
104:101–115. 2004. View Article : Google Scholar
|
|
7
|
Das SK, Bhutia SK, Sokhi UK, et al: Raf
kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis
in melanoma. Cancer Res. 72:6217–6226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu Y and Schachner M: Syntenin-a promotes
spinal cord regeneration following injury in adult zebrafish. Eur J
Neurosci. 38:2280–2289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gangemi R, Mirisola V, Barisione G, et al:
Mda-9/syntenin is expressed in uveal melanoma and correlates with
metastatic progression. PLoS One. 7:e299892012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Hong Q, Shi P, Liu Z, Luo J and
Shao Z: Elevated expression of syntenin in breast cancer is
correlated with lymph node metastasis and poor patient survival.
Breast Cancer Res. 15:R502013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Das SK, Bhutia SK, Kegelman TP, et al:
MDA-9/syntenin: a positive gatekeeper of melanoma metastasis. Front
Biosci. 17:1–15. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boukerche H, Su ZZ, Prévot C, Sarkar D and
Fisher PB: mda-9/syntenin promotes metastasis in human
melanoma cells by activating c-Src. Proc Natl Acad Sci.
105:15914–15919. 2008. View Article : Google Scholar
|
|
13
|
Koo TH, Lee JJ, Kim EM, Kim KW, Kim HD and
Lee JH: Syntenin is overexpressed and promotes cell migration in
meta-static human breast and gastric cancer cell lines. Oncogene.
21:4080–4088. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dasgupta S, Menezes ME, Das SK, et al:
Novel role of MDA-9/syntenin in regulating urothelial cell
proliferation by modulating EGFR signaling. Clin Cancer Res.
19:4621–4633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han W, Pan H, Chen Y, et al: EGFR tyrosine
kinase inhibitors activate autophagy as a cytoprotective response
in human lung cancer cells. PLoS One. 6:e186912011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spano J, Fagard R, Soria JC, Rixe O,
Khayat D and Milano G: Epidermal growth factor receptor signaling
in colorectal cancer: preclinical data and therapeutic
perspectives. Ann Oncol. 16:189–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kannangai R, Sahin F and Torbenson MS:
EGFR is phosphorylated at Ty845 in hepatocellular carcinoma. Mod
Pathol. 19:1456–1461. 2006.PubMed/NCBI
|
|
18
|
Mitsudomi T and Yatabe Y: Epidermal growth
factor receptor in relation to tumor development: EGFR gene
and cancer. FEBS J. 277:301–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Lu N, Niu B, Chen X, Xie J and
Cheng N: Overexpression of Aurora-A enhances invasion and matrix
metalloproteinase-2 expression in esophageal squamous cell
carcinoma cells. Mol Cancer Res. 10:588–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim H and Lim HY: Novel EGFR-TK inhibitor
EKB-569 inhibits hepatocellular carcinoma cell proliferation by AKT
and MAPK pathways. J Korean Med Sci. 26:1563–1568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boukerche H, Aissaoui H, Prévost C, et al:
OC-13 enhanced expression of Mda-9/syntenin induced through the
tissue-factor pathway in melanoma promotes tumor cell migration and
invasion. Thromb Res. 125(Suppl 2): S1642010. View Article : Google Scholar
|
|
22
|
Kim WY, Jang JY, Jeon YK, Chung DH, Kim YG
and Kim CW: Syntenin increases the invasiveness of small cell lung
cancer cells by activating p38, AKT, focal adhesion kinase and SP1.
Exp Mol Med. 46:e902014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bazley LA and Gullick WJ: The epidermal
growth factor receptor family. Endocr Relat Cancer. 12(Suppl 12):
S17–S27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
da Cunha Santos G, Shepherd FA and Tsao
MS: EGFR mutations and lung cancer. Annu Rev Pathol. 6:49–69.
2011.
|
|
25
|
Hirsch FR, Varella-Garcia M and Cappuzzo
F: Predictive value of EGFR and HER2 overexpression in advanced
non-small-cell lung cancer. Oncogene. 28:S32–S37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37:9–15. 2001. View Article : Google Scholar
|
|
27
|
Duffy MJ, McGowan PM and Gallagher WM:
Cancer invasion and metastasis: changing views. J Pathol.
214:283–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lara-Pezzi E, Gómez-Gaviro MV, Gálvez BG,
et al: The hepatitis B virus X protein promotes tumor cell invasion
by inducing membrane-type matrix metalloproteinase-1 and
cyclooxygenase-2 expression. J Clin Invest. 110:1831–1838. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giannelli G, Bergamini C, Marinosci F, et
al: Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular
carcinoma. Int J Cancer. 97:425–431. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
del Barco Barrantes I and Nebreda A: Roles
of p38 MAPKs in invasion and metastasis. Biochem Soc Trans.
40:79–84. 2012.PubMed/NCBI
|