Introduction
Breast cancer is one of the most common malignant
cancers in women, and is one of the leading causes of
cancer-related deaths worldwide. Distant metastasis is the main
cause of breast cancer-related mortality (1). However, the mechanisms leading to
breast cancer metastasis remain poorly understood. Breast cancer
commonly metastasizes to certain organs such as the lymph node,
bone marrow, and lung, which exhibit enhanced secretion of
cytokines, but rarely to other organs with low expression of
cytokines, such as the kidney and skin. This characteristic feature
highlights the important role played by chemokines and their
receptors in breast cancer metastasis (2,3).
CXCR4 and its ligand CXCL12 (also called SDF-1 α)
play a critical role in breast cancer carcinogenesis and
metastasis, and targeting the CXCL12-CXCR4 signaling pathway is a
potential therapeutic strategy for the treatment of breast cancer
(3,4). Activation of CXCL12-CXCR4 signaling
has been reported to promote survival, proliferation, adhesion,
chemotaxis and migration of breast cancer cells (5). The binding of CXCL12 to CXCR4 results
in activation of several signaling pathways in breast cancer
including the MAPK/ ERK1/2 (6,7) and
PI3K/AKT (8,9) pathways. It has been reported that
CXCL12-CXCR4 signaling activates the Janus kinase 2 (JAK2) signal
transducer and activator of transcription 3 (STAT3) pathway
(10,11), and STAT3 inactivation inhibits
murine breast cancer metastasis (12). However, it remains unclear whether
activation of the JAK2/STAT3 pathway is involved in the
CXCL12-CXCR4 signaling axis in human breast cancer.
STAT3, a transcription factor that belongs to the
STAT family, has been found to be overexpressed and constitutively
activated in many malignant tumors (13–15).
STAT3 has been regarded as an oncogene, and constitutive activation
of STAT3 can lead to abnormal cell proliferation and malignant
transformation (16,17). JAK2 can recruit and activate STAT3,
which translocates to the nucleus and regulates transcription of a
variety of genes that are associated with proliferation,
differentiation, apoptosis and metastasis of many types of cancer
cells (18,19). However, it remains unclear whether
the JAK2/STAT3 pathway is coupled to CXCL12-CXCR4 signaling. It has
been reported that in response to CXCL12, CXCR4 activates the
JAK2/STAT3 pathway (10,11,20).
However, Moriguchi et al reported that CXCL12-CXCR4
signaling is independent of the JAK2/STAT3 pathway in primary
lymphocytes (21). Furthermore,
STAT3 activation is associated with the CXCR4 signaling pathway in
many types of cancers such as cervical carcinoma (22), small cell lung cancer (23) and bladder cancer (24). However, Lee et al reported
that CXCL12 failed to activate STAT3 in gastric cancer cells
(25). Constitutively activated
STAT3 was found in primary tumors from high risk breast cancer
patients (26), and is associated
with breast cancer growth and metastasis in xenograft models
(27,28). However, it remains unclear whether
CXCL12-CXCR4 signaling is coupled to the JAK2/STAT3 pathway in
human breast cancer.
In the present study, we aimed to investigate the
association between CXCR4 and STAT3, particularly p-STAT3, in
breast cancer tissues from Chinese patients, and to identify the
JAK2/STAT3 pathway in CXCL12-CXCR4 signaling in human breast cell
lines. We performed immunohistochemistry to examine the expression
of CXCR4, STAT3 and p-STAT3 in 208 patients with breast cancer, and
analyzed their correlation with clinicopathological characteristics
of these patients. We found that the expression levels of CXCR4 and
STAT3, particularly p-STAT3, were increased with increased TNM
stage and lymph node metastasis. The combined expression of both
CXCR4 and p-STAT3 was correlated with breast cancer malignancy.
Furthermore, we found that inhibition of CXCR4 or JAK2 prevented
CXCL12-induced phosphorylation of STAT3 in breast cancer cell
lines. Our results suggest that activation of the JAK2/STAT3
pathway by CXCL12-CXCR4 signaling may play an important role in
breast cancer malignancy, and targeting the CXCL12-CXCR4/JAK2/STAT3
signaling pathway may be a potential therapeutic strategy for the
treatment of breast cancer.
Materials and methods
Patients and tissue samples
The Ethics Committee of China Medical University
approved this study. Human breast cancer samples of 208 female
patients with primary breast cancer were collected at the
Department of Surgical Oncology and The Department of General
Surgery of the First Affiliated Hospital of China Medical
University between 2003 and 2010. Some of the samples were used in
our previous studies (29,30). The median age of the breast cancer
patients was 51 years (range, 29 to 85 years). Of the 208 patients,
the stage and the histological grading of the cancer were evaluated
according to the TNM staging system and the Elston-Ellis
modification of Scarff-Bloom-Richardson grading system,
respectively. Clinicopathological data including patient age,
menopausal status, tumor size, tumor type, lymph node metastasis,
and the status of the estrogen receptor (ER), progesterone receptor
(PR) and human epidermal growth factor receptor (HER2) were
retrospectively retrieved from the medical records. Of the 208
patients, 182 patients had invasive ductal carcinoma, and 10 had
invasive lobular carcinoma. Other patients presenting with tumors
of less incidence were categorized into one group, including 3
patients with cribriform carcinoma, 3 patients with micropapillary
carcinoma, 2 patients with mucinous carcinoma, 2 patients with
medullary carcinoma, 2 patients with papillary carcinoma, 1 patient
with tubular carcinoma and 3 patients with ductal carcinoma in
situ. This study included 26 tumor-adjacent samples as
controls. The breast tissues outside the cancer loci were selected
as tumor-adjacent samples. The diagnosis of breast cancer was
confirmed by pathological staining. The tumor-adjacent samples
exhibited no tumor texture histologically. All patients did not
undergo radiation therapy, chemotherapy and hormonal therapy.
Cell culture
Human breast cancer cell lines (MDA-MB-231, BT-549,
and MCF-7) were obtained from the American Type Culture Collection
(ATCC), and maintained according to ATCC’s recommendation. To
investigate the effect of CXCR4 on the phosphorylation of STAT3,
human breast cancer cells were treated with 100 ng/ml CXCL12
(350-NF/ CF; R&D Systems Inc.) for 0, 2, 5, 15 and 30 min after
serum-starvation for 4 h. To investigate whether CXCR4 or JAK2
mediates activation of STAT3 by CXCL12, cells were pretreated with
the CXCR4 antagonist AMD3100 (10 μM, A5602; Sigma-Aldrich St.
Louis, MO, USA) or the JAK2 inhibitor AG490 (25 μM, 658401;
Calbiochem) for 2 h before treatment with CXCL12.
Immunohistochemistry
Tissue sections (4-μm thick) were obtained from
formalin-fixed and paraffin-embedded tissue blocks from the control
and breast cancer samples. Sections were washed in xylene to remove
the paraffin, and rehydrated with serial dilutions of alcohol,
followed by a wash in PBS solution. Endogenous peroxidase activity
was blocked by 3% hydrogen peroxide in methanol at 37°C for 20 min.
Sections were then incubated with primary antibodies against CXCR4
(1:100 dilution, ab2074; Abcam), STAT3 (1:50 dilution, #4904; Cell
Signaling Technology) and p-STAT3 (Tyr705) (1:200 dilution, #9145;
Cell Signaling Technology) overnight at 4°C. After the primary
antibody was washed off, sections were incubated with goat
anti-rabbit biotin-conjugated secondary antibodies (1:1000
dilution; Dako, Glostrup, Denmark) for 30 min at 37°C. The tissue
sections were then incubated with streptavidin horseradish
peroxidase for 30 min at 37°C. DAB (3,3-diaminobenzidine) substrate
was applied to the sections, and then sections were counterstained
with hematoxylin. Sections in which primary antibodies were omitted
were used as negative control.
Immunostaining was examined under a light microscope
by two pathologists blinded to the experimental conditions.
Agreement on the scores between the two pathologists was nearly
100%. In cases in which the pathologists disagreed to the score,
the immunohistochemical scoring was repeated by both pathologists
until the same score was achieved. The immunoreactivity was
evaluated using a scoring system according to the percentage of
stained cells and the intensity of the immunoreactivity. The
intensity of immunoreactivity was scored as follows: 0 for no
staining, 1 for weak staining, 2 for moderate staining, and 3 for
strong staining. The percentage of stained cells was scored as
follows: 0 for <10%, 1 for 10–29%, 2 for 30–49%, 3 for 50–74%,
and 4 for ≥75%. The final immunoreactive score was determined by
multiplying the intensity score with the score for the percentage
of positively stained cells. The minimum score was 0 and the
maximum score was 12. Low and high expression of CXCR4, STAT3 and
p-STAT3 was defined by a final score of <6 and ≥6,
respectively.
Flow cytometry
Flow cytometric analysis was performed on a
FACSCalibur (Becton-Dickinson, Franklin Lakes, NJ, USA). Cells
(1×106 cells/ml) were fixed with 4% paraformaldehyde,
and then washed and resuspended in PBS containing 0.5% bovine serum
albumin. Cells were permeabilized with 0.1% Triton-X 100, and
incubated with the primary antibodies against CXCR4 (1:100
dilution) for 30 min at room temperature. Isotype rabbit IgG was
used as a control. After washing, cells were labeled with Alexa
Fluor 488-conjugated secondary antibodies (Molecular Probes,
A21206; Invitrogen) for 1 h at 4°C. Cells were subsequently
analyzed by flow cytometry.
Western blot analysis
Human breast cancer cells were homogenized on ice in
RIPA lysis buffer (Beyotime, Nantong, China) containing a cocktail
of protease and phosphatase inhibitors (Sigma-Aldrich). Proteins
were resolved by SDS-PAGE, and transferred onto polyvinylidene
fluoride membranes by electroblotting. The membranes were incubated
with primary antibodies against CXCR4 (1:2,000 dilution), STAT3
(1:4,000 dilution) and p-STAT3 (1:2,000 dilution) at 4°C with
gentle shaking overnight. GAPDH was used as a loading control. The
membranes were then incubated with horseradish peroxidase-linked
goat anti-rabbit secondary antibodies (dilution 1:5,000) at room
temperature for 2 h. Bands were visualized using a
chemiluminescence detection system.
Immunoprecipitation
MCF-7 cells were solubilized on ice with lysis
buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.5, 1% Triton X-100)
supplemented with a cocktail of phosphatase and proteinase
inhibitors (Sigma-Aldrich). Lysates were centrifuged at 10,000 × g
for 15 min at 4°C. The supernatants were incubated with antibodies
against CXCR4 (1:100 dilution) or non-specific rabbit IgG
(control), and protein A-agarose at 4°C overnight. The
immunoprecipitates were collected by centrifugation, and the
agarose pellet was suspended in 2× sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer. The
expression of JAK2 was determined by western blotting, using
antibodies against JAK2 (1:1,000 dilution, #3230; Cell Signaling
Technology).
Statistical analysis
Statistical analyses were performed using SPSS 11.5.
The numerical data are presented as mean and standard deviation.
Student t-test or one-way analysis of variance (ANOVA) was used to
compare the difference in the means among two or more groups,
respectively. Categorical data were compared with Pearson Chi
squared tests. The Spearman’s correlation analysis was applied to
assess the association of the expression of CXCR4 with the
expression of STAT3 and p-STAT3. Probability (P)-values <0.05
were considered to indicate statistically significant results.
Results
Expression of CXCR4, STAT3 and p-STAT3 in
the breast cancer tissues
The clinicopathological characteristics of 208
patients with breast cancer are shown in Table I. Of the 208 breast cancer patients,
the TNM stage, tumor size, lymph node metastasis, and histological
grading were recorded in 194, 193, 186 and 177 patients,
respectively. ER, PR, and HER2 were examined in 179, 179 and 164
patients, respectively.
 | Table ICorrelation of the expression of
CXCR4, STAT3 and p-STAT3 with the clinicopathological parameters of
the breast cancer cases. |
Table I
Correlation of the expression of
CXCR4, STAT3 and p-STAT3 with the clinicopathological parameters of
the breast cancer cases.
| | CXCR4 expression n
(%) | | STAT3 expression n
(%) | | p-STAT3 expression
n (%) | |
|---|
| |
| |
| |
| |
|---|
| Parameters | Na/208 | Low | High | P-valueb | Low | High | P-valueb | Low | High | P-valueb |
|---|
| Age (years) | 208/208 | | | 0.563 | | | 0.749 | | | 0.189 |
| <50 | 86 | 27 (31.4) | 59 (68.6) | | 25 (29.1) | 61 (70.9) | | 53 (61.6) | 33 (38.4) | |
| ≥50 | 122 | 43 (35.2) | 79 (64.8) | | 33 (27.0) | 89 (73.0) | | 64 (52.5) | 58 (47.5) | |
| Menopause
status | 196/208 | | | 0.590 | | | 0.160 | | | 0.080 |
|
Pre-menopausal | 83 | 31 (37.3) | 52 (62.7) | | 29 (34.9) | 54 (65.1) | | 53 (63.9) | 30 (36.1) | |
|
Post-menopausal | 113 | 38 (33.6) | 75 (66.4) | | 29 (25.7) | 84 (74.3) | | 58 (51.3) | 55 (48.7) | |
| Tumor type | 208/208 | | | 0.850 | | | 0.080 | | | 0.970 |
| Ductal | 182 | 60 (33.0) | 122 (67.0) | | 46 (25.3) | 136 (74.7) | | 102 (56.0) | 80 (44.0) | |
| Lobular | 10 | 4 (40.0) | 6 (60.0) | | 5 (50.0) | 5 (50.0) | | 6 (60.0) | 4 (40.0) | |
| Others | 16 | 6 (37.5) | 10 (62.5) | | 7 (43.8) | 9 (56.2) | | 9 (56.2) | 7 (43.8) | |
| TNM stage | 194/208 | | | 0.002 | | | 0.045 | | | 0.000 |
| I | 29 | 16 (64.7) | 13 (35.3) | | 11 (37.9) | 18 (62.1) | | 21 (72.4) | 8 (27.6) | |
| II | 100 | 38 (38.0) | 62 (62.0) | | 32 (32.0) | 68 (68.0) | | 65 (65.0) | 35 (35.0) | |
| III–IV | 65 | 13 (20.0) | 52 (80.0) | | 11 (16.9) | 54 (83.1) | | 22 (33.8) | 43 (66.2) | |
| Tumor size
(cm) | 193/208 | | | 0.193 | | | 0.999 | | | 0.080 |
| ≤2.0 | 53 | 23 (43.4) | 30 (56.6) | | 15 (28.3) | 38 (71.7) | | 31 (58.5) | 22 (41.5) | |
| >2.0 to
≤5.0 | 98 | 32 (33.5) | 66 (64.5) | | 28 (28.6) | 70 (71.4) | | 62 (63.3) | 36 (36.7) | |
| >5.0 | 42 | 11 (26.2) | 31 (73.8) | | 12 (28.6) | 30 (71.4) | | 18 (42.9) | 24 (57.1) | |
| LNM | 186/208 | | | 0.025 | | | 0.040 | | | 0.023 |
| No | 78 | 34 (43.6) | 44 (56.4) | | 28 (35.9) | 50 (64.1) | | 50 (64.1) | 28 (35.9) | |
| Yes | 108 | 30 (27.8) | 78 (72.2) | | 24 (22.2) | 84 (77.8) | | 51 (51.0) | 57 (57.0) | |
| Histological
grade | 177/208 | | | 0.277 | | | 0.072 | | | 0.000 |
| I | 25 | 10 (40.0) | 15 (60.0) | | 11 (44.0) | 14 (56.0) | | 22 (88.0) | 3 (12.0) | |
| II | 131 | 46 (35.1) | 85 (64.9) | | 35 (26.7) | 96 (73.3) | | 71 (54.2) | 60 (45.8) | |
| III | 21 | 4 (19.0) | 17 (81.0) | | 3 (14.3) | 18 (85.7) | | 6 (28.6) | 15 (71.4) | |
| ER | 179/208 | | | 0.640 | | | 0.964 | | | 0.312 |
| Negative | 68 | 25 (36.8) | 43 (63.2) | | 20 (29.4) | 48 (70.6) | | 42 (61.8) | 26 (38.2) | |
| Positive | 111 | 37 (33.3) | 74 (66.7) | | 33 (29.7) | 78 (70.3) | | 60 (54.1) | 51 (45.9) | |
| PR | 179/208 | | | 0.415 | | | 0.978 | | | 0.752 |
| Negative | 72 | 22 (30.6) | 50 (69.4) | | 21 (29.2) | 51 (70.8) | | 40 (55.6) | 32 (44.4) | |
| Positive | 107 | 39 (36.4) | 68 (63.6) | | 31 (29.0) | 76 (71.0) | | 62 (57.9) | 45 (42.1) | |
| HER2 | 164/208 | | | 0.307 | | | 0.852 | | | 0.584 |
| Negative | 53 | 21 (39.6) | 32 (60.4) | | 17 (32.1) | 36 (67.9) | | 32 (60.4) | 21 (39.6) | |
| Positive | 111 | 35 (31.5) | 76 (68.5) | | 34 (30.6) | 77 (69.4) | | 62 (55.9) | 49 (44.1) | |
We studied the expression of CXCR4, STAT3, and
p-STAT3 in 208 tumor samples and in 26 tumor-adjacent samples from
patients with breast cancer, using immunohistochemistry (Fig. 1). The expression levels of CXCR4,
STAT3, and p-STAT3 were higher in the breast cancer samples than
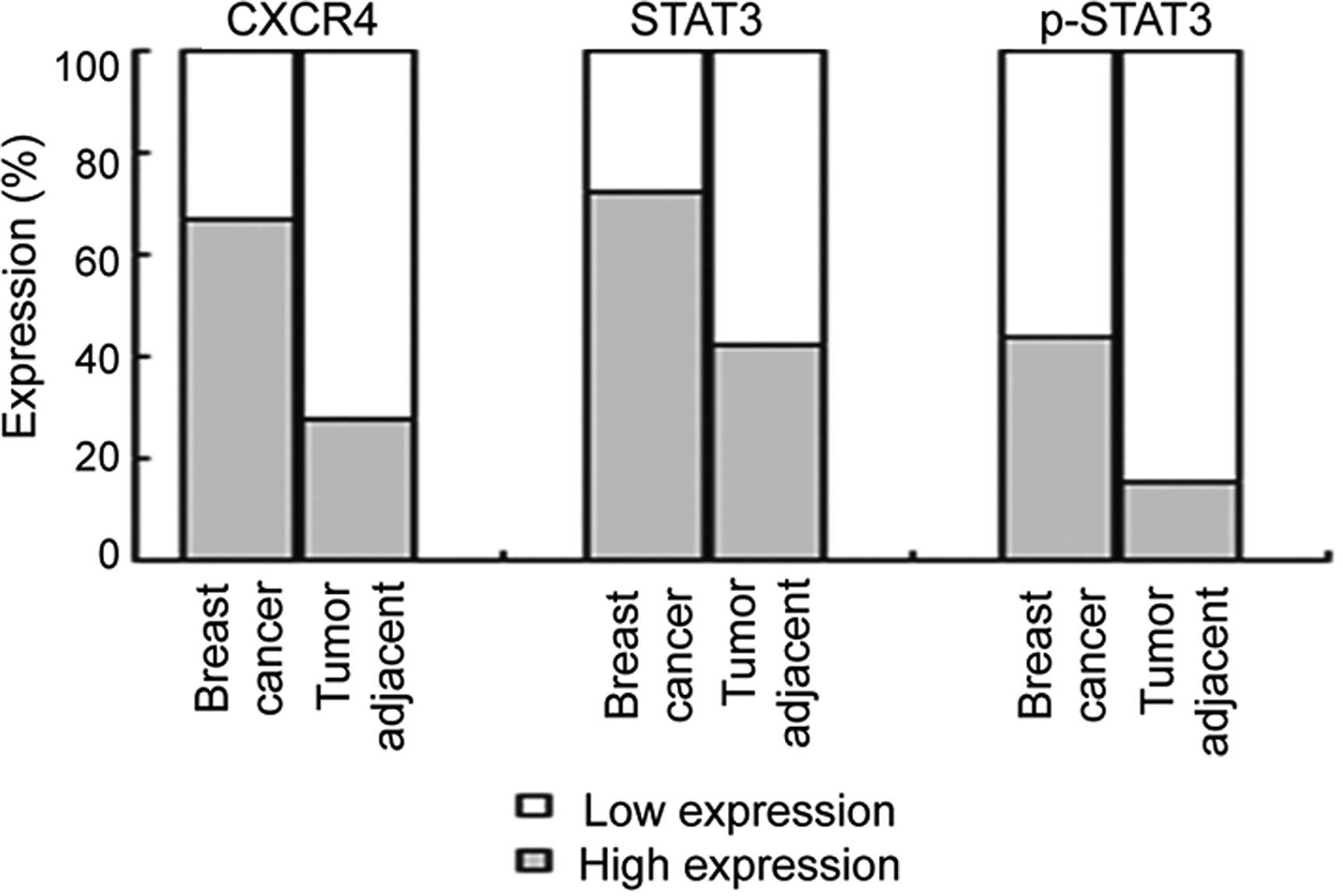
these levels in the tumor-adjacent samples (Fig. 2). CXCR4 immunoreactivity showed low
expression and high expression in 70 (33.6%) and 138 (66.4%) of the
208 breast cancer samples, respectively, and in 19 (70.1%) and 7
(26.9%) of the 26 tumor-adjacent samples, respectively (Fig. 2, P<0.001). The expression level
of CXCR4 increased with increased TNM stage (P=0.025) and lymph
node metastasis (P=0.039), but not with tumor size (P=0.193) and
histological grade (P=0.277) (Table
I). STAT3 immunoreactivity showed low expression and high
expression in 58 (27.9%) and 150 (72.1%) of the 208 breast cancer
samples, respectively and in 15 (57.7%) and 11 (42.3%) of 26
tumor-adjacent samples, respectively (Fig. 2, P=0.002). The expression level of
STAT3 increased with increased TNM stage (P=0.045) and lymph node
metastasis (P=0.040), but not with the tumor size (P=0.999) and
histological grade (P=0.072) (Table
I). p-STAT3 immunoreactivity showed low expression and high
expression in 117 (56.2%) and 91 (43.8%) of the 208 breast cancer
samples, respectively and in 22 (84.6%) and 4 (15.4%) of the 26
tumor-adjacent samples, respectively (Fig. 2, P=0.003). The expression of p-STAT3
was increased with increased TNM stage (P<0.001), lymph node
metastasis (P=0.023) and histological grade (P<0.001), but not
with the tumor size (P=0.080) (Table
I). In addition, the expression levels of CXCR4, STAT3, and
p-STAT3 did not significantly differ in regards to patient age,
menstruation status, tumor type, ER status, PR status and HER2
status.
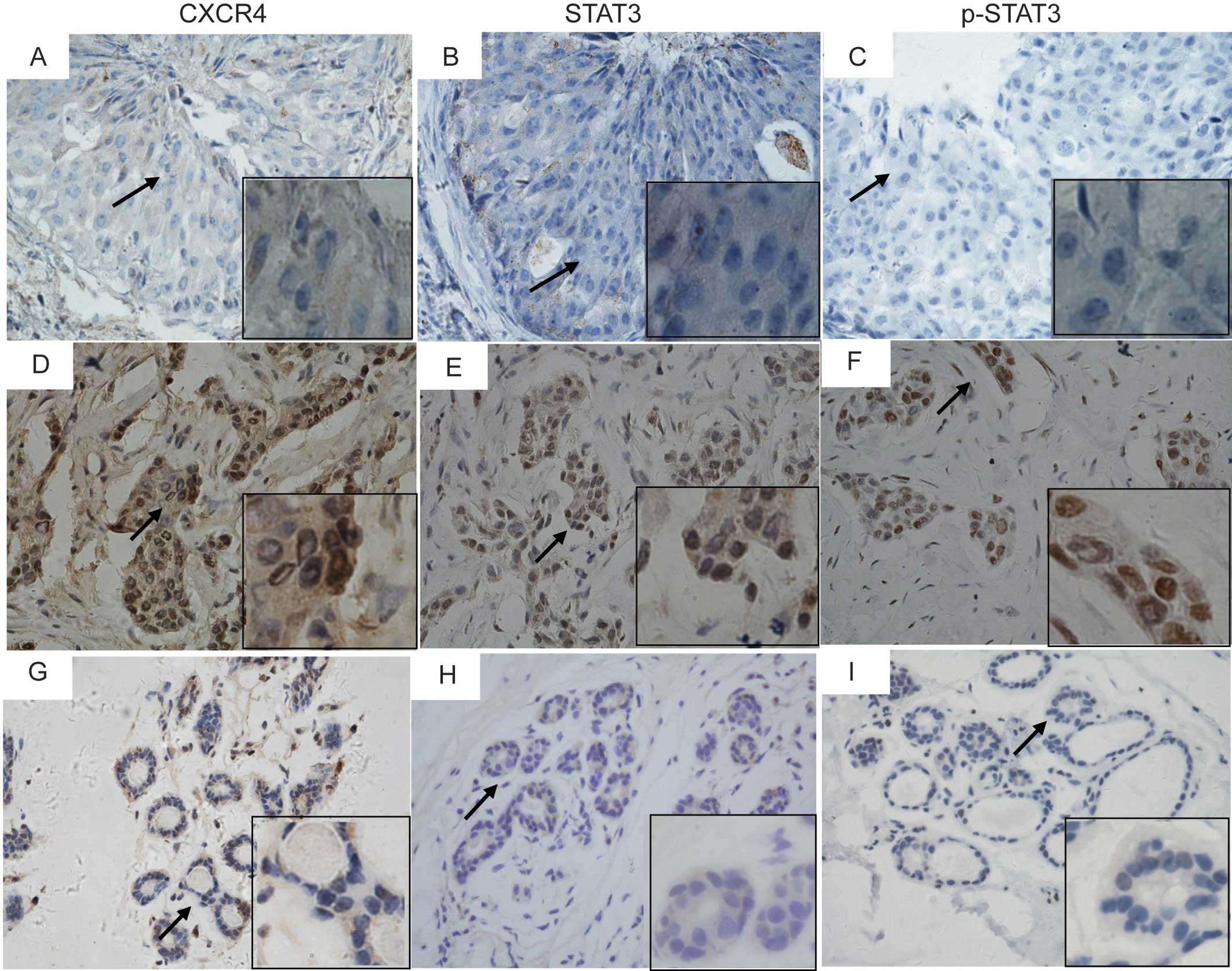 | Figure 1Immunohistochemical staining of CXCR4
(A, D and G), STAT3 (B, E and H), and phospho-STAT3 (Tyr705) (C, F
and I) in breast cancer tissues (A–F) and tumor-adjacent tissues
(G–I). Representative micrographs of low expression (A–C), and high
expression (D–F) of CXCR4, STAT3 and p-STAT3 in consecutive
sections of breast cancer tissues, and the expression of CXCR4,
STAT3, and p-STAT3 in tumor-adjacent tissues (G–I). Magnification;
×400. Areas indicated by an arrow are magnified in inserts
(x1,000). |
Association of the combined expression of
CXCR4/STAT3 and CXCR4/p-STAT3 with clinicopathological
characteristics of the breast cancer
We further investigated the correlation of the
expression of CXCR4 with the expression of STAT3 and p-STAT3. A
positive correlation was observed between the expression of CXCR4
and STAT3 (r=0.260, P<0.001) and between the expression of CXCR4
and p-STAT3 (r=0.300, P<0.001). These results suggest that the
signaling pathway that involves CXCR4 and STAT3 may play a role in
breast cancer carcinogenesis.
We then investigated the association of the combined
expression of CXCR4 and STAT3 (CXCR4/STAT3) or CXCR4 and p-STAT3
(CXCR4/p-STAT3) with the clinicopathological characteristics of the
breast cancer cases (Table II).
Compared with the combined low expression of CXCR4 and STAT3
(Low/Low) and the high expression of either CXCR4 or STAT3
(High/Low or Low/High), the combined high expression of CXCR4/STAT3
(High/High) was highly correlated with TNM stage (P=0.002) and
lymph node metastasis (P=0.002), but not with the tumor size
(P=0.348) and histological grade (P=0.078). Compared with the
combined low expression of CXCR4 and p-STAT3 (Low/Low) and the high
expression of either CXCR4 or p-STAT3 (High/Low or Low/High), the
combined high expression of CXCR4/p-STAT3 (High/High) was highly
correlated with TNM stage (P<0.001), tumor size (P=0.043), lymph
node metastasis (P=0.013) and histological grade (P=0.027).
 | Table IICorrelation of the combined
expression of CXCR4/STAT3 or CXCR4/pSTAT3 with TNM stage, tumor
size, metastasis and histological grade in the breast cancer
cases. |
Table II
Correlation of the combined
expression of CXCR4/STAT3 or CXCR4/pSTAT3 with TNM stage, tumor
size, metastasis and histological grade in the breast cancer
cases.
| CXCR4/STAT3
expression, n (%) | | CXCR4/p-STAT3
expression, n (%) | |
|---|
|
| |
| |
|---|
| Parameters | Low/Low | Low/High or
High/Low | High/High | P-valueb | Low/Low | High/Low | Low/High or
High/High | P-valueb |
|---|
| TNM stage | | | | | | | | |
| I | 7 (24.1) | 14 (48.3) | 8 (27.6) | 0.002 | 12 (41.4) | 13 (44.8) | 4 (13.8) |
<0.001 |
| II | 18 (18.0) | 34 (34.0) | 48 (48.0) | | 29 (29.0) | 45 (45.0) | 26 (26.0) | |
| III–IV | 5 (7.7) | 14 (21.5) | 46 (70.8) | | 10 (15.4) | 17 (26.2) | 38 (58.5) | |
| Tumor size
(cm) | | | | | | | | |
| ≤2.0 | 10 (18.9) | 20 (37.7) | 23 (43.4) | 0.348 | 16 (30.2) | 24 (45.3) | 13 (24.5) | 0.043 |
| >2.0 to
<4.0 | 13 (13.3) | 34 (34.7) | 51 (52.0) | | 25 (25.5) | 43 (43.9) | 30 (30.6) | |
| ≥4.0 | 7 (16.7) | 9 (21.4) | 26 (61.9) | | 10 (23.8) | 10 (23.8) | 22 (52.4) | |
| LNM | | | | | | | | |
| No | 15 (19.2) | 33 (42.3) | 30 (38.5) | 0.002 | 24 (30.8) | 35 (44.9) | 19 (24.4) | 0.013 |
| Yes | 15 (13.9) | 24 (22.2) | 69 (63.9) | | 25 (23.1) | 34 (31.5) | 49 (45.4) | |
| Histological
grade | | | | | | | | |
| I | 7 (28.0) | 8 (32.0) | 10 (40.0) | 0.078 | 10 (40.0) | 12 (48.0) | 3 (12.0) | 0.027 |
| II | 19 (14.5) | 44 (33.6) | 68 (51.9) | | 33 (25.2) | 52 (35.7) | 46 (35.1) | |
| III | 1 (4.8) | 4 (19.0) | 16 (76.2) | | 3 (14.3) | 6 (28.6) | 12 (57.1) | |
CXCR4-mediated activation of JAK2/STAT3
in human breast cancer cell lines
The positive correlation of CXCR4 expression with
the expression level of STAT3 and p-STAT3 in breast cancer suggests
that p-STAT3 may be regulated by CXCR4. To investigate the
signaling pathway that is involved in activation of STAT3 by CXCR4,
three human breast cancer cell lines (MDA-MB-231, BT-549 and MCF-7)
were analyzed for their expression of CXCR4, STAT3 and p-STAT3,
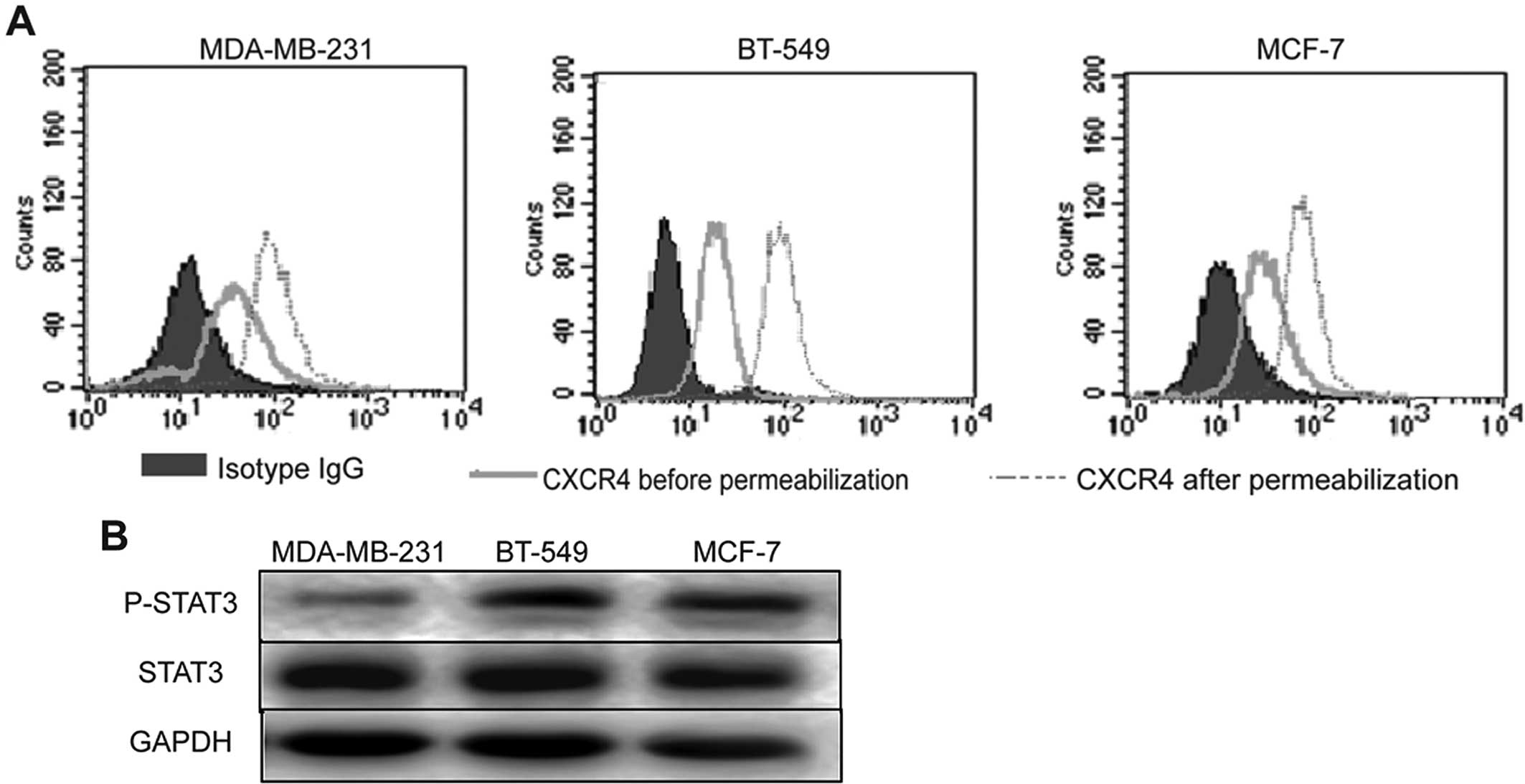
using flow cytometry and western blot analysis. Flow cytometric
analysis showed that CXCR4 was endogenously expressed in the cell
membrane and cytoplasm of the MDA-MB-231, BT-549 and MCF-7 cells
(Fig. 3A). Western blot analysis
showed constitutive expression of STAT3 and p-STAT3 in all the
breast cancer cell lines (Fig.
3B).
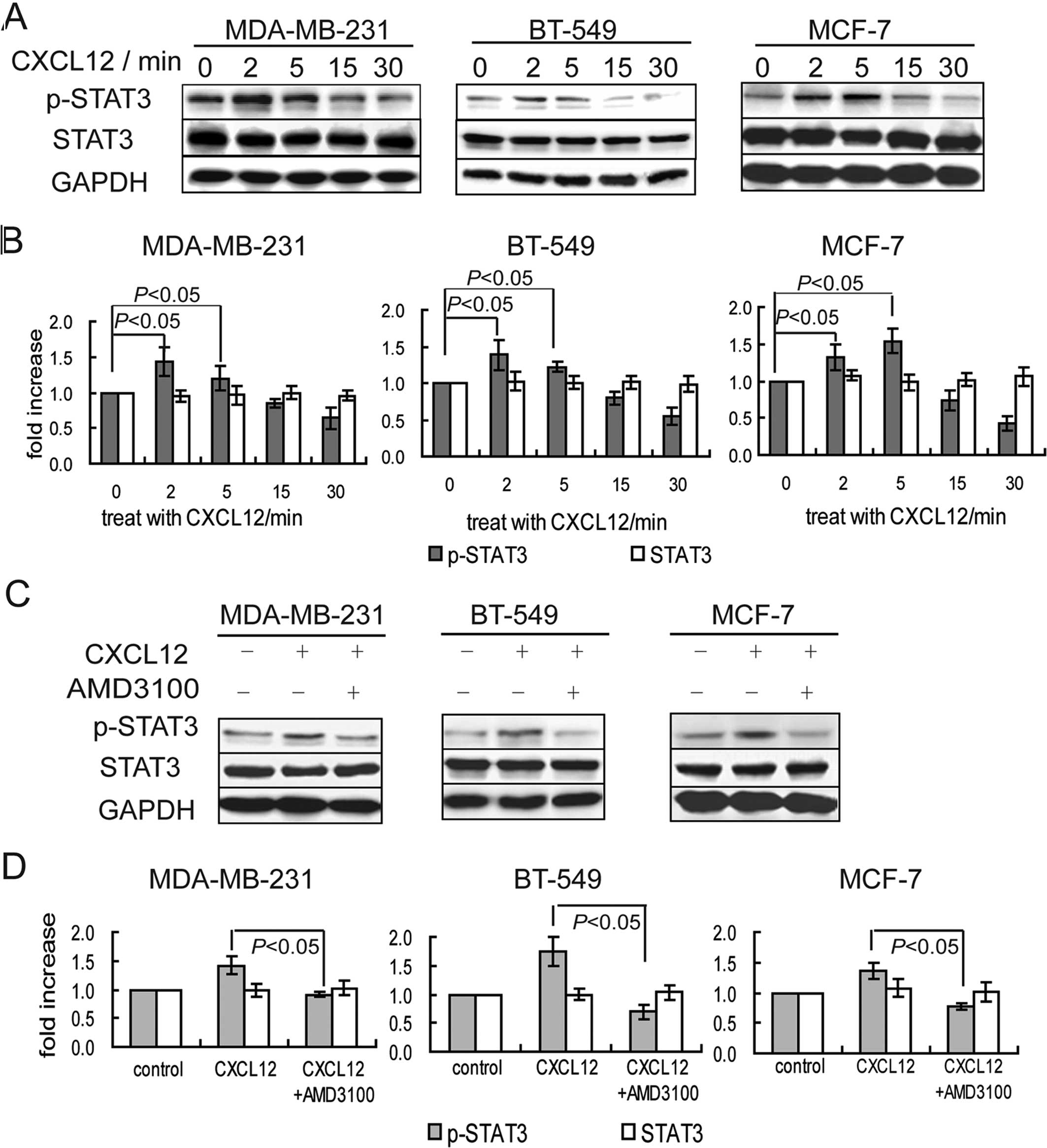
To test whether CXCR4 promotes the phosphorylation
of STAT3, human breast cancer cells were treated with the CXCR4
agonist CXCL12 for 0, 2, 5, 15 and 30 min. The expression of
p-STAT3 in these cells was significantly increased at 2 to 5 min
after CXCL12 treatment, and gradually declined at 15 to 30 min
after CXCL12 treatment (Fig. 4A and
B). Pretreatment with the CXCR4 antagonist AMD3100 inhibited
the CXCL12-induced increase in the phosphorylation of STAT3
(Fig. 4C and D). These results
suggest that the activation of CXCR4 promoted the expression of
p-STAT3 in breast cancer cells.
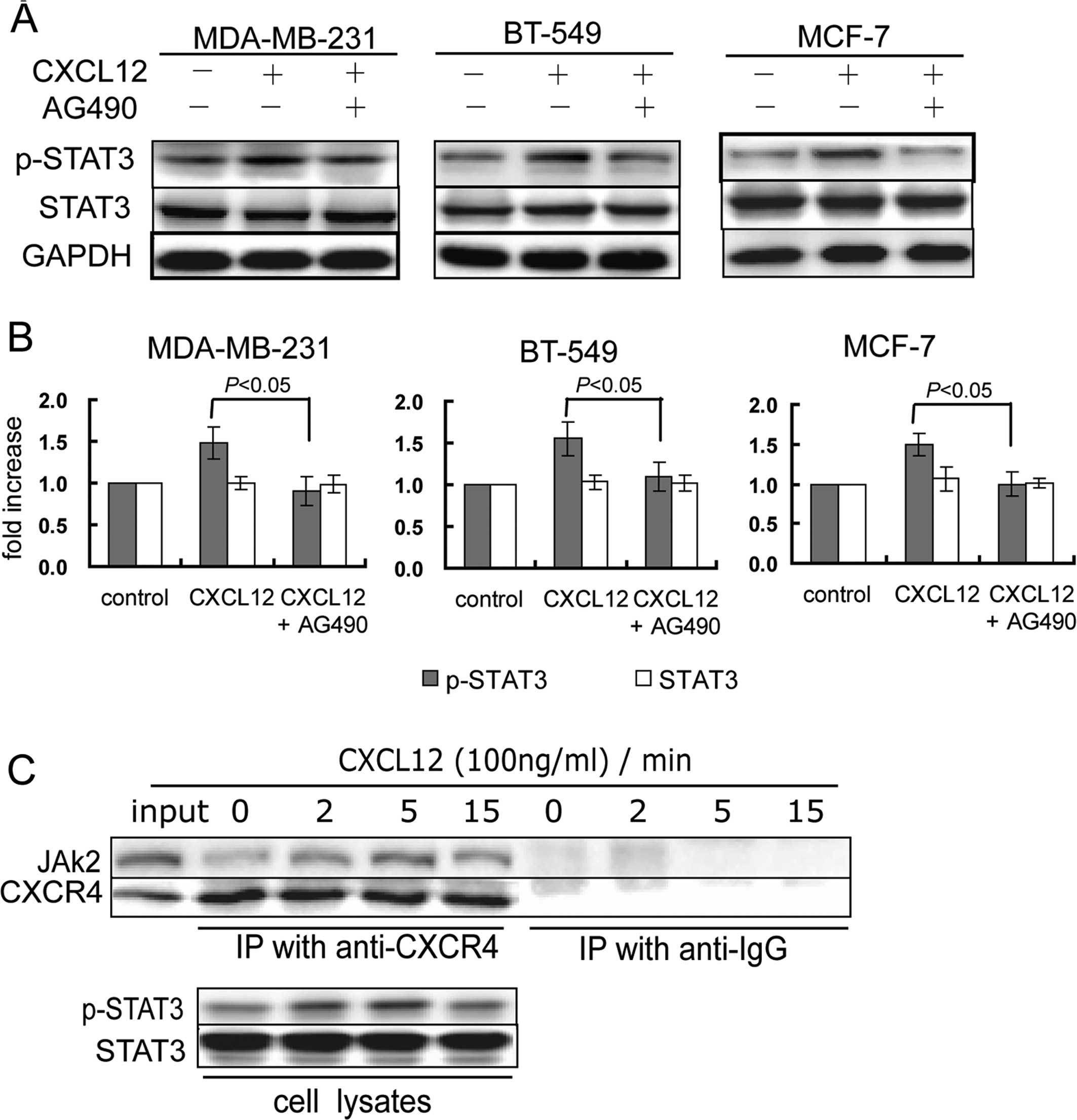
STAT3 has been shown to be activated by JAK2
(11,31). We then investigated whether CXCR4
mediates activation of STAT3 via JAK2. Pretreatment with the JAK2
inhibitor AG490 inhibited CXCL12-induced phosphorylation of STAT3
(Fig. 5A and B), suggesting that
JAK2 mediated CXCR4-induced activation of STAT3. Furthermore, we
investigated whether CXCR4 directly interacted with JAK2 in MCF-7
cells, using immunoprecipitation. In the absence of CXCL12, JAK2
was immunoprecipitated with CXCR4. After CXCL12 treatment for 2 to
5 min, the binding of JAK2 to CXCR4 was increased, accompanied by
an increase in the expression of p-STAT3 (Fig. 5C), suggesting that CXCR4 directly
activated JAK2. These results suggest that CXCR4 mediates the
JAK2/STAT3 activation in breast cancer cells.
Discussion
CXCR4 and STAT3 have been known to play an important
role in growth, progression, angiogenesis and metastasis of many
tumor types (3,18,32).
In breast cancer, high CXCR4 expression has been found to be
associated with poor prognosis (2,33), and
the CXCL12-CXCR4 signaling axis mediates breast cancer metastasis
(34,35). It is known that constitutively
activated STAT3 is associated with breast cancer growth and
metastasis (27,28). In addition, it has been reported
that CXCR4 contributes to murine breast cancer growth and
metastasis via modulation of STAT3 (36). However, the association of CXCR4 and
STAT3 has not been studied in breast cancer patients. In the
present study, we investigated the expression of CXCR4, STAT3 and
p-STAT3 in 208 breast cancer patients, and analyzed their
correlations with clinicopathological features of these patients.
We found that the expression of CXCR4, STAT3 and p-STAT3 was higher
in the breast cancer tissues than that in the tumor-adjacent
tissues. The combined expression levels of CXCR4/p-STAT3 were
positively associated with TNM stage, tumor size, lymph node
metastasis and histological grade. Furthermore, we found that
CXCL12-CXCR4 induced phosphorylation of STAT3 via JAK2 in the
breast cancer cell lines. Our results suggest that the
CXCL12-CXCR4/JAK2/ STAT3 signaling pathway may play an important
role in breast cancer malignancy and metastasis.
CXCR4 expression has been found to be upregulated in
primary breast cancers, and is associated with breast cancer
metastasis (37–39). Consistent with previous studies, we
found that CXCR4 was highly expressed in breast cancer tissues, and
the CXCR4 expression was correlated with TNM stage and lymph node
metastasis. These results suggest that increased receptor numbers,
at least in part, contribute to breast cancer metastasis. It has
been reported that upregulation of CXCR4 receptors is induced by
several oncogenic events such as hypoxia (40), HER2 overexpression (41), EGFR variant-mediated invasion
(42) and TGFβ1 signaling (43), suggesting that CXCR4 mediates many
oncogenic signals that lead to breast cancer metastasis. CXCL12,
the CXCR4 ligand, attracts tumor cells expressing CXCR4 to organs
such as the lymph node and bone marrow that produce CXCL12, thereby
mediating metastasis. Our finding that CXCR4 expression is
correlated with the lymph node metastasis in breast cancers
highlights the important role played by CXCL12-CXCR4 signaling in
breast cancer metastasis.
Several downstream signaling pathways including the
JAK2/STAT3 pathway have been reported to be activated by
CXCL12-CXCR4 signaling in breast cancer cells and xenograft animal
models, and contribute to tumor angiogenesis, tumorigenesis and
metastasis (10–12). In the present study, we found that
the expression level of STAT3 was correlated with that of CXCR4,
and the combined high expression of CXCR4 and STAT3, especially
CXCR4 and p-STAT3, was correlated with TNM stage and lymph node
metastasis of breast cancer, suggesting that the signaling pathway
that involves STAT3 is critical for CXCR4-mediated breast cancer
metastasis. This finding is in accord with a previous study by Ling
et al showing that STAT3 was involved in CXCR4-mediated
breast cancer growth and metastasis in a xenograft animal model
(36). Furthermore, we also found
that the combined high expression level of CXCR4 and p-STAT3 was
correlated with tumor size and histological grade. This finding is
consistent with evidence that constitutively activated STAT3
participates in breast carcinogenesis in cell lines and xenograft
animal models (27,28,44).
Our study suggests that activation of STAT3 by CXCR4 is important
in human breast cancer malignancy.
In the present study, we investigated the potential
signaling pathway that involves activation of STAT3 by CXCR4 in
human breast cancer cell lines after treatment with CXCL12. We
found that inhibition of either CXCR4 or JAK2 prevented
CXCL12-induced phosphorylation of STAT3, suggesting that
CXCL12-CXCR4 activated STAT3 via JAK2 in breast cancer cells. Our
study agrees with previous studies that the JAK2/STAT3 pathway is
activated by binding of CXCL12 to CXCR4 in human T cells (10), HEK cells expressing CXCR4 (11), and human acute lymphoblastic
leukemia cells (20). However, it
has been reported that CXCL12-CXCR4 cannot activate JAK2 in primary
lymphocytes (21). The discrepancy
may be due to different coupling mechanisms underlying activation
of JAK2/STAT3 in different cells. In breast cancer cells, it is
known that the JAK2/STAT3 pathway is activated by interleukin-6
(45,46) and osteopontin (47). In the present study, we found that
the JAK2 antagonist inhibited CXCL12-induced phosphorylation of
STAT3 in three breast cancer cell lines, suggesting that the
JAK2/STAT3 pathway was coupled to CXCL12-CXCR4 signaling in human
breast cancer cells. Furthermore, we found that JAK2 was
immunoprecipitated with CXCR4, further suggesting that
CXCR4-mediated activation of JAK2 acts via direct interaction
between JAK2 and CXCR4. The N-terminal part of the third
intracellular loop in CXCR4, which is critical for CXCR4-mediated
JAK2 activation, may be the binding site for JAK2 (11).
In summary, we found that the expression of CXCR4,
STAT3 and p-STAT3 was higher in breast cancer tissues than in
tumor-adjacent tissues. The expression levels of STAT3 and p-STAT3
were correlated with the expression levels of CXCR4 and
clinicopathological characteristics of the breast cancer patients.
The combined expression level of CXCR4 and p-STAT3 was correlated
with TNM stage, tumor size, lymph node metastasis and histological
grade, suggesting that activation of STAT3 by CXCR4 may play an
important role in breast cancer malignancy. In addition, in breast
cancer cell lines, we found that CXCL2-CXCR4 signaling activated
the JAK2/STAT3 pathway. The CXCR4 or JAK2 inhibitor effectively
prevented CXCL12-induced activation of STAT3. Targeting the
CXCL12-CXCR4/JAK2/STAT3 signaling pathway may represent a potential
therapeutic strategy for the treatment of breast cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81173092), Liaoning
S&T Projects (no. 2011415052), and the Liaoning Province Doctor
Startup Fund Program (no. 20111107).
References
|
1
|
Lu J, Steeg PS, Price JE, et al: Breast
cancer metastasis: challenges and opportunities. Cancer Res.
69:4951–4953. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mukherjee D and Zhao J: The role of
chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer
Res. 3:46–57. 2013.PubMed/NCBI
|
|
4
|
Gil M, Seshadri M, Komorowski MP, Abrams
SI and Kozbor D: Targeting CXCL12/CXCR4 signaling with oncolytic
virotherapy disrupts tumor vasculature and inhibits breast cancer
metastases. Proc Natl Acad Sci USA. 110:E1291–E1300. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luker KE and Luker GD: Functions of CXCL12
and CXCR4 in breast cancer. Cancer Lett. 238:30–41. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandis AZ, Prasad A, Band H, Klosel R
and Ganju RK: Regulation of CXCR4-mediated chemotaxis and
chemoinvasion of breast cancer cells. Oncogene. 23:157–167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rhodes LV, Short SP, Neel NF, et al:
Cytokine receptor CXCR4 mediates estrogen-independent
tumorigenesis, metastasis, and resistance to endocrine therapy in
human breast cancer. Cancer Res. 71:603–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prasad A, Fernandis AZ, Rao Y and Ganju
RK: Slit protein- mediated inhibition of CXCR4-induced chemotactic
and chemoinvasive signaling pathways in breast cancer cells. J Biol
Chem. 279:9115–9124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang Z, Brooks J, Willard M, et al:
CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through
Akt signaling pathway. Biochem Biophys Res Commun. 359:716–722.
2007. View Article : Google Scholar
|
|
10
|
Vila-Coro AJ, Rodriguez-Frade JM, Martin
De Ana A, Moreno-Ortiz MC, Martinez AC and Mellado M: The chemokine
SDF-1alpha triggers CXCR4 receptor dimerization and activates the
JAK/STAT pathway. FASEB J. 13:1699–1710. 1999.PubMed/NCBI
|
|
11
|
Ahr B, Denizot M, Robert-Hebmann V, Brelot
A and Biard-Piechaczyk M: Identification of the cytoplasmic domains
of CXCR4 involved in Jak2 and STAT3 phosphorylation. J Biol Chem.
280:6692–6700. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ling X, Konopleva M, Zeng Z, et al: The
novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1,
9-dien-28-oic acid inhibits metastatic murine breast tumor growth
through inactivation of STAT3 signaling. Cancer Res. 67:4210–4218.
2007. View Article : Google Scholar
|
|
13
|
Alvarez JV, Febbo PG, Ramaswamy S, Loda M,
Richardson A and Frank DA: Identification of a genetic signature of
activated signal transducer and activator of transcription 3 in
human tumors. Cancer Res. 65:5054–5062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Germain D and Frank DA: Targeting the
cytoplasmic and nuclear functions of signal transducers and
activators of transcription 3 for cancer therapy. Clin Cancer Res.
13:5665–5669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al Zaid Siddiquee K and Turkson J: STAT3
as a target for inducing apoptosis in solid and hematological
tumors. Cell Res. 18:254–267. 2008.PubMed/NCBI
|
|
16
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
et al: Stat3 as an oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar
|
|
17
|
Turkson J: STAT proteins as novel targets
for cancer drug discovery. Expert Opin Ther Targets. 8:409–422.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Crowe PJ, Goldstein D and Yang JL:
STAT3 inhibition, a novel approach to enhancing targeted therapy in
human cancers (Review). Int J Oncol. 14:1181–1191. 2012.PubMed/NCBI
|
|
19
|
Groner B, Lucks P and Borghouts C: The
function of Stat3 in tumor cells and their microenvironment. Semin
Cell Dev Biol. 19:341–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soriano SF, Serrano A, Hernanz-Falcon P,
et al: Chemokines integrate JAK/STAT and G-protein pathways during
chemotaxis and calcium flux responses. Eur J Immunol. 33:1328–1333.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moriguchi M, Hissong BD, Gadina M, et al:
CXCL12 signaling is independent of Jak2 and Jak3. J Biol Chem.
280:17408–17414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Majka M, Drukala J, Lesko E, Wysoczynski
M, Jenson AB and Ratajczak MZ: SDF-1 alone and in co-operation with
HGF regulates biology of human cervical carcinoma cells. Folia
Histochem Cytobiol. 44:155–164. 2006.PubMed/NCBI
|
|
23
|
Pfeiffer M, Hartmann TN, Leick M, Catusse
J, Schmitt-Graeff A and Burger M: Alternative implication of CXCR4
in JAK2/STAT3 activation in small cell lung cancer. Br J Cancer.
100:1949–1956. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen B, Zheng MQ, Lu JW, Jiang Q, Wang TH
and Huang XE: CXCL12-CXCR4 promotes proliferation and invasion of
pancreatic cancer cells. Asian Pac J Cancer Prev. 14:5403–5408.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HJ, Kim SW, Kim HY, et al: Chemokine
receptor CXCR4 expression, function, and clinical implications in
gastric cancer. Int J Oncol. 34:473–480. 2009.PubMed/NCBI
|
|
26
|
Diaz N, Minton S, Cox C, et al: Activation
of stat3 in primary tumors from high-risk breast cancer patients is
associated with elevated levels of activated SRC and survivin
expression. Clin Cancer Res. 12:20–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ling X and Arlinghaus RB: Knockdown of
STAT3 expression by RNA interference inhibits the induction of
breast tumors in immunocompetent mice. Cancer Res. 65:2532–2536.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Selander KS, Li L, Watson L, et al:
Inhibition of gp130 signaling in breast cancer blocks constitutive
activation of Stat3 and inhibits in vivo malignancy. Cancer Res.
64:6924–6933. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao L, Wang L, Jin F, et al: Silencing of
estrogen receptor alpha (ERalpha) gene by promoter hypermethylation
is a frequent event in Chinese women with sporadic breast cancer.
Breast Cancer Res Treat. 117:253–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai X, Jin F, Fu Y, et al:
Clinicopathological significance and prognostic value of Xeroderma
pigmentosum complementary group C (XPC) expression in sporadic
breast cancer patients. Med Oncol. 29:1543–1553. 2012. View Article : Google Scholar
|
|
31
|
Pellegrini S and Dusanter-Fourt I: The
structure, regulation and function of the Janus kinases (JAKs) and
the signal transducers and activators of transcription (STATs). Eur
J Biochem. 248:615–633. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu TP, Shen H, Liu LX and Shu YQ: The
impact of chemokine receptor CXCR4 on breast cancer prognosis: A
meta-analysis. Cancer Epidemiol. 37:725–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin F, Brockmeier U, Otterbach F and
Metzen E: New insight into the SDF-1/CXCR4 axis in a breast
carcinoma model: hypoxia-induced endothelial SDF-1 and tumor cell
CXCR4 are required for tumor cell intravasation. Mol Cancer Res.
10:1021–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang M, Liu HX, Teng XD, et al: The
differences in CXCR4 protein expression are significant for the
five molecular subtypes of breast cancer. Ultrastruct Pathol.
36:381–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ling X, Spaeth E, Chen Y, et al: The CXCR4
antagonist AMD3465 regulates oncogenic signaling and invasiveness
in vitro and prevents breast cancer growth and metastasis in vivo.
PLoS One. 8:e584262013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hao L, Zhang CH, Qiu YH, et al:
Recombination of CXCR4, VEGF, and MMP-9 predicting lymph node
metastasis in human breast cancer. Cancer Lett. 253:34–42. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Andre F, Xia WY, Conforti R, et al: CXCR4
expression in early breast cancer and risk of distant recurrence.
Oncologist. 14:1182–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cabioglu N, Yazici MS, Arun BK, et al:
Expression of CXCR4 predicts lymph node metastasis in early breast
cancer. J Clin Oncol. 22:841s. 2004.
|
|
40
|
Schioppa T, Uranchimeg B, Saccani A, et
al: Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp
Med. 198:1391–1402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li YM, Pan Y, Wei Y, et al: Upregulation
of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer
Cell. 6:459–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rahimi M, George J and Tang C: EGFR
variant-mediated invasion by enhanced CXCR4 expression through
transcriptional and post-translational mechanisms. Int J Cancer.
126:1850–1860. 2010.PubMed/NCBI
|
|
43
|
Zhao XP, Huang YY, Huang Y, et al:
Transforming growth factor-beta1 upregulates the expression of CXC
chemokine receptor 4 (CXCR4) in human breast cancer MCF-7 cells.
Acta Pharmacol Sin. 31:347–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dechow TN, Pedranzini L, Leitch A, et al:
Requirement of matrix metalloproteinase-9 for the transformation of
human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci USA.
101:10602–10607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Berishaj M, Gao SP, Ahmed S, et al: Stat3
is tyrosine-phosphorylated through the interleukin-6/glycoprotein
130/Janus kinase pathway in breast cancer. Breast Cancer Res.
9:R322007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Marotta LL, Almendro V, Marusyk A, et al:
The JAK2/STAT3 signaling pathway is required for growth of
CD44+CD24− stem cell-like breast cancer cells
in human tumors. J Clin Invest. 121:2723–2735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Behera R, Kumar V, Lohite K, Karnik S and
Kundu GC: Activation of JAK2/STAT3 signaling by osteopontin
promotes tumor growth in human breast cancer cells. Carcinogenesis.
31:192–200. 2010. View Article : Google Scholar : PubMed/NCBI
|