Introduction
Pancreatic cancer represents <3% of all new
cancer cases in the United States; however, it is the fourth
leading cause of cancer-related mortality (1). With a 5-year survival rate of ~6%, the
prognosis of this disease is considerably poor (1). Although surgical resection offers the
only chance for cure, the 5-year survival rate is only 20–25% and
~80–85% of patients present with advanced unresectable disease
(2). In addition, the front-line
gemcitabine-based therapy offers marginal efficacy, thus
highlighting the need to develop new therapies for this aggressive
disease.
Cancer cells employ several mechanisms of survival,
including overexpression of prosurvival Bcl-2 family members,
making them attractive targets for the treatment of numerous
malignancies. Since 88% of invasive ductal carcinomas express
Bcl-xL and Mcl-1, while 23% express Bcl-2 (3), small molecule inhibitors that target
these proteins could be promising therapeutic agents for the
treatment of pancreatic cancer.
GX15-070 (obatoclax) is a pan-Bcl-2 inhibitor that
has been demonstrated to directly induce apoptosis as well as
non-apoptotic cell death in solid tumors and hematologic
malignancies (4–8). In our most recent study, we
demonstrated that clinically achievable concentrations of GX15-070
suppressed the growth of pancreatic cancer cell lines. However,
they only induced minimal levels of apoptosis in the cells
(9). We hypothesized that GX15-070
induced autophagy which prevented apoptosis in pancreatic cancer
cells since Bcl-2 family proteins also play an important role in
autophagy. Beclin 1 is a Bcl-2 binding protein that regulates the
initial steps of autophagy (10).
Bcl-2, Mcl-1, and Bcl-xL have been shown to interact with Beclin 1,
binding through the BH3 domain, and inhibit autophagy (10,11).
In line with this, treatment with Bcl-2 inhibitors, such as
GX15-070, have been shown to induce autophagy in various
malignancies (12–15). Although autophagy has been shown to
be involved in tumor suppression as well as therapeutic resistance,
in pancreatic cancer it has been demonstrated to play a
cytoprotective role against anticancer drugs (16,17).
Therefore, combining an autophagy inhibitor with GX15-070 may
enhance GX15-070-induced apoptosis in pancreatic cancer cells.
In the present study, we investigated the
combination of GX15-070 and the autophagy inhibitor chloroquine
(CQ) in pancreatic cancer cell lines. GX15-070 treatment alone
induced autophagy in a dose-dependent manner. Addition of CQ
resulted in additive to synergistic growth inhibition as well as
enhanced apoptosis. These results suggest that combined GX15-070
and CQ might be a promising treatment for pancreatic cancer.
Materials and methods
Chemicals
GX15-070 (Obatoclax) was purchased from Selleck
Chemicals LLC (Houston, TX, USA). CQ was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The AsPC-1, BxPC-3, CFPAC-1, HPAC, MIA PaCa-2 and
PANC-1 human pancreatic cancer cell lines were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
HPAC, MIA PaCa-2 and PANC-1 cell lines were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Life Technologies, Carlsbad, CA,
USA); AsPC-1 and BxPC-3 were cultured in RPMI-1640 medium (Life
Technologies); and CFPAC-1 in Iscove’s modified Dulbecco’s medium
(IMDM; Life Technologies) with 10% heat-inactivated fetal bovine
serum (FBS; Hyclone Labs, Logan, UT, USA) plus 100 U/ml penicillin
and 100 μg/ml streptomycin in a 37°C humidified atmosphere
containing 5% CO2/95% air. Cell lines were authenticated
by the University of Arizona Genetics Core Facility (Tucson, AZ,
USA).
Western blot analysis
Whole cell lysates from AsPC-1, BxPC-3, CFPAC-1,
HPAC, MIA PaCa-2 or PANC-1 cells treated with vehicle control or
drugs for 72 h were subjected to SDS-polyacrylamide gel
electrophoresis. Separated proteins were electrophoretically
transferred to polyvinylidene difluoride (PVDF) membranes (Thermo
Fisher Inc., Rockford, IL, USA) and immunoblotted with anti-LC3B,
-PARP, -Mcl-1,-Bcl-2, -Bcl-xL (Cell Signaling Technology, Beverly,
MA, USA), or -β-actin (Sigma-Aldrich) antibody, as previously
described (18). Immunoreactive
proteins were visualized using the Odyssey Infrared Imaging System
(Li-Cor, Lincoln, NE, USA), as described by the manufacturer.
Western blot analyses were repeated at least three times and one
representative image is shown.
Transmission electron microscopy
(TEM)
Briefly, PANC-1 cells were treated with vehicle
control or 200 nM GX15-070 for 72 h. Then, the cells were
trypsinized and washed with PBS twice. The cells were fixed with
2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4). The
samples were treated with 1% osmium acid, dehydrated in a graded
series of ethanol, and embedded in Epon812 epoxy resin (SPI
Supplies/Structure Probe, Inc., West Chester, PA, USA). Semi-thin
sections were made using an LKB-III ultramicrotome (LKB Bromma,
Stockholm, Sweden). Then ultrathin sections were made and picked up
with naked 200 mesh copper grids. Grids were stained with uranyl
acetate and lead citrate. The ultrathin sections were then observed
using a Hitachi H-7650 transmission electron microscope (Hitachi
High-Technologies Corporation, Tokyo, Japan) at 80 kV.
In vitro cytotoxicity assays
In vitro CQ or GX15-070 cytotoxicities were
determined using
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT)
assays (Sigma-Aldrich), as previously described (19,20).
Briefly, cells were cultured in 100 μl of complete medium in the
presence of variable concentrations of GX15-070 or CQ alone or in
combination. The cells were incubated at 37°C in 96-well plates for
96 h. MTT was added to a final concentration of 1 mM. After 4 h,
formazan crystals were dissolved by the addition of 100 μl of 10%
SDS in 10 mM HCl. Optical densities were measured using a visible
light microplate reader at 590 nm. IC50 values were
calculated as drug concentrations necessary to inhibit 50% growth
compared to vehicle control-treated cells. The data are presented
as mean values ± standard errors from at least 3 independent
experiments. The extent and direction of antitumor interactions
were evaluated using CompuSyn software (ComboSyn, Inc., Paramus,
NJ, USA). Drug interactions were quantified by determining the
combination index (CI), where CI<1, CI=1 and CI>1 indicate
synergistic, additive and antagonistic effects, respectively
(21).
Apoptosis and cell cycle analysis
Cells were treated with CQ or GX15-070, alone or in
combination for 72 h. Then, cells were fixed with ice-cold 80%
(v/v) ethanol for 24 h, washed with PBS and resuspended in PBS
containing propidium iodide (50 μg/ml), Triton X-100 (0.1%, v/v),
and DNase-free RNase (1 μg/ml). DNA content was determined by flow
cytometry analysis, as previously described (22). Cell cycle analysis was performed
using Multicycle software (Phoenix Flow Systems, Inc., San Diego,
CA, USA). Apoptotic events were recorded as PI+ events
with sub-G1 DNA content. The data are presented as mean values ±
standard errors from one representative experiment, which was
repeated at least 3 independent times.
Statistical analysis
The data are expressed as the mean values ± standard
errors. The differences in CQ IC50 between
GX15-070-treated and vehicle control-treated cells and differences
in cell apoptosis between CQ and GX15-070 combination-treated and
GX15-070-treated cells were compared using the pairwise two-sample
t-test. Statistical analyses were performed using GraphPad Prism
5.0.
Results
GX15-070 induces autophagy in pancreatic
cancer cell lines
Bcl-2 family proteins play an important role in
autophagy. Others have demonstrated that GX15-070, a pan-Bcl-2
inhibitor, induces autophagy in various cancer cell lines (12–15,23).
To begin to determine if GX15-070 induces autophagy in pancreatic
cancer cells, we determined if GX15-070 treatment results in
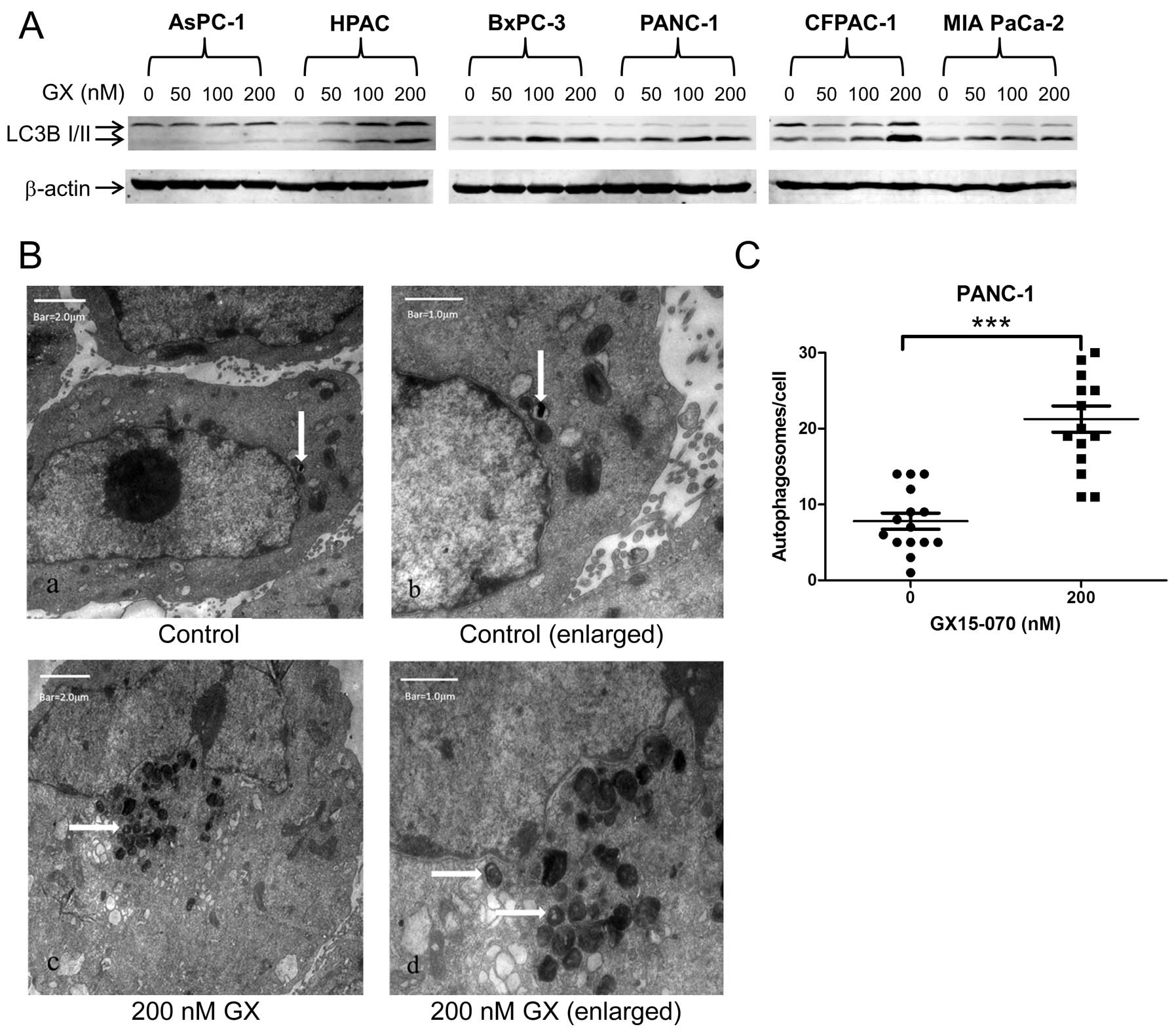
increased conversion of LC3B-I to LC3B-II. There was a
dose-dependent increase in LC3B-II protein levels in all the cell
lines tested, except AsPC-1 (Fig.
1A). To further confirm that GX15-070 induces autophagy in the
pancreatic cancer cells, we treated PANC-1 cells with or without
200 nM GX15-070 and examined autophagosomes by TEM. As shown in
Fig. 1B, GX15-070 treatment induced
autophagosome formation. The number of autophagosomes per cell was
significantly higher in the GX15-070-treated cells compared to no
drug treatment (p<0.0001, Fig.
1C).
Synergistic antitumor interactions of CQ
and GX15-070 in pancreatic cancer cells
Autophagy has been shown to be protective when cells
encounter stress and without it cells can succumb to such cellular
stresses leading to cell death (11,16).
Therefore, inhibiting autophagy might inhibit the adaptive response
to GX15-070 treatment and enhance apoptosis. To test this, we
determined the extent and direction of the antitumor interactions
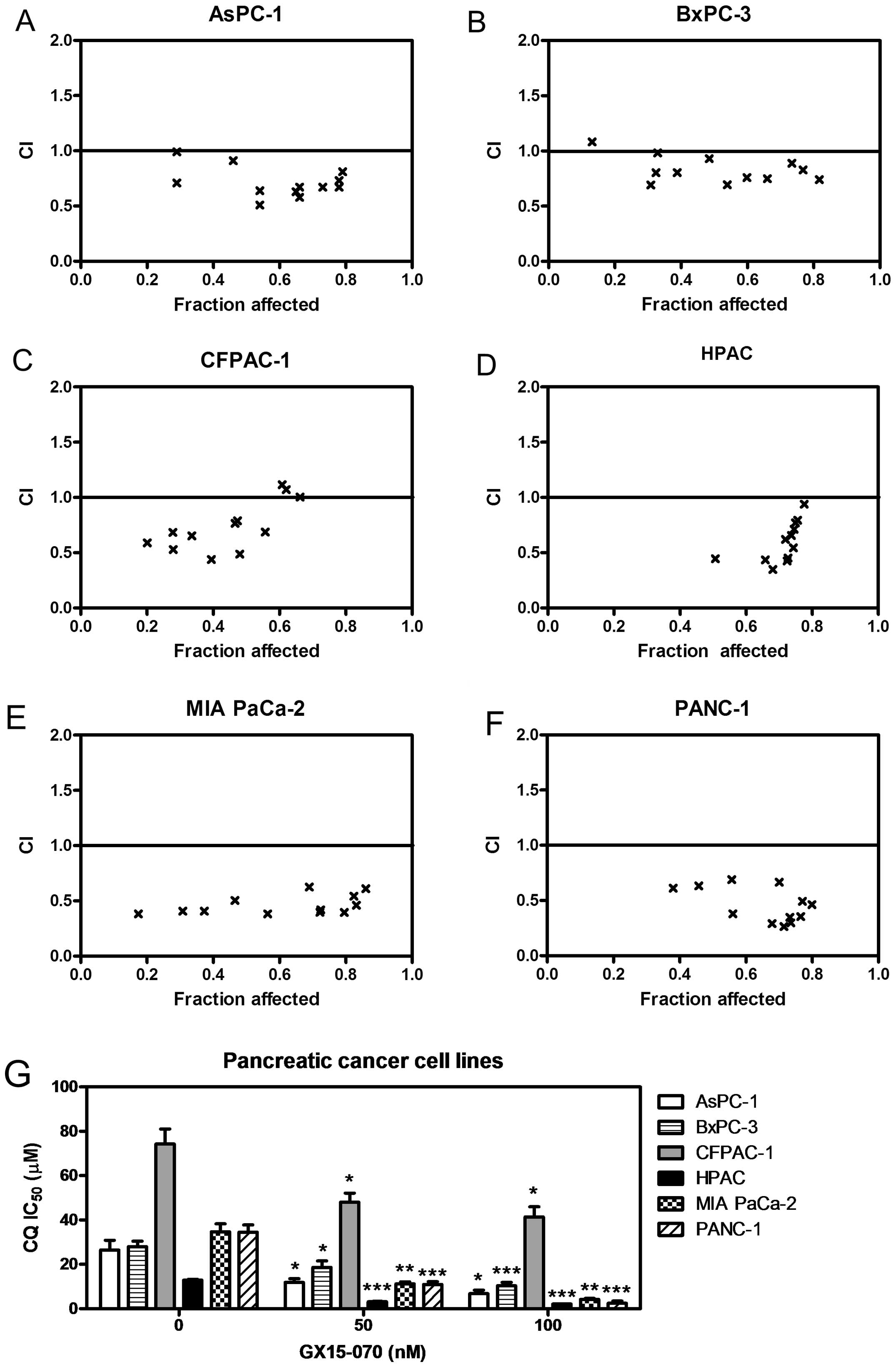
of CQ and GX15-070 in pancreatic cancer cell lines by MTT assays.
In all six cell lines tested, we found that the combination
resulted in additive to synergistic antitumor interactions, as
indicated by the combination index values <1 (Fig. 2A–F). CQ IC50 was
significantly lower in the presence of GX15-070 (Fig. 2G). The reduction in IC50
ranged from 1.5-fold (BxPC-3) to 13.9-fold (PANC-1).
CQ and GX15-070 cooperatively induce
apoptosis in pancreatic cancer cells
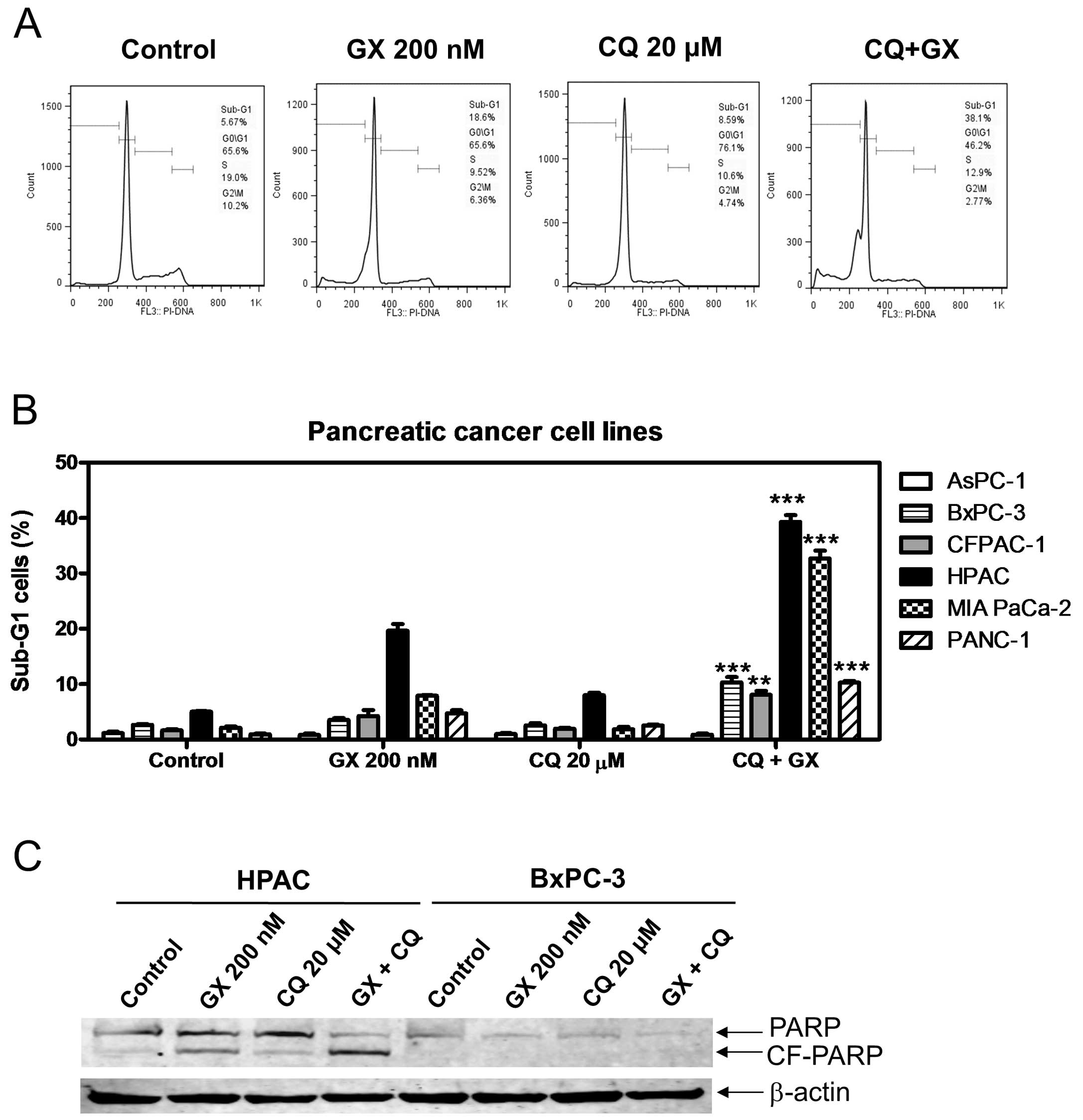
We then sought to determine if CQ and GX15-070
treatment induces apoptosis. As indicated in Fig. 3A–C, treatment with GX15-070 or CQ
alone induced minimal apoptosis; however, in the combination,
significant increase of apoptotic cells was observed in all the
cell lines tested except AsPC-1. This was accompanied by cleavage
of PARP, as demonstrated in the HPAC cells (Fig. 3C). Although increase of cleaved PARP
was not detectable in the BxPC-3 cells, there was a decrease in the
full-length PARP. The lack of detection of the cleaved form might
be due to the detection limits of the assay. In addition to
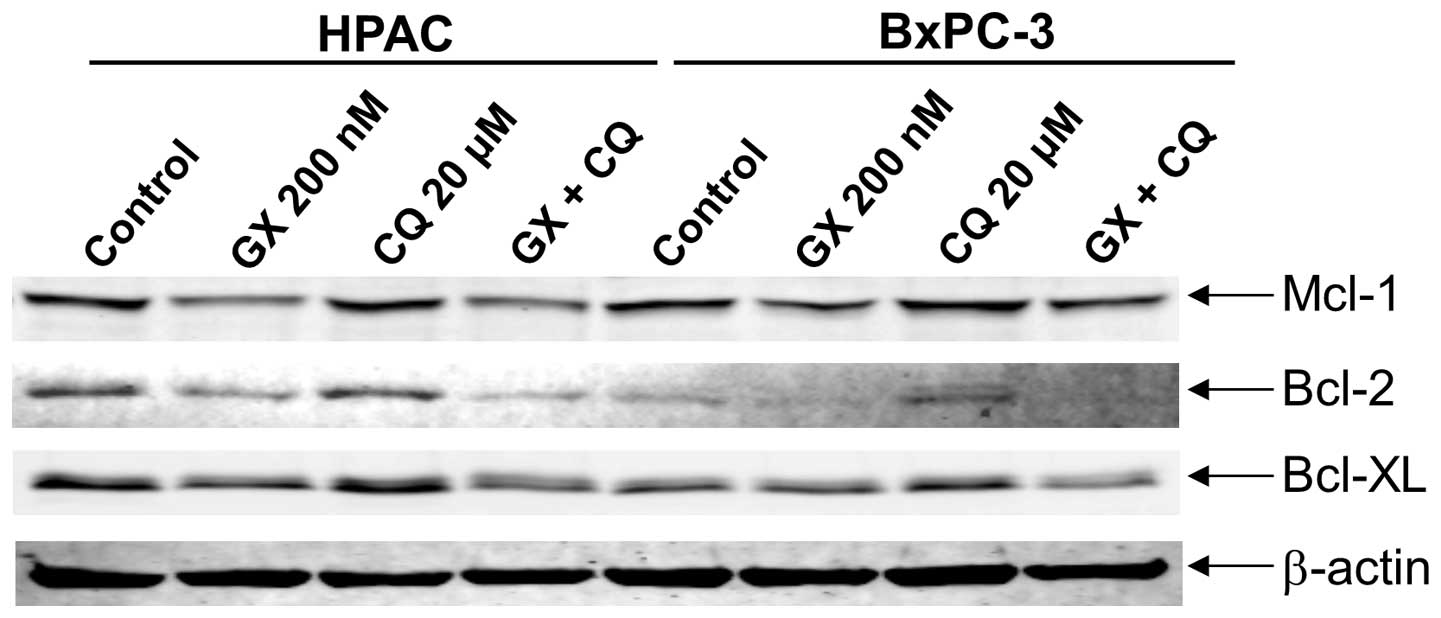
inducing apoptosis, combined drug treatment also resulted in
decreased levels of Bcl-2, Bcl-xL and Mcl-1 (Fig. 4).
Discussion
Bcl-2 inhibitors have demonstrated promising
anticancer activity in a variety of malignancies, including
pancreatic cancer (4–8,12,13,15).
In our previous study, we demonstrated that clinically achievable
concentrations of GX15-070 induced significant growth arrest in
pancreatic cancer cell lines. Notably, they only induced minimal
levels of apoptosis (9), suggesting
that other mechanisms must exist preventing apoptosis from
occurring. In this study, we found that GX15-070 induced autophagy
in the pancreatic cancer cell lines, which is in agreement with
numerous studies using Bcl-2 inhibitors in various malignancies
(4,12,13,15).
Pancreatic cancer cells require autophagy for growth
and protection from cytotoxic anticancer drugs (16,17),
suggesting that it might be an effective drug target for treating
pancreatic cancer. Recently, the autophagy inhibitor CQ was used in
combination with gemcitabine to treat pancreatic cancer cell lines
(16). Results of that study found
that gemcitabine induced autophagy, which was protective against
growth inhibition, and combination with CQ resulted in significant
growth inhibition. In line with that study, we demonstrated that
GX15-070 induces autophagy and synergizes with CQ to induce growth
arrest and enhances apoptosis. In addition to pancreatic cancer,
Pan et al demonstrated similar results in esophagus cancer
cells indicating that GX15-070 induces cytoprotective autophagy
(13).
In summary, in the present study we demonstrated
that GX15-070 in combination with CQ may be a promising strategy
for treating pancreatic cancer. Our results suggest that GX15-070
induces cytoprotective autophagy which can be overcome by the
addition of CQ. CQ has long been used as anti-malarial treatment as
well as anti-arthritis treatment and the safety profiles are
already established, making it an attractive drug for use in
combination with chemotherapy drugs. As the combination of GX15-070
and CQ induced variable levels of apoptosis, it may be advantageous
to investigate some of the newer more potent Bcl-2 inhibitors in
combination with CQ. Although our study involved a limited number
of cell lines, it provides a compelling rationale for the further
study of Bcl-2 inhibitors in combination with CQ in mouse models
and holds promise as a potential effective treatment strategy for
pancreatic cancer.
Acknowledgements
This study was funded by a Start-up Fund from Jilin
University, Changchun, China, and grants from the National Natural
Science Foundation of China (NSFC 31271477 and 81200363).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar
|
|
3
|
Miyamoto Y, Hosotani R, Wada M, et al:
Immunohistochemical analysis of Bcl-2, Bax, Bcl-X, and Mcl-1
expression in pancreatic cancers. Oncology. 56:73–82. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei Y, Kadia T, Tong W, et al: The
combination of a histone deacetylase inhibitor with the Bcl-2
homology domain-3 mimetic GX15-070 has synergistic antileukemia
activity by activating both apoptosis and autophagy. Clin Cancer
Res. 16:3923–3932. 2010. View Article : Google Scholar
|
|
5
|
McCoy F, Hurwitz J, McTavish N, et al:
Obatoclax induces Atg7-dependent autophagy independent of beclin-1
and BAX/BAK. Cell Death Dis. 1:e1082010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Konopleva M, Watt J, Contractor R, et al:
Mechanisms of anti-leukemic activity of the novel Bcl-2 homology
domain-3 mimetic GX15-070 (obatoclax). Cancer Res. 68:3413–3420.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen M, Marcellus RC, Roulston A, et al:
Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes
MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA.
104:19512–19517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahmani M, Aust MM, Attkisson E, Williams
DC Jr, Ferreira-Gonzalez A and Grant S: Inhibition of Bcl-2
antiapoptotic members by obatoclax potently enhances
sorafenib-induced apoptosis in human myeloid leukemia cells through
a Bim-dependent process. Blood. 119:6089–6098. 2012. View Article : Google Scholar
|
|
9
|
Chen S, Wang G, Niu X, et al: Combination
of AZD2281 (Olaparib) and GX15-070 (Obatoclax) results in
synergistic antitumor activities in preclinical models of
pancreatic cancer. Cancer Lett. 348:20–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Z and Klionsky DJ: Mammalian
autophagy: core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malik SA, Orhon I, Morselli E, et al: BH3
mimetics activate multiple pro-autophagic pathways. Oncogene.
30:3918–3929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan J, Cheng C, Verstovsek S, Chen Q, Jin
Y and Cao Q: The BH3-mimetic GX15-070 induces autophagy,
potentiates the cytotoxicity of carboplatin and 5-fluorouracil in
esophageal carcinoma cells. Cancer Lett. 293:167–174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vogler M, Weber K, Dinsdale D, et al:
Different forms of cell death induced by putative BCL2 inhibitors.
Cell Death Differ. 16:1030–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maiuri MC, Criollo A, Tasdemir E, et al:
BH3-only proteins and BH3 mimetics induce autophagy by
competitively disrupting the interaction between Beclin 1 and
Bcl-2/Bcl-X(L). Autophagy. 3:374–376. 2007. View Article : Google Scholar
|
|
16
|
Hashimoto D, Blauer M, Hirota M, Ikonen
NH, Sand J and Laukkarinen J: Autophagy is needed for the growth of
pancreatic adenocarcinoma and has a cytoprotective effect against
anti-cancer drugs. Eur J Cancer. 50:1382–1390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang S, Wang X, Contino G, et al:
Pancreatic cancers require autophagy for tumor growth. Genes Dev.
25:717–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edwards H, Xie C, LaFiura KM, et al: RUNX1
regulates phosphoinositide 3-kinase/AKT pathway: role in
chemotherapy sensitivity in acute megakaryocytic leukemia. Blood.
114:2744–2752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie C, Edwards H, Xu X, et al: Mechanisms
of synergistic anti-leukemic interactions between valproic acid and
cytarabine in pediatric acute myeloid leukemia. Clinical cancer
research: an official journal of the American Association for
Cancer Research. 16:5499–5510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu X, Xie C, Edwards H, Zhou H, Buck SA
and Ge Y: Inhibition of histone deacetylases 1 and 6 enhances
cytarabine-induced apoptosis in pediatric acute myeloid leukemia
cells. PloS One. 6:e171382011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang G, He J, Zhao J, et al: Class I and
class II histone deacetylases are potential therapeutic targets for
treating pancreatic cancer. PloS One. 7:e520952012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maiuri MC, Le Toumelin G, Criollo A, et
al: Functional and physical interaction between Bcl-X(L) and a
BH3-like domain in Beclin-1. EMBO J. 26:2527–2539. 2007. View Article : Google Scholar : PubMed/NCBI
|