Introduction
Breast cancer is the most common type of non-skin
human malignancy and the second leading cause of cancer-related
mortality among women in Western countries, accounting for 23% of
total cancer cases and 14% of cancer-related deaths. The incidence
of breast cancer is the highest in North America and Europe and the
lowest in Asia. However, the incidence of and mortality from breast
cancer in poorly developed countries are expected to increase
considering the trend in westernization of living (1,2).
Although several etiological factors are associated with the
occurrence of breast cancer, type of diet, hormone-associated
reproductive factors, obesity and atypical hyperplasia of the
mammary gland are the most prevalent. Approximately 50% of breast
cancers arise in the absence of known common risk factors, and
surgery remains the primary treatment with chemotherapy and
radiotherapy used as adjuvants (3,4).
Despite the extensive use of multimodal therapies, breast cancer
remains the leading cause of cancer-related mortality among women
in the world, emphasizing the need for novel therapeutic
approaches. To overcome these challenges, novel therapeutic
strategies are required for more effective treatment of this
serious disease.
Apoptosis, or programmed cell death, is an essential
tool for the body to eliminate excess, damaged or harmful cells. In
general, cells undergo apoptosis through the extrinsic (death
receptor-mediated) and intrinsic (mitochondrial-mediated) pathways
(5,6). The extrinsic pathway is triggered by
binding of the cell surface death receptors with their relevant
ligands, which leads to activation of caspase-8 and/or -10. During
the intrinsic apoptotic pathway, internal and external stressors
lead to the release of apoptogenic proteins from the mitochondria,
which along with Apaf-1, activate caspase-9. Caspase-8, -9 and -10
are the initiator caspases, as they initiate activation of the
effector caspase-3 and -7, triggering the apoptotic caspase cascade
(6,7). Caspase-8 alone is sufficient in some
cases to activate caspase-3 in response to receptor-mediated
apoptotic stimuli, however caspase-8 requires the assistance of the
intrinsic apoptotic pathway for enhancing apoptotic stimuli. This
can be achieved through caspase-8-mediated proteolytic activation
of the pro-apoptotic Bcl-2 homology domain (BH)3-only protein
BH3-interacting domain death agonist (Bid), which then causes
mitochondrial outer membrane permeabilization and leads to
mitochondrial release of apoptogenic proteins including cytochrome
c (8,9). During the past decade, accumulating
evidence has suggested that many cancer therapeutic agents induce
apoptosis. Although the anticancer agent mechanisms of the
apoptotic pathway are controversial, the killing of cancer cells
through the induction of apoptosis has been recognized as a novel
strategy for identifying chemotherapeutic as well as
chemopreventive agents (10–12).
Cyperus rotundus Linn, a sedge in the
Cyperaceae family, is a well documented medicinal herb, with a
worldwide distribution in tropical and temperate regions. The tuber
part of C. rotundus is a commonly used traditional Oriental
medicine for treatment of dysmenorrhea and other menstrual
irregularities. This herb is also widely used as an analgesic,
nerve tonic, nootropic, sedative, anti-spasmodic, anti-malarial,
anti-diarrheal and a reliever of stomach disorders (13–17).
Previous pharmacological investigations indicated that the extracts
and/or components of C. rotundus rhizomes have marked
hypotensive, anti-mutagenic, anti-obese, neuroprotective,
antioxidant, anti-inflammatory and anti-pyretic effects (18–26).
In addition, this herb is often prescribed in combination with
other herbs to treat stomach ache, inflammation and peptic ulcer
disease (27,28). However, despite its valuable
effects, little is known about the biochemical basis of the
anticancer potential of C. rotundus. In the present study,
as part of our search for novel biologically active substances to
prevent and treat cancer from traditional medicinal resources, we
evaluated whether the extracts of C. rotundus rhizomes could
inhibit cell growth and trigger apoptosis in human breast carcinoma
cells.
Materials and methods
Reagents and antibodies
Fetal bovine serum (FBS), Dulbecco’s modified
Eagle’s medium (DMEM), penicillin, streptomycin and
trypsin-ethylenediaminetetraacetic acid (EDTA) were purchased from
Gibco-BRL (Gaithersburg, MD, USA).
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT), trypan blue, 4,6-diamidino-2-phenyllindile (DAPI), propidium
iodide (PI), paraformaldehyde,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
(JC-1) and N-acetyl L-cysteine (NAC) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The pan-caspase inhibitor
z-VAD-fmk and inhibitors of phosphatidylinositol 3-kinase
(PI3K)/Akt and mitogen-activated protein kinase (MAPK) were
obtained from Calbiochem (San Diego, CA, USA). DNA ladder size
markers were purchased from Invitrogen (Carlsbad, CA, USA).
2′,7′-Dichlorofluorescein diacetate (DCFDA) and
fluorescein-isothiocyanate (FITC)-Annexin V were purchased from
Molecular Probes (Eugene, OR, USA) and BD Pharmingen (San Diego,
CA, USA), respectively. Caspase activity assay kits and an enhanced
chemiluminescence (ECL) kit were purchased from R&D Systems
(Minneapolis, MN, USA) and Amersham (Arlington Heights, IL, USA),
respectively. Antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA), Chemicon (Temecula, CA, USA)
and Sigma-Aldrich. Peroxidase-labeled donkey anti-rabbit and sheep
anti-mouse immunoglobulin were purchased from Amersham. All other
chemicals were purchased from Sigma-Aldrich.
Preparation of C. rotundus rhizome
extracts
C. rotundus rhizomes were obtained from the
Dongeui Oriental Hospital, Dongeui University College of Oriental
Medicine, Busan, Republic of Korea. They were cut into small
pieces, ground to powder, passed through a 20-mesh sieve, and
transferred to a flask in a 1:10 proportion (drug: solvent, w/v),
using methanol, ethanol and water. The materials were protected
from light with aluminum foil, and the flasks were shaken at 50 rpm
for 24 h at 60°C. The extracts were filtered through Whatman paper
no. 1 and dried in a Rotavapor at 40°C, resulting in methanol
(MECR), ethanol (EECR) and water (WECR) extracts. The extracts were
weighed, resuspended into dimethyl sulfoxide to a final
concentration of 100 mg/ml (extract stock solution) and then
diluted with medium to the desired concentration prior to use.
Cell culture
The MDA-MB-231 human breast carcinoma cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and maintained in DMEM supplemented with 10% FBS, 1%
L-glutamine and penicillin/streptomycin. The cells were cultured in
an incubator with 5% CO2 at 37°C.
Cell growth and cell viability assay
Cell growth was assessed using the trypan blue dye
exclusion assay. In brief, cells (2×104 cells/well) were
seeded in 6-well plates. After treatment with the indicated
concentrations of the C. rotundus extracts for 24 h, the
cells were trypsinized and viable cells were counted by trypan blue
dye exclusion using a hemocytometer under an inverted microscope
(Carl Zeiss, Jena, Germany). Cell viability was determined using
the MTT assay. In brief, cells (2×104 cells/well) were
seeded in 24-well plates and exposed to the extracts of C.
rotundus for 24 h. After treatment, 5 mg/ml MTT solution was
added, followed by a 3-h incubation at 37°C in the dark. Absorbance
of the formazan product was measured at a wavelength of 540 nm with
an enzyme-linked immunosorbent assay (ELISA) reader (Molecular
Devices, Sunnyvale, CA, USA). Cells were photographed directly for
a morphological study using an inverted microscope.
DAPI nuclear staining
The cells were washed with phosphate-buffered saline
(PBS) and fixed with 3.7% paraformaldehyde in PBS for 10 min at
room temperature. The fixed cells were permeabilized with 0.1%
Triton X-100 for 10 min, the supernatant was removed and the cells
were washed three times with PBS. The cells were incubated with 2.5
μg/ml DAPI for 10 min at room temperature in the dark. The cells
were then washed twice with PBS and DAPI-stained cell nuclei
(apoptotic nuclei stained intensely) and were observed with a
fluorescence microscope (Carl Zeiss).
Agarose gel electrophoresis
Cells were lysed in a buffer containing 10 mM
Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA and 0.5% Triton X-100 for
1 h at room temperature for the DNA fragmentation assay. Lysates
were vortexed and cleared by centrifugation at 19,000 × g for 30
min at 4°C. A 25:24:1 (v/v/v) equal volume of neutral
phenol:chloroform:isoamyl alcohol was used to extract DNA from the
supernatant, followed by electrophoretic analysis on 1.5% agarose
gels containing 0.1 μg/ml ethidium bromide.
Flow cytometric detection of
apoptosis
A combination of Annexin V-FITC and PI staining
distinguishes between early (Annexin V+/PI−)
and late (Annexin V+/PI+) apoptotic, necrotic
and live cells. After the cells were treated with the EECR for 24
h, they were trypsinized, washed with PBS, and resuspended in 100
μl binding buffer containing 5 μl Annexin V-FITC and 5 μl PI for 15
min at room temperature in the dark. The cells were immediately
analyzed by flow cytometry (FACSCalibur; Becton-Dickinson, San
Jose, CA, USA). The percentages of apoptotic cells (Annexin
V+ cells) are presented as the means ± standard
deviation as previously described (29).
Protein extraction and western blot
analysis
The cells were collected by trypsin-EDTA and lysed
with lysis buffer (20 mM sucrose, 1 mM EDTA, 20 μM Tris-Cl, pH 7.2,
1 mM DTT, 10 mM KCl, 1.5 mM MgCl2, 5 μg/ml pepstatin A,
10 μg/ml leupeptin and 2 μg/ml aprotinin) containing protease
inhibitors for 30 min at 4°C. The mixtures were centrifuged (10,000
× g) for 10 min at 4°C, and the supernatants were collected as
whole-cell extracts. Protein content was determined using the
Bio-Rad protein assay reagent (Hercules, CA, USA) and bovine serum
albumin as the standard according to the manufacturer’s
instructions to determine protein concentrations. After
normalization, an equal amount of protein was subjected to
electrophoresis on sodium dodecyl sulfate-polyacrylamide gels and
transferred to nitrocellulose membranes (Schleicher & Schuell,
Keene, NH, USA) by electroblotting. The blots were blocked with 5%
skim milk for 1 h at room temperature. The blots were incubated
overnight with primary antibodies, followed by horseradish
peroxidase-conjugated donkey anti-rabbit and sheep anti-mouse
immunoglobulin for 1 h. The immunoreactive bands were revealed by
enhanced chemiluminescence with a commercially available ECL
kit.
Measurement of mitochondrial membrane
potential (MMP)
MMP values were determined using the dual-emission
potential-sensitive probe, JC-1. Briefly, cells were collected and
incubated with 10 μM JC-1 for 30 min at 37°C in the dark. After
JC-1 was removed, the cells were washed with PBS to remove unbound
dye, and the amount of JC-1 retained by 10,000 cells/sample was
measured at 488 and 575 nm using a flow cytometer.
Caspase activity assay
Caspase activities were determined with colorimetric
assay kits, which utilize synthetic tetrapeptides [Asp-Glu-Val-Asp
(DEAD) for caspase-3, Ile-Glu-Thr-Asp (IETD) for caspase-8 and
Leu-Glu-His-Asp (LEHD) for caspase-9] labeled with p-nitroaniline
(pNA). Briefly, EECR-treated and untreated cells were lysed in the
supplied lysis buffer. The supernatants were collected and
incubated with the supplied reaction buffer containing
dithiothreitol and DEAD-pNA, IETD-pNA or LEHD-pNA as substrates at
37°C. The reactions were measured by changes in absorbance at 405
nm using an ELISA reader.
Measurement of reactive oxygen species
(ROS)
ROS production was monitored using the stable
nonpolar dye DCFDA. The cells were seeded at a density of
1×105 cells/ml, allowed to attach for 24 h and exposed
to 200 μg EECR for various periods. The cells were incubated with
10 mM DCFDA for 20 min at 37°C in the dark. ROS production in the
cells was monitored with a flow cytometer using CellQuest Pro
software.
Statistical analysis
Data are reported as means ± standard deviation of
three independent experiments. A one-way analysis of variance was
performed to determine differences. p<0.05 was considered to
indicate a statistically significant difference.
Results
Antiproliferative activity of C. rotundus
rhizome extracts in MDA-MB-231 cells
To investigate the effects of C. rotundus
rhizome extracts on MDA-MB-231 cell growth, the cells were treated
with various concentrations of the MECR, EECR and WECR for 24 h,
and cell number and viability were measured by trypan blue
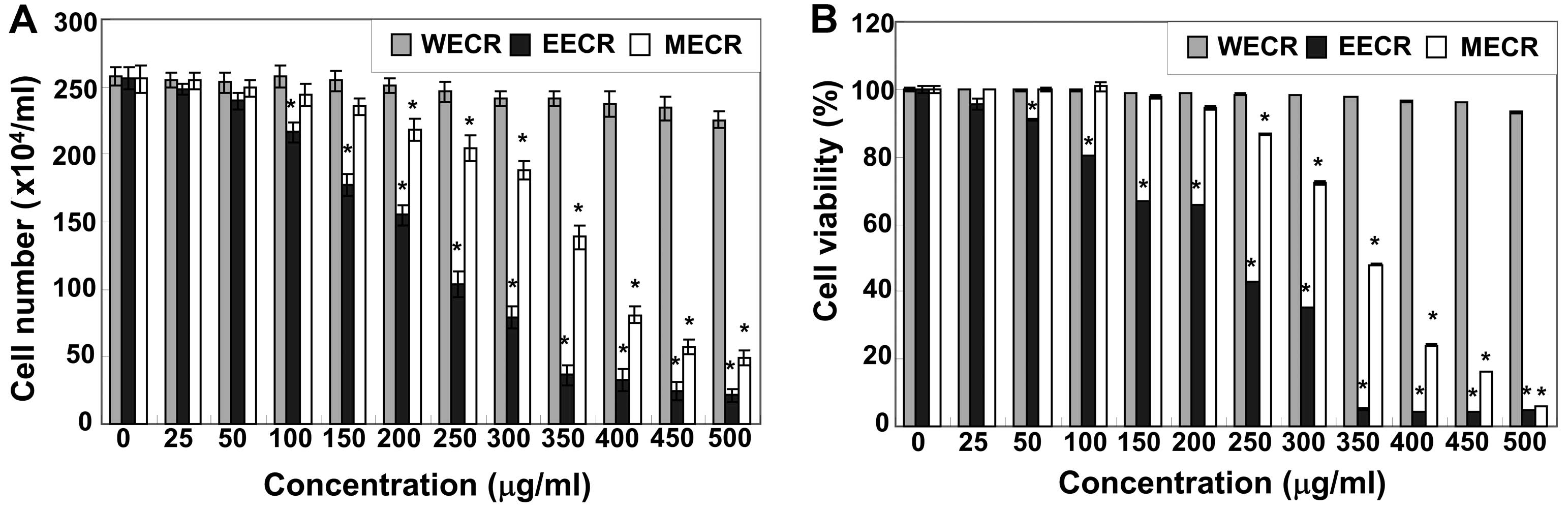
exclusion and the MTT assay, respectively. As shown in Fig. 1, the MECR and EECR markedly
inhibited cell proliferation and viability of MDA-MB-231 cells in a
concentration-dependent manner. For example, cell viability was
inhibited by >30 or 65% in cells exposed to 150 or 300 μg/ml of
the EECR, respectively, compared to that in untreated controls. In
contrast, the WECR was not cytotoxic under the same experimental
conditions. The EECR possessed stronger cytotoxicity than MECR;
thus, we used the EECR for further studies.
Induction of apoptosis in MDA-MB-231
cells by EECR treatment
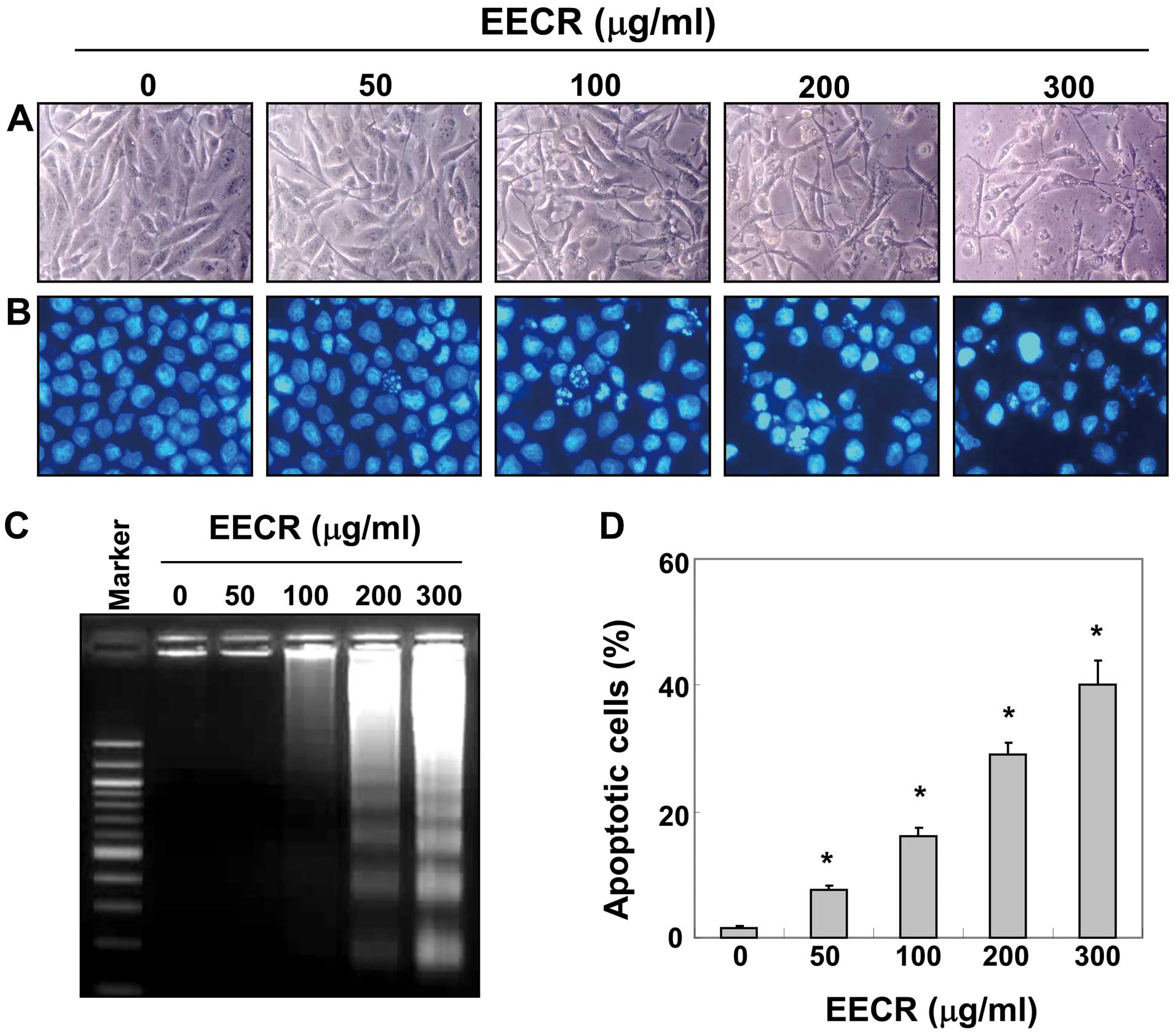
Cell morphology was assessed by DAPI staining. The
results indicated that the nuclear structure of control cells
remained intact, whereas nuclear chromatin condensation and
fragmentation, characteristics of apoptosis, were observed in cells
treated with the EECR and were associated with numerous cellular
morphological changes (Fig. 2A and
B). In particular, cell shrinkage and cytoplasm condensation
appeared in a dose-dependent manner after EECR treatment. In
addition, treatment with the EECR induced progressive accumulation
of fragmented DNA, which appeared as a typical ladder pattern of
DNA fragmentation due to internucleosomal cleavage associated with
apoptosis, in a concentration-dependent manner (Fig. 2C). Furthermore, we evaluated
apoptotic induction by flow cytometry analysis with Annexin V and
PI staining. As shown in Fig. 2D,
treatment with the EECR resulted in an increased accumulation of
apoptotic cells compared with that of untreated control cells.
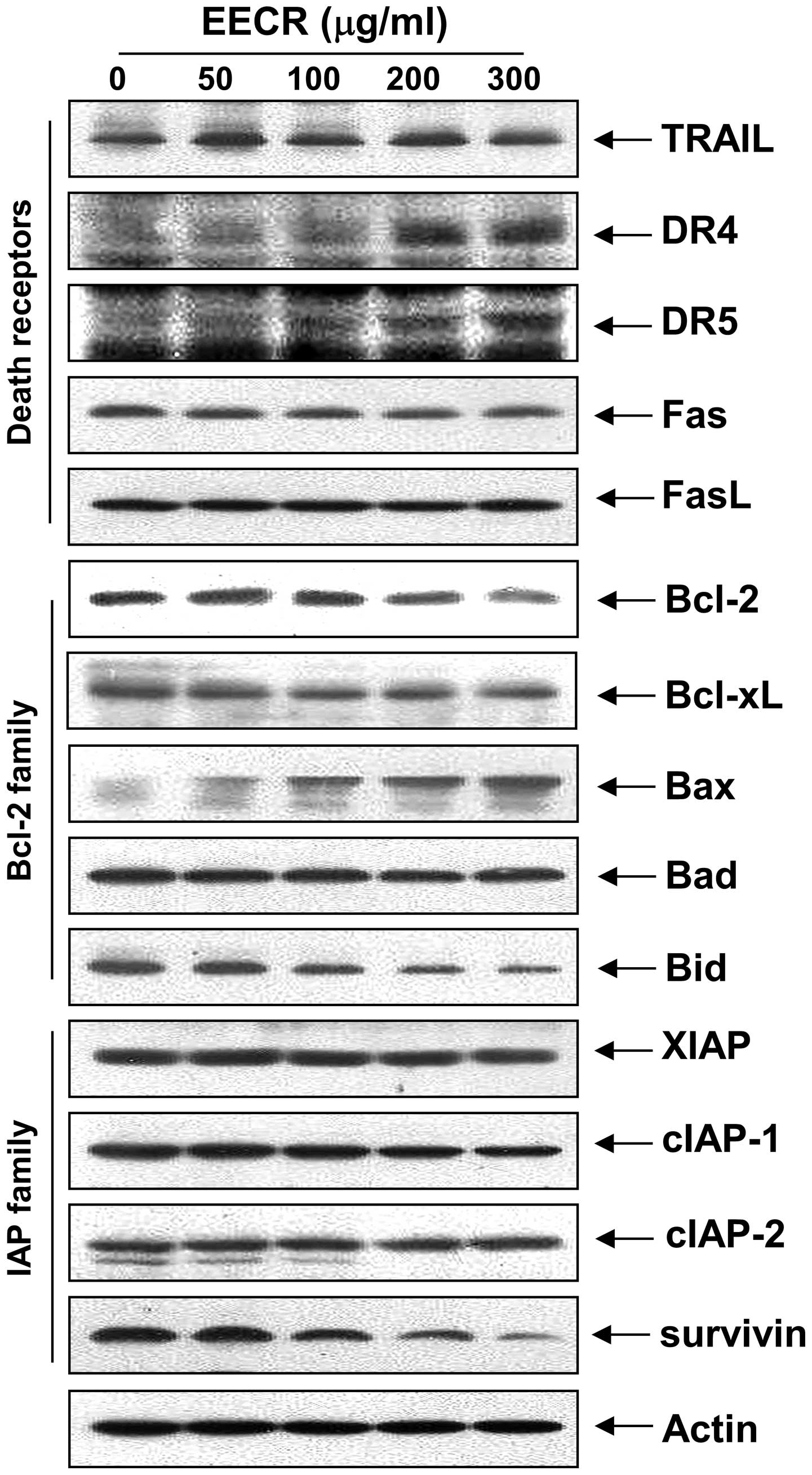
Effects of EECR treatment on expression
of apoptosis-related genes
The death receptor and corresponding pro-apoptotic
ligands were examined by western blot analysis to determine which
apoptosis pathway contributes to EECR-induced apoptosis. The
results showed that the levels of TRAIL, Fas and FasL expression
were relatively unchanged in response to EECR treatment; however,
the levels of death receptor (DR) 4 and DR5 markedly increased in
response to EECR treatment (Fig.
3). Next, we examined the effects of the EECR on the levels of
Bcl-2 family proteins. The results of immunoblotting revealed
downregulation of anti-apoptotic Bcl-2, but not Bcl-xL, in
MDA-MB-231 cells (Fig. 3). However,
treatment with the EECR caused a marked increase in pro-apoptotic
Bax expression, but not Bad. In addition, relative protein
expression of anti-apoptotic survivin decreased in a
concentration-dependent manner compared to that in control cells,
whereas expression of XIAP, cIAP-1 and cIAP-2 was relatively
constant in EECR-treated MDA-MB-231 cells (Fig. 3).
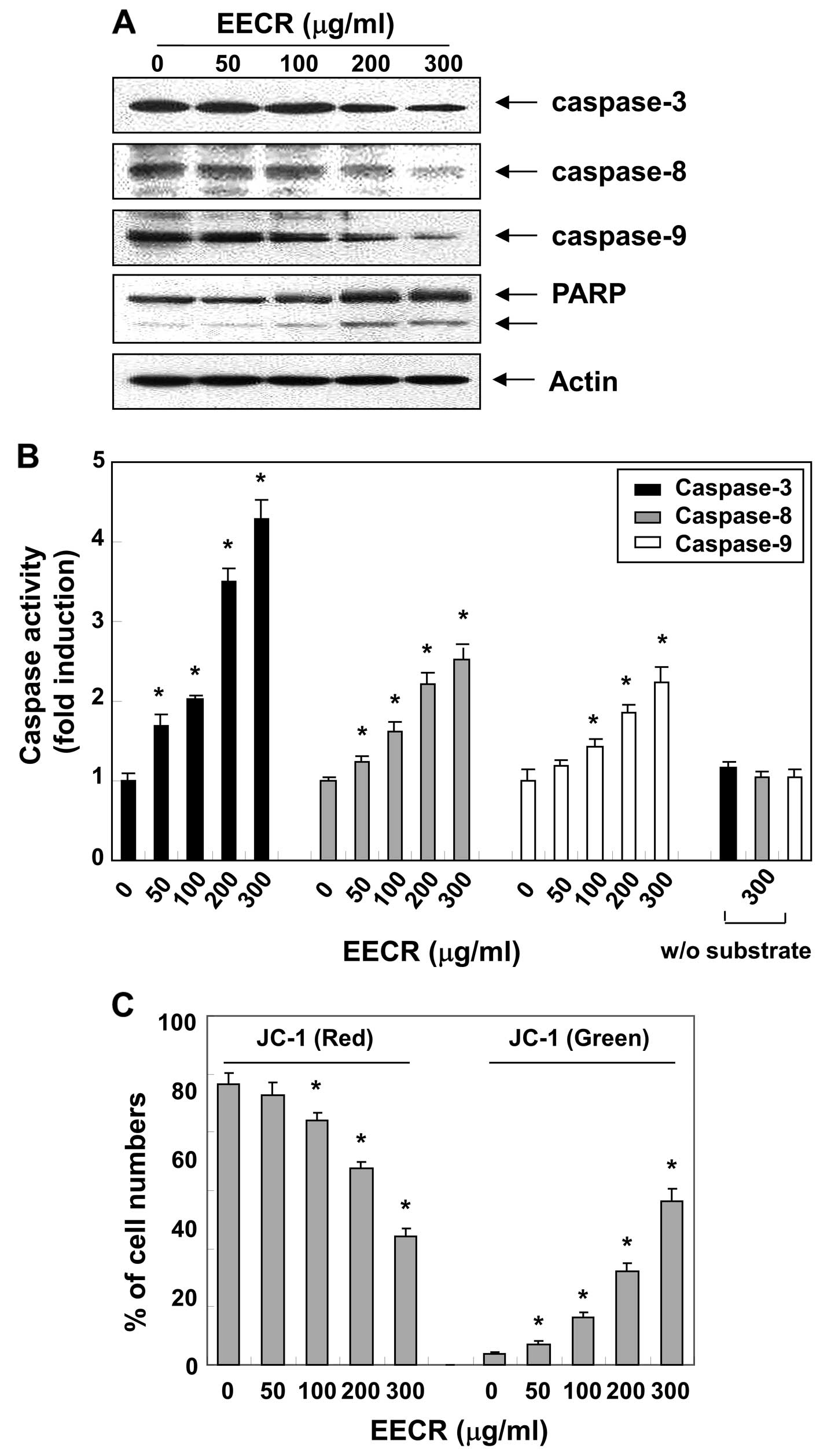
Activation of caspases and degradation of
poly(ADP-ribose) polymerase (PARP) by EECR treatment
We next examined the effect of the EECR on caspase
activity to determine whether caspase activation occurs during
EECR-induced apoptosis. Fig. 4A
shows that treatment of MDA-MB-231 cells with the EECR decreased
pro-caspase-8 and -9 levels, which are initiator caspases of the
extrinsic and intrinsic apoptotic pathways, respectively. In
conjunction with the decrease in pro-caspase-8 and -9 expression,
western blotting revealed that EECR treatment of MDA-MB-231 cells
also resulted in the downregulation of pro-caspase-3. Furthermore,
activation of caspases by the EECR was confirmed by measuring
enzyme activity using the specific synthetic substrates for each
caspase. As indicated in Fig. 4B,
we observed a dose-dependent gradual increase in caspase-3, -8 and
-9 activities in EECR-treated MDA-MB-231 cells similar to the
proteolytic processing of pro-caspases. Activation of caspase-3 was
further evidenced from cleavage of PARP from a 116 kDa band to a 89
kDa fragment, a substrate of active caspase-3, which also serves as
a marker of cells undergoing apoptosis (30).
Effects of the EECR on apoptosis
induction for mitochondrial signaling
Since the EECR activated caspase-9, we investigated
whether it would affect EECR-induced apoptosis associated with
mitochondrial signaling. Thus, we examined the effects of the EECR
on mitochondrial membrane integrity, one of the early events
leading to apoptosis, using the JC-1 fluorescent probe. The results
in Fig. 4C indicate that treatment
with the EECR clearly elicited dissipation of the MMP when compared
to that in control cells. The extrinsic apoptotic signaling cascade
starts with activation of caspase-8 and truncation of Bid (tBid), a
BH3 pro-apoptotic protein, which translocates to the mitochondrial
membrane, allowing activation of pro-apoptotic proteins (8,9).
Therefore, we further examined the effect of the EECR on the level
of Bid. As indicated in Fig. 3,
EECR treatment caused a decrease in the amount of the Bid pro-form,
which is indirect evidence of protein truncation and activation,
suggesting that EECR-induced apoptosis in MDA-MB-231 cells may
occur via activation of caspase-8 and Bid truncation.
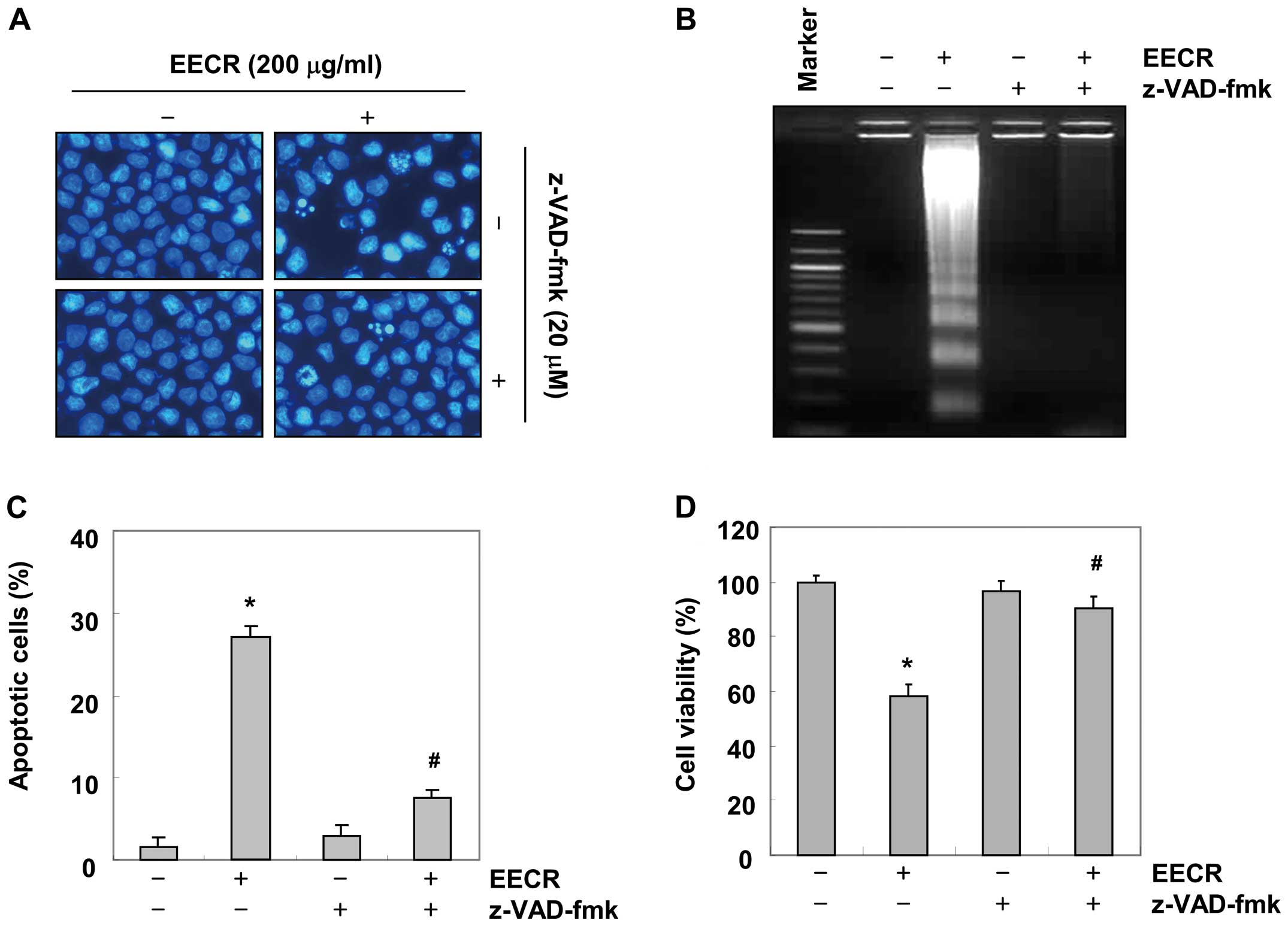
Inhibition of EECR-induced apoptosis by a
caspase inhibitor
To further confirm the significance of caspase
activation in EECR-induced apoptosis, we examined the effects of
the general caspase inhibitor, z-VAD-fmk. As shown in Fig. 5A and B, pre-treatment with z-VAD-fmk
completely abrogated the appearance of cells with apoptotic
features, such as chromatin condensation and formation of apoptotic
bodies, and attenuated the accumulation of fragmented DNA. A flow
cytometric analysis and MTT assay indicated that pretreatment of
cells with z-VAD-fmk prevented EECR-induced accumulation of
apoptotic cell population and growth inhibition (Fig. 5C and D). These results show that
EECR-induced apoptosis in MDA-MB-231 cells occurred via a
caspase-dependent pathway.
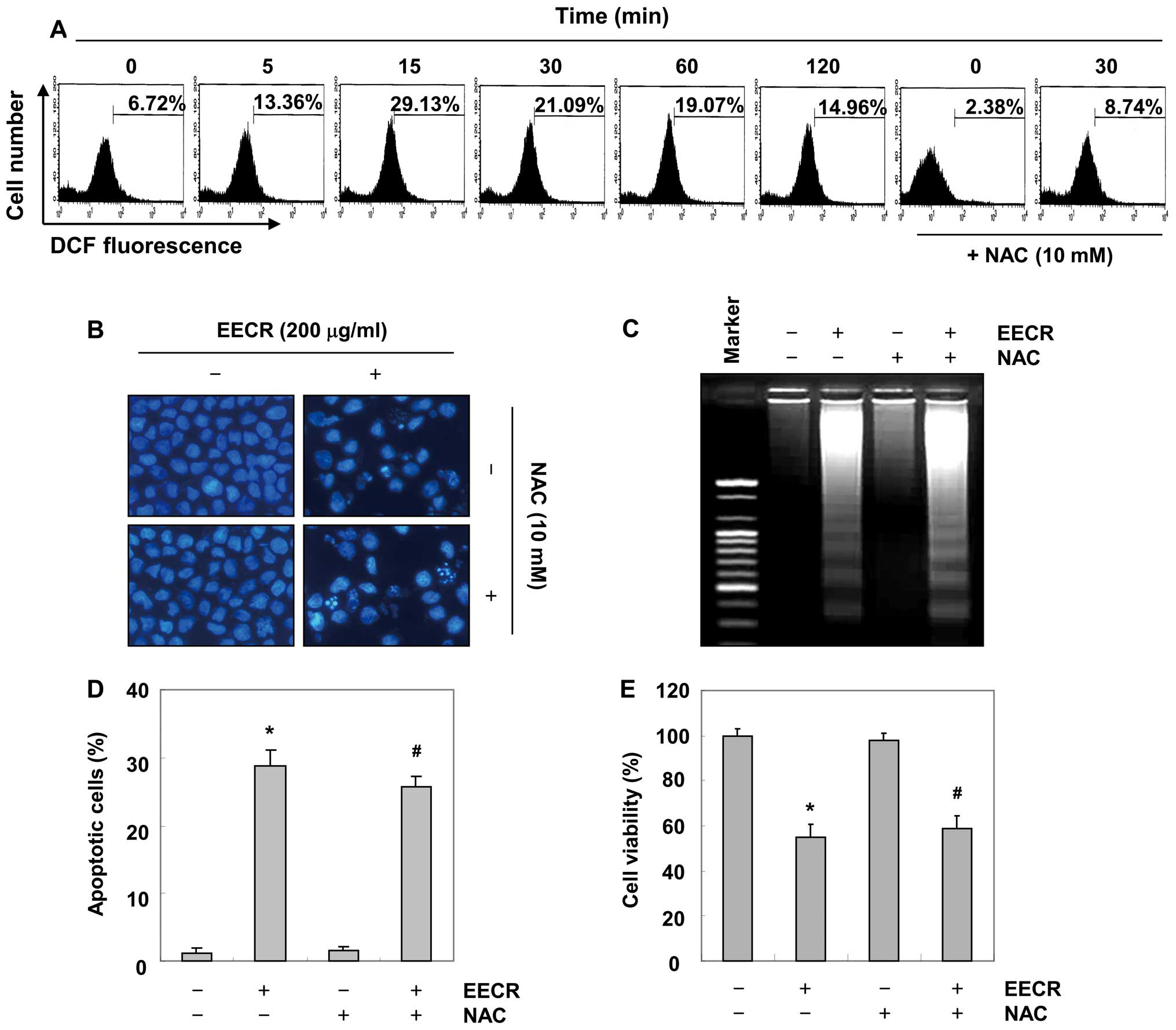
EECR-induced apoptosis is independent of
ROS generation in MDA-MB-231 cells
Oxidative stress occurs due to an imbalance in
pro-oxidant and antioxidant levels in cells. Excess ROS generation
through an imbalance between pro-oxidants and antioxidants leads to
damage of cellular macromolecules including DNA, proteins and
lipids, and eventually results in physical and chemical damage to
tissues that may lead to cell death (31,32).
Therefore, we investigated whether ROS generation was also involved
in EECR-induced apoptosis. As shown in Fig. 6A, the EECR markedly increased
intracellular ROS levels within 15 min, and pretreatment with NAC,
a well known ROS scavenger, markedly attenuated EECR-induced ROS
generation. However, pretreatment with NAC did not block
EECR-induced apoptotic morphological changes or DNA fragmentation
(Fig. 6B and C). Moreover, NAC did
not inhibit EECR-induced apoptosis and growth inhibition (Fig. 6D and E). Collectively, these results
clearly indicate that the EECR-induced apoptosis in MDA-MB-231
cells is not mediated by ROS generation.
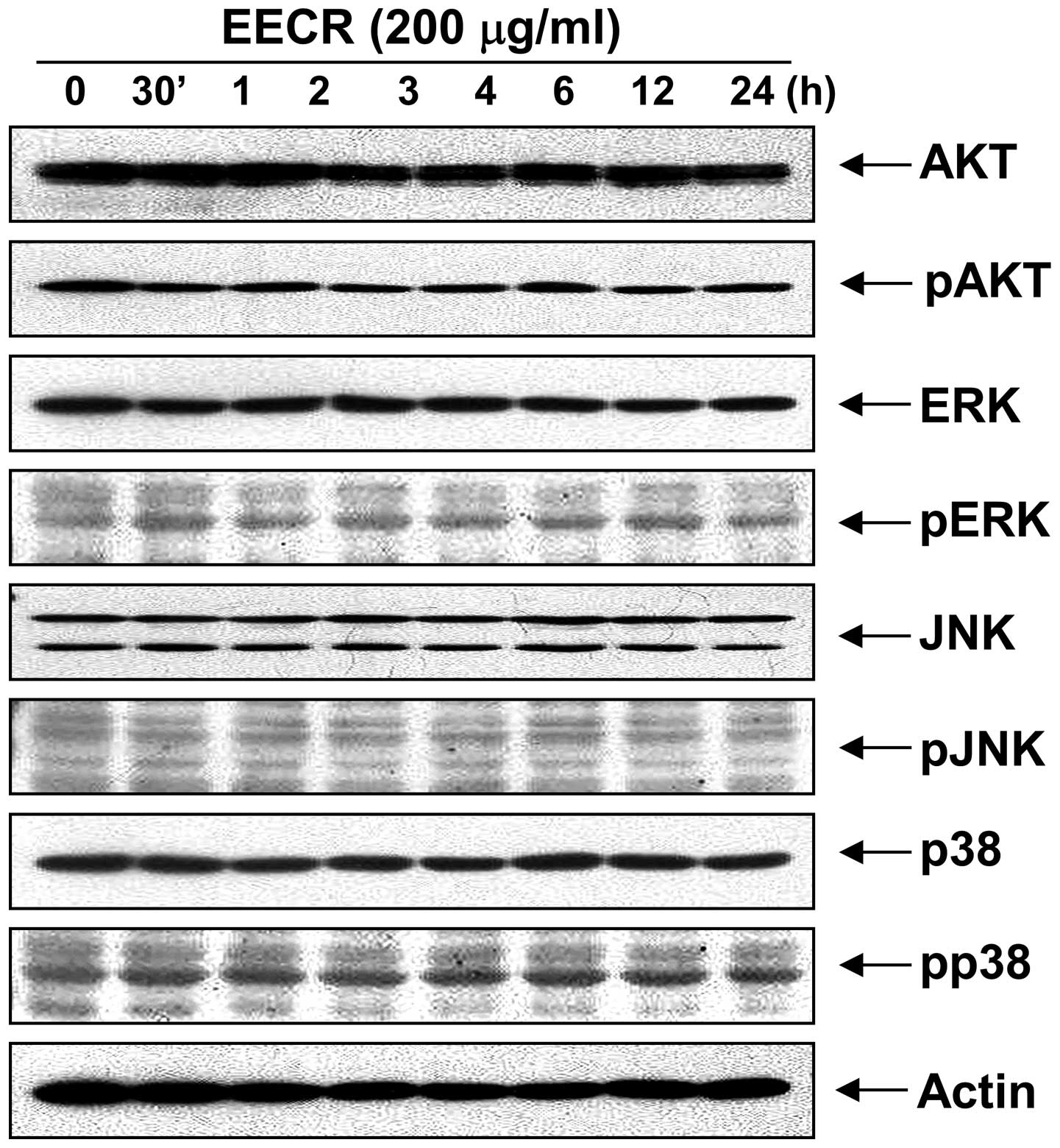
Activation of Akt and MAPKs is not
involved in EECR-induced apoptosis in MDA-MB-231 cells
Next, we investigated the effect of EECR treatment
on the expression and activities of PI3K/Akt and MAPKs to determine
whether these signaling pathways play a role in mediating the
observed apoptotic response. Western blot analysis showed that
total Akt protein and phosphorylated Akt levels did not change
significantly in response to EECR treatment. In addition, the EECR
did not lead to phosphorylation of the three MAPKs including
extracellular signal-regulated kinase (ERK), p38 MAPK and c-Jun
NH2-terminal kinase (JNK) (Fig. 7),
and there was also no effect on steady-state levels of total MAPK
proteins in MDA-MB-231 cells treated with EECR. Moreover,
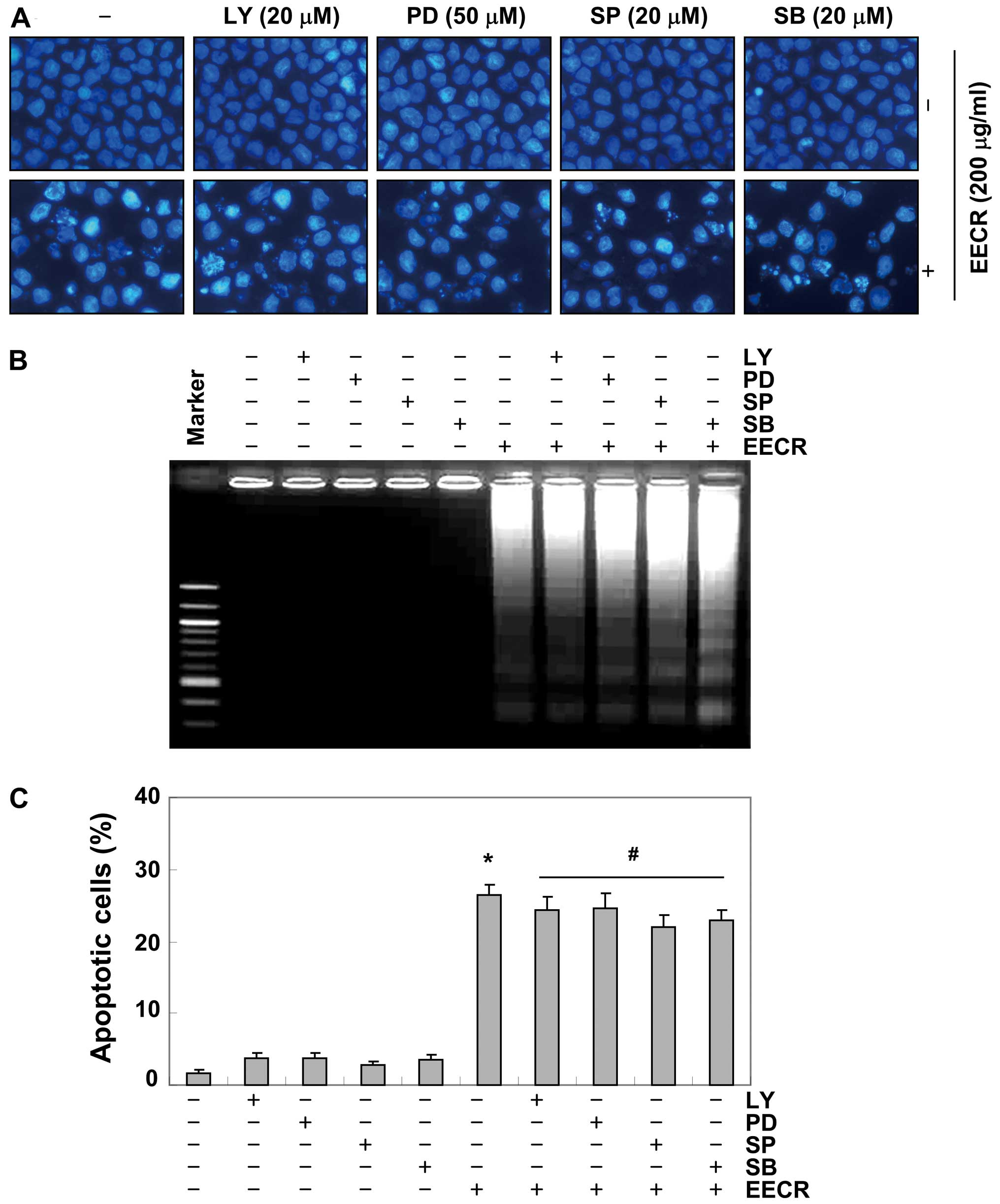
pretreatment with a representative PI3K/Akt inhibitor, LY294002 and
inhibitors of MAPKs (PD98059, a potent inhibitor of ERK; SP600125,
a potent inhibitor of JNK; SB203589, a specific inhibitor of the
p38 MAPK) did not have a significant effect on EECR treatment
(Fig. 8). Collectively, these
results suggest that the PI3K/Akt and MAPK pathways did not play a
role in regulating EECR-induced apoptosis of MDA-MB-231 cells.
Discussion
Although the extracts and components isolated from
C. rotundus possess a wide range of biological activities
that may contribute to health beneficial effects, the mechanisms of
the antiproliferative actions on malignant cell growth have not yet
been elucidated. In our search for new natural sources of
apoptosis-inducing agents from traditional medicinal plants, we
studied the efficiency of extracts from C. rotundus. In the
present study, using the MDA-MB-231 human breast cancer cell line,
we demonstrated inhibited cell growth and viability by the EECR and
the MECR as well as changes in cell morphology in a
concentration-dependent manner (Figs.
1 and 2A). To further confirm
that the EECR-induced antiproliferative effects were related to
apoptotic cell death, induction of apoptosis by the EECR was
confirmed by measuring nuclear chromatin condensation, DNA
fragmentation and accumulation of apoptotic cells (Fig. 2B–D).
Dysregulated apoptosis induces a number of
pathological conditions, including cancer, and failure of apoptosis
leads to an imbalance in cell number, which in turn leads to
tumorigenesis. Therefore, induction of apoptosis in malignant cells
has emerged as an important strategy for cancer therapy (5,6). Our
data demonstrated that EECR-induced apoptosis was associated with
induction of DR4 and DR5, inhibition of survivin, and cleavage of
Bid (Fig. 3). The data also
indicated that treatment with the EECR induced activation of
caspases-3, -8, and -9 and concomitant proteolytic degradation of
PARP and loss of MMP (Fig. 4).
Among the two major apoptotic pathways, the extrinsic pathway
requires recruitment of the death receptor-associated death domain
and caspase-8/-10, which results in caspase-8/-10 activation and
subsequent activation of downstream executioner caspases including
caspase-3 and -7 and apoptosis (33,34).
The intrinsic pathway is triggered by cell stressors and many
chemotherapeutic agents, resulting in the induction of
mitochondrial dysfunction. Mitochondrial dysfunction induces
activation of caspase-9 and subsequently activates effector
caspases. Following activation of caspase-3, several specific
substrates including PARP are cleaved, eventually leading to
apoptosis (30). In some cells,
caspase-8 also mediates the intrinsic pathway via cleavage of the
pro-apoptotic Bid protein (8,9). In
particular, caspases are regulated by various molecules, including
members of the Bcl-2 and IAP families. Bcl-2 family proteins are
involved in the control of the apoptotic process by interactions
between pro-apoptotic (such as Bax and Bad) and anti-apoptotic
(such as Bcl-2 and Bcl-xL) members, particularly those of the
intrinsic pathway with mitochondrial dysfunction. Cellular proteins
in the IAP family (including XIAP, cIAP-1, cIAP-2 and survivin)
specifically inhibit caspase-3 and -9 activities, yet they do not
inhibit caspase-8 (6,7). In the intrinsic pathway, members of
the IAP family bind directly to a principal caspase, such as
pro-caspase-3 and -9, and inhibit apoptosis induced by Bcl-2 family
proteins. Therefore, the downregulation of IAP family proteins
relieves the triggering block of pro-apoptotic signaling and the
execution caspases, thus activating cell death (35,36).
In addition to activation of caspases, EECR treatment resulted in a
significant increase in Bax expression and a decrease in Bcl-2
expression (Fig. 3), suggesting
that changes in the ratio of pro-apoptotic and anti-apoptotic Bcl-2
family proteins may contribute to the apoptosis-promoting activity
of the EECR. Our results also revealed that pretreatment with the
pan-caspase inhibitor, z-VAD-fmk, significantly attenuated
EECR-induced apoptosis (Fig. 5).
These data indicate that caspases are the key molecules mediating
EECR-induced apoptosis, supporting the hypothesis that the
caspase-dependent pathway may be involved in EECR-induced apoptosis
of MDA-MB-231 cells.
Accumulating evidence indicates that the redox state
of cells is involved in cell fate and suggests the possibility that
ROS-mediated oxidative DNA damage and depolarization of
mitochondrial membranes play an important role in apoptosis
(32,37). In the present study, intracellular
ROS levels increased markedly within 15 min by EECR treatment in
MDA-MB-231 cells; however, the quenching of ROS generation with the
antioxidant NAC, a widely used ROS scavenger, did not confer
significant protection against EECR-elicited apoptosis,
demonstrating that the generation of ROS is not required for
apoptosis induced by EECR treatment of MDA-MB-231 cells. In
addition to ROS generation, it has been demonstrated that the
phosphorylation states of some regulatory proteins are also crucial
events along the pathways controlling cell survival and death.
Among them, the PI3K/Akt pathway plays an important role
controlling the balance between cell survival and apoptosis. This
pathway is normally activated in a wide variety of cancers and
results in enhanced resistance to apoptosis through multiple
mechanisms. Therefore, inhibiting PI3K/Akt decreases cell survival
and enhances the effects of chemotherapeutic drugs in many types of
cancer cells (38,39). Another well-established apoptotic
signaling cascade is regulated by MAPKs, including JNK, ERK and p38
MAPK. In general, they are activated in response to various stimuli
and participate in a variety of signaling pathways that regulate
diverse cellular processes including cell growth, differentiation
and stress responses (40,41). However, PI3K/Akt as well as MAPKs
were not activated by EECR treatment, and specific inhibitors of
these pathways did not reduce or increase apoptosis. These results
demonstrate that activation of PI3K/Akt as well as MAPKs is not a
necessary step in EECR-induced apoptosis in MDA-MB-231 cells.
In summary, the results of the present study
demonstrate that the death receptor-mediated pathway is initiated
by ligation of the transmembrane death receptor to activate
membrane-proximal caspases (caspase-8), which in turn activate
effector caspases such as caspase-3. Second, the
mitochondrial-mediated pathway requires disruption of the
mitochondrial membrane, which induces activation of caspase-9 and
thereby initiates the apoptotic caspase cascade. In addition,
crosstalk between the two pathways may be mediated by truncation of
Bid, which may act as a potential feedback loop to amplify
EECR-induced caspase-dependent apoptosis. Although further studies
are required to identify the active compounds, these novel
phenomena have not been previously described and provide important
new insight into the anticancer effects of the EECR.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) grant funded by the Korea government (no. 2012046358).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: a
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Senkus E, Kyriakides S, Penault-Llorca F,
Poortmans P, Thompson A, Zackrisson S and Cardoso F; ESMO
Guidelines Working Group. Primary breast cancer: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 24(Suppl 6): vi7–vi23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spector D, Deroo LA and Sandler DP:
Lifestyle behaviors in black and white women with a family history
of breast cancer. Prev Med. 52:394–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
7
|
Caroppi P, Sinibaldi F, Fiorucci L and
Santucci R: Apoptosis and human diseases: mitochondrion damage and
lethal role of released cytochrome c as proapoptotic protein. Curr
Med Chem. 16:4058–4065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zha J, Weiler S, Oh KJ, Wei MC and
Korsmeyer SJ: Posttranslational N-myristoylation of BID as a
molecular switch for targeting mitochondria and apoptosis. Science.
290:1761–1765. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung KM and Yu SW: Interplay between
autophagy and programmed cell death in mammalian neural stem cells.
BMB Rep. 46:383–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mansilla S, Llovera L and Portugal J:
Chemotherapeutic targeting of cell death pathways. Anticancer
Agents Med Chem. 12:226–238. 2012. View Article : Google Scholar
|
|
12
|
Rufini A and Melino G: Cell death
pathology: the war against cancer. Biochem Biophys Res Commun.
414:445–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thebtaranonth C, Thebtaranonth Y,
Wanauppathamkul S and Yuthavong Y: Antimalarial sesquiterpenes from
tubers of Cyperus rotundus: structure of
10,12-peroxycalamenene, a sesquiterpene endoperoxide.
Phytochemistry. 40:125–128. 1995.PubMed/NCBI
|
|
14
|
Zhu M, Luk HH, Fung HS and Luk CT:
Cytoprotective effects of Cyperus rotundus against ethanol
induced gastric ulceration in rats. Phytother Res. 11:392–394.
1997.
|
|
15
|
Uddin SJ, Mondal K, Shilpi JA and Rahman
MT: Antidiarrhoeal activity of Cyperus rotundus.
Fitoterapia. 77:134–136. 2006. View Article : Google Scholar
|
|
16
|
Sharma R and Gupta R: Cyperus
rotundus extract inhibits acetylcholinesterase activity from
animal and plants as well as inhibits germination and seedling
growth in wheat and tomato. Life Sci. 80:2389–2392. 2007.
View Article : Google Scholar
|
|
17
|
Kilani-Jaziri S, Neffati A, Limem I,
Boubaker J, Skandrani I, Sghair MB, Bouhlel I, Bhouri W, Mariotte
AM, Ghedira K, Dijoux Franca MG and Chekir-Ghedira L: Relationship
correlation of antioxidant and antiproliferative capacity of
Cyperus rotundus products towards K562 erythroleukemia
cells. Chem Biol Interact. 181:85–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yazdanparast R and Ardestani A: In vitro
antioxidant and free radical scavenging activity of Cyperus
rotundus. J Med Food. 10:667–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ardestani A and Yazdanparast R: Cyperus
rotundus suppresses AGE formation and protein oxidation in a
model of fructose-mediated protein glycoxidation. Int J Biol
Macromol. 41:572–578. 2007. View Article : Google Scholar
|
|
20
|
Gupta MB, Palit TK, Singh N and Bhargava
KP: Pharmacological studies to isolate the active constituents from
Cyperus rotundus possessing anti-inflammatory, anti-pyretic
and analgesic activities. Indian J Med Res. 59:76–82.
1971.PubMed/NCBI
|
|
21
|
Seo WG, Pae HO, Oh GS, Chai KY, Kwon TO,
Yun YG, Kim NY and Chung HT: Inhibitory effects of methanol extract
of Cyperus rotundus rhizomes on nitric oxide and superoxide
productions by murine macrophage cell line, RAW 264.7 cells. J
Ethnopharmacol. 76:59–64. 2001.PubMed/NCBI
|
|
22
|
Kilani S, Ben Ammar R, Bouhlel I,
Abdelwahed A, Hayder N, Mahmoud A, Ghedira K and Chekir-Ghedira L:
Investigation of extracts from (Tunisian) Cyperus rotundus
as antimutagens and radical scavengers. Environ Toxicol Pharmacol.
20:478–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsoyi K, Jang HJ, Lee YS, Kim YM, Kim HJ,
Seo HG, Lee JH, Kwak JH, Lee DU and Chang KC: (+)-Nootkatone and
(+)-valencene from rhizomes of Cyperus rotundus increase
survival rates in septic mice due to heme oxygenase-1 induction. J
Ethnopharmacol. 137:1311–1317. 2011.PubMed/NCBI
|
|
24
|
Lemaure B, Touché A, Zbinden I, Moulin J,
Courtois D, Macé K and Darimont C: Administration of Cyperus
rotundus tubers extract prevents weight gain in obese Zucker
rats. Phytother Res. 21:724–730. 2007.
|
|
25
|
Kumar KH and Khanum F: Hydroalcoholic
extract of Cyperus rotundus ameliorates
H2O2-induced human neuronal cell damage via
its anti-oxidative and anti-apoptotic machinery. Cell Mol
Neurobiol. 33:5–17. 2013.
|
|
26
|
Hemanth Kumar K, Tamatam A, Pal A and
Khanum F: Neuroprotective effects of Cyperus rotundus on
SIN-1 induced nitric oxide generation and protein nitration:
ameliorative effect against apoptosis mediated neuronal cell
damage. Neurotoxicology. 34:150–159. 2013.
|
|
27
|
Fu XC, Shan HL, Bai HB and Hu R:
Protective effect of Jiangbaiweiyan tablet on ethanol-induced
gastric mucosa injury in rats. Zhejiang Da Xue Xue Bao Yi Xue Ban.
40:391–394. 2011.(In Chinese).
|
|
28
|
He CL, Yi PF, Fan QJ, Shen HQ, Jiang XL,
Qin QQ, Song Z, Zhang C, Wu SC, Wei XB, Li YL and Fu BD:
Xiang-Qi-Tang and its active components exhibit anti-inflammatory
and anticoagulant properties by inhibiting MAPK and NF-κB signaling
pathways in LPS-treated rat cardiac microvascular endothelial
cells. Immunopharmacol Immunotoxicol. 35:215–224. 2013.PubMed/NCBI
|
|
29
|
Lee SJ, Hwang SO, Noh EJ, Kim DU, Nam M,
Kim JH, Nam JH and Hoe KL: Transactivation of bad by
vorinostat-induced acetylated p53 enhances doxorubicin-induced
cytotoxicity in cervical cancer cells. Exp Mol Med. 46:e762014.
|
|
30
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matés JM, Segura JA, Alonso FJ and Márquez
J: Intracellular redox status and oxidative stress: implications
for cell proliferation, apoptosis, and carcinogenesis. Arch
Toxicol. 82:273–299. 2008.PubMed/NCBI
|
|
32
|
Trueba GP, Sánchez GM and Giuliani A:
Oxygen free radical and antioxidant defense mechanism in cancer.
Front Biosci. 9:2029–2044. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peter ME and Krammer PH: The
CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roy N, Deveraux QL, Takahashi R, Salvesen
GS and Reed JC: The c-IAP-1 and c-IAP-2 proteins are direct
inhibitors of specific caspases. EMBO J. 16:6914–6925. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schumacker PT: Reactive oxygen species in
cancer cells: live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoeli-Lerner M and Toker A: Akt/PKB
signaling in cancer: a function in cell motility and invasion. Cell
Cycle. 5:603–605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu L, Hofmann J, Lu Y, Mills GB and Jaffe
RB: Inhibition of phosphatidylinositol 3′-kinase increases efficacy
of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer
Res. 62:1087–1092. 2002.
|
|
40
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|