Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer in the world and the third most common cause of
cancer-related death (1–3). Although most cases of this malignancy
are associated with viral infections such as hepatitis B virus
(HBV) and hepatitis C virus (HCV) infections, a substantial
proportion of HCC patients are negative for markers of HBV surface
antigen (HBsAg) and HCV antibody (HCVAb) [non-B non-C HCC
(NBNC-HCC)]. The frequency of NBNC-HCC has been reported to range
from 5 to 15%, and the number of NBNC-HCC patients in Japan has
recently been gradually increasing (4–7). It is
noteworthy that the proportion of NBNC-HCC patients was ~30% in
2011, 2012 and 2013 in our hospital (1).
Curative therapies for HCC consist of liver
transplantation, surgical resection (SR) and radiofrequency
ablation (RFA) (1–7). The clinical outcome of HCC patients
undergoing these therapies has improved substantially in recent
years due to their advances. However, HCC often recurs even after
curative therapies, leading to high mortality. Recurrence only
occurs at intrahepatic sites in 68–96% of patients (1,8–10).
Hence, the identification of predictive factors and effective
management of HCC recurrence are essential for improving survival,
even after curative treatment.
Recently, several noninvasive tools have been
introduced to evaluate the degree of hepatic fibrosis in patients
with chronic liver disease; these include serum markers such as
aspartate aminotransferase to platelet ratio index (APRI), FIB-4
index, aspartate aminotransferase (AST) to alanine aminotransferase
(ALT) ratio or modalities such as acoustic radiation force impulse,
transient elastography and magnetic resonance elastography
(11–18). Serum markers and developed scores
are of rising significance in noninvasive diagnosis of liver
fibrosis owing to the easy availability in field practice. Among
these tools, the FIB-4 index is a simple formula used for
predicting liver fibrosis based on standard biochemical values
(platelet count, AST and ALT) and age, and is demonstrated to be
highly helpful for predicting advanced liver fibrosis (19–22).
However, this test has seldom been applied for the evaluation of
liver function prior to SR and for prediction of clinical outcomes
for NBNC-HCC patients who undergo SR, although there are several
reports regarding the clinical significance of preoperative APRI on
survival in HBV-related HCC patients treated with SR (23,24).
The aims of the present study were to examine the
relationship between preoperative FIB-4 index and background liver
fibrosis in non-tumor parts obtained from extracted surgical
specimens and to investigate whether the preoperative FIB-4 index
can be a useful predictor for NBNC-HCC patients treated with
SR.
Patients and methods
Patients
A total of 128 treatment-naïve NBNC-HCC patients
received SR at our institution between June 2004 and June 2014 with
curative intent. Curative surgery was defined as resection of all
tumors detectable using imaging modalities. NBNC-HCC was defined as
HCC negative for both HBsAg and HCVAb. Patients with severe
alcoholic cirrhosis (n=7), a patient with autoimmune hepatitis
(n=1) and patients with primary biliary cirrhosis (n=2) were
excluded from the present study. A total of 118 NBNC-HCC patients
were thus analyzed in the present study. A diagnosis of diabetes
mellitus (DM) was based on past medical history or 75-g oral
glucose tolerance test results (25). We examined predictive factors
associated with overall survival (OS) and recurrence-free survival
(RFS) in univariate and multivariate analyses.
Written informed consent was obtained from all
patients prior to SR, and the study protocol complied with all of
the provisions of the Declaration of Helsinki. The present study
was approved by the Ethics Committee of Osaka Red Cross Hospital,
Japan. The present study comprised a retrospective analysis of
patient records registered in our database, and all treatments were
conducted in an open-label manner.
Calculated scores
The APRI score was calculated using Wai’s formula:
(AST/upper limit of normal)/platelet count (expressed as platelets
× 109/l) × 100 (26).
The FIB-4 score was calculated using Sterling’s formula as: [age
(years) × AST (IU/l)/platelet count (×109/l) ×
ALT½ (IU/l)] (27).
HCC diagnosis
HCC was diagnosed using abdominal ultrasound and
dynamic CT scans (hyperattenuation during the arterial phase in all
or some part of the tumor and hypoattenuation in the portal-venous
phase) and/or magnetic resonance imaging (MRI), based mainly on the
recommendations of the American Association for the Study of Liver
Diseases (28). Arterial- and
portal-phase dynamic CT images were obtained at ~30 and 120 sec,
respectively, after the injection of the contrast material. HCC
stage was determined using the Liver Cancer Study Group of Japan
staging system (29). All HCC was
confirmed pathologically except for two cases with complete
necrosis due to preoperative transcatheter arterial
chemoembolization (TACE).
Hepatectomy and surgical procedure
All surgical procedures were performed by one of
four surgeons with at least 10 years experience of SR. Anatomical
SR was defined as a resection in which tumors were completely
removed anatomically on the basis of Couinaud’s classification
(segmentectomy, sectionectomy and hemihepatectomy, or extended
hemihepatectomy). Non-anatomical partial SR was carried out as a
limited resection or tumor enucleation. Anatomical SR was performed
in 68 patients (57.6%) and non-anatomical SR was performed in 50
patients (42.4%) in the present study. Conventional open
hepatectomy was performed in 95 patients (80.5%), and laparoscopic
hepatectomy was performed in 23 patients (19.5%) in the present
study.
Histological evaluation of the extracted
liver specimens
All extracted liver specimens were reviewed by a
single pathologist in our hospital. Background liver fibrosis was
staged as F0 to F4: F0, no fibrosis; F1, portal fibrosis without
septa; F2, portal fibrosis and a few septa; F3, numerous septa
without cirrhosis; and F4, cirrhosis. The degree of differentiation
of HCC in each resected specimen was determined as
well-differentiated HCC, moderately differentiated HCC, poorly
differentiated HCC or combined type of HCC and cholangiocellular
carcinoma (CCC) (30).
Follow-up
Follow-up after each therapy consisted of periodic
blood tests and monitoring of tumor markers, including
α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP), using
chemiluminescent enzyme immunoassays (Lumipulse PIVKA-II Eisai;
Eisai, Tokyo, Japan). Dynamic CT scans and/or MRI were obtained
every 2–4 months after each therapy. Chest CT, whole abdominal CT,
brain MRI, and bone scintigraphy were performed when extrahepatic
HCC recurrence was suspected. When HCC recurred, the most
appropriate therapy for HCC recurrence was performed considering
tumor status, liver function or performance status of the
patients.
Statistical analysis
Data were analyzed using univariate and multivariate
analyses. Continuous variables were compared between groups by the
Mann-Whitney U test. Receiver operating characteristic (ROC) curve
analysis was performed for calculating the area under the ROC
(AUROC) for the FIB-4 index, APRI, AST to ALT ratio, serum albumin,
total bilirubin and platelet count and selecting the optimal
cut-off value that maximized the sum of sensitivity and specificity
for liver cirrhosis (F4). Time to recurrence was defined as the
interval between initial therapy and first confirmed recurrence.
For analysis of RFS, follow-up ended at the time of first
recurrence; other patients were censored at their last follow-up
visit or the time of death from any cause without recurrence. For
analysis of OS, follow-up ended at the time of death from any
cause, and the remaining patients were censored at the last
follow-up visit. The cumulative OS and RFS rates were calculated
using the Kaplan-Meier method, and tested using the log-rank test.
Factors with a P-value <0.05 in univariate analysis were
subjected to multivariate analysis using the Cox proportional
hazards model. These statistical methods were used to estimate the
interval from initial treatment. Data were analyzed using SPSS
software (SPSS, Inc., Chicago, IL, USA) for Microsoft Windows. Data
are expressed as means ± standard deviation (SD). Values of
P<0.05 were considered to indicate statistically significant
differences.
Results
Baseline characteristics
The baseline characteristics of the analyzed
patients (n=118) are shown in Table
I. The mean age was 68.9±9.0 years. The median observation
period was 3.2 years (range, 0.1–10.1 years). There were 93 males
and 25 females. The mean maximum tumor size was 5.7±3.2 cm. The
mean body mass index (BMI) was 24.3±3.9 kg/m2. As for
the histological findings, in terms of the degree of liver fibrosis
in the non-tumor portion, F4 was observed in 39 patients, F3 in 20,
F2 in 22, F1 in 14 and F0 in 23, whereas in terms of HCC histology,
well-differentiated HCC was observed in 11 patients, moderately
differentiated HCC in 69, poorly differentiated HCC in 33, combined
type of HCC and CCC in 3 and complete necrosis due to preoperative
TACE in 2 patients.
 | Table IBaseline characteristics of the
patients with non-B and non-C hepatocellular carcinoma (n=118). |
Table I
Baseline characteristics of the
patients with non-B and non-C hepatocellular carcinoma (n=118).
| Variables | Data (N=118) |
|---|
| Age (years) | 68.9±9.0 |
| Gender,
male/female | 93/25 |
| Body mass index
(kg/m2) | 24.3±3.9 |
| Diabetes mellitus,
yes/no | 53/65 |
| HCC stage,
I/II/III/IV | 4/74/33/7 |
| Maximum tumor size
(cm) | 5.7±3.2 |
| Tumor number,
single/multiple | 75/43 |
| AST (IU/l) | 43.4±26.0 |
| ALT (IU/l) | 37.8±29.4 |
| ALP (IU/l) | 343.8±186.8 |
| GGT (IU/l) | 158.8±170.6 |
| Serum albumin
(g/dl) | 4.0±0.5 |
| Total bilirubin
(mg/dl) | 0.8±0.4 |
| Prothrombin time
(%)a | 92.5±18.7 |
| Platelets
(×104/mm3) | 17.0±8.1 |
| AFP (ng/ml) | 1,713±11,917 |
| DCP
(mAU/ml)b | 8,629±48,077 |
| Histological
findings (extracted surgical specimen) | |
| Background liver
fibrosis, F4/3/2/1/0 | 39/20/22/14/23 |
| Tumor
differentiation, well/moderate/poor/combined/necrosis | 11/69/33/3/2 |
Comparison of the area under receiver
operating curves for serum markers for liver cirrhosis
We evaluated the correlation between serum markers
including FIB-4 index, APRI, AST to ALT ratio, platelet count,
serum albumin and total bilirubin and liver cirrhosis (F4).
Receiver operating curves of the serum markers used for predicting
liver cirrhosis are demonstrated in Fig. 1. FIB-4 index, APRI and platelet
count exhibited reliable discriminative ability for predicting
liver cirrhosis. Among these, the FIB-4 index yielded the highest
AUROC with a level of 0.887 at an optimal cut-off value of 2.97
(sensitivity, 92.3%; specificity, 69.6%) (Table II). The FIB-4 index in patients
with liver cirrhosis (F4, n=39) was significantly higher than that
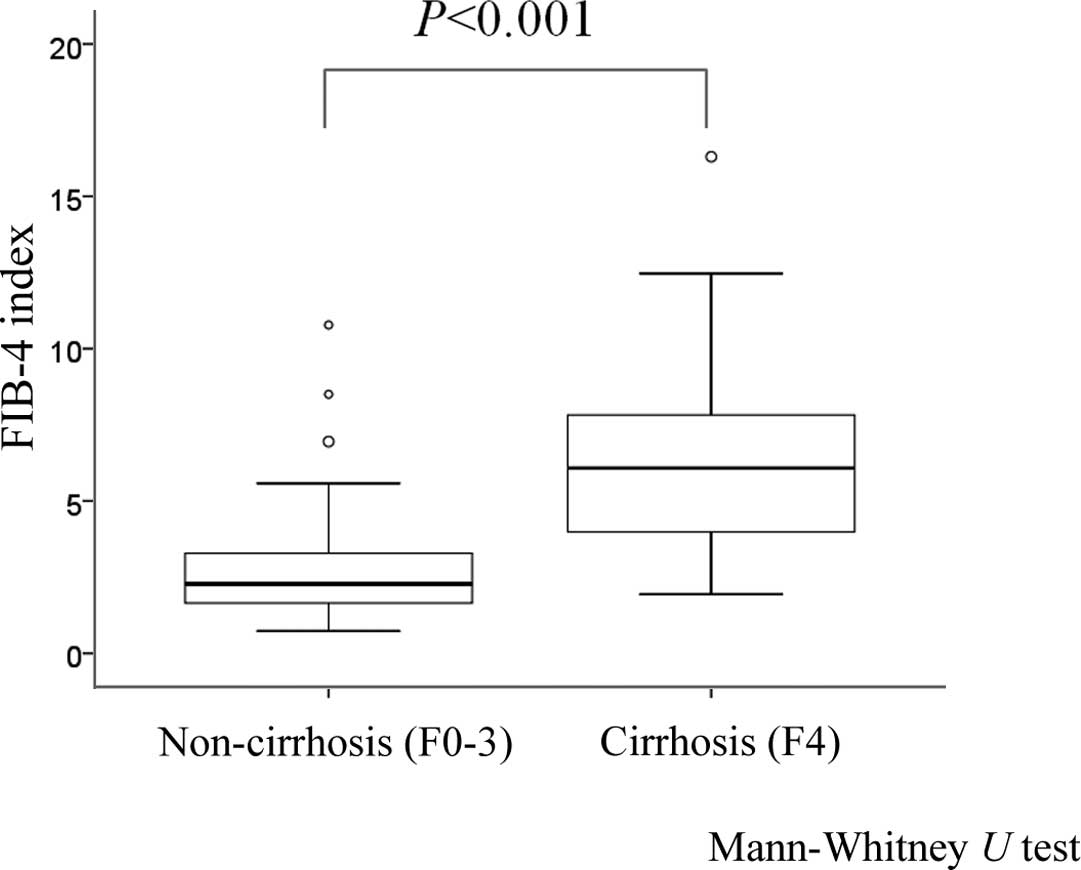
in the patients with non-liver cirrhosis (F0–3, n=79) (P<0.001,
Mann-Whitney U test) (Fig. 2).
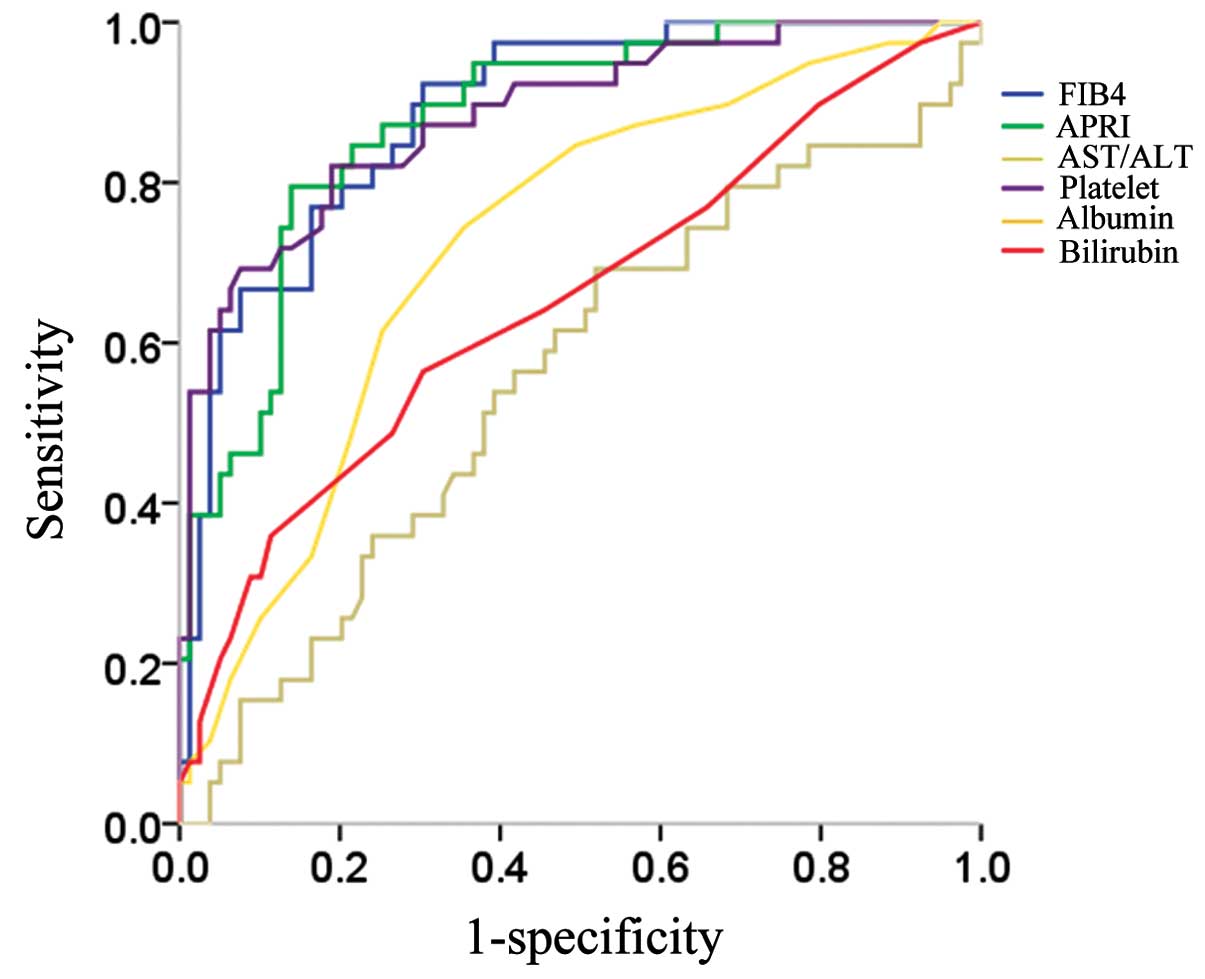 | Figure 1Correlation between serum markers
including FIB-4 index, AST to platelet ratio index (APRI), AST to
ALT ratio, platelet count, serum albumin and total bilirubin and
liver cirrhosis (F4). FIB-4 index yielded the highest AUROC with a
level of 0.887 at an optimal cut-off value of 2.97 (sensitivity,
92.3%; specificity, 69.6%). AST, aspartate aminotransferase; ALT,
alanine aminotransferase; AUROC, area under the ROC; ROC, receiver
operating characteristic. |
 | Table IIComparison of the area under the
receiver operating curves (AUROCs) for FIB-4 index, APRI, AST to
ALT ratio, platelet count, serum albumin and total bilirubin for
liver cirrhosis. |
Table II
Comparison of the area under the
receiver operating curves (AUROCs) for FIB-4 index, APRI, AST to
ALT ratio, platelet count, serum albumin and total bilirubin for
liver cirrhosis.
| AUROC | 95% CI | Standard error | P-value |
|---|
| FIB-4 index | 0.887 | 0.828–0.947 | 0.030 | <0.001 |
| APRI | 0.877 | 0.814–0.941 | 0.032 | <0.001 |
| AST to ALT
ratio | 0.559 | 0.446–0.671 | 0.057 | 0.302 |
| Platelet count
(/mm3) | 0.883 | 0.818–0.949 | 0.034 | <0.001 |
| Serum albumin
(g/dl) | 0.724 | 0.629–0.820 | 0.049 | <0.001 |
| Total bilirubin
(mg/dl) | 0.652 | 0.543–0.761 | 0.056 | 0.007 |
Cumulative OS and RFS rates for all
patients
For all patients (n=118), the 1-, 3- and 5-year
cumulative OS rates were 92.0, 76.6 and 67.6%, respectively. The
corresponding RFS rates were 71.9, 37.8 and 30.5%,
respectively.
Cumulative OS and RFS rates according to
FIB-4 index
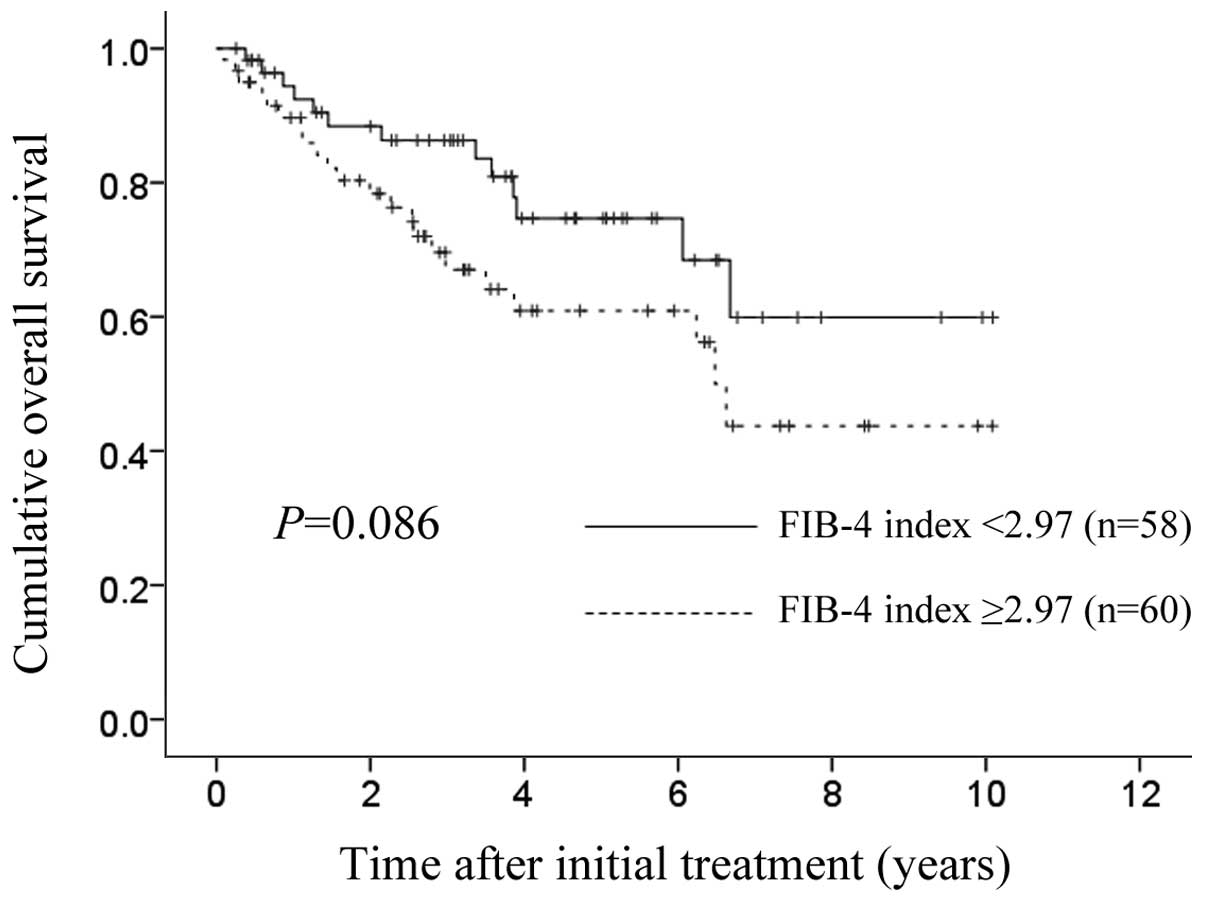
The 1-, 3- and 5-year cumulative OS rates in
patients with FIB-4 index >2.97 (optimal cut-off value) (n=60)
were 89.7, 67.0 and 60.9%, respectively, and the corresponding
cumulative OS rates in patients with FIB-4 index <2.97 (n=58)
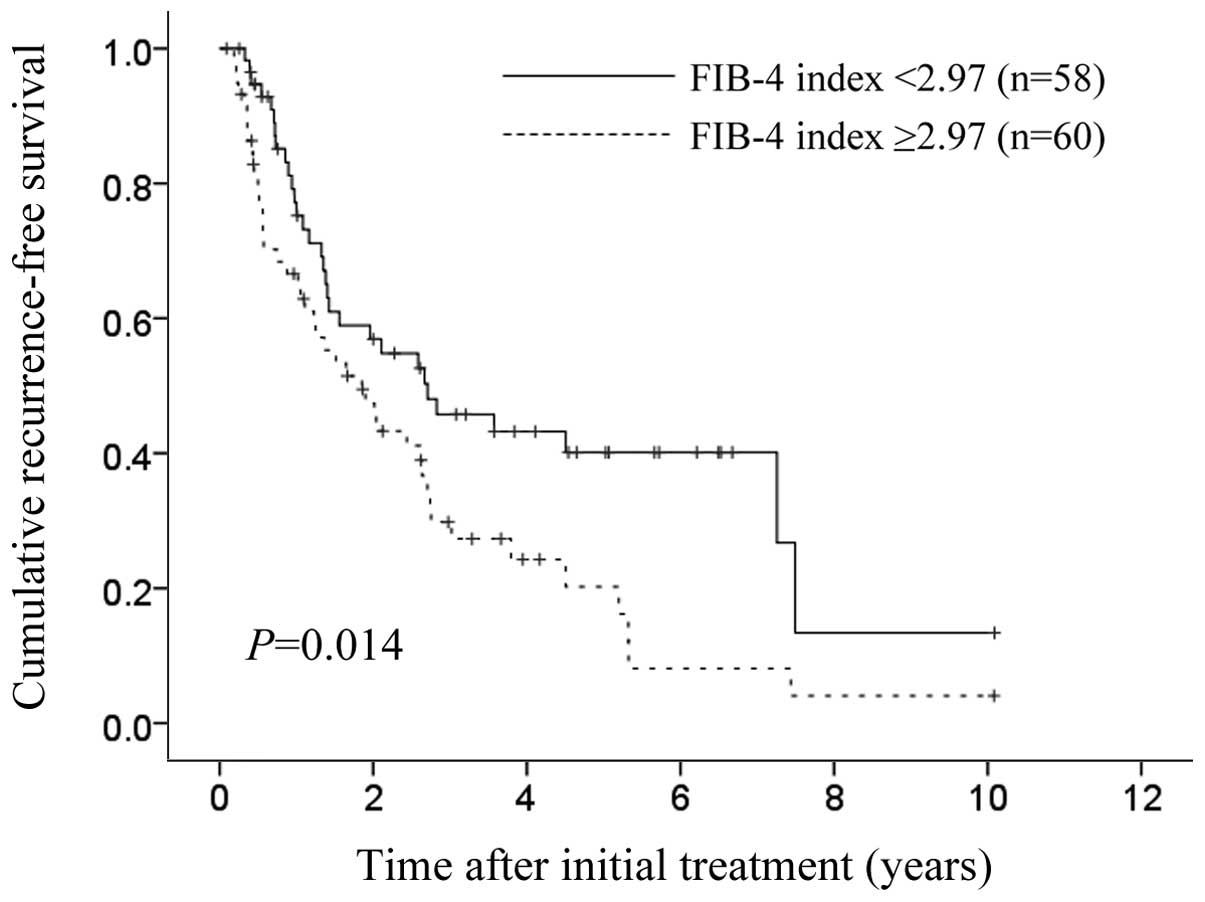
were 94.4, 86.3 and 74.7%, respectively (P=0.086) (Fig. 3). The 1-, 3- and 5-year cumulative
RFS rates in patients with FIB-4 index >2.97 were 66.6, 29.8 and
20.2%, respectively, and the corresponding cumulative RFS rates in
patients with FIB-4 index <2.97 were 75.2, 45.8 and 40.1%,
respectively (P=0.014) (Fig.
4).
Univariate and multivariate analyses of
factors contributing to OS
Univariate analysis identified the following factors
as significantly associated with OS for all cases (n=118): tumor
number (single or multiple) (P=0.006); alkaline phosphatase (ALP)
>300 IU/l (P=0.037); serum albumin >4.0 g/dl (P=0.010); and
AFP >40 ng/ml (P=0.048) (Table
III). The hazard ratios (HRs) and 95% confidence intervals
(CIs) calculated using multivariate analysis for the four factors
with P-values of <0.05 in univariate analysis are detailed in
Table III. Only the AFP value was
found to be a significant predictor linked to OS in multivariate
analysis (P=0.026).
 | Table IIIUnivariate and multivariate analysis
of factors contributing to overall survival. |
Table III
Univariate and multivariate analysis
of factors contributing to overall survival.
| Variables | n | Univariate
analysis | Multivariate
analysis |
|---|
|
|---|
| Hazard ratio (95%
CI) | P-valuea |
|---|
| Gender, male vs.
female | 93/25 | 0.330 | | |
| Age (years), >70
vs. ≤70 | 58/60 | 0.802 | | |
| Tumor number,
single vs. multiple | 75/43 | 0.006 | 0.544
(0.271–1.092) | 0.087 |
| Maximum tumor size
(cm), ≥4.5 vs. <4.5 | 59/59 | 0.386 | | |
| Microscopic
vascular invasion, yes vs. no | 52/66 | 0.100 | | |
| AST (IU/l), ≥40 vs.
<40 | 53/65 | 0.561 | | |
| ALT (IU/l), ≥40 vs.
<40 | 34/84 | 0.196 | | |
| ALP (IU/l), ≥300
vs. <300 | 56/62 | 0.037 | 0.514
(0.239–1.104) | 0.088 |
| GGT (IU/l), ≥100
vs. <100 | 58/60 | 0.370 | | |
| FIB-4 index ≥2.97,
yes vs. no | 60/58 | 0.086 | | |
| Serum albumin level
(g/dl), ≥4.0 vs. <4.0 | 74/44 | 0.010 | 1.991
(0.986–4.022) | 0.055 |
| Total bilirubin
(mg/dl), ≥1.0 vs. <1.0 | 23/95 | 0.465 | | |
| Platelet count
(×104/mm3), ≥15 vs. <15 | 64/54 | 0.205 | | |
| Prothrombin time
(%),≥80 vs. <80b | 98/18 | 0.051 | | |
| Diabetes mellitus,
yes vs. no | 53/65 | 0.150 | | |
| Body mass index
(kg/m2), ≥25 vs. <25 | 49/69 | 0.322 | | |
| Serum AFP (ng/ml),
≥40 vs. <40 | 31/87 | 0.048 | 0.427
(0.202–0.904) | 0.026 |
| DCP (mAU/ml), ≥200
vs. <200c | 61/55 | 0.406 | | |
Univariate and multivariate analyses of
factors contributing to RFS
Univariate analysis identified the following factors
as significantly associated with RFS for all cases (n=118): tumor
number (single or multiple) (P<0.001); presence of microscopic
vascular invasion (P=0.024); ALP >300 IU/l (P=0.002); γ-glutamyl
transpeptidase (GGT) >100 IU/l (P=0.010); and FIB-4 index
>2.97 (P=0.014) (Table IV). The
HRs and 95% CIs calculated using multivariate analysis for the five
factors with P-values of <0.05 in univariate analysis are
detailed in Table IV. Tumor number
(P=0.002) and FIB-4 index (P=0.044) were found to be significant
prognostic factors linked to RFS.
 | Table IVUnivariate and multivariate analysis
of factors contributing to recurrence-free survival. |
Table IV
Univariate and multivariate analysis
of factors contributing to recurrence-free survival.
| Variables | n | Univariate
analysis | Multivariate
analysis |
|---|
|
|---|
| Hazard ratio (95%
CI) | P-valuea |
|---|
| Gender, male vs.
female | 93/25 | 0.407 | | |
| Age (years), >70
vs. ≤70 | 58/60 | 0.396 | | |
| Tumor number,
single vs. multiple | 75/43 | <0.001 | 0.468
(0.291–0.751) | 0.002 |
| Maximum tumor size
(cm), >4.5 vs. ≤4.5 | 59/59 | 0.335 | | |
| Microscopic
vascular invasion, yes vs. no | 52/66 | 0.024 | 0.648
(0.406–1.032) | 0.068 |
| AST (IU/l), ≥40 vs.
<40 | 53/65 | 0.035 | | |
| ALT (IU/l), ≥40 vs.
<40 | 34/84 | 0.880 | | |
| ALP (IU/l), ≥300
vs. <300 | 56/62 | 0.002 | 0.827
(0.492–1.391) | 0.474 |
| GGT (IU/l), ≥100
vs. <100 | 58/60 | 0.010 | 0.680
(0.399–1.159) | 0.156 |
| FIB-4 index ≥2.97,
yes vs. no | 60/58 | 0.014 | 0.640
(0.390–0.988) | 0.044 |
| Serum albumin level
(g/dl), ≥4.0 vs. <4.0 | 74/44 | 0.933 | | |
| Total bilirubin
(mg/dl), ≥1.0 vs. <1.0 | 23/95 | 0.817 | | |
| Platelet count
(×104/mm3), ≥15 vs. <15 | 64/54 | 0.063 | | |
| Prothrombin time
(%),≥80 vs. <80b | 98/18 | 0.052 | | |
| Diabetes mellitus,
yes vs. no | 53/65 | 0.652 | | |
| Body mass index
(kg/m2), ≥25 vs. <25 | 49/69 | 0.916 | | |
| Serum AFP (ng/ml),
≥40 vs. <40 | 31/87 | 0.116 | | |
| DCP (mAU/ml), ≥200
vs. <200c | 61/55 | 0.686 | | |
Causes of death
Thirty-five patients (29.7%) died during the
follow-up period. The causes of death were HCC recurrence in 24
patients, liver failure in 6 patients and miscellaneous causes in 5
patients.
HCC recurrence
In the present study, 75 patients (63.6%) had HCC
recurrences during the follow-up period. The patterns of HCC
recurrence after initial treatment were: single HCC recurrence in
the liver in 32 patients; multiple HCC recurrences in the liver in
28 patients; multiple HCC recurrences in the liver with lung
metastases in three patients; multiple lung metastases in 5
patients; multiple HCC recurrences in the liver with lymph node
metastases in 2 patients; multiple HCC recurrences in the liver
with peritoneal dissemination in one patient; multiple HCC
recurrences in the liver with bone metastases in one patient;
multiple bone metastases in one patient; single HCC recurrence in
the liver with duodenal invasion in one patient; and local tumor
progression (recurrence in the SR site) in one patient. Treatment
methods for the first HCC recurrence were: SR in 11 patients; RFA
in 29 patients; TACE in 16 patients; systemic chemotherapy such as
sorafenib in 7 patients; radiation therapy in 2 patients and no
specific treatment in 10 patients.
In patients with a preoperative FIB-4 index >2.97
(n=60), HCC recurrence was found in 44 patients (73.3%), while in
patients with a preoperative FIB-4 index <2.97 (n=58), HCC
recurrence was observed in 38 patients (65.5%). Fifteen patients
(25.0%) had late first confirmed HCC recurrence (>2 years after
initial SR) in patients with a preoperative FIB-4 index >2.97,
whereas 9 patients (15.5%) had late first confirmed HCC recurrence
in patients with a preoperative FIB-4 index <2.97.
Discussion
To the best of our knowledge, this is the first
reported study to investigate the relationship between preoperative
FIB-4 index and clinical outcomes in NBNC-HCC patients treated with
SR. Although the FIB-4 index has been demonstrated to be a useful
noninvasive serum marker for predicting liver fibrosis in many
previous studies, the effect of this marker on clinical outcomes in
NBNC-HCC patients who undergo SR remains obscure (11–18).
Hence, we conducted the current analyses.
In our results, the FIB-4 index yielded the highest
AUROC with a level of 0.887 for cirrhosis and in the multivariate
analysis, FIB-4 index was significantly associated with RFS
although in terms of OS, FIB-4 index was not a significant
predictor. These results revealed that the FIB-4 index has the
highly discriminative ability for predicting liver cirrhosis and it
could be a useful predictor for NBNC-HCC patients treated with SR.
Liver biopsy, which has been considered as the ‘golden standard’
for defining liver fibrosis, carries some drawbacks: sampling error
and inter-observer variability, which have raised questions on its
value, whereas in our current analyses, we examined the effect of
FIB-4 index on liver cirrhosis using non-tumor parts of extracted
surgical specimens, which had sufficient amount of liver specimens
for an exact assessment of extension of liver fibrosis (13,31).
Thus, our current data are highly reliable. One possible reason
that the FIB-4 index was not a significant predictor linked to OS
in the present analysis is that HCC patients with extremely poor
hepatic reserve were excluded from our candidates for SR.
In our baseline characteristics, the proportion of
patients with DM was 44.9% (53/118). Wang et al performed a
meta-analysis including a total of 25 cohort studies to investigate
the relationship between DM and HCC development and reported that
DM was associated with an increased incidence of HCC (HR=2.01, 95%
CI=1.61–2.51) compared with individuals without DM, and it was also
positively associated with HCC mortality (HR=1.56, 95%
CI=1.30–1.87) (32). Our high
proportion of diabetic patients may be associated with their
results. On the other hand, the mean BMI in the present study was
24.3±3.9 kg/m2 and the proportion of patients with BMI
>30 kg/m2 that indicated obesity was only 7.6%
(9/118). Obesity and its related metabolic abnormalities, including
chronic inflammatory condition, have been shown to increase the
risk of HCC development (33).
Although the reasons for these discrepancies are unclear, other
carcinogenic factors than obesity may be closely associated with
NBNC-HCC development in Japanese populations. Furthermore, it is of
note that the proportion of patients with F0 or F1 that indicated
minimal fibrosis was 31.4% (37/118) in the present study, although
advanced fibrosis was found to be a significant risk factor of HCC
development in many previous reports (1–7). The
carcinogenic mechanisms between NBNC-HCC and virus-related HCC may
be different.
In the multivariate analysis in terms of OS, AFP was
an independent predictor (P=0.026) and serum albumin had marginal
significance (P=0.055). Both tumor-related factors and liver
function-related factors may be essential for the management of
NBNC-HCC patients treated with SR. In that sense, branched-chain
amino acid therapy for background liver disease may improve
clinical outcomes (34). On the
other hand, as for RFS, tumor number (P=0.002) and FIB-4 index
(P=0.044) were found to be independent significant predictors and
the presence of microvascular invasion had marginal significance
(P=0.068) in the multivariate analysis. Particularly in patients
with these risk factors, close observation for HCC recurrence after
SR is needed. Furthermore, in our results, the proportion of late
HCC recurrence in patients with FIB-4 index >2.97 was higher
than that in patients with FIB-4 index <2.97 (25.0 vs. 15.5%).
Several investigators have demonstrated that advanced fibrosis is
related to late de novo HCC recurrence after SR in the
remnant fibrotic liver (35,36).
Our results were consistent with their reports.
The present study included several limitations.
Firstly, this is a retrospective observational study. Secondly, the
sample size in the present study was relatively small for
statistical analyses. Thirdly, our study cohort included only
Japanese HCC patients, who in general had lower BMI than
populations in Western countries (37,38).
Hence, caution should be exercised in interpreting our results and
further larger prospective studies are necessary. However, our
results demonstrated that the FIB-4 index had high discriminative
ability for predicting liver cirrhosis and it could identify
patients with a high risk of HCC recurrence in NBNC-HCC patients
treated with SR.
In conclusion, the FIB-4 index is a useful serum
marker for predicting liver cirrhosis and a useful predictor of
clinical outcomes for NBNC-HCC patients who undergo SR.
Acknowledgements
The authors would like to thank Haruko Takada for
the data collection.
References
|
1
|
Osaki Y and Nishikawa H: Treatment for
hepatocellular carcinoma in Japan over the last three decades: our
experience and published work review. Hepatol Res. Jun
26–2014.(Epub ahead of print). View Article : Google Scholar
|
|
2
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Lope CR, Tremosini S, Forner A, Reig M
and Bruix J: Management of HCC. J Hepatol. 56(Suppl 1): S75–S87.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaibori M, Ishizaki M, Matsui K and Kwon
AH: Clinicopathologic characteristics of patients with non-B non-C
hepatitis virus hepatocellular carcinoma after hepatectomy. Am J
Surg. 204:300–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nishikawa H and Osaki Y: Non-B, non-C
hepatocellular carcinoma (Review). Int J Oncol. 43:1333–1342.
2013.PubMed/NCBI
|
|
6
|
Utsunomiya T and Shimada M: Molecular
characteristics of non-cancerous liver tissue in non-B non-C
hepatocellular carcinoma. Hepatol Res. 41:711–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Umemura T and Kiyosawa K: Epidemiology of
hepatocellular carcinoma in Japan. Hepatol Res. 37(Suppl 2):
S95–S100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP,
Pan ZY, Lau WY and Wu MC: A prospective, randomized, controlled
trial of preoperative transarterial chemoembolization for
resectable large hepatocellular carcinoma. Ann Surg. 249:195–202.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa H, Osaki Y, Kita R, Kimura T,
Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, Henmi S,
Hatamaru K, Saito S and Nasu A: Transcatheter arterial infusion
chemotherapy prior to radiofrequency thermal ablation for single
hepatocellular carcinoma reduces the risk of intrahepatic distant
recurrence. Int J Oncol. 41:903–909. 2012.PubMed/NCBI
|
|
10
|
Nishikawa H, Osaki Y, Kita R, Kimura T,
Ohara Y, Takeda H, Sakamoto A, Saito S, Nishijima N, Nasu A,
Komekado H and Nishiguchi S: Comparison of transcatheter arterial
chemoembolization and transcatheter arterial chemotherapy infusion
for patients with intermediate-stage hepatocellular carcinoma.
Oncol Rep. 31:65–72. 2014.
|
|
11
|
Castera L: Invasive and non-invasive
methods for the assessment of fibrosis and disease progression in
chronic liver disease. Best Pract Res Clin Gastroenterol.
25:291–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chrostek L and Panasiuk A: Liver fibrosis
markers in alcoholic liver disease. World J Gastroenterol.
20:8018–8023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sumida Y, Nakajima A and Itoh Y:
Limitations of liver biopsy and non-invasive diagnostic tests for
the diagnosis of nonalcoholic fatty liver disease/nonalcoholic
steatohepatitis. World J Gastroenterol. 20:475–485. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith JO and Sterling RK: Systematic
review: non-invasive methods of fibrosis analysis in chronic
hepatitis C. Aliment Pharmacol Ther. 30:557–576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
D’Onofrio M, Crosara S, De Robertis R,
Canestrini S, Demozzi E, Gallotti A and Pozzi Mucelli R: Acoustic
radiation force impulse of the liver. World J Gastroenterol.
19:4841–4849. 2013. View Article : Google Scholar
|
|
16
|
Mariappan YK, Glaser KJ and Ehman RL:
Magnetic resonance elastography: a review. Clin Anat. 23:497–511.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu ML, Lin SM, Lee CM, Dai CY, Chang WY,
Chen SC, Lee LP, Lin ZY, Hsieh MY, Wang LY, Chuang WL and Liaw YF:
A simple noninvasive index for predicting long-term outcome of
chronic hepatitis C after interferon-based therapy. Hepatology.
44:1086–1097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Castera L: Noninvasive methods to assess
liver disease in patients with hepatitis B or C. Gastroenterology.
142:1293–1302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: an inexpensive and accurate marker of fibrosis in HCV
infection. Comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Angulo P, Bugianesi E, Bjornsson ESP,
Mills PR, Barrera F, Haflidadottir S, Day CP and George J: Simple
noninvasive systems predict long-term outcomes of patients with
nonalcoholic fatty liver disease. Gastroenterology. 145:782–789.e4.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tamaki N, Kurosaki M, Matsuda S, Muraoka
M, Yasui Y, Suzuki S, Hosokawa T, Ueda K, Tsuchiya K, Nakanishi H,
Itakura J, Takahashi Y, Asahina Y and Izumi N: Non-invasive
prediction of hepatocellular carcinoma development using serum
fibrosis marker in chronic hepatitis C patients. J Gastroenterol.
Dec 15–2013.(Epub ahead of print). PubMed/NCBI
|
|
22
|
Chon YE, Jung ES, Park JY, Kim do Y, Ahn
SH, Han KH, Chon CY, Jung KS and Kim SU: The accuracy of
noninvasive methods in predicting the development of hepatocellular
carcinoma and hepatic decompensation in patients with chronic
hepatitis B. J Clin Gastroenterol. 46:518–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen SL, Fu SJ, Chen B, Kuang M, Li SQ,
Hua YP, Liang LJ, Guo P, Hao Y and Peng BG: Preoperative aspartate
aminotransferase to platelet ratio is an independent prognostic
factor for hepatitis B-induced hepatocellular carcinoma after
hepatic resection. Ann Surg Oncol. May 22–2014.(Epub ahead of
print). View Article : Google Scholar
|
|
24
|
Hung HH, Su CW, Lai CR, Chau GY, Chan CC,
Huang YH, Huo TI, Lee PC, Kao WY, Lee SD and Wu JC: Fibrosis and
AST to platelet ratio index predict post-operative prognosis for
solitary small hepatitis B-related hepatocellular carcinoma.
Hepatol Int. 4:691–699. 2010. View Article : Google Scholar
|
|
25
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabet Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wai CT, Greenson JK, Fontana RJ,
Kalbfleisch JD, Marrero JA, Conjeevaram HS and Lok AS: A simple
noninvasive index can predict both significant fibrosis and
cirrhosis in patients with chronic hepatitis C. Hepatology.
38:518–526. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, Sulkowski MS, Torriani FJ, Dieterich DT,
Thomas DL, Messinger D and Nelson M: APRICOT Clinical
Investigators: Development of a simple noninvasive index to predict
significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
No authors listed. The general rules for
the clinical and pathological study of primary liver cancer. Liver
Cancer Study Group of Japan. Jpn J Surg. 19:98–129. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Utsunomiya T, Shimada M, Kudo M, Ichida T,
Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y,
Takayama T and Kokudo N; for the Liver Cancer Study Group of Japan.
A Comparison of the surgical outcomes among patients with
HBV-positive, HCV-positive, and non-B non-C hepatocellular
carcinoma: a nationwide study of 11,950 patients. Ann Surg. Jul
28–2014.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nalbantoglu I and Brunt EM: Role of liver
biopsy in nonalcoholic fatty liver disease. World J Gastroenterol.
20:9026–9037. 2014.PubMed/NCBI
|
|
32
|
Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen
Y, Li G and Wang L: Increased risk of hepatocellular carcinoma in
patients with diabetes mellitus: a systematic review and
meta-analysis of cohort studies. Int J Cancer. 130:1639–1648. 2012.
View Article : Google Scholar
|
|
33
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishikawa H and Osaki Y: Clinical
significance of therapy using branched-chain amino acid granules in
patients with liver cirrhosis and hepatocellular carcinoma. Hepatol
Res. 44:149–158. 2014. View Article : Google Scholar
|
|
35
|
Jung KS, Kim SU, Choi GH, Park JY, Park
YN, Kim do Y, Ahn SH, Chon CY, Kim KS, Choi EH, Choi JS and Han KH:
Prediction of recurrence after curative resection of hepatocellular
carcinoma using liver stiffness measurement
(FibroScan®). Ann Surg Oncol. 19:4278–4286. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Imamura H, Matsuyama Y, Tanaka E, Ohkubo
T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T,
Kawasaki S and Makuuchi M: Risk factors contributing to early and
late phase intrahepatic recurrence of hepatocellular carcinoma
after hepatectomy. J Hepatol. 38:200–207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McCurry J: Japan battles with obesity.
Lancet. 369:451–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Examination Committee of Criteria for
‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity.
New criteria for ‘Obesity Disease’ in Japan. Circ J. 66:987–992.
2002. View Article : Google Scholar
|