Introduction
Liver cancer is the fifth most common cancer in men
and the seventh in women; it accounts for 9% of all cancer-related
deaths worldwide and 12% in developing countries (1). Epidemiologic studies have shown that
the main risk factors for hepatocellular carcinoma (HCC) are
chronic hepatitis B virus (HBV) and hepatitis C virus (HCV)
infections (2,3). Among primary live cancers, HCC
accounts for almost 70–85% of the total liver cancer burden. Until
recently, there is still a lack of systemic chemotherapy in
treating HCC efficaciously. Although sorafenib has been approved by
FDA for the treatment of advanced HCC, it causes several adverse
effects including diarrhea, hand-foot skin reaction,
hypophosphatemia and the risk of bleeding (4,5).
Therefore, it is urgent to develop alternative therapeutic
strategies for liver cancer.
Resveratrol, a naturally occurring polyphenolic
compound, is commonly present in the skin of grapes and in red wine
(6). Due to its high toxicity
toward tumor cells, resveratrol appears to be a good candidate drug
for cancer therapy. Resveratrol can delay or prevent all stages of
carcinogenesis in vitro and in vivo, including
initiation, promotion and progression (6,7). When
resveratrol is combined with other anticancer agents, such as
5-fluorouracil and curcumin, they display synergistic anticancer
properties (8–10). Previous studies have also
demonstrated that resveratrol inhibits cell proliferation and
induces apoptotic cell death in HCC cells in vivo and in
vitro (7).
Matrine is an important ingredient of a traditional
Chinese herb Sophora flavescens Ait., which has widely been
used in China for the treatment of viral hepatitis, liver
cirrhosis, cardiac arrhythmia and skin inflammations without any
obvious side-effects (11).
Recently, its anticancer activity has been extensively
investigated; it inhibits proliferation and metastasis and induces
apoptosis in a variety of malignant cells (12–15).
It also reduces the occurrence of multidrug-resistant tumor cells
induced by chemotherapy and displays synergistic activities with
other anticancer agents (16,17).
Matrine inhibits the growth of HCC cells by inducing apoptosis via
upregulation of the ratio of Bax/Bcl-2 (13). However, matrine alone weakly
inhibits proliferation of cancer cell lines with an IC50
value of 2–16 mM (17).
In the present study, we first evaluated the
antiproliferative effect of resveratrol on HepG2 cells and then
explored the underlying molecular mechanisms. Since both
resveratrol and matrine independently exert anticancer activities
against liver cancer cells, it led us to hypothesize that an
increased benefit would result when using the combination of these
two agents when compared with each single agent alone. Thus, the
anticancer effect of the combination treatment of resveratrol and
matrine was also evaluated in HepG2 cells.
Materials and methods
Cell culture and reagents
Matrine (purity >98%) was obtained from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). Resveratrol (purity ≥99%),
3-(4,5-dimethyl-2-thiazoyl)-2,5-di-phenyl-2H-tetrazolium bromide
(MTT) and propidium iodide (PI) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). The Annexin V/PI double staining kit was
purchased from Nanjing KeyGen Biotech., Co., Ltd. (Nanjing, China).
The HepG2 cells were purchased from the American Type Culture
Collection (Rockville, MD, USA). The cells were maintained in DMEM
supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin
and 100 U/ml penicillin at 37°C in a humidified atmosphere with 5%
CO2.
Cell viability assay
MTT assay was used to assess cell viability. HepG2
cells were seeded into 96-well plates at a cell density of
5,000/well and allowed to adhere for 24 h, followed by resveratrol
and/or matrine treatment for 48 h. Then 5 μl of 5 mg/ml MTT was
added to the medium and incubated for 2 h at 37°C. After removing
the culture medium, 100 μl of DMSO was added. The plates were read
using an enzyme-linked immunosorbent assay plate reader at 570 nm.
All experiments were performed in triplicate, and the cell
viability of HepG2 cells was calculated as the ratio of each
experimental condition to the control. The IC50 value
was calculated from the nonlinear regression analysis.
Cell cycle analysis
To determine cell cycle distribution, HepG2 cells
were treated with resveratrol for 24 h. The cells were trypsinized
and fixed with cold 70% ethanol overnight at 4°C. Then the fixed
cells were washed twice with phosphate-buffered saline (PBS) and
incubated with 100 μg/ml of ribonuclease A at 37°C for 30 min and
then stained with 50 μg/ml PI for 1 h. The fluorescence intensity
was detected using BD FACSCalibur cytometer, and the cell cycle
distribution was assayed using ModFit LT software (both from BD
Biosciences, San Jose, CA, USA).
Colony formation assay
HepG2 cells were seeded into 6-well plates at a
density of 400 cells/well. After overnight incubation, the cells
were exposed to resveratrol and/or matrine for 48 h. Thereafter,
the drugs were removed by replacing the medium with fresh medium,
and the cells were then maintained in culture for another 10 days
and the medium was replaced every three days, during which time the
surviving cells produced colonies. The colonies were visualized by
staining for 4 h with 1% methylene blue (in 100% methanol), and the
colonies that contained >50 cells were counted. The colony
formation efficacy was calculated according to the following
formula: Colony formation efficacy = colony counts/seeded cells ×
100%. All experiments were performed in triplicate.
Synergy between the resveratrol and
matrine combination
The nature of the combined effect of resveratrol and
matrine was determined using the method described by George et
al (9), based on the principles
described by Chou and Talalay (18). In brief, the expected value of the
combined effect between agent 1 and 2 is calculated as: [(observed
agent 1 value)/(control value)] × [(observed agent 2
value)/(control value)] × (control value); and the ratio is
calculated as (expected value)/(observed value). A ratio of >1
indicates a synergistic effect, and a ratio of <1 indicates a
less than additive effect.
Quantification of apoptosis
HepG2 cells were seeded in 6-well plates at a
density of 3×105 cells/well and exposed to resveratrol
and/or matrine treatment for 48 h. The cells were harvested and
washed twice in PBS, and then resuspended in 500 μl binding buffer
at a density of 1×106 cells/ml. The cell suspension was
incubated with 5 μl Annexin V and 5 μl PI in the dark for 15 min at
room temperature. Finally, apoptotic cells were detected by flow
cytometry. The amount of apoptosis was evaluated as the percentage
of Annexin V+/PI− and Annexin
V+/PI+ cells.
Western blot analysis
Western blot analysis was performed as previously
described (19). In brief, after
treatment with resveratrol and/or matrine, HepG2 cells were
collected, lysed and subjected to 7.5–12.5% sodium dodecyl
sulfate-polyacrylamide gel and transferred onto a polyvinylidene
fluoride membrane. After blocking with 5% non-fat milk in the
blocking buffer (PBS containing 0.1% Tween-20, pH 7.5), the
membrane was probed with designated first and second antibodies.
The immunoreactive bands were visualized using the ECL Plus Western
Blotting Detection System (Piscataway, NJ, USA). The level of
β-actin was used as a loading control. The antibody against
caspase-3 and caspase-9, poly(ADP-ribose) polymerase-1 (PARP-1),
p53, survivin, Bax, Bcl-2 were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The antibodies against β-actin
were purchased from Sigma-Aldrich.
Reactive oxygen species (ROS) and
mitochondrial membrane potential (Δψm) assays
HepG2 cells were seeded into 6-well plates at a
density of 3×105 cells/well. After overnight incubation,
the cells were treated with resveratrol and/or matrine for 24 h.
Then the HepG2 cells for the detection of ROS were incubated with
10 μM H2DCFDA at 37°C for 30 min in the dark. For the
Δψm assay, the HepG2 cells were incubated with 0.5 mM
Rhodamine 123 at 37°C for 30 min in the dark. The intracellular
fluorescence intensity was measured with a BD FACSCalibur
cytometer.
Statistical analysis
All experiments were repeated as least three times.
One-way analysis of variance (ANOVA) was used to analyze the
variance for the means of multiple groups. Statistical analysis was
performed using SPSS, and significant differences were considered
at values of P<0.05.
Results
Resveratrol inhibits HepG2 cell
proliferation
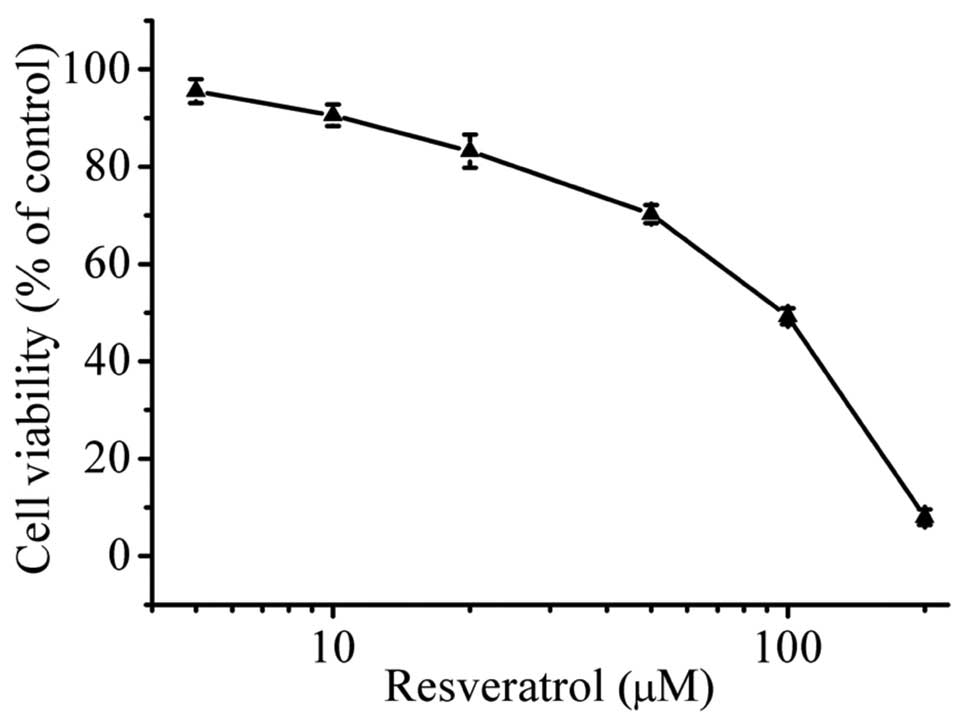
The effect of resveratrol on the cell proliferation
of HepG2 cells was evaluated by the MTT assay. The viability of
HepG2 cells was decreased significantly when resveratrol was used
at concentrations >10 μM (P<0.05) (Fig. 1). The cell viabilities at 10, 20, 50
and 100 μM concentrations of resveratrol were recorded as 91, 83,
70 and 46%, respectively. The results showed that resveratrol
inhibited the growth of HepG2 cells in a dose-dependent manner. The
IC50 value was 70 μM after incubation for 48 h.
Resveratrol arrests HepG2 cells in the
G1 and S phase in HepG2 cells
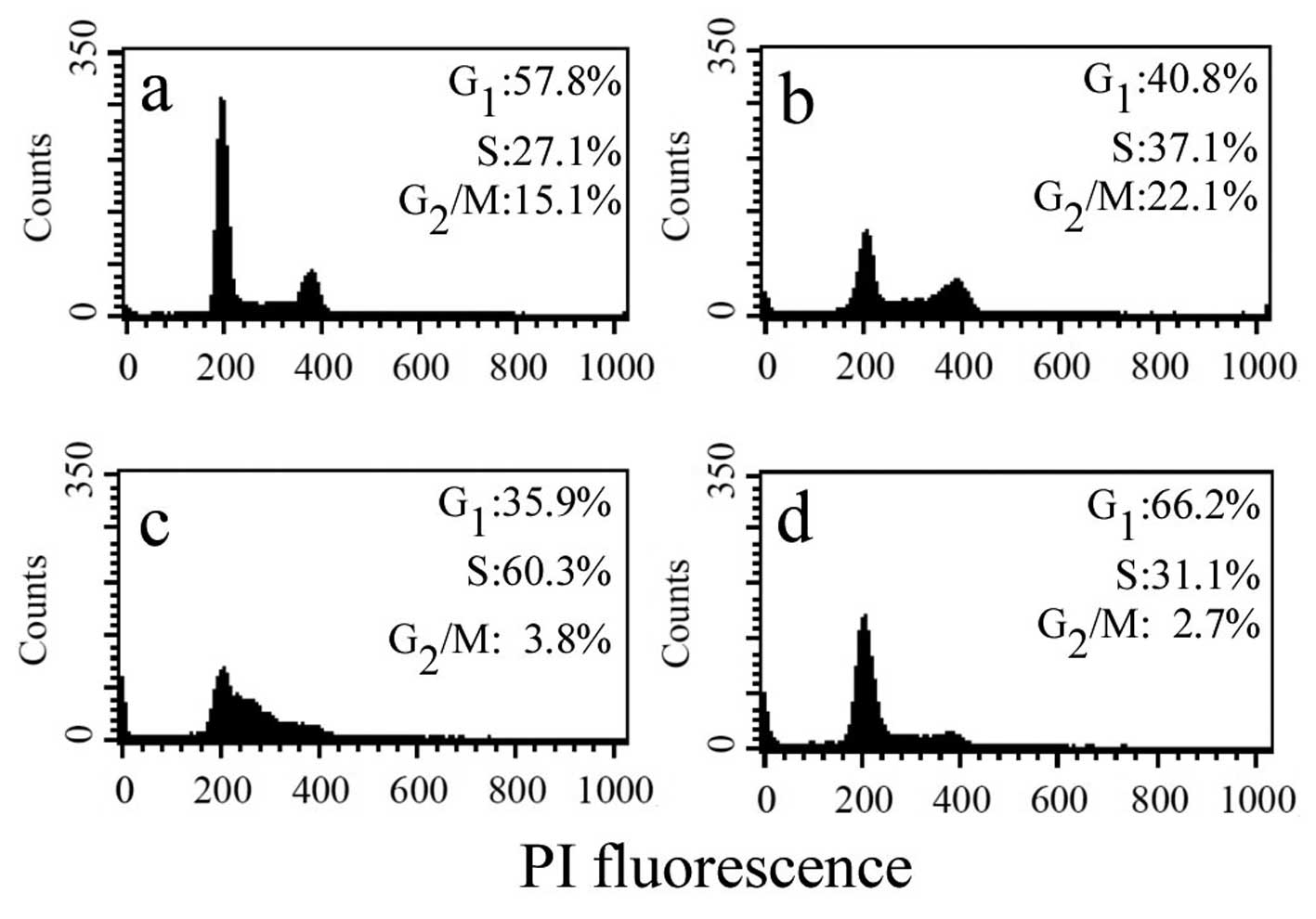
To explore the growth-inhibitory mechanisms of
resveratrol, the effect of resveratrol on cell cycle perturbations
was examined. The cell cycle profile was assessed in HepG2 cells
after exposure to 0, 20, 50 and 100 μM resveratrol for 24 h. A
clear dose-dependent cell cycle arrest was observed in the HepG2
cells (Fig. 2). At lower
concentrations of resveratrol (20 and 50 μM), the number of HepG2
cells increased in the S phase. However, when resveratrol was used
at a higher concentration (100 μM), there was a considerable
accumulation of cells in the G1 phase. These results
indicate that resveratrol arrests the cell cycle in the
G1 and S phase in a concentration-dependent manner.
Resveratrol triggers apoptosis in HepG2
cells
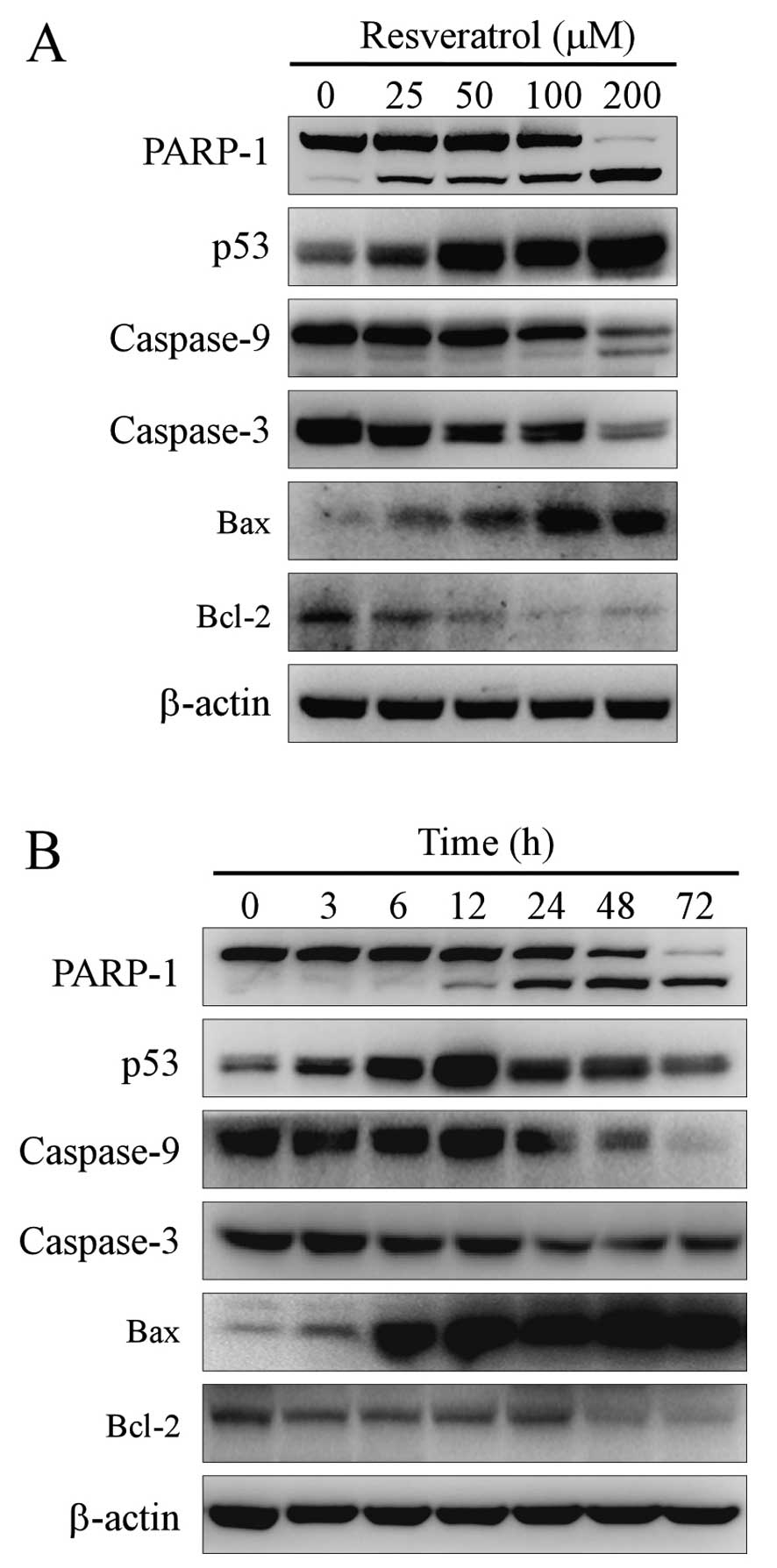
In order to determine whether apoptosis participates
in resveratrol-induced cell death, the expression levels of PARP-1,
Bcl-2, Bax, p53, caspase-3 and -9 were measured by western
blotting. Resveratrol increased PARP-1 cleavage and caspase-3 and
caspase-9 activation, which are hallmarks of an increase in
apoptosis, in a dose- and time-dependent manner (Fig. 3). Resveratrol also inhibited
anti-apoptotic protein Bcl-2 expression and upregulated expression
of pro-apoptotic protein Bax and tumor suppressor protein p53.
Therefore, resveratrol induced apoptotic cell death via a caspase-
and p53-dependent pathway.
Resveratrol and matrine synergistically
inhibit the growth of HepG2 cells
The effect of matrine on the growth of HepG2 cells
was evaluated by the MTT assay. Matrine inhibited the growth of
HepG2 cells in a dose-dependent manner (Fig. 4A). However, matrine treatment at
concentrations <2 mM only resulted in a slight decrease in cell
survival rate. To assess whether matrine can enhance the anticancer
activity of resveratrol, we treated HepG2 cells with increasing
concentrations of resveratrol (25, 50, 100 and 200 μM) in the
presence or absence of matrine (1 or 2 mM) for 48 h. The MTT assay
results showed that the combination treatment was more effective in
inhibiting the proliferation of HepG2 cells when compared with
either agent alone (Fig. 4B).
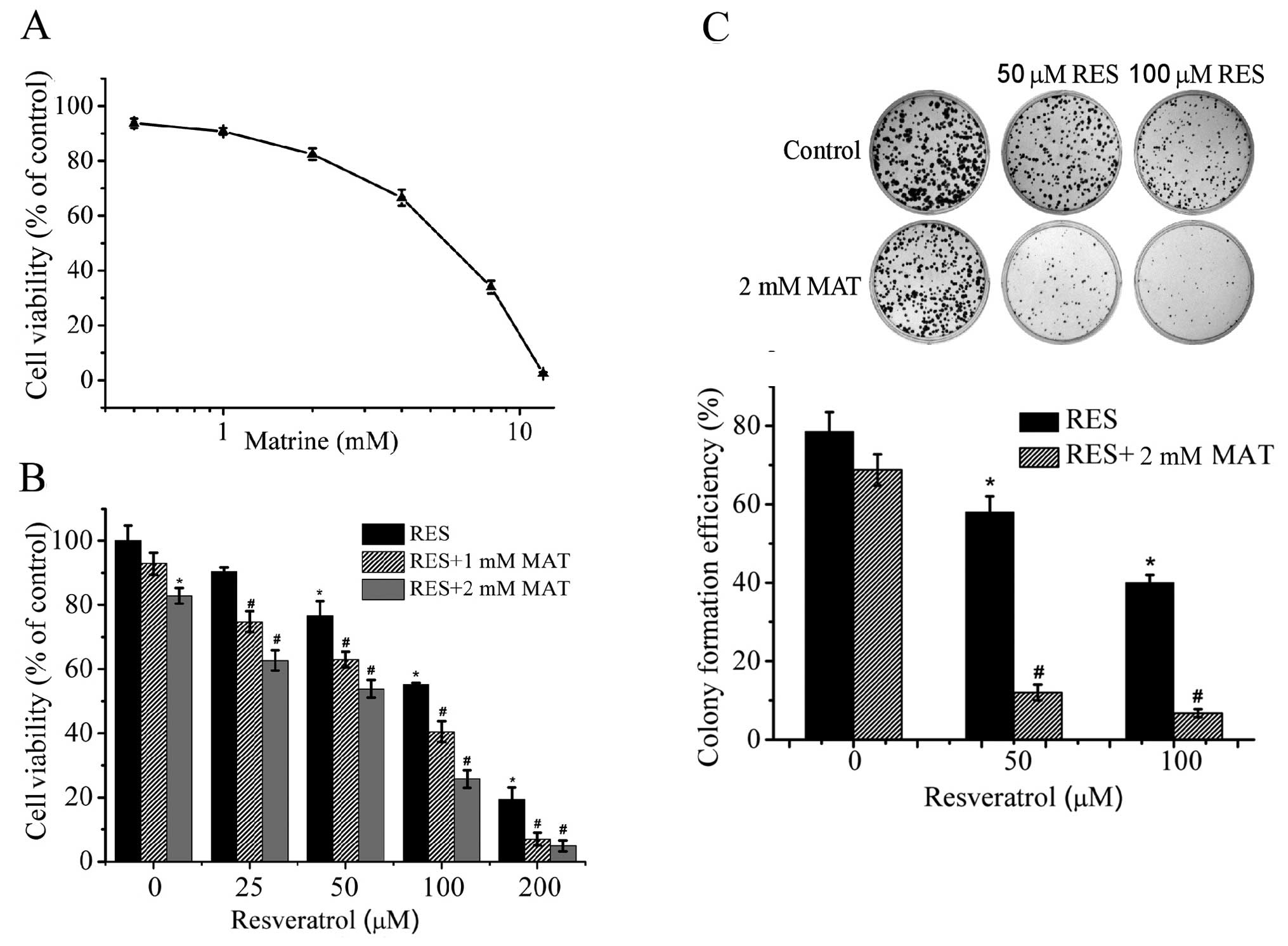 | Figure 4Combined effect of resveratrol and
matrine on HepG2 cell proliferation. (A) HepG2 cells were treated
with increasing concentrations of matrine (0, 0.5, 1.0, 2, 4, 8 and
12 mM) for 48 h, and the percentage of viable cells was then
determined using the MTT assay. (B) HepG2 cells were treated with
matrine (1 or 2 mM) and increasing concentrations of resveratrol
(0, 25, 50, 100 and 200 μM), alone or in combination, and then the
percentage of viable cells was determined by the MTT assay. (C)
Colony formation assay was used to confirm the growth-inhibitory
effect of resveratrol (0, 50 and 100 μM) with or without 2 mM
matrine. Error bars represent the means ± SEM for three independent
experiments. *P<0.05 compared with the control,
#P<0.05 compared with each agent alone. MAT, matrine.
RES, resveratrol. |
In order to confirm the growth-inhibitory effect of
the combination treatment of resveratrol and matrine, a colony
formation assay was also used to further study the combined
treatment of resveratrol and matrine. HepG2 cells were treated with
increasing concentrations of resveratrol (50 and 100 μM) with or
without 2 mM matrine. When resveratrol was combined with matrine,
the colony formation efficacy of the HepG2 cells was significantly
reduced when compared with either agent alone (P<0.05) (Fig. 4C). The precise nature of this
combination was further analyzed by the method described by George
et al (9). The expected
effect of the combination treatment on the cell proliferation was
greater than the observed combination, suggesting a synergistic
effect between resveratrol and matrine on HepG2 cells (Table I). Based on these results, we
selected 50 μM resveratrol and 100 μM resveratrol in the presence
or absence of matrine (2 mM) to carry out the subsequent
studies.
 | Table ISynergistic antiproliferative effect
between resveratrol and matrine combination on HepG2 cells. |
Table I
Synergistic antiproliferative effect
between resveratrol and matrine combination on HepG2 cells.
| Treatment | Observed value | Expected value | Ratioa |
|---|
| 50 μM
resveratrol+matrine | 0.12 | 0.51 | 4.25 |
| 100 μM
resveratrol+matrine | 0.07 | 0.35 | 5.00 |
Matrine enhances resveratrol-induced
apoptosis in HepG2 cells
To understand the molecular basis of the
growth-inhibitory mechanism caused by resveratrol and matrine,
Annexin V/PI double staining was used to quantify the extent of
apoptosis after 48 h treatment with resveratrol and/or matrine.
When matrine was combined with resveratrol, matrine significantly
enhanced resveratrol-induced apoptosis of HepG2 cells (P<0.05)
(Fig. 5A). We further detected the
effects of resveratrol and/or matrine on apoptosis-related
proteins. The combination treatment of resveratrol and matrine
significantly enhanced the cleavage of PARP-1, activation of
caspase-3 and caspase-9 when compared to either agent alone
(Fig. 5B). In addition, the
combined treatment significantly inhibited survivin expression in
HepG2 cells compared to the expression following treatment with
either agent alone.
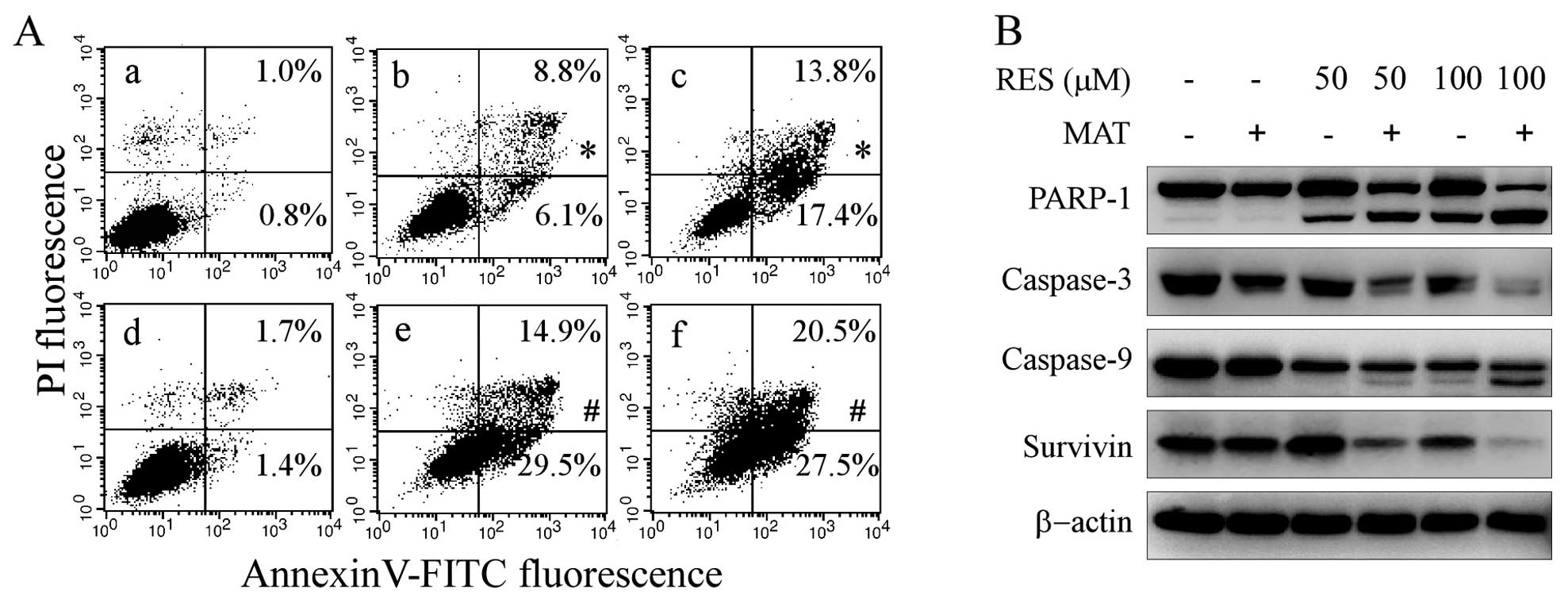 | Figure 5Enhancing resveratrol-induced
apoptosis by matrine in HepG2 cells. HepG2 cells were treated with
(a) 0, (b) 50 μM RES, (c) 100 μM RES, (d) 2 mM MAT, (e) 2 mM MAT+50
μM RES, (f) 2 mM MAT+100 μM RES for 48 h. (A) Annexin V/PI double
staining was used to quantify apoptosis. The amount of apoptosis
was evaluated as the percentage of Annexin
V+/PI− and Annexin
V+/PI+ cells. (B) Western blotting was used
to analyze the status of PARP-1, caspase- 3, -9 and survivin.
β-actin was used as a loading control. *P<0.05
compared with the control, #P<0.05 compared with each
agent alone. RES, resveratrol; MAT, matrine; PARP-1,
poly(ADP-ribose) polymerase-1. |
Combined treatment of resveratrol and
matrine induces ROS generation and decreases Δψm in
HepG2 cells
In order to study the mechanisms of the apoptosis
induced by the combined treatment, we further examined the combined
effect of resveratrol and matrine on ROS production in HepG2 cells
after 24 h treatment. The combined treatment significantly induced
ROS generation in the HepG2 cells when compared with either agent
alone (P<0.05) (Fig. 6A). To
better characterize the apoptotic cell death induced by resveratrol
and matrine in HepG2, the role of mitochondria was also evaluated
after the treatment of resveratrol and/or matrine for 24 h. The
combined treatment of resveratrol and matrine caused a marked loss
of Δψm in the HepG2 cells in respect to each agent alone
(P<0.05) (Fig. 6B).
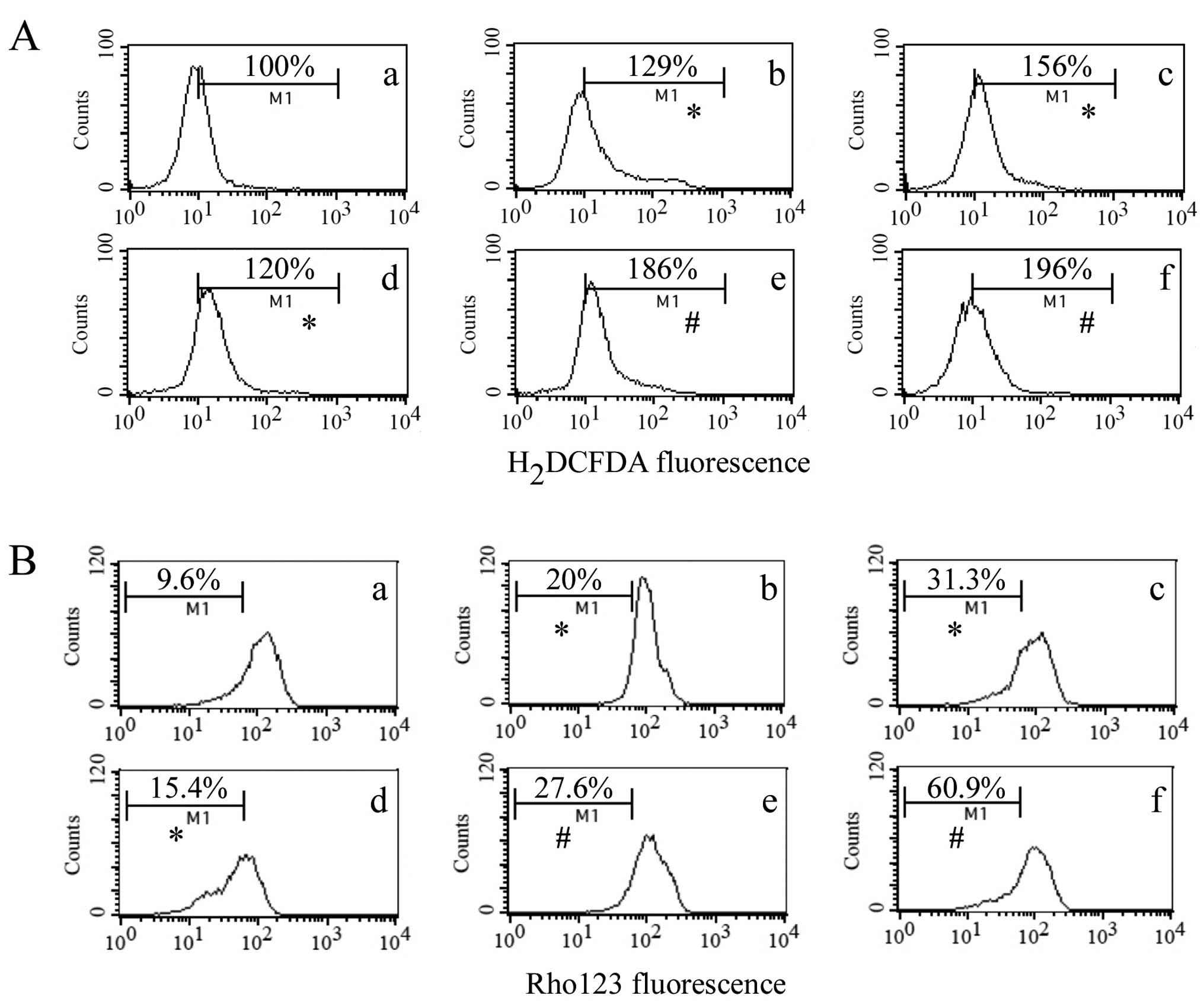 | Figure 6Induction of ROS generation and
disruption of Δψm by the combined treatment of
resveratrol and matrine in HepG2 cells. HepG2 cells were treated
with (a) 0, (b) 50 μM RES, (c) 100 μM RES, (d) 2 mM MAT, (e) 2 mM
MAT+50 μM RES, (f) 2 mM MAT+100 μM RES for 48 h. (A) After
treatment, the cells were stained with H2DCFDA for 30
min and then analyzed by flow cytometry. (B) After staining with
Rhodamine 123 for 30 min, the cells were assayed with a
FACSCalibur. *P<0.05 compared with the control,
#P<0.05 compared with each agent alone. ROS, reactive
oxygen species; MAT, matrine. RES, resveratrol. |
Discussion
Previous studies have demonstrated that resveratrol
inhibits the proliferation of various types of tumor cells in
vivo and in vitro (20–22).
In the present study, we showed that resveratrol decreased the
viability of HepG2 cells in a dose-dependent manner. In addition,
we further dissected the mechanisms underlying the antitumor effect
of resveratrol. Resveratrol arrested HepG2 cells in the
G1 and S phase which is consistent with reported
findings (23), suggesting that
retardation of cell cycle progression may be one of the mechanisms
underlying the antitumor effect of resveratrol.
Apoptosis, or programed cell death, is a
well-documented phenomenon in many cellular systems, which has been
recognized as a major anticancer therapeutic response (24). Our findings are consistent with
previous reports that resveratrol induces apoptosis in cancer cells
(25–27). In the present study, we confirmed
that resveratrol induced apoptosis in HepG2 cells, as shown by the
cleavage of PARP-1, the upregulation of p53 expression and the
activation of caspase-9 and caspase-3. Caspase-9 is an initiator in
the mitochondrial death pathway (the intrinsic pathway), which
could be activated by apoptosomes formed by cytochrome c,
Apaf-1 and pro-caspase-9 (28).
Then activated caspase-9 can cleave and activate caspase-3.
Caspase-3, as an effector caspase, initiates the hallmark of the
degradation process of apoptosis, such as cell shrinkage, membrane
blebbing, DNA fragmentation and finally the breakdown of the cell
into smaller units (apoptotic bodies) (29). The Bcl-2 family members Bax and
Bcl-2 serve as critical regulators of the mitochondrial-dependent
apoptotic pathway. Bcl-2 that negatively regulates apoptosis
promotes cell survival, whereas Bax that positively regulates
apoptosis stimulates mitochondrial damage (30). Consistent with the ability of
resveratrol to kill HepG2 cells via apoptotic processes,
resveratrol upregulated the ratio of Bax/Bcl-2, indicating that the
increased ratio of Bax/Bcl-2 may trigger resveratrol-induced
apoptosis in HepG2 cells.
Matrine has been approved as an adjuvant drug for
the treatment of various malignant cancers in China (11). However, matrine alone weakly
inhibits proliferation of cancer cell lines with an IC50
value of 2–16 mM (17). Combination
of anticancer agents for cancer therapy and prevention has been
extensively studied in numerous in vivo and in vitro
models (31,32). Since each agent may have its own
targets and also share common targets, the combination of two
anticancer agents may exert a synergistic effect. Thus, the effect
of the combined treatment of resveratrol and matrine on HepG2 cells
was also evaluated here. The combined treatment significantly
enhanced the antiproliferative effect when compared with either
agent alone. The ratio of expected value/observed value was >1
for the concentrations tested, indicating that the combined
treatment of resveratrol and matrine exhibited a synergistic
antiproliferative effect. To our knowledge, the present study was
the first to investigate the effect of the combination treatment of
resveratrol and matrine on cancer cells. The combination treatment
significantly induced apoptotic cell death in HepG2 cells as
compared to either agent alone, indicating that induction of
apoptosis is an important mechanism of enhancing the anticancer
effects of resveratrol by matrine.
Survivin is a member of the inhibitors of
apoptosis-related proteins, which has been found to be frequently
overexpressed in most types of cancer cells, including HCC cells.
Therefore, survivin has emerged as a potential therapeutic target
for natural anticancer compounds (33,34).
Our results showed that the combination treatment of resveratrol
and matrine significantly reduced the expression of survivin in
HepG2 cells compared with the control or either drug alone. These
data suggest that downregulation of survivin expression is also
involved in the antiproliferative effects of the combined
treatment.
ROS are known to disrupt Δψm, and
therefore trigger a series of mitochondrial-associated events
(35). A high level of ROS leads to
oxidative stress, loss of cell function and ultimately apoptosis or
necrosis (36). Natural products
may exert anticancer effects by inducing ROS-mediated apoptosis.
Both resveratrol and matrine were found to induce ROS production in
cancer cell lines (37–39). However, there is no evidence of the
combined effect of resveratrol and matrine on ROS generation. In
the present study, we demonstrated that the combination treatment
significantly enhanced the generation of ROS in HepG2 cells when
compared with either agent alone. Furthermore, the combined
treatment also resulted in loss of Δψm. Therefore,
induction of ROS generation and disruption of Δψm are
involved in potentiating resveratrol-induced apoptosis by
matrine.
Taken together, resveratrol exhibits multiple
anticancer effects by inducing cell growth inhibition, cell cycle
arrest and apoptosis in HepG2 cells. Moreover, the present study is
the first to demonstrate that the combination treatment of
resveratrol and matrine exhibits a synergistic antiproliferative
effect on HepG2 cells. Therefore, the combined treatment of
resveratrol and matrine is an effective and promising strategy for
the prevention and treatment of liver cancer.
Acknowledgements
This study was supported by the Central Research
Institutes of Basic Research and Public Service Special Operations
in 2013.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2001. View Article : Google Scholar
|
|
2
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Je Y, Schutz FA and Choueiri TK: Risk of
bleeding with vascular endothelial growth factor receptor
tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic
review and meta-analysis of clinical trials. Lancet Oncol.
10:967–974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang M, Cai L, Udeani GO, et al: Cancer
chemopreventive activity of resveratrol, a natural product derived
from grapes. Science. 275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bishayee A, Politis T and Darvesh AS:
Resveratrol in the chemoprevention and treatment of hepatocellular
carcinoma. Cancer Treat Rev. 36:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu SL, Sun ZJ, Yu L, Meng KW, Qin XL and
Pan CE: Effect of resveratrol and in combination with 5-FU on
murine liver cancer. World J Gastroenterol. 10:3048–3052.
2004.PubMed/NCBI
|
|
9
|
George J, Singh M, Srivastava AK, et al:
Resveratrol and black tea polyphenol combination synergistically
suppress mouse skin tumors growth by inhibition of activated MAPKs
and p53. PLoS One. 6:e233952011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwuchukwu OF, Tallarida RJ and Nagar S:
Resveratrol in combination with other dietary polyphenols
concomitantly enhances antiproliferation and UGT1A1 induction in
Caco-2 cells. Life Sci. 88:1047–1054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Zhang J, Wang Y, et al: Matrine, a
novel autophagy inhibitor, blocks trafficking and the proteolytic
activation of lysosomal proteases. Carcinogenesis. 34:128–138.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S, Zhang Y, Zhuang Y, et al: Matrine
induces apoptosis in human acute myeloid leukemia cells via the
mitochondrial pathway and Akt inactivation. PLoS One. 7:e468532012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou H, Xu M, Gao Y, et al: Matrine
induces caspase-independent program cell death in hepatocellular
carcinoma through bid-mediated nuclear translocation of apoptosis
inducing factor. Mol Cancer. 13:592014. View Article : Google Scholar
|
|
14
|
Zhang L, Wang T, Wen X, et al: Effect of
matrine on HeLa cell adhesion and migration. Eur J Pharmacol.
563:69–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang T, Zhu Y, Luo C, et al: Matrine
inhibits the activity of translation factor eIF4E through
dephosphorylation of 4E-BP1 in gastric MKN45 cells. Planta Med.
73:1176–1181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun M, Cao H, Sun L, et al: Antitumor
activities of kushen: literature review. Evid Based Complement
Alternat Med. 2012:3732192012.PubMed/NCBI
|
|
17
|
Yu Q, Chen B, Zhang X, Qian W, Ye B and
Zhou Y: Arsenic trioxide-enhanced, matrine-induced apoptosis in
multiple myeloma cell lines. Planta Med. 79:775–781. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao N, Shang B, Zhang X, et al: Potent
antitumor actions of the new antibiotic boningmycin through
induction of apoptosis and cellular senescence. Anticancer Drug.
22:166–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis, and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903.
2002.PubMed/NCBI
|
|
21
|
Garvin S, Ollinger K and Dabrosin C:
Resveratrol induces apoptosis and inhibits angiogenesis in human
breast cancer xenografts in vivo. Cancer Lett. 231:113–122. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: the in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stervbo U, Vang O and Bonnesen C: Time-
and concentration-dependent effects of resveratrol in HL-60 and
HepG2 cells. Cell Prolif. 39:479–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong RS: Apoptosis in cancer: from
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang H, Zhang L, Kuo J, et al:
Resveratrol-induced apoptotic death in human U251 glioma cells. Mol
Cancer Ther. 4:554–561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kai L, Samuel SK and Levenson AS:
Resveratrol enhances p53 acetylation and apoptosis in prostate
cancer by inhibiting MTA1/NuRD complex. Int J Cancer.
126:1538–1548. 2010.PubMed/NCBI
|
|
27
|
Scarlatti F, Sala G, Somenzi G, Signorelli
P, Sacchi N and Ghidoni R: Resveratrol induces growth inhibition
and apoptosis in metastatic breast cancer cells via de novo
ceramide signaling. FASEB J. 17:2339–2341. 2003.PubMed/NCBI
|
|
28
|
Cepero E, King AM, Coffey LM, Perez RG and
Boise LH: Caspase-9 and effector caspases have sequential and
distinct effects on mitochondria. Oncogene. 24:6354–6366.
2005.PubMed/NCBI
|
|
29
|
Kumar S: Caspase function in programmed
cell death. Cell Death Differ. 14:32–43. 2007. View Article : Google Scholar
|
|
30
|
Bagci EZ, Vodovotz Y, Billiar TR,
Ermentrout GB and Bahar I: Bistability in apoptosis: roles of bax,
bcl-2, and mitochondrial permeability transition pores. Biophys J.
90:1546–1559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du Q, Hu B, An HM, et al: Synergistic
anticancer effects of curcumin and resveratrol in Hepa1–6
hepatocellular carcinoma cells. Oncol Rep. 29:1851–1858.
2013.PubMed/NCBI
|
|
32
|
Pan X, Zhang X, Sun H, Zhang J, Yan M and
Zhang H: Autophagy inhibition promotes 5-fluorouraci-induced
apoptosis by stimulating ROS formation in human non-small cell lung
cancer A549 cells. PLoS One. 8:e566792013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh RP, Dhanalakshmi S, Agarwal C and
Agarwal R: Silibinin strongly inhibits growth and survival of human
endothelial cells via cell cycle arrest and downregulation of
survivin, Akt and NF-κB: implications for angioprevention and
antiangiogenic therapy. Oncogene. 24:1188–1202. 2005.PubMed/NCBI
|
|
34
|
Wang T, Wei J, Qian X, Ding Y, Yu L and
Liu B: Gambogic acid, a potent inhibitor of survivin, reverses
docetaxel resistance in gastric cancer cells. Cancer Lett.
262:214–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kirkland RA and Franklin JL: Bax, reactive
oxygen, and cytochrome c release in neuronal apoptosis.
Antioxid Redox Signal. 5:589–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miki H, Uehara N, Kimura A, et al:
Resveratrol induces apoptosis via ROS-triggered autophagy in human
colon cancer cells. Int J Oncol. 40:1020–1028. 2012.PubMed/NCBI
|
|
38
|
Tan C, Qian X, Jia R, Wu M and Liang Z:
Matrine induction of reactive oxygen species activates p38 leading
to caspase-dependent cell apoptosis in non-small cell lung cancer
cells. Oncol Rep. 30:2529–2535. 2013.PubMed/NCBI
|
|
39
|
Hussain AR, Uddin S, Bu R, et al:
Resveratrol suppresses constitutive activation of AKT via
generation of ROS and induces apoptosis in diffuse large B cell
lymphoma cell lines. PLoS One. 6:e247032011. View Article : Google Scholar : PubMed/NCBI
|