Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer-related mortality in females
worldwide (1). While significant
treatment advances have been made, all patients face the risk of
disease recurrence, which is the main cause of death from breast
cancer (2). In recent years,
studies on cancer development and recurrence have been influenced
by side population (SP) cells (3).
SP cells are a small subpopulation of cells with enriched stem cell
activity (4). Dye exclusion is a
valuable technique successful in isolating and identifying SP
cells, based on stem cells possessing a high ability to exclude
fluorescent DNA-binding dye, Hoechst 33342 (5–7).
Gemcitabine [2′,2′-difluorodeoxycytidine (dFdC)] is
a pyrimidine nucleoside analogue of deoxycytidine commonly used in
non-small-cell lung cancer (NSCLC) and breast cancer (8,9).
Gemcitabine is phosphorylated by deoxycytidine kinases, which then
incorporate into DNA to inhibit synthesis and cell proliferation,
while promoting apoptosis in cancer cells (10). Doxorubicin is one of the most
effective and widely used chemotherapeutic agents for the treatment
of various human malignancies (11,12).
To the best of our knowledge, the effects of
doxorubicin and gemcitabine on breast cancer cells are poorly
understood. The present study addressed, for the first time, the
potential killing roles of doxorubicin and gemcitabine in breast
cancer SP and main population (MP) cells, as well as the mechanisms
of doxorubicin and gemcitabine. This finding may be useful in
improving the clinical effectiveness of biotherapy for the
treatment of malignant tumors.
Materials and methods
Cell culture
MCF7 cells were purchased from the American Type
Culture Collection (ATCC; Rockville, MD, USA) and maintained in
RPMI-1640 containing 10% heat-inactivated fetal bovine serum (FBS)
and 1% penicillin-streptomycin. Cells were maintained in a
humidified cell incubator with 5% CO2 at 37°C.
SP cell analysis
MCF7 cells were suspended at 1×106
cells/ml and then incubated at 37°C for 60 min with 5 μg/ml Hoechst
33342 (Sigma Chemicals, St. Louis, MO, USA). The control cells were
cultured in the presence of 500 μM verapamil (Sigma). Analysis and
sorting of the SP cells was performed using a FACS VantageSE
cytometer (Becton-Dickinson, San Jose, CA, USA). Hoechst 33342 was
excited using a UV laser at 350 nm and fluorescence emission was
measured at 402–446 and 640 nm for Hoechst blue and red,
respectively.
RT-PCR
Total RNA was isolated from MP and SP cells using an
RNeasy Mini kit (Biomed, Beijing, China). cDNA was reverse
transcribed with 1 μg of total RNA using a Takara Reverse
Transcription kit (Takara, Dalian, China) and was amplified using
the following primers: CD133: 5′-ACCGACTGAGACCCAACATC-3′
(sense), and 5′-GGTGCTGTTCATGTTCTCCA-3′ (antisense) and
ABCG2: 5′-AGCTGCAAGGAAAGATCCAA-3′ (sense), and
5′-TCCAGACACACCACGGATAA-3′ (antisense). GAPDH primers were:
5′-AGAAGGCTGGGGCTCATTTG-3′ (sense), and 5′-AGGGGCCATCCACAGTCTTC-3′
(antisense), and used as an internal control. The PCR products were
electrophoresed on a 1.5% agarose gel, and visualized by ethidium
bromide staining under a UV imaging system (UVP, LLC, Upland, CA,
USA).
Immunofluorescence
Cells were fixed with 4% paraformaldehyde for 15–20
min, washed twice in phosphate-buffered saline (PBS) at room
temperature for 5 min and permeabilized in PBS containing 2% Triton
X-100 for 30 min. Non-specific binding sites were blocked with 3%
bovine serum albumin (BSA) in PBS for 1 h. The primary monoclonal
antibody CD133 or ABCG2, diluted in 3% BSA/PBS, was applied
overnight at 4°C. The cells were washed twice with PBS and then
exposed to the secondary antibody diluted at 1:100 in 3% BSA/PBS
for 1 h. For every coverslip, the cells were observed and
photographed in 5 random fields using an Olympus CX71 fluorescence
microscope (Olympus, Tokyo, Japan).
Sphere assay
The SP and MP cells were plated at a density of
6×104 cells/well in 6-well, ultra-low attachment plates
under serum-free, sphere-specific conditions described by Gibbs
et al (13). Fresh aliquots
of epidermal growth factor (EGF) and basic fibroblast growth factor
(bFGF) were added each day. After culture for 7 days, the spheres
were visible under a fluorescence microscope, as described
above.
Drug and treatment
Doxorubicin (D1515) and gemcitabine (Y0000676) were
purchased from Sigma. MCF7 MP cells were designed as group 1
(control group, no treatment with drugs); group 2 (1 μg/ml,
doxorubicin-treated MP cells); group 3 (1 μg/ml,
gemcitabine-treated MP cells); MCF-7 SP cells were designed as
group 4 (control group, no treatment with drugs); group 5 (1 μg/ml,
doxorubicin-treated SP cells); and group 6 (1 μg/ml,
gemcitabine-treated SP cells).
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay
The proliferation rate of the treated and control
cells was measured by MTT assay. Briefly, for the MTT assay,
treated or control cells were plated at a density of
1×103/well in 96-well plates and incubated for 48 h
under complete culture medium containing 0.5 mg/ml MTT (Sigma).
Four hours later, the medium was replaced with 100 μl dimethyl
sulfoxide (DMSO) (Sigma) and vortexed for 10 min to dissolve the
crystals. Absorbance optical density (OD) of each well was
determined at a wavelength of 490 nm with subtraction of baseline
reading.
Apoptosis assay
For the apoptosis assay, equal numbers of cells were
seeded in 6-cm plates. Following the manufacturer’s instructions
(Apoptosis Detection kit; KeyGen, Nanjing, China), the cells were
trypsinized, washed twice with cold PBS, and resuspended in 200 μl
binding buffer. Annexin V-FITC was added to a final concentration
of 0.5 μg/ml. Samples were incubated at room temperature in the
dark. After 20 min, 300 μl binding buffer containing 0.5 μg/ml PI
was added and samples were immediately analyzed on a FACSCalibur
flow cytometer (Becton-Dickinson Medical Devices, Shanghai, China).
Cells in the stages of early apoptosis were defined as
FITC+/PI− cells.
Determination of mitochondrial membrane
potential (MMP)
MMP was analyzed using the fluorescent dye
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolycarbocyanine
iodide (JC-1) following the manufacturer’s instructions (KeyGen).
Briefly, the cells were plated in 6-well culture plates. After
treatment for 24 h, the cells were washed twice with PBS, harvested
and incubated with 20 nM JC-1 for 30 min in the dark. MMP was
subsequently analyzed using the FACSCalibur machine, as described
above.
Quantification of cellular reactive
oxygen species (ROS)
Cells (5×105) were cultured in 12-well
tissue culture plates overnight, and then co-treated with drugs and
2′,7′-dichlorofluorescein diacetate, a ROS-sensitive dye (KeyGen).
After drug treatment, the cells were harvested and suspended in
PBS. Relative fluorescence intensities of cells were quantified
using the FACSCalibur machine, as described above.
Adhesion assay
The adhesion ability of cancer cells was examined
using the adhesion assay. Six-well plates were coated with collagen
I (10 μg/ml), fibronectin (10 μg/ml) or growth factor-reduced
Matrigel (10 μg/ml) (BD Biosciences), with 1% BSA as the control.
The cells were harvested with trypsin-EDTA and resuspended in
serum-free medium. The cells were allowed to attach at 37°C for 1
h. Unbound cells were removed by washing twice with PBS. Attached
cells were fixed in 4% paraformaldehyde and counted. Cell counts
were obtained by averaging the cell numbers from five wells. The
percentage of cells adhering was calculated as: % bindings = (OD of
treated surface-only ECM component)/OD of total surface × 100.
Cell invasion assay
For the invasive assay, the cells were resuspended
in serum-free RPMI-1640 and seeded in the control-membrane insert
on the top portion of the Matrigel-coated chamber (BD Biosciences).
The lower compartment of the chamber contained 10% FBS as a
chemoattractant. After incubation for 24 h, the cells on the
membrane were scrubbed, washed with PBS and fixed in 100% methanol
and stained with Giemsa dye (KeyGen).
Transplantation experiment
Sorted SP and MP cells were collected, and the cells
were resuspended in HBSS. Cell suspension was then mixed with
Matrigel (1:1). This cell-Matrigel suspension was then
subcutaneously injected into 4-week-old BALB/C-nu/nu nude mice
(male) obtained from the Shanghai Laboratory Animal Center of China
under anesthesia. Groups of mice were inoculated with SP cells at
1×105 or MP cells at 1×107. The mice were
examined once every 5 days and tumor growth was evaluated by
measuring the length and width of the tumor. The mice were
sacrificed and tumor masses were removed and fixed in 10%
neutral-buffered formalin solution for histological
preparations.
Immunohistochemistry (IHC)
Sections (4 μm) were baked at 65°C for 30 min, and
then deparaffinized with xylene and rehydrated. The sections were
submerged into EDTA (pH=8.0), autoclaved for antigen retrieval, and
treated with 3% hydrogen peroxide, followed by incubation with 1%
FBS. The primary antibody was added and incubated overnight at 4°C.
Horseradish peroxidase (HRP)-labeled secondary antibody in the
MaxVision™ HRP-Polymer anti-Mouse/Rabbit IHC kit (KIT-5930; Maixin
Biology, Fuzhou, China) was applied and incubated for 30 min,
followed by 5 min incubation with DAB, provided in the kit for
color development. The sections were then counterstained with
hematoxylin and mounted. The results were visualized and
photographed under a light microscope.
PHYRE database was used to generate
predicted structural models
The protein sequence of ABCG2 was obtained from the
PubMed (http://www.ncbi.nlm.nih.gov/protein/AAG52982.1) and
submitted to the Protein Homology/analogY Recognition Engine
(PHYRE; version 2). Based on the homology sequence in the PHYRE
server, the three-dimensional structure of the ABCG2 protein was
predicted.
Preparation of proteins and ligand
structures for docking
We applied this approach to the predicted structural
model of ABCG2. The molecular structures of doxorubicin (CID 31703)
and gemcitabine (CID 60750) were downloaded from PubChem Compound
(http://www.ncbi.nlm.nih.gov/pccompound). Data were
imported into the modeling software SYBYL-X 1.3 (Tripos
International, St. Louis, MO, USA). Non-protein components such as
water molecules, metal ions and lipids were deleted, and hydrogen
atoms were added to the protein structures. The interaction of
doxorubicin or gemcitabine and ABCG2 was analyzed by SYBYL-X
1.3.
SDS-PAGE and immunoblotting
Proteins (30 μg/lane) were separated by 10%
SDS-polyacrylamide gel electrophoresis and transferred to PVDF
membranes (Millipore Corporation, Billerica, MA, USA). Western
blotting was performed using primary antibodies: anti-ABCG2 (4477;
1:200) was purchased from Cell Signaling Technology (Beverly, MA,
USA), and anti-CD133 (MAB4310; 1:200) was purchased from Millipore
Corporation. Anti-Bax (sc-7480; 1:500), anti-Bcl-xL (sc-8392;
1:500), anti-Bcl-2 (sc-783; 1:500), anti-phospho-Bcl-2 (Ser 87)
(sc-16323; 1:500) and β-actin (sc-47778; 1:1,000) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Incubation with antibodies was performed in 1.5% BSA in TBS, 0.1%
Tween. Detection of the immune complexes was performed with the ECL
Plus Western Blotting Detection System (Amersham Biosciences,
Piscataway, NJ, USA).
Statistical analysis
Data are presented as means ± SD. The statistical
significance of differences was determined by Student’s two-tailed
t-test in two groups and one-way ANOVA in multiple groups.
Kaplan-Meier survival plots were generated and comparisons between
survival curves were made with the log-rank statistic. P<0.05
was considered to indicate a statistically significant result. Data
were analyzed with GraphPad Prism 5 (San Diego, CA, USA).
Results
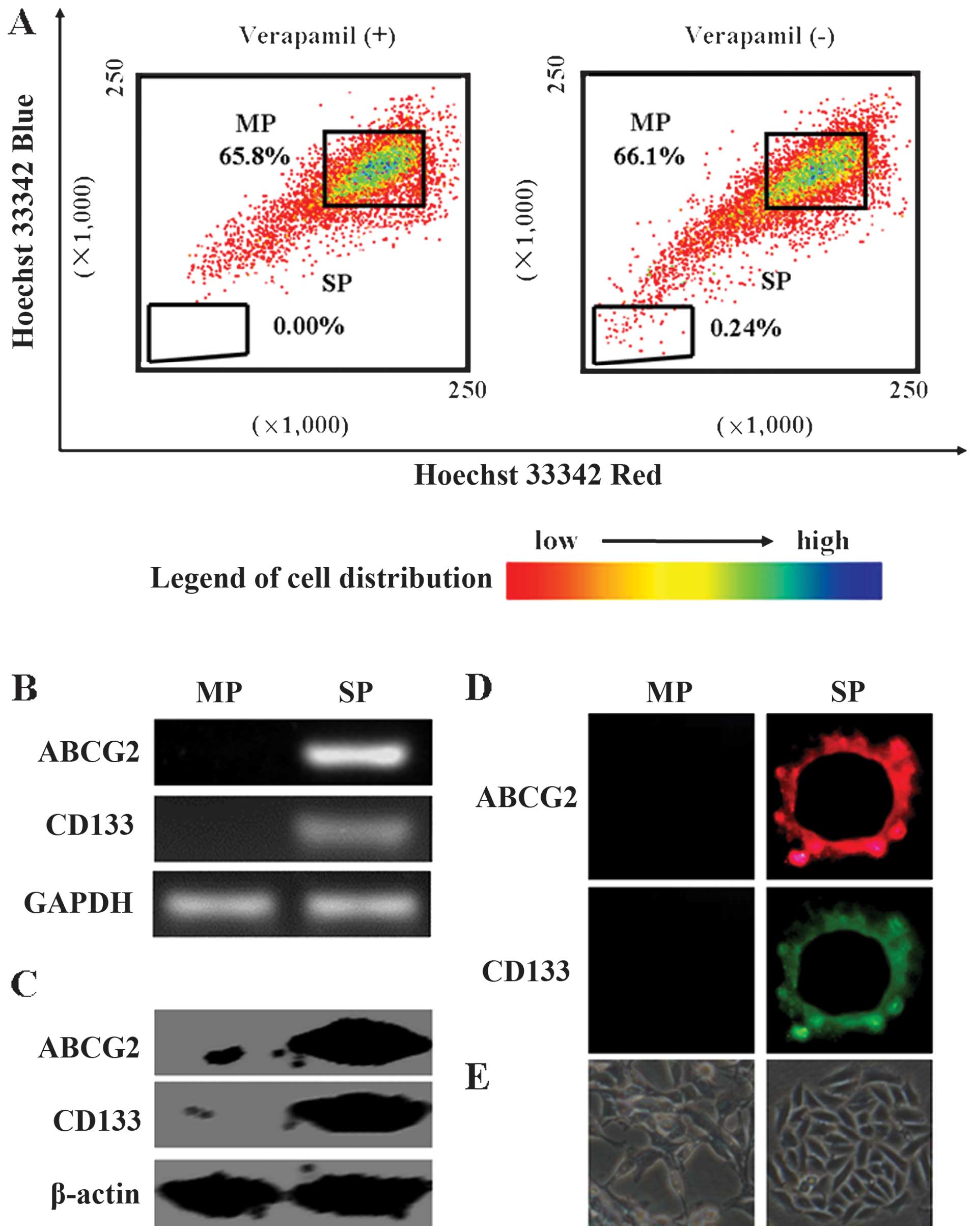
SP fraction in MCF7 cells
The SP cell fraction comprised 0.24% of the total
MCF7 cells, and this population disappeared following treatment
with the selective ABCG2 transporter inhibitor, verapamil (Fig. 1A). In addition, we showed that
CSC-specific markers, ABCG2 and CD133 mRNA, and proteins were
significantly increased in the SP cells when compared with those in
MP cells (Fig. 1B and C).
Immunofluorescence results showed that ABCG2 and CD133 were
localized in the membrane of the SP cells (Fig. 1D). After 7 days of culture, sphere
clusters were clearly observed in the SP cultures, while MP cells
did not form spheres (Fig. 1E).
Effects of doxorubicin or gemcitabine on
the proliferation, apoptosis, MMP and mobility of MCF7 cells
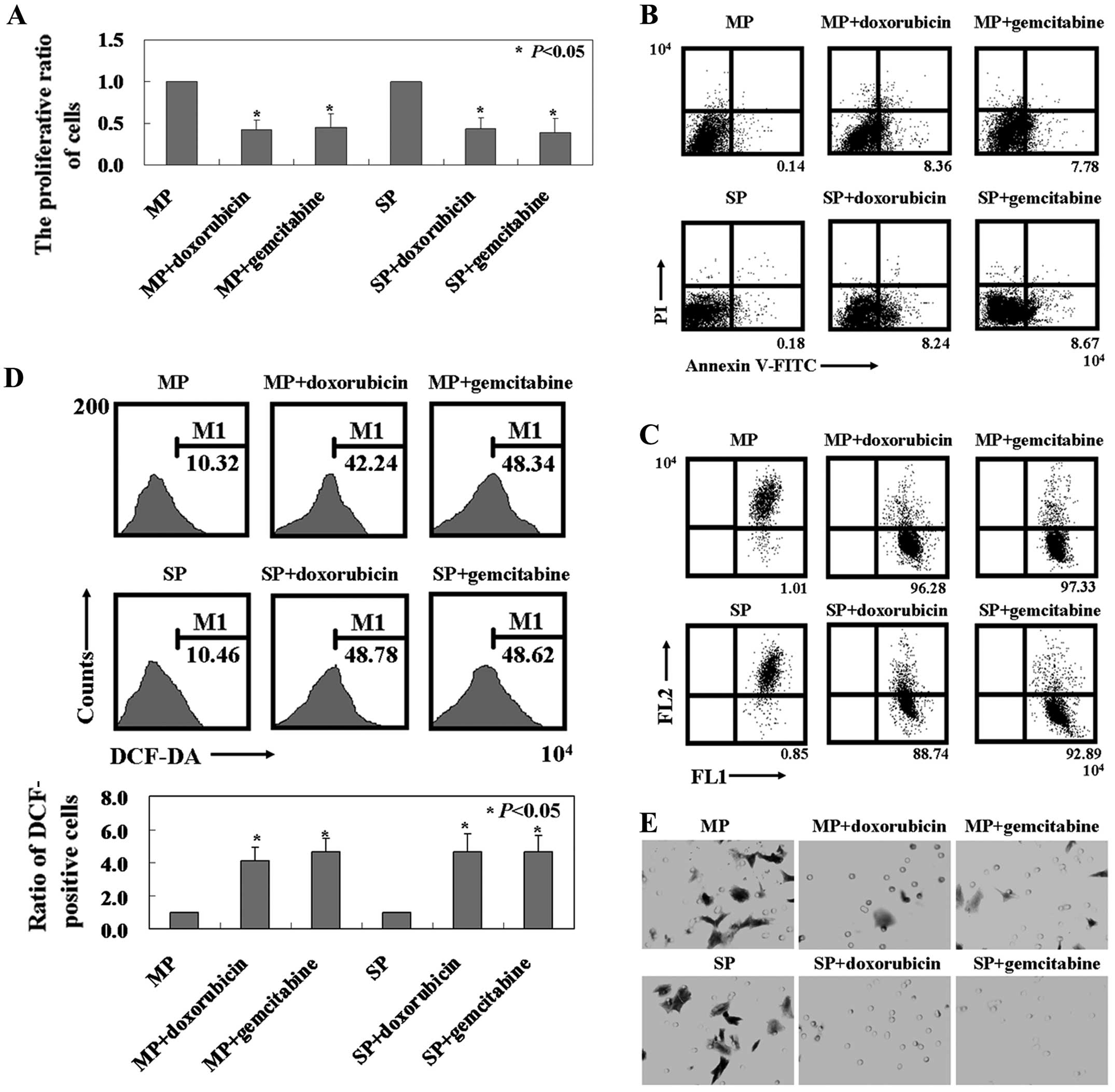
As shown in Fig. 2A,
doxorubicin and gemcitabine decreased the cell viability of MCF7 SP
and MP cells (P<0.05). In addition, doxorubicin or
gemcitabine-induced apoptosis was detected in the SP and MP cells
by Annexin V/PI double staining (Fig.
2B). Changes in MMP were detected in MCF7 MP and SP cells after
doxorubicin or gemcitabine treatment by using flow cytometry
(Fig. 2C). Furthermore, the level
of ROS content was significantly increased in MCF7 MP and SP cells
after doxorubicin or gemcitabine treatment compared to untreated MP
and SP cells using the fluorescent dye DCF-DA (P<0.05, Fig. 2D). Based on these results, we
hypothesized that doxorubicin- or gemcitabine-induced apoptosis in
MCF7 MP and SP cells was associated with the mitochondrial
apoptotic signaling pathway. Since breast cancer has a high rate of
metastasis, we determined whether there were any mobility changes
in MCF7 MP and SP cells after doxorubicin or gemcitabine treatment
using the Transwell assay. We found that significantly less MCF7 MP
and SP cells with doxorubicin or gemcitabine treatment migrated to
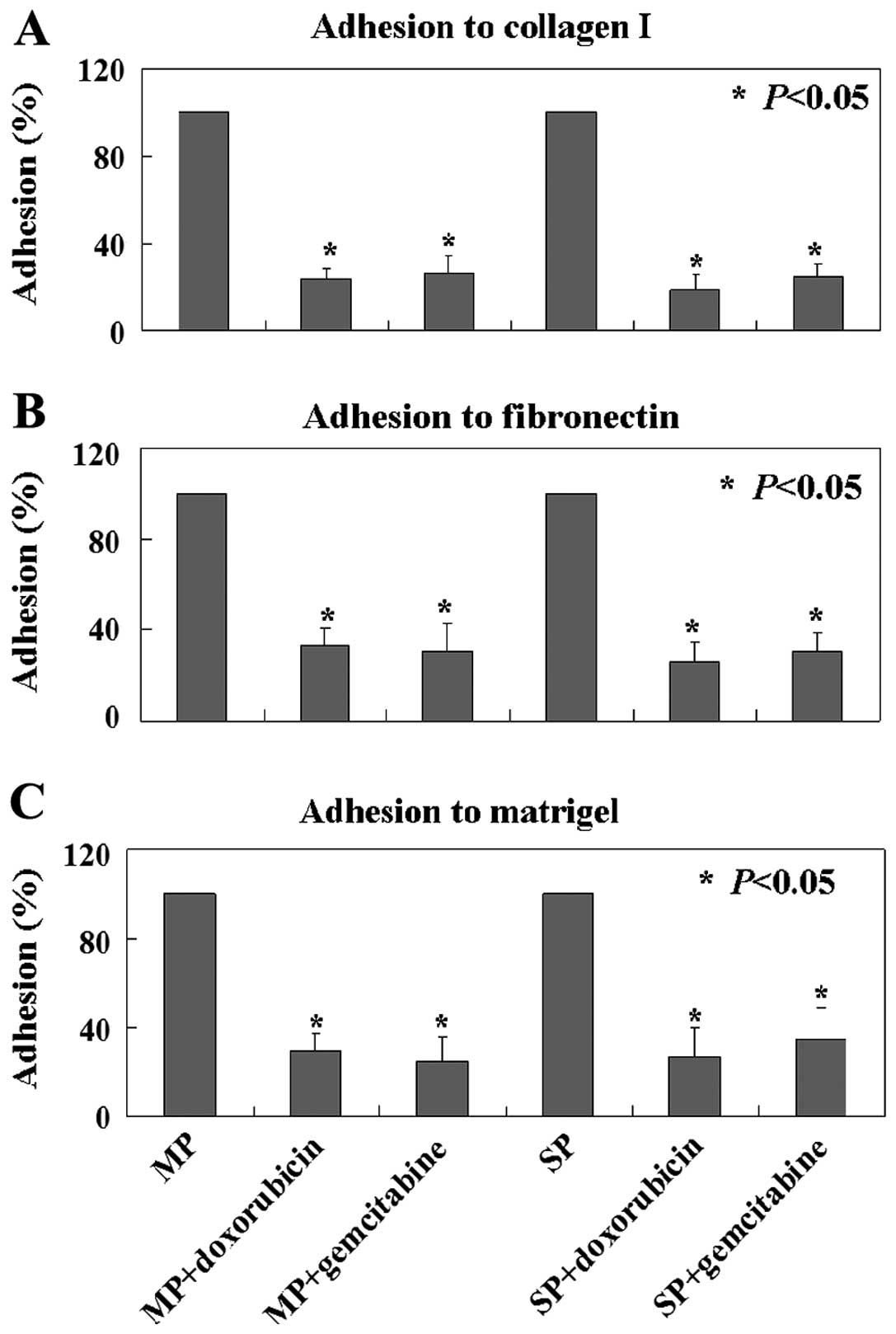
the lower membrane compared to control cells (Fig. 2E). In ECM-mediated adhesion, MCF7 MP
and SP cells with doxorubicin or gemcitabine treatment showed
significantly less adhesion to type I collagen, fibronectin and
Matrigel compared to the untreated MCF7 MP and SP cells (Fig. 3).
Doxorubicin or gemcitabine inhibits tumor
growth and improves survival rate of mice in vivo
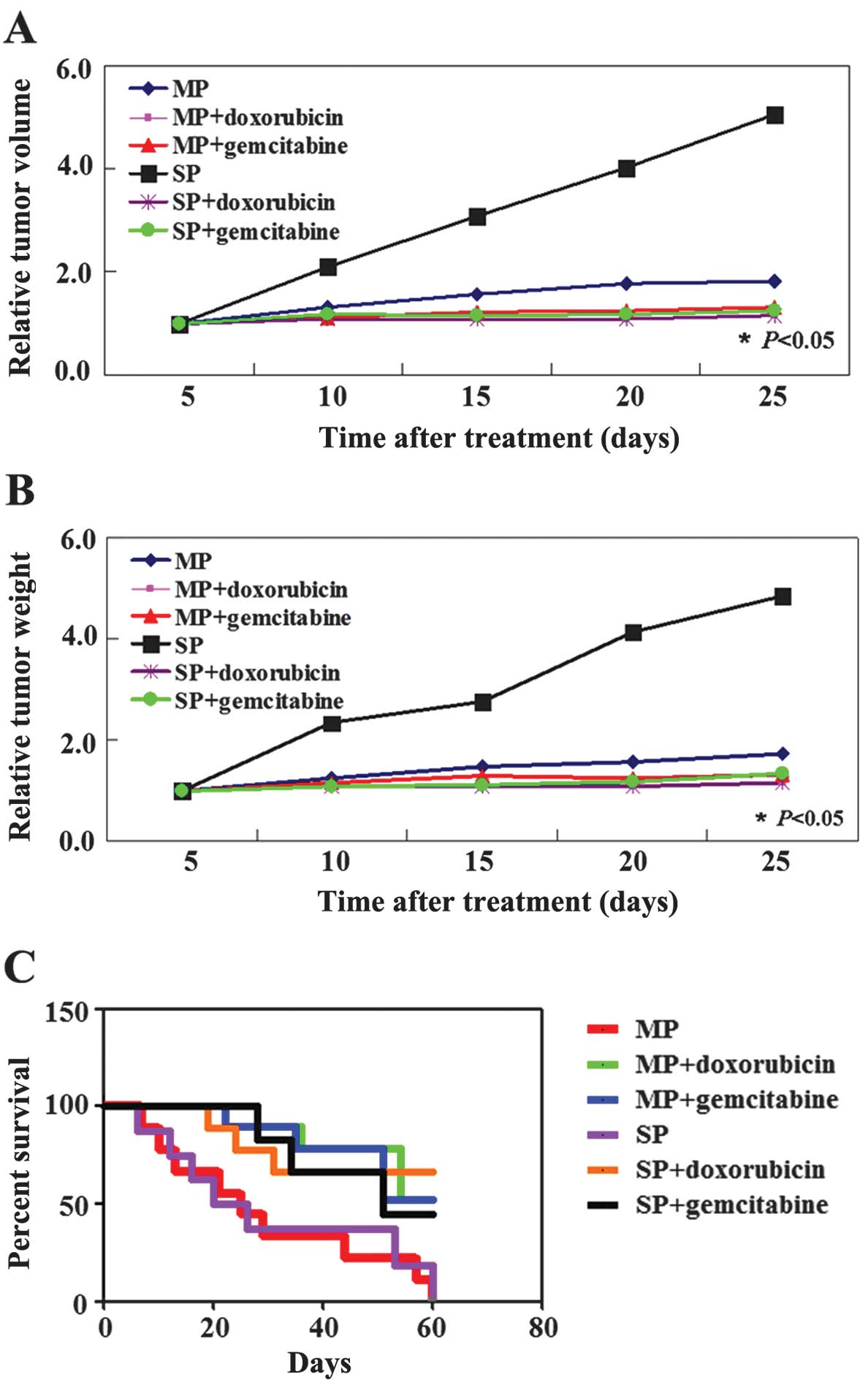
We determined whether doxorubicin or gemcitabine
exhibits antitumor properties in established xenograft tumor
models. As shown in Fig. 4A and B,
the tumor volume of doxorubicin or gemcitabine-treated MP mice was
less than that of the untreated MP mice (P<0.05). Tumor weight
was significantly decreased in the doxorubicin or
gemcitabine-treated MP group compared to the untreated group by 20
days after treatment (P<0.05). Similar results were observed in
the SP group (P<0.05, Fig. 4A and
B). We also found that doxorubicin or gemcitabine-treated mice
had an improved survival rate compared to the untreated mice
(P<0.05, Fig. 4C). In the
untreated groups, the mice needed to be sacrificed due to tumor
burden or organ failure beginning 5 days after treatment. However,
mice in the doxorubicin- or gemcitabine-treated group did not need
to be sacrificed until 20 days after treatment. The survival rate
of the doxorubicin or gemcitabine-treated group was 50% when
compared to the control groups.
Doxorubicin or gemcitabine binds to ABCG2
and activates the mitochondrial apoptotic signaling pathway in MCF
MP and SP cells
To examine the possible mechanisms whereby
doxorubicin or gemcitabine could play roles in SP and MP cells, we
applied the modeling software SYBYL-X 1.3 and found that
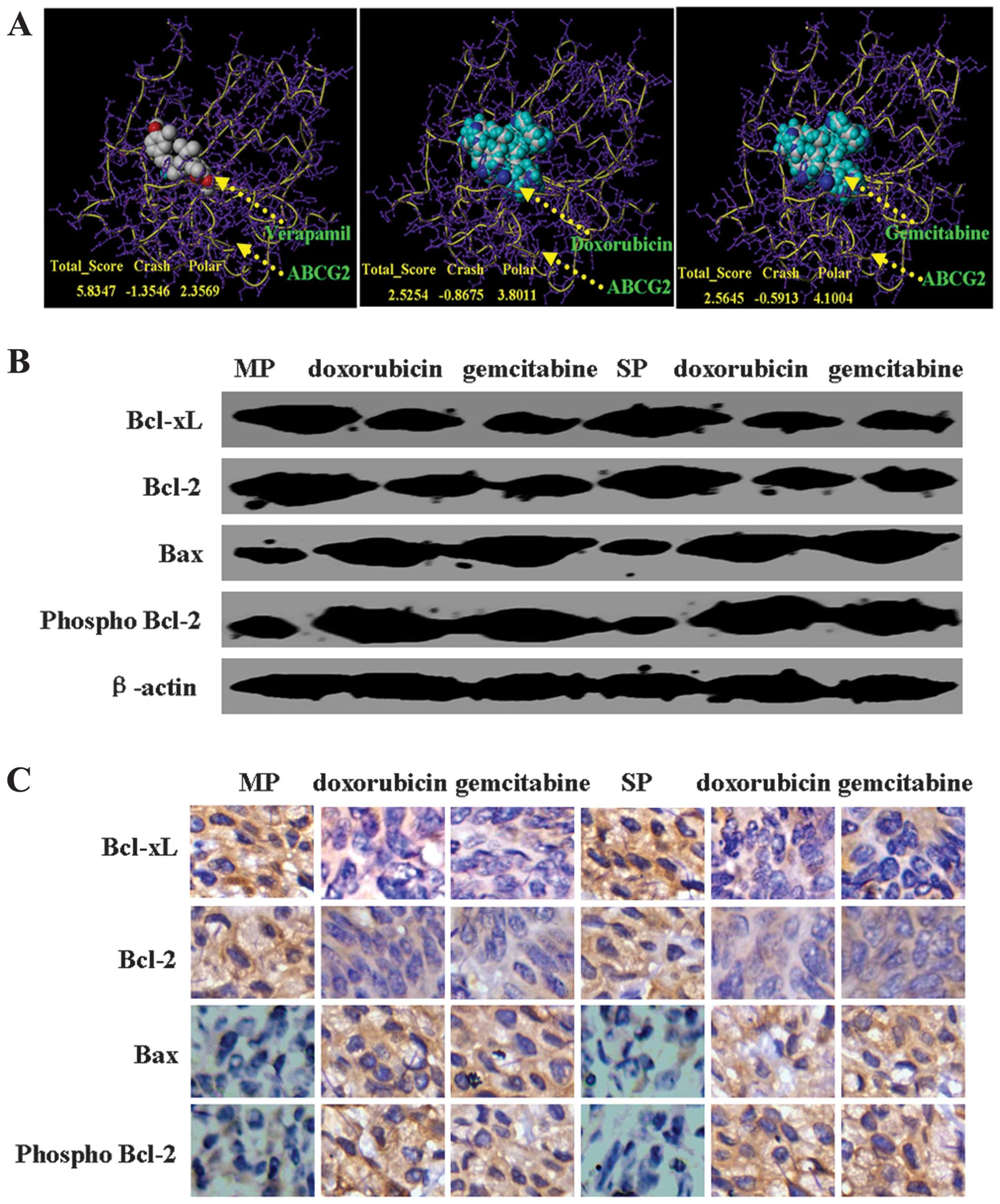
doxorubicin or gemcitabine was able to dock into ABCG2 (Fig. 5A). Interesting, the docking position
of doxorubicin or gemcitabine in ABCG2 was similar to verapamil
(Fig. 5A). In order to identify the
mechanism of action of doxorubicin or gemcitabine in MCF7 MP and SP
cells, we detected the protein expression of Bax, Bcl-2, p-Bcl-2
and Bcl-xL by western blot analysis. We found a decrease in Bcl-2
and Bcl-xL protein as well as an increase in p-Bcl-2 and Bax
protein levels in MCF7 MP and SP cells with doxorubicin or
gemcitabine treatment (Fig. 5B).
Furthermore, we confirmed our results in vivo by using IHC
(Fig. 5C).
Discussion
ABCG2 is one of the human ABC transporters that are
involved in multidrug resistance (MDR) in cancer chemotherapy
(14,15). The ABCG2 gene, located on
chromosome 4, is >66 kb and contains 16 exons and 15 introns
(16). ABCG2 is a 655 amino acid,
72-kDa protein with a single ABC signature domain within the
nucleotide-binding domain and six transmembrane domains (17). Previous studies of the role of drug
efflux in resistance to doxorubicin or gemcitabine have yielded
controversial results. Zhou et al (18) suggested that the expression of ABCB1
and ABCG2 (BCRP) transporters may contribute to gemcitabine
resistance and tumor relapse. However, Bergman et al
(19) reported that the expression
of ABCB1 and ABCC1 (MRP1) enhances gemcitabine sensitivity.
Doxorubicin or gemcitabine have been shown to possess a broad
antitumor activity against breast, lung, ovarian, bladder and
pancreatic cancer (10–12). In the present study, we found that
doxorubicin or gemcitabine inhibited MCF7 MP and SP cells. We
hypothesized that the high intracellular accumulation of
doxorubicin or gemcitabine in MCF7 MP and SP cells was a
consequence of the decreased activity of ABCG2. Notably, we found
that the docking position of doxorubicin or gemcitabine in ABCG2
was similar to that of verapamil. Verapamil is a non-selective
pharmacological inhibitor of ABC transporter family members
(20). Future studies should be
conducted to determine the roles of doxorubicin or gemcitabine in
ABCG2.
Furthermore, we found that doxorubicin- or
gemcitabineinduced apoptosis in MP and SP cells were associated
with loss of mitochondrial membrane potential (MMP). The disruption
of MMP has been reported to be affected by reactive oxygen species
(ROS) (21). Consistent with recent
studies (22,23), in the present study, we found that
ROS is significantly increased in MP and SP cells with doxorubicin
or gemcitabine treatment. Bcl-2 and Bcl-xL are two anti-apoptotic
proteins and Bax is a pro-apoptotic protein in the mitochondrial
apoptotic pathway (22–24). In concordance with the earlier
findings, we observed reductions in the expression of Bcl-2 and
Bcl-xL in cells treated with doxorubicin or gemcitabine. Consistent
with the results by Xia et al (22), we also found that p-Bcl-2, an
inactivated form of Bcl-2, was significantly increased.
In summary, doxorubicin and gemcitabine decreased
the cell viability, induced apoptosis and mitochondrial damage in
MCF7 SP and MP cells. Consequently, the mitochondrial apoptotic
pathway was activated. Therefore, the present study has expanded
our understanding of the role of doxorubicin and gemcitabine in
MCF7 SP and MP cells. However, whether the high intracellular
accumulation of doxorubicin or gemcitabine in MCF7 MP and SP cells
was a consequence of the decreased activity of ABCG2 remains
unclear.
Acknowledgements
We would like to thank Miss Ying Zhang for her
valuable comments and excellent technical assistance.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Glück S and Gorouhi F: Clinical and
economic benefits of aromatase inhibitor therapy in early-stage
breast cancer. Am J Health Syst Pharm. 68:1699–1706.
2011.PubMed/NCBI
|
|
3
|
Noto A, Raffa S, De Vitis C, et al:
Stearoyl-CoA desaturase-1 is a key factor for lung
cancer-initiating cells. Cell Death Dis. 4:e9472013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia P, Gou WF, Zhao S and Zheng HC:
Crizotinib may be used in Lewis lung carcinoma: A novel use for
crizotinib. Oncol Rep. 30:139–148. 2013.PubMed/NCBI
|
|
6
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haraguchi N, Utsunomiya T, Inoue H, et al:
Characterization of a side population of cancer cells from human
gastrointestinal system. Stem Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
View Article : Google Scholar
|
|
9
|
Spielmann M, Llombart-Cussac A, Kalla S,
et al: Single-agent gemcitabine is active in previously treated
breast cancer. Oncology. 60:303–307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Plunkett W, Huang P, Xu YZ, et al:
Gemcitabine: metabolism, mechanisms of action, and
self-potentiation. Semin Oncol. 22(Suppl 11): S3–S10. 1995.
|
|
11
|
Chatterjee K, Zhang J, Honbo N and
Karliner JS: Doxorubicin cardiomyopathy. Cardiology. 115:155–162.
2010. View Article : Google Scholar
|
|
12
|
Takemura G and Fujiwara H:
Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms
to management. Prog Cardiovasc Dis. 49:330–352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu J, Peng H and Zhang JT: Human multidrug
transporter ABCG2, a target for sensitizing drug resistance in
cancer chemotherapy. Curr Med Chem. 14:689–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang JT: Biochemistry and pharmacology of
the human multidrug resistance gene product, ABCG2. Zhong Nan Da
Xue Xue Bao Yi Xue Ban. 32:531–541. 2007.PubMed/NCBI
|
|
16
|
Kanzaki A, Toi M, Neamati N, et al:
Copper-transporting P-type adenosine triphosphatase (ATP7B) is
expressed in human breast carcinoma. Jpn J Cancer Res. 93:70–77.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robey RW, Ierano C, Zhan Z and Bates SE:
The challenge of exploiting ABCG2 in the clinic. Curr Pharm
Biotechnol. 12:595–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Wang CY, Liu T, et al: Persistence
of side population cells with high drug efflux capacity in
pancreatic cancer. World J Gastroenterol. 14:925–930. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergman AM, Pinedo HM, Talianidis I, et
al: Increased sensitivity to gemcitabine of P-glycoprotein and
multidrug resistance-associated protein-overexpressing human cancer
cell lines. Br J Cancer. 88:1963–1970. 2003. View Article : Google Scholar
|
|
20
|
Nobili S, Landini I, Giglioni B and Mini
E: Pharmacological strategies for overcoming multidrug resistance.
Curr Drug Targets. 7:861–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu ZH and Lenardo MJ: Reactive oxygen
species regulate autophagy through redox-sensitive proteases. Dev
Cell. 12:484–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia P, Gou WF, Wang JJ, et al: Distinct
radiosensitivity of lung carcinoma stem-like side population and
main population cells. Cancer Biother Radiopharm. 28:471–478. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xing YN, Deng P and Xu HM: Canstatin
induces apoptosis in gastric cancer xenograft growth in mice
through mitochondrial apoptotic pathway. Biosci Rep. Apr
2–2014.(Epub ahead of print).
|
|
24
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24−/low/CD44+ breast
cancer-initiating cells to radiation. J Natl Cancer Inst.
98:1777–1785. 2006.PubMed/NCBI
|