Introduction
Breast cancer remains one of the most common
malignancies and is the second leading cause of cancer-related
mortality in women (1). Standard
treatment modalities have improved the overall survival and the
quality of life of patients. However, more personalized therapies
or improvement of existing treatment are needed due to the
heterogeneity of breast cancer and resistance to existing
therapies. The molecular profiling of human breast cancers has
identified at least 5 subtypes with distinct clinical outcomes
(2–5). One of the common subgroups is the
ErbB2-positive subtype, which occurs in 20–30% of breast cancer
cases. It also has been demonstrated that elevated
expression/amplification of ErbB2 correlates with poor prognosis
(6–8).
Mutation or deficiency of the tumor-suppressor
phosphatase and tensin homologue (PTEN) has been reported to occur
in 5–10% of human breast cancer cases (9). Loss of PTEN function results in
hyperactivation of the PI3K/AKT pathway and induction of basal-like
breast cancers (10,11). Furthermore, numerous studies have
indicated that loss of PTEN confers resistance to trastuzumab
(Herceptin), a humanized monoclonal antibody targeting ErbB2
(12–14). The above findings suggest that PTEN
disruption may play a critical role in ErbB2-positive human breast
cancer. To directly evaluate the effect of PTEN loss on
ErbB2-induced mammary tumorigenesis and progression, Shade et
al generated the PTEN-deficient/NIC genetically engineered
mouse model. This novel model utilized the murine mammary tumor
virus (MMTV) promoter to drive co-expression of activated ErbB2/Neu
and Cre recombinase coupling PTEN conditional depletion in the same
mammary epithelial cells (15).
PTEN-deficient/NIC mice exhibited rapid formation of highly
metastatic mammary tumors and displayed histopathological and
molecular features characteristic of the luminal subtype of primary
human breast cancer (15,16). We found previously that loss of both
PTEN alleles (PTEN−/−/NIC mice) resulted in significant
resistance to Neu antibody treatment (17). Therefore, PTEN−/−/NIC
mice represent a valuable tool for biological study and drug
discovery for trastuzumab-resistant ErbB2/Neu-positive breast
cancer.
However, there are several disadvantages and
challenges of using the PTEN−/−/NIC model to
preclinically evaluate novel therapeutics. First, it has a high
cost, is time consuming and is labor intensive to obtain cohorts of
female PTEN−/−/NIC mice. Second, it may be very
difficult to quantify tumor size during preclinical investigations
due to the fact that the PTEN−/−/NIC model develops
multifocal and aggressive mammary tumors. To partially overcome the
major drawbacks and complement the genetic engineering approach, we
attempted to establish cell lines from spontaneous mammary tumors
that arose in the PTEN−/−/NIC mice. In the present
study, two cell lines derived from PTEN−/−/NIC mammary
tumors were generated and characterized both in vitro and
in vivo, providing an alternative and useful resource for
future studies.
Materials and methods
Cell lines and cell culture
Cells were cultured in growth medium [DMEM/F12 with
10% FBS, 5 μg/ml insulin, 10 ng/ml epidermal growth
factor(EGF),1μg/mlhydrocortisone,35μg/ml bovine pituitary extract
and 100 U penicillin/streptomycin]. The cell number was evaluated
on a cell counter. TM15 cells were established from a spontaneous
ErbB2/Neu-positive/ PTEN wild-type
(PTEN+/+/ErbB2KI) mammary tumor (18,19).
MT104T is an immortalized ErbB2/Neu-positive/PTEN-deficienct
carcinoma cell line isolated from
PTEN−/−/ErbB2KI mammary tumors (17,20).
NICP20 and NICP21 cells were established from mammary tumors of
PTEN−/−/NIC genetically engineered FVB/N female mice,
following protocols essentially as described previously (21). Briefly, fresh mammary tumors from
the PTEN−/−/NIC mice were excised and minced to roughly
1 mm3 pieces, and then digested in collagenase (2 mg/ml;
Sigma) containing DMEM/F12 medium. The cells were resuspended and
cultured in DMEM/F12 containing 40% Matrigel (BD Biosciences).
After 2 weeks, cells were dissociated by dispase (Sigma) treatment
and cultured in growth medium for 24 h. Single cells, generated by
digestion with 0.05% trypsin (Invitrogen) were plated at 1,000
cells/10-cm dish. Pooled colonies were expanded. Cells were then
digested and resuspended in 100 μl phosphate-buffered saline
(PBS)/Matrigel (1:1) for mammary fat pad injection. Eight weeks
post-injection, the cells from the resulting orthotopic tumors were
explanted for a second round in vitro culture. The NICP20
and NICP21 cells were derived from selected colonies that
originated from two separate PTEN−/−/NIC mice.
Mammary fat pad and tail vein injection
of cells
Confluent cells (70–80%) were digested with trypsin
and washed with PBS. Cells were counted and resuspended to a final
concentration. For mammary fat pad injection, 104,
5×104, 105, 5×105 or
106 cells in 100 μl PBS/Matrigel (1:1) were injected
into the #2 inguinal mammary gland of FVB/N female mice at 8 weeks
of age. For tail vein injection, mice were anesthetized and
2×104 cells in 100 μl PBS were injected using a 1-ml
syringe (29 G; BD insulin syringe). FVB/N mice were obtained from
the Harlan Laboratory. All animal experiments were performed
according to the Guidelines for the Institutional Animal Care and
Use Committee of The University of Texas M.D. Anderson Cancer
Center and School of Medicine, Nanjing University.
Polymerase chain reaction (PCR) for
genotyping
Genomic DNA was extracted from the cells at 70–80%
confluency by digestion with proteinase K (Sigma) in 200 μl of
lysis buffer in a 60°C incubator for 2 h, followed by heating at
95°C for 10 min to inactivate the enzyme. After centrifugation, 1
μl of the supernatant was used for PCR reaction. Primer sequences
for NEU were: 5′-TTCCGGAACCCACATCAGGCC-3′ and 5′-GT
TTCCTGCAGCAGCCTACGC-3′; for CRE, 5′-TGCTCTGTC CGTTTGCCG-3′ and
5′-ACTGTGTCCAGACCAGGC-3′.
Immunoblotting
Cells in monolayer were washed with ice cold-PBS and
harvested by scraping in lysis buffer (1% Triton X-100, 50 mM HEPES
pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 100 mM
NaF, 10 mM Na pyruvate, 1 mM Na3VO4, 10%
glycerol) containing protease inhibitors (Sigma) and phosphatase
inhibitors (Roche). The lysate was incubated on ice for 20 min and
centrifuged at 15,000 × g for 15 min at 4°C. The protein
concentration of the supernatant was determined by BCA assay
(Thermo Scientific), and 30 μg total protein was electrophoresed on
10% SDS-PAGE and then transferred onto a nitrocellulose membrane
(Bio-Rad). Pten, pAkt S473 and Akt antibodies were obtained from
Cell Signaling Technology. Antibodies for ErbB2/Neu and β-actin
were obtained from Santa Cruz Biotechnology and Sigma,
respectively. The blots were incubated with HRP-conjugated
secondary antibodies and visualized by ECL (Amersham).
Whole mount and histology
Number 4 mammary gland was excised from 4- to 6-week
old virgin female PTEN−/−/NIC mice and spread on glass
slides for overnight fixation in 4% paraformaldehyde. The next day
the samples were hydrated and stained in a filtered solution of
0.2% carmine (Sigma) and 0.5% aluminum potassium sulfate for 1–2
days. Glands were then dehydrated sequentially through 70, 90 and
100% ethanol for 15 min each, precleared in toluene and stored in
methyl-salicylate. Lung whole mount preparations were harvested and
infused with formalin. Tumor tissues and lungs were fixed in
formalin, processed routinely and embedded in paraffin. For
histological analysis, paraffin sections (4-μm) were stained with
hematoxylin and eosin according to standard protocols.
Immunohistochemistry
Paraffin-embedded tumor sections (4-μm) were
subjected to antigen retrieval in a pressure cooker with sodium
citrate buffer (pH, 6.0) and incubated with antibodies specific for
phospho-Akt S473 (1:100; Cell Signaling), Ki-67 (1:200; Dako), CD34
(1:50; eBioscience), CD3 (1:300; Epitomics) overnight at 4°C.
Biotin-conjugated secondary antibodies were used. Remaining steps
were performed using Vectastain ABC kits (Vector Laboratories).
Slides were counterstained with hematoxylin. The stained slides
were evaluated by two pathologists, and images were acquired using
a Zeiss microscope with Axiovision software (Carl Zeiss, Inc.).
Results
PTEN−/−/NIC mice develop
multifocal and aggressive mammary tumors
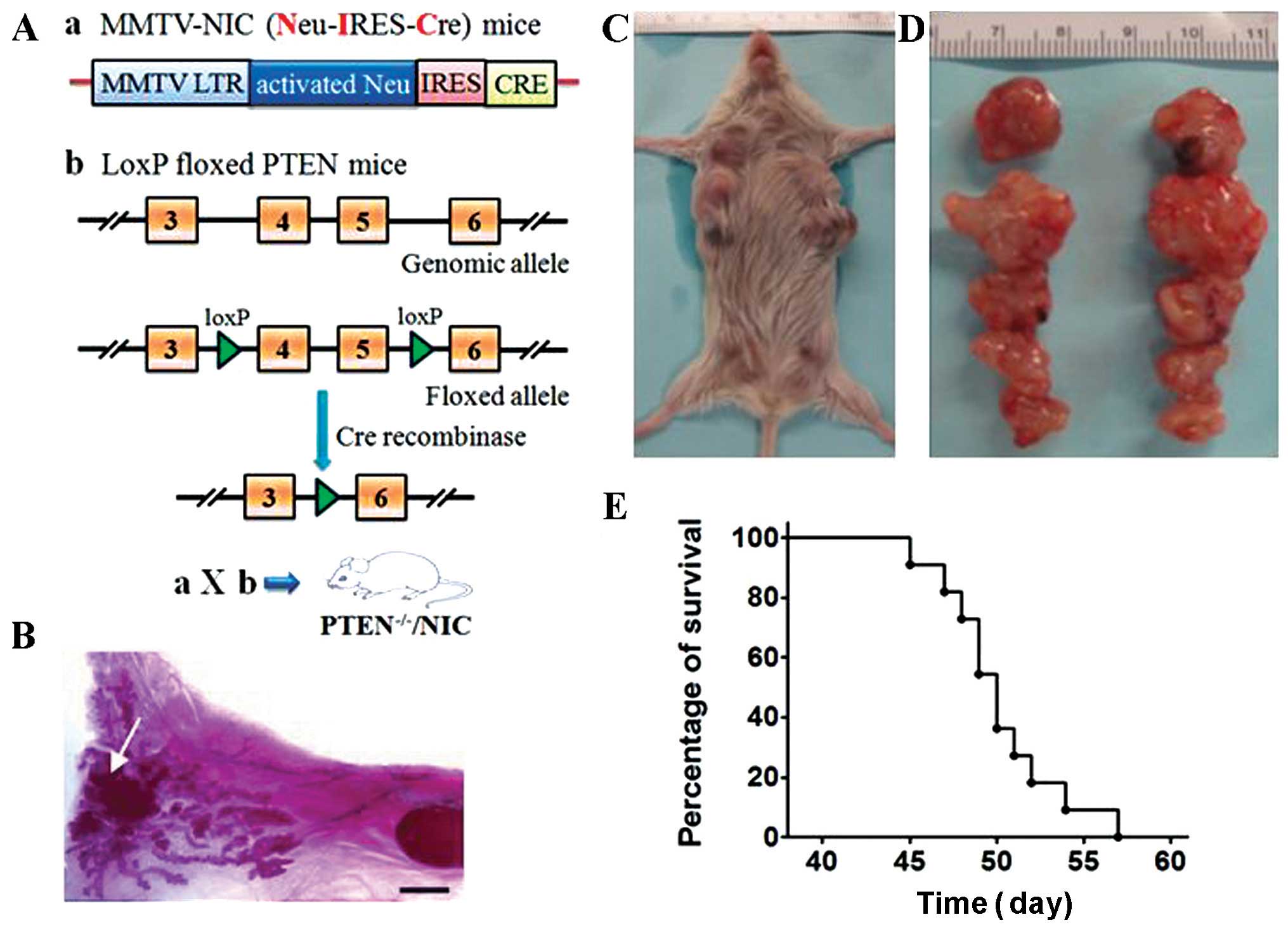
Genetic structure and general procedures to generate
the PTEN−/−/NIC mice are depicted in Fig. 1A. PTEN−/−/NIC mice
developed tumors rapidly with a latency of 28 to 43 days (data not
shown), which was consistent with previous results (15,17).
As shown in Fig. 1B, a tumor mass
was found in the inguinal mammary gland from a female
PTEN−/−/NIC mouse at 30-days old. All female
PTEN−/−/NIC mice developed multifocal mammary tumors
(Fig. 1C and D). Due to the rapid
tumor growth and progression, most female PTEN−/−/NIC
mice had to be euthanized three to four weeks after initial tumor
detection (tumor size >15 mm in diameter). The overall survival
curve of these female mice is shown in Fig. 1E with a median survival time of 50
days.
Establishment and characterization of the
mammary tumor cells from PTEN−/−/NIC mice in vitro
Given the multifocal and aggressive features of the
PTEN−/−/NIC tumors, it was difficult to follow tumor
growth. Meanwhile, to partially overcome the drawbacks of the
genetic engineered model (e.g. high cost, time consuming and labor
intensive), we attempted to establish cell lines from tumors that
arose in the PTEN−/−/NIC mice. According to protocols as
previously described (21), we
obtained two cell lines, named NICP20 and NICP21, respectively.
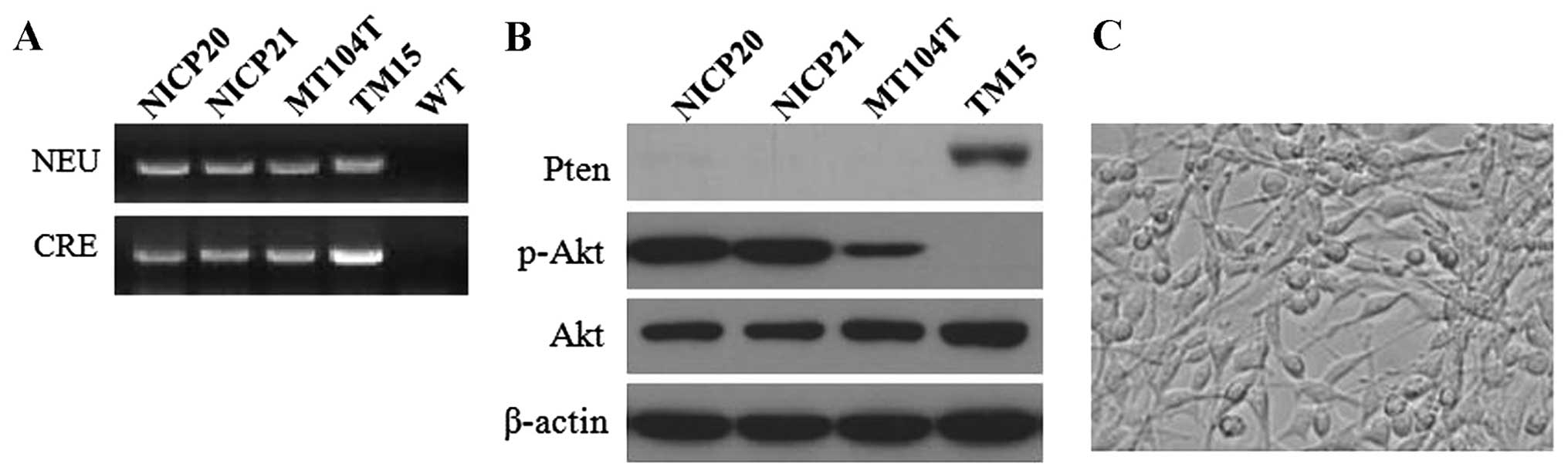
Firstly, the NEU and CRE transgenes in the NICP20 and NICP21 cells
were confirmed by PCR (Fig. 2A). As
expected, western blot analysis showed no detectable PTEN
expression and a high level of activated Akt both in the NICP20 and
NICP21 cell lines (Fig. 2B). These
results revealed that the NICP20 and NICP21 cells retained the
critical molecular phenotype similar to the origin. Moreover, gross
appearance of the cultured cells displayed epithelial morphology
over subsequent passages (Fig.
2C).
Tumorigenesis and lung metastasis of the
NICP20 and NICP21 cells in syngeneic mice
After generation of the above two
ErbB2/Neu-positive/PTEN-deficient cell lines, the tumorigenic
ability of these cells in immune intact, syngeneic FVB/N female
mice was initially evaluated. The initial round of intramammary
injections utilized 106, 5×105,
105, 5×104 or 104 cells for each
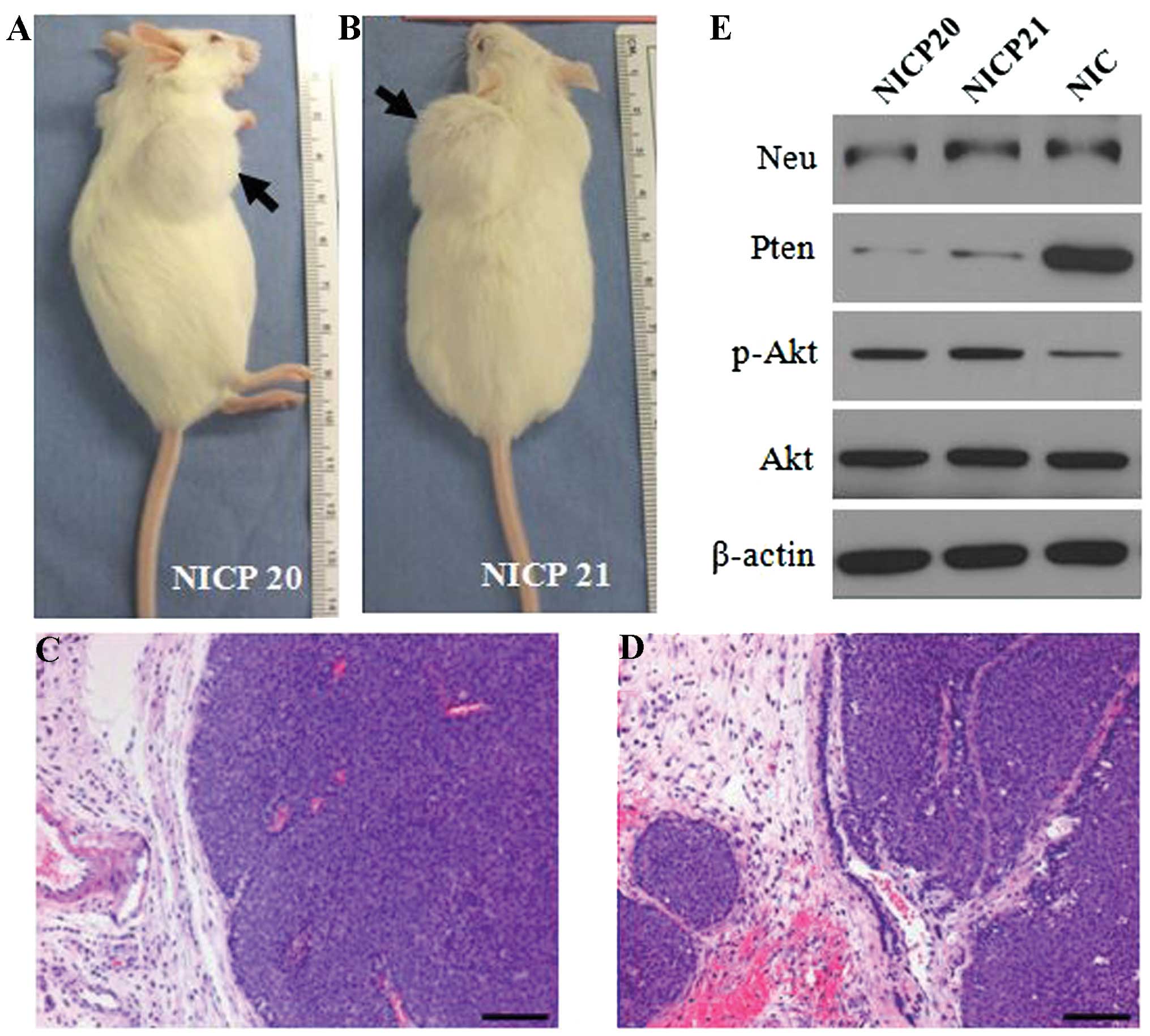
cell line. By 2–5 weeks post injection, all mice had palpable tumor
nodules in the mammary glands. Seven weeks post injection of
105 NICP20 cells (Fig.
3A) and 8 weeks post injection of 5×104 NICP21 cells
(Fig. 3B), the tumors were >15
mm in diameter and the mice were euthanized. Histological analysis
revealed that the tumors induced by the NICP20 and NICP21 cell
lines had large, solid nodular nests that resembled luminal-like
histologic features (Fig. 3C and D)
similar to the morphology of the original PTEN−/−/NIC
tumors. Expression of ErbB2/Neu and PTEN protein in the tumors
induced by NICP20 and NICP21 cells was also determined by western
blotting (Fig. 3E). The weak bands
of PTEN protein confirmed the expression of PTEN in the stromal
tissue but not in the tumor cells. As expected, loss of PTEN
increased Akt phosphorylation relative to the PTEN wild-type
MMTV-NIC tumors (Fig. 3E).
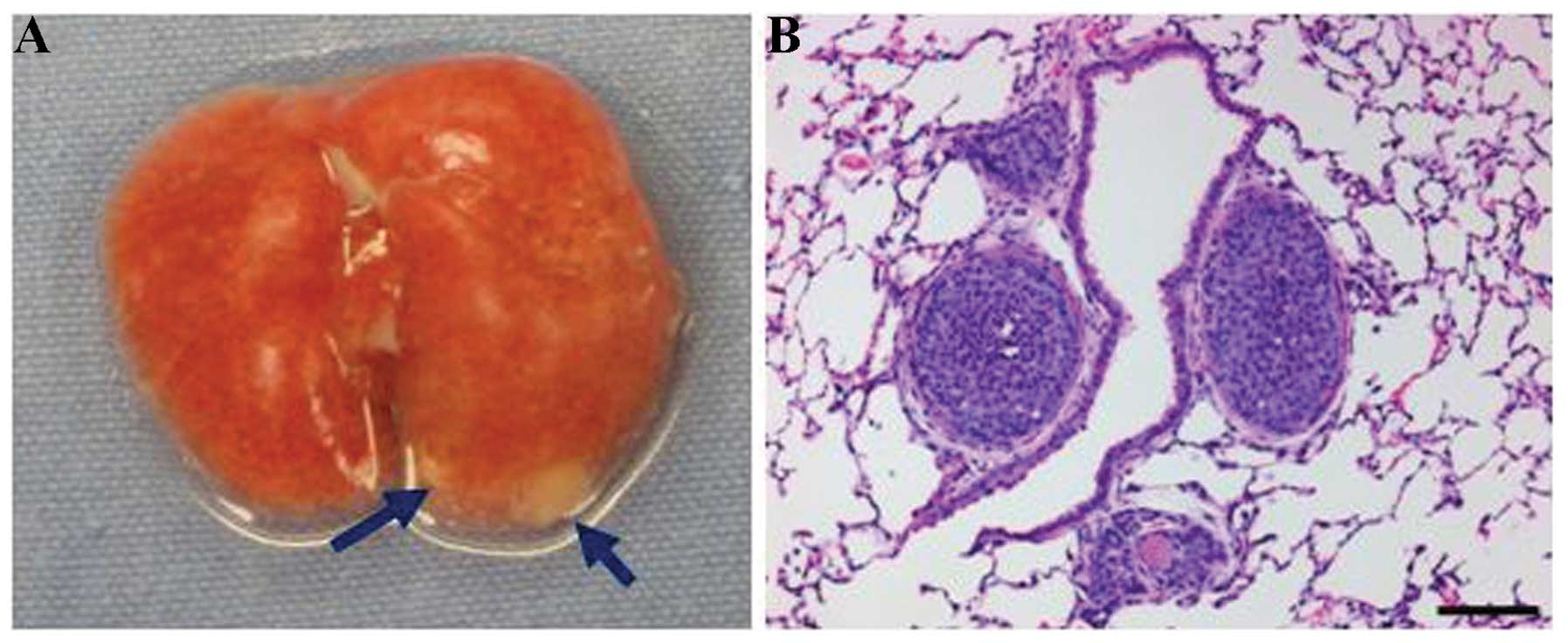
Meanwhile, the lung tissues of all the tumor-bearing mice (induced
by NICP20 or NICP21 cells) were examined macroscopically and
microscopically when euthanized, and no metastatic lesion was
found. Base on this findings, tail vein injections were performed
using different cell concentrations. Both cell lines produced lung
metastasis when only 104 cells were injected through the
tail vein (Fig. 4).
Akt hyperactivation, high proliferation,
angiogenesis and immune-cell infiltration of tumors formed by
NICP20 and NICP21 cells
To determine whether the in vivo
characteristics of the NICP20 and NICP21 cells resemble the
features of the original PTEN−/−/NIC tumors, serial
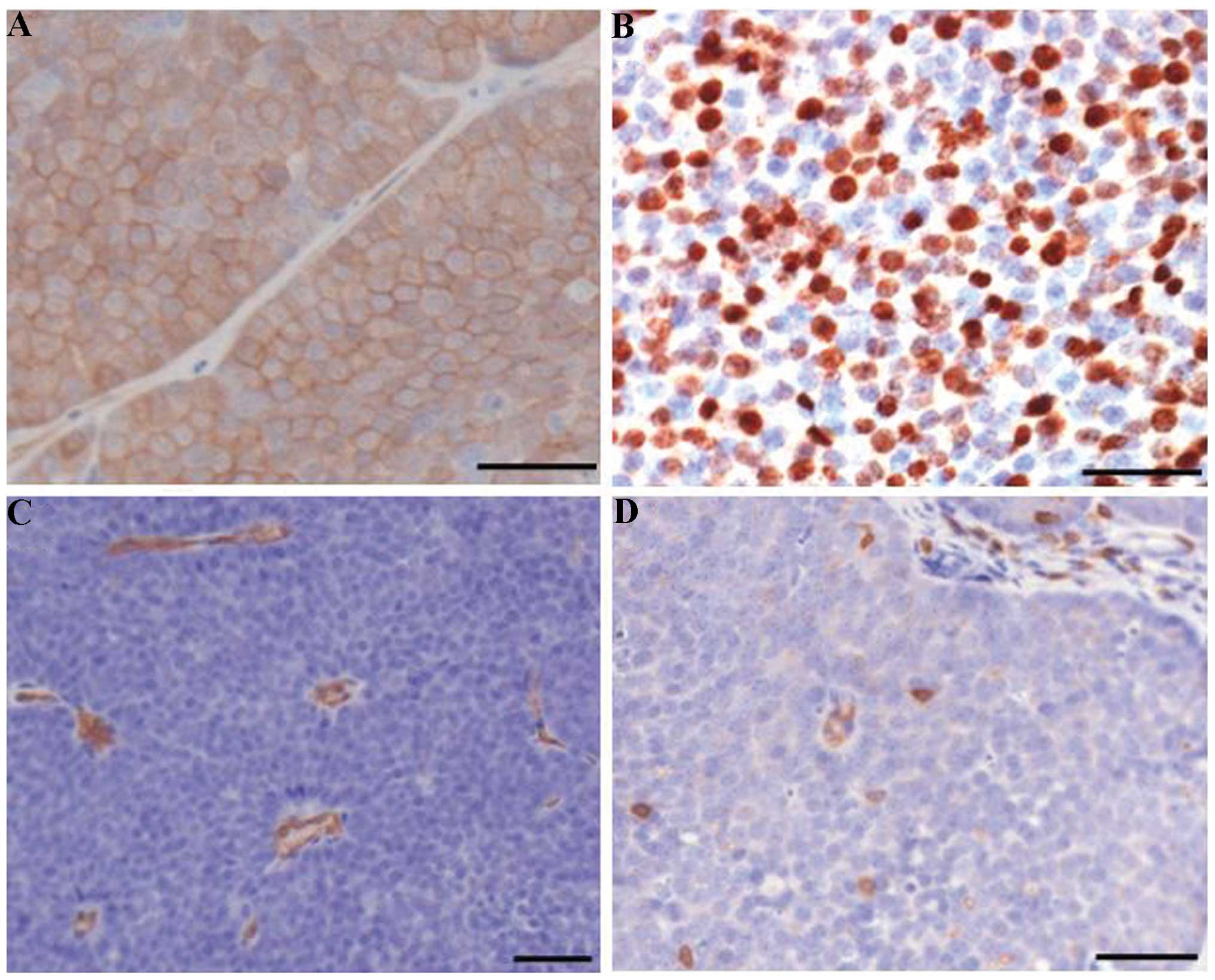
immunohistological analyses were further performed. Examination of
phospho-Akt staining revealed that tumors formed by NICP20 or
NICP21 cells displayed hyperactivation of Akt (Fig. 5A). As shown in Fig. 5B, these tumors displayed a high
proliferative level. Moreover, microvessel density reflected by
CD34-positive endothelium (Fig. 5C)
and CD3-positive cells (Fig. 5D)
per area of tumor epithelium in the cell line-induced tumors also
revealed comparable angiogenesis and T-cell infiltration properties
similar to the in PTEN−/−/NIC tumors (15,17).
Discussion
Genetically engineered mouse models of human breast
cancer have contributed substantially to our understanding of the
initiation and progression of breast cancer and have emerged as
valuable tools for preclinical research (22–24).
Breast cancer is a complex and heterogeneous disease that has
distinct histopathological features, genetic variability and
diverse clinical outcomes. Overexpression of the ErbB2 oncogene and
loss of tumor-suppressor PTEN are both observed in certain human
breast cancer cases. The FVB/N PTEN−/−/NIC genetically
engineered mouse was originally generated to better study the role
of PTEN in ErbB2/Neu-induced mammary tumorigenesis (15). Notably, the PTEN−/−/NIC
model developed multifocal, highly invasive mammary tumors with
histopathological and molecular features relevant to the luminal
subtype of primary human breast cancer (15). Our data confirmed the aggressive
features of this model with an extremely short lifespan (Fig. 1).
To facilitate our understanding and ease the future
application of this well-defined model, cell lines were generated
from PTEN−/−/NIC mammary tumors in the present study.
With the generation of the in vitro cell lines from
PTEN−/−/NIC mammary tumors and development of the
syngeneic xenograft model, an additional model system for the
investigation of the interplay between ErbB-2/Neu and PTEN
function/signaling in mammary tumor occurrence, progression and
therapies is available, possessing the advantages of easy use,
trouble-free monitoring of tumor growth, relatively inexpensive
cost as well as the potential for in vitro manipulation.
Similarly, Sahin et al reported that syngeneic
transplantation of tumors from PTEN−/−/NIC mice is an
effective way to generate large cohorts to test therapeutics
(25). Thus, the availability of
our cell line model and the syngeneic transplantation approach may
provide tractable alternatives for optimizing
PTEN−/−/NIC mice as powerful preclinical models.
Given that PTEN is a negative regulator of the
PI3K/Akt pathway, its loss can lead to hyperactivation of Akt. As
expected, the NICP20 and NICP21 cells had high levels of activated
Akt in vitro (Fig. 2B) and
immunohistochemical staining also localized the activated Akt on
tumor cells in vivo (Fig.
5A). Hyperactivation of Akt has been associated with
trastuzumab resistance and Akt has been proposed as a potential
target to overcome trastuzumab resistance (12,26–28).
Novel inhibitors can be tested and evaluated using these NICP20 and
NICP21 cells in vitro and in vivo in immunocompetent
mice. In addition to inhibiting oncogenic signaling of tumor cells
(29) (autonomous mechanism),
studies have revealed that the immune response or stroma-tumor
interactions in the tumor microenvironment (non-autonomous
mechanism) are involved in the therapeutic activities of
trastuzumab alone or in combination with other agents (17,30–32).
Since the NICP20 and NICP21 cell-induced tumors showed comparable
angiogenesis and T-cell infiltration properties as those of
PTEN−/−/NIC tumors (Fig.
5), they may also be useful to investigate the roles of
non-autonomous mechanisms on the therapeutic response of potential
treatment, by working with syngeneic wild-type or related knockout
mice.
In summary, we provide an additional valuable mouse
mammary carcinoma cell resource to enhance our understanding of the
nature of ErbB2-positive breast cancers, particularly accompanying
PTEN loss, and to facilitate experimental therapeutic studies.
Acknowledgements
We thank Dr William Muller at McGill University for
kindly providing the mouse strains and the TM15 cells, and Dr Dihua
Yu at the University of Texas MD Anderson Cancer Center for her
full support of this study. The present study was supported by a
grant from the National Natural Science Foundation of China (no.
81302241), the Key Project of Medical Science and Technology
Development Foundation from Nanjing Department of Health
(YKK13066), and funding from the Scientific Research Service of the
Central Universities (Q.W.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Network. Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perou CM, Sørlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sørlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sotiriou C and Piccart MJ: Taking
gene-expression profiling to the clinic: when will molecular
signatures become relevant to patient care? Nat Rev Cancer.
7:545–553. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andrulis IL, Bull SB, Blackstein ME, et
al: neu/erbB-2 amplification identifies a poor-prognosis group of
women with node-negative breast cancer. Toronto Breast Cancer Study
Group. J Clin Oncol. 16:1340–1349. 1998.PubMed/NCBI
|
|
9
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hollander MC, Blumenthal GM and Dennis PA:
PTEN loss in the continuum of common cancers, rare syndromes and
mouse models. Nat Rev Cancer. 11:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saal LH, Gruvberger-Saal SK, Persson C, et
al: Recurrent gross mutations of the PTEN tumor suppressor gene in
breast cancers with deficient DSB repair. Nat Genet. 40:102–107.
2008. View Article : Google Scholar
|
|
12
|
Esteva FJ, Guo H, Zhang S, et al: PTEN,
PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab
response and survival in patients with HER2-positive metastatic
breast cancer. Am J Pathol. 177:1647–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gajria D and Chandarlapaty S:
HER2-amplified breast cancer: mechanisms of trastuzumab resistance
and novel targeted therapies. Expert Rev Anticancer Ther.
11:263–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagata Y, Lan KH, Zhou X, et al: PTEN
activation contributes to tumor inhibition by trastuzumab, and loss
of PTEN predicts trastuzumab resistance in patients. Cancer Cell.
6:117–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schade B, Rao T, Dourdin N, et al: PTEN
deficiency in a luminal ErbB-2 mouse model results in dramatic
acceleration of mammary tumorigenesis and metastasis. J Biol Chem.
284:19018–19026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andrechek ER, Laing MA, Girgis-Gabardo AA,
Siegel PM, Cardiff RD and Muller WJ: Gene expression profiling of
neu-induced mammary tumors from transgenic mice reveals genetic and
morphological similarities to ErbB2-expressing human breast
cancers. Cancer Res. 63:4920–4926. 2003.PubMed/NCBI
|
|
17
|
Wang Q, Li SH, Wang H, et al: Concomitant
targeting of tumor cells and induction of T-cell response
synergizes to effectively inhibit trastuzumab-resistant breast
cancer. Cancer Res. 72:4417–4428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andrechek ER, Hardy WR, Siegel PM,
Rudnicki MA, Cardiff RD and Muller WJ: Amplification of the
neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc
Natl Acad Sci USA. 97:3444–3449. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ursini-Siegel J, Rajput AB, Lu H, et al:
Elevated expression of DecR1 impairs ErbB2/Neu-induced mammary
tumor development. Mol Cell Biol. 27:6361–6371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dourdin N, Schade B, Lesurf R, et al:
Phosphatase and tensin homologue deleted on chromosome 10
deficiency accelerates tumor induction in a mouse model of ErbB-2
mammary tumorigenesis. Cancer Res. 68:2122–2131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Ding H, Liu B, et al: Addition of
the Akt inhibitor triciribine overcomes antibody resistance in
cells from ErbB2/ Neu-positive/PTEN-deficient mammary tumors. Int J
Oncol. 44:1277–1283. 2014.PubMed/NCBI
|
|
22
|
Frese KK and Tuveson DA: Maximizing mouse
cancer models. Nat Rev Cancer. 7:645–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vargo-Gogola T and Rosen JM: Modelling
breast cancer: one size does not fit all. Nat Rev Cancer.
7:659–672. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gopinathan A and Tuveson DA: The use of
GEM models for experimental cancer therapeutics. Dis Model Mech.
1:83–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sahin O, Wang Q, Brady SW, et al:
Biomarker-guided sequential targeted therapies to overcome therapy
resistance in rapidly evolving highly aggressive mammary tumors.
Cell Res. 24:542–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berns K, Horlings HM, Hennessy BT, et al:
A functional genetic approach identifies the PI3K pathway as a
major determinant of trastuzumab resistance in breast cancer.
Cancer Cell. 12:395–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kruser TJ and Wheeler DL: Mechanisms of
resistance to HER family targeting antibodies. Exp Cell Res.
316:1083–1100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hernandez-Aya LF and Gonzalez-Angulo AM:
Targeting the phosphatidylinositol 3-kinase signaling pathway in
breast cancer. Oncologist. 16:404–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosen LS, Ashurst HL and Chap L: Targeting
signal transduction pathways in metastatic breast cancer: a
comprehensive review. Oncologist. 15:216–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferris RL, Jaffee EM and Ferrone S: Tumor
antigen-targeted, monoclonal antibody-based immunotherapy: clinical
response, cellular immunity, and immunoescape. J Clin Oncol.
28:4390–4399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park S, Jiang Z, Mortenson ED, et al: The
therapeutic effect of anti-HER2/neu antibody depends on both innate
and adaptive immunity. Cancer Cell. 18:160–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stagg J, Loi S, Divisekera U, et al:
Anti-ErbB-2 mAb therapy requires type I and II interferons and
synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad
Sci USA. 108:7142–7147. 2011. View Article : Google Scholar : PubMed/NCBI
|