Introduction
Cancer is the leading cause of death in most
developed countries, and breast cancer is one of the leading causes
of cancer-related mortality among women (1). Although surgery and follow-up
treatment have been successful in improving the prognosis of breast
cancer patients, patients with metastatic tumors still suffer from
poor prognosis. Therefore, developing novel therapeutics for breast
cancer is an absolute necessity. For this purpose, understanding
the molecular mechanism of breast carcinogenesis is essential.
Microarray technology which provides quantitative genome-wide gene
expression profiling has been widely used to analyze the pathways
associated with cancer development and progression. (2). Through the screening of genes which
showed enhanced expression in breast cancer tissues, we identified
several molecular targets that are essential for breast cancer cell
proliferation (3–6). For example, brefeldin A-inhibited
guanine nucleotide-exchange protein 3 (BIG3), which was found to be
frequently upregulated in breast cancer tissues, interacts with
prohibitin 2/repressor of estrogen receptor activity (PHB2/REA)
protein. This binding inhibits PHB2/REA nuclear translocation and
subsequently activates ERα signaling pathways (7). In addition, a synthesized peptide
which inhibits the interaction between BIG3 and PHB2/REA is able to
suppress E2-dependent breast cancer cell growth (8).
Similarly, identification of genes which exhibit low
expression in cancer tissues is also important for the
understanding of human carcinogenesis. Tumor-suppressor genes
(TSGs) act as guardians against malignant transformation. Genomic
alteration or promoter hypermethylation are common causes of TSG
inactivation. In breast cancer tissues, hypermethylation of TSGs is
considered to be an early event during tumorigenesis (9). Overexpression of DNA methyltransferase
(DNMT) 1, 3a, and 3b is frequently observed in breast
fibroadenoma (22–44%) (10), which
may result in TSG promoter hypermethylation including APC,
BRCA1, p16, p21 and TIMP3 (11–13).
Several studies have demonstrated that hypermethylated DNA of TSGs
in serum could be a potential biomarker for disease prediction and
therapeutic response in breast cancer (14). In addition, DNMT inhibitors are used
for the treatment of myelodysplastic syndrome and solid cancers
(15–17). Therefore, identification of novel
TSGs would not only provide a fundamental understanding of cancer
biology, but may also contribute to breast cancer diagnosis or more
effective therapeutics. Recently, we reported a TSG candidate,
HSPB7, which was found to be downregulated in renal cancer
samples by epigenetic abnormalities (18). In the present study, we used
microarray technology and identified peptidase domain containing
associated with muscle regeneration 1 (PAMR1) whose expression
was frequently suppressed in breast cancer tissues by promoter
hypermethylation.
Materials and methods
Breast cancer cell lines and clinical
cancer samples
Human breast cancer cell lines including BSY1,
BT-20, BT-474, BT-549, HBC4, HBC5, HBL-100, HCC1143, HCC1395,
HCC1500, HCC1599, HCC1937, MCF7, MDA-MB-231, MDA-MB-435s,
MDA-MB-453, OCUB-F, SK-BR-3, T-47D, YMB-1 and ZR-75-1 were obtained
and cultured as previously reported (4). The cell lines BST1, HBC4 and HBC5 were
kindly provided by Dr Takao Yamori of the Division of Molecular
Pharmacology, Cancer Chemotherapy Center, Japanese Foundation for
Cancer Research. The other cell lines were purchased from the
American Type Culture Collection (ATCC, USA). Human mammary
epithelial cells (HMECs) were purchased from Lonza Switzerland and
were cultured in mammary epithelial cell growth medium supplemented
with bovine pituitary extract, hEGF, hydrocortisone, GA-1000 and
insulin (Lonza). The HMECs used for all experiments were under
passage 15. All cells were maintained at 37°C in an atmosphere of
humidified air with 5% CO2 except for MDA-MB-231 and
MDA-MB-435s which were maintained at 37°C in an atmosphere of
humidified air without CO2. Primary breast normal and
cancer tissues were obtained with informed consent from patients
who received treatment at the Department of Breast Surgery, Cancer
Institute Hospital, Tokyo. All tissue samples underwent
laser-microbeam micro-dissection (19).
Plasmid construction
The two PAMR1 isoforms were amplified from
HMEC cDNA by KOD plus DNA polymerase (Toyobo, Japan). The sequences
of the cloning primers are listed in Table I. The amplified DNAs were then
subsequently cloned into the pCAGGS vector with HA-tagged at the
C-terminal.
 | Table IList of primers used in the present
study. |
Table I
List of primers used in the present
study.
| Forward primer | Reverse primer |
|---|
| Cell line RT-PCR |
| C2orf88 |
GCTTAATCACAATGCCCTCAAC |
CTGAACTAATGCCCACAGCTC |
| CSRNP3 |
AGTGGGGACAGTGTCAATCC |
CCTTGCCTCCTGGTGAAGTA |
| PAMR1 |
CCTTCTCATCTCGTCCTTGC |
AACCCACGACTTCCCTCTTT |
| PDLIM3 |
CTCAGGGGGCATAGACTTC |
ATCTCCAGGACACAGGTTGG |
|
PPP1R12B |
TGACCAGCCGTGTAGAAGAAG |
CTGGGCTTCCTGAAGTTTTG |
| SAMD5 |
GCTACCCCAAACTGAAGCTG |
AGCGGCTCTGTGATGACTTC |
| Tissue RT-PCR |
AGGGAAGATCTGGGCTTCATG |
GGGAAGGAAAAGGACCAGAC |
| Cloning PCR |
TTAAGAATTCGCGGCAAGGATGGAGCTGGG |
CGCGCTCGAGTTTCATATTTCTTTCAATCC |
| Isoform PCR |
TTACAAGTGTGCCTGCTTGG |
GCCCCCTGTTATTTTCTGGT |
| Bisulfite
sequencing |
TTAATTTGTGATTATTTGGAGTAAA |
CTCATCTAAAAAAAACCACCTTCAA |
cDNA microarray
cDNA microarray analysis was performed as previously
described (19). In brief, tumor
cells obtained from 81 breast cancer patients (12 ductal carcinomas
in situ and 69 T2 invasive ductal carcinomas) underwent
laser micro-beam microdissection. The total RNAs were extracted
using the RNeasy Mini kit (Qiagen, Germany) and treated with DNase
I digestion according to the manufacturer’s manual. The RNAs were
then reverse-transcribed and hybridized with the microarray slide.
The microarray slide contained 23,040 cDNAs selected from the
UniGene database (build #131), including 52 housekeeping genes and
two types of negative control genes. A mixture of normal breast
ductal cell RNAs isolated from 15 pre-menopausal breast cancer
patients was used as the normal control.
Real-time quantitative PCR
The mRNAs of human normal tissues were purchased
from Takara (Takara Bio, Japan). Total RNAs from the cell lines
were extracted using the RNeasy Mini kit and reverse transcribed
into cDNA by SuperScript III (Life Technologies, USA) according to
the manufacturer’s instructions. Real-time quantitative PCR (qPCR)
was performed using SYBR-Green I Master Mix on LightCycler 480
(Roche, Germany). The primer sequences are listed in Table I.
DNA isolation, sodium bisulfite treatment
and DNA sequencing
Genomic DNAs were isolated by DNeasy Blood &
Tissue kit (Qiagen) according to the instruction manual. Bisulfite
treatment and DNA sequencing was performed as previously reported
(20). In brief, 2 μg of DNA was
digested by XhoI for 16 h at 37°C. The digested DNA was then
denatured by 0.3 M NaOH and treated with 3.12 M sodium bisulfite
and 0.5 mM hydroquinone for 16 h at 55°C. Following incubation, DNA
was purified and desulfoned by 0.3 M of NaOH at 37°C for 20 min,
followed by ethanol precipitation. Finally the DNA was amplified by
PCR with the specific primers (Table
I) and subcloned into the pCR 2.1 vector by TA cloning kit
(Invitrogen, USA). The cloned plasmids were transformed into
competent cells. For each treated DNA, 10 individual colonies were
chosen and plasmid extractions were performed. DNA sequencing of
the isolated plasmids was performed by the ABI sequencing system
(Applied Biosystems, USA) according to the manufacturer’s
instructions.
Demethylation drug treatment
The demethylation drug 5-aza-2′ deoxycytidine
(5-aza-dC) was purchased from Sigma (Sigma-Aldrich, USA). The drug
was dissolved in dimethyl sulphoxide (DMSO) and freshly prepared
before use. Breast cancer cells were cultured in 6-well plates one
day before drug treatment. Fresh medium containing various
concentrations of 5-aza-dC was replaced daily for 3 consecutive
days. The RNA from each treated cell line was isolated 72 h post
drug treatment. Cells treated with DMSO served as the negative
controls.
Western blotting
Breast cancer cells (5×105) were cultured
in a 60-mm dish under normal conditions and allowed to attach for
24 h. The culture medium was then removed and the cells were washed
twice by PBS. A total of 2 ml of fresh medium without FBS was then
replaced, and the cells were allowed to grow for another 24 h.
After incubation, 1 ml of conditioned medium was collected from
each sample, followed by centrifugation at 15,000 rpm for 15 min at
4°C twice to remove all floating cells. The conditioned medium was
then mixed with an equal volume of ice-cold acetone and stored at
−80°C for 1 h. The protein was harvested by centrifugation at
15,000 rpm for 15 min at 4°C. The precipitated protein was
dissolved using Laemmli sample buffer and analyzed by western
blotting following standard protocols (Bio-Rad, USA). Rat anti-HA
antibody (Roche) and sheep anti-PAMR1 antibody (R&D Systems,
USA) were used to detect PARM1 protein in the conditioned medium.
Mouse anti-β-actin antibody (Santa Cruz, USA) was used as the
loading control.
Colony formation assay
Breast cancer cells were cultured in 6-well plates
for 24 h before transfection. One hundred and fifty million copies
of plasmid from the vector alone control (pCAGGS), and two variants
of PAMR1 were transfected into each well individually by
FuGene HD (Roche) in a 1:3 (μg:μl) ratio. Transfection was
performed according to the user manual. G418 (Life Technologies)
was added to the cells one day after transfection. The
drug-resistant cells were allowed to grow for three weeks until
colonies formed. Finally the cells were fixed by 10% formamide and
stained with 0.1% crystal violet solution. The number of colonies
was counted by Image J software.
Results
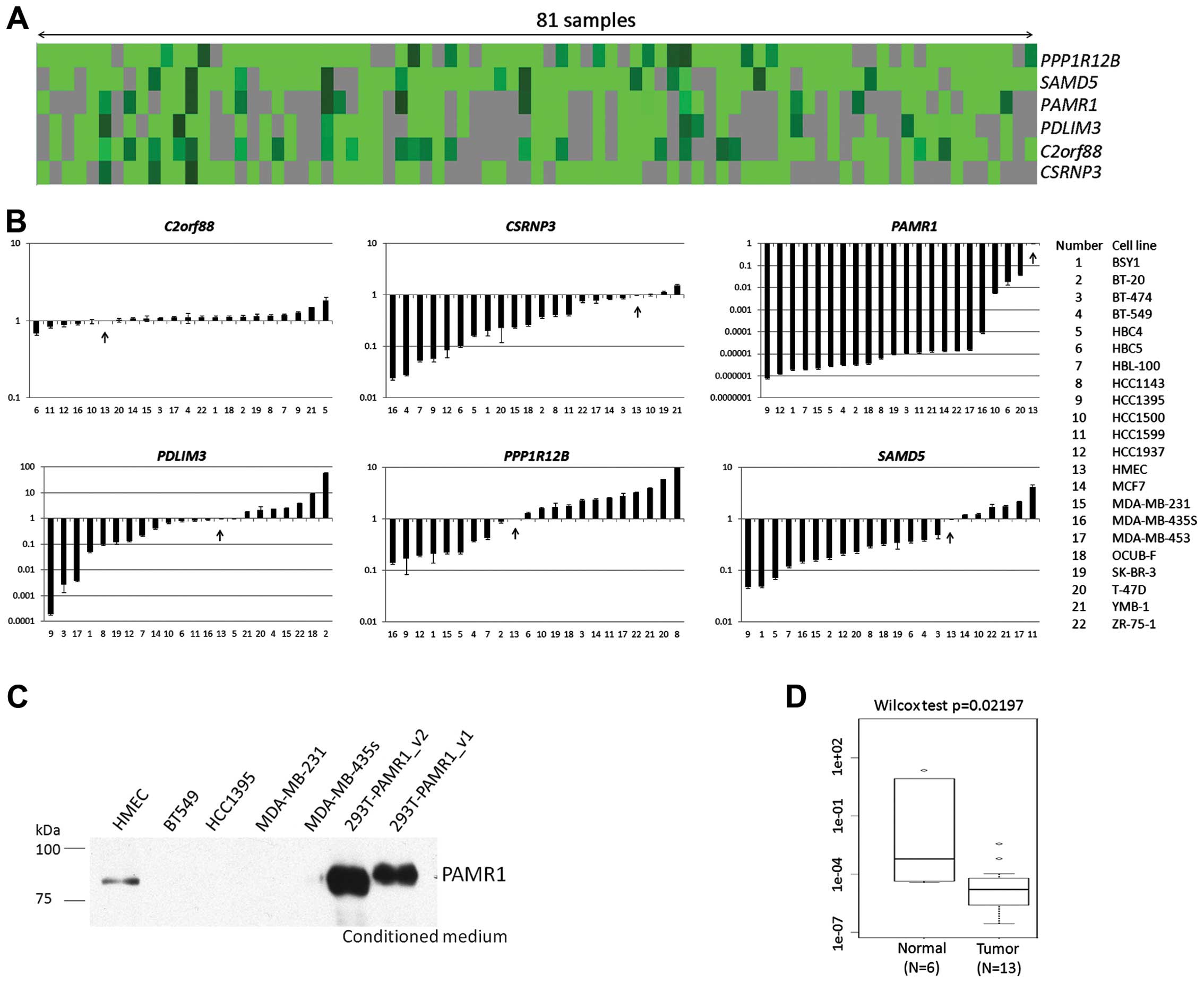
Identification of genes frequently
downregulated in breast cancer tissues
We previously performed cDNA microarray analyses of
81 breast tumor samples (19). All
the tumor cells and normal breast epithelial cells were purified by
laser microbeam microdissection. In order to identify novel genes
which are commonly downregulated in breast cancer tissues, we
screened the cDNA microarray database consisting of 23,040 probes
using the following criteria: i) genes for which we were able to
obtain expression signal in >50% of total examined samples; ii)
genes whose expression ratio (cancer/normal) was <0.2 in more
than 90% of informative samples; iii) genes whose association with
human carcinogenesis had not been reported to date. Finally, we
selected 6 candidate genes, namely chromosome 2 open reading
frame 88 (C2orf88), cysteine-serine-rich nuclear protein 3
(CSRNP3), PAMR1, PDZ and LIM domain 3 (PDLIM3), protein phosphatase
1 regulatory subunit 12B (PPP1R12B) and sterile α motif
domain containing 5 (SAMD5) (Fig.
1A, Table II).
 | Table IIMicroarray study results of the 6
novel candidate genes with downregulated expression in breast
cancer tissues. |
Table II
Microarray study results of the 6
novel candidate genes with downregulated expression in breast
cancer tissues.
| Gene | Valid sample
(n) | Ratio <0.2
(n) | Downregulated
(%) |
|---|
| C2orf88 | 54 | 54 | 100 |
| CSRNP3 | 46 | 45 | 98 |
| PAMR1 | 46 | 42 | 91 |
| PDLIM3 | 44 | 42 | 95 |
|
PPP1R12B | 67 | 63 | 94 |
| SAMD5 | 70 | 66 | 94 |
Downregulation of PAMR1 in breast cancer
cell lines and tissues
We then examined the expression of these genes in 21
breast cancer cell lines by qPCR analyses (Fig. 1B). Human mammary epithelial cells
(HMECs) served as a normal control. Among the 6 candidate genes,
PAMR1 expression was reduced in all breast cancer cell
lines. To confirm this result, we conducted western blot analysis
using conditioned medium from the cultured cell lines, as PAMR1 was
shown to be a secreted protein (21). As a result, PAMR1 protein was
detectable only in the culture medium of HMECs but not in those of
the cancer cell lines (Fig. 1C).
The conditioned media from HEK293T cells transfected with the
plasmid designed to express PAMR1 were used as a positive control.
We also examined PAMR1 expression in 13 breast cancer
tissues and 6 normal breast tissues by qPCR analysis. The cancer
tissues showed reduced expression of PAMR1, concordant with
the result of the cDNA microarray analysis (Fig. 1D).
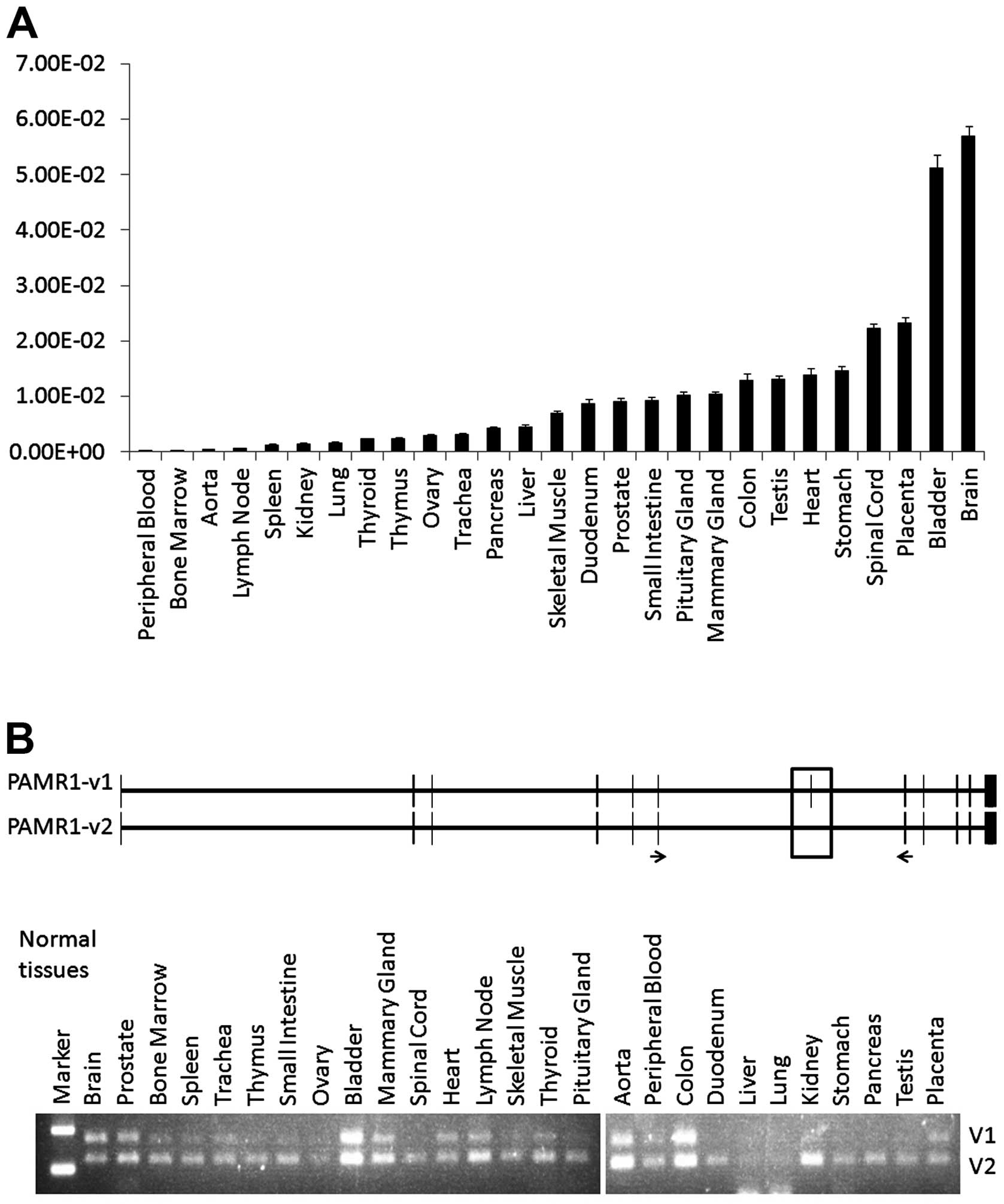
Expression of PAMR1 in mammary gland
PAMR1 was originally identified as a
regulator of muscle regeneration. PAMR1 was found to be
downregulated in the muscles of Duchenne muscular dystrophy (DMD)
patients and DMD mice (22). Our
qPCR analysis revealed that PAMR1 showed the highest
expression in brain tissue and moderate expression in breast and
skeletal muscle tissues among the 27 normal human tissues (Fig. 2A), concordant with a previous report
(22). Therefore, we hypothesized
that PAMR1 may have unique functions in different tissues.
PAMR1 has two isoforms, and variant 2 which lacks exon 7 (51
bp) encodes a 17-amino acid shorter protein compared with variant
1. To investigate expression of the two isoforms in each tissue, we
designed a pair of primers flanking exons 6 and 8. After PCR
amplification and gel electrophoresis, DNA fragments corresponding
to variant 1 and variant 2 showed similar intensity in the brain,
prostate, bladder, heart, colon and placenta, while the intensity
of the DNA fragment corresponding to variant 2 was dominant in the
other tissues including the mammary gland (Fig. 2B).
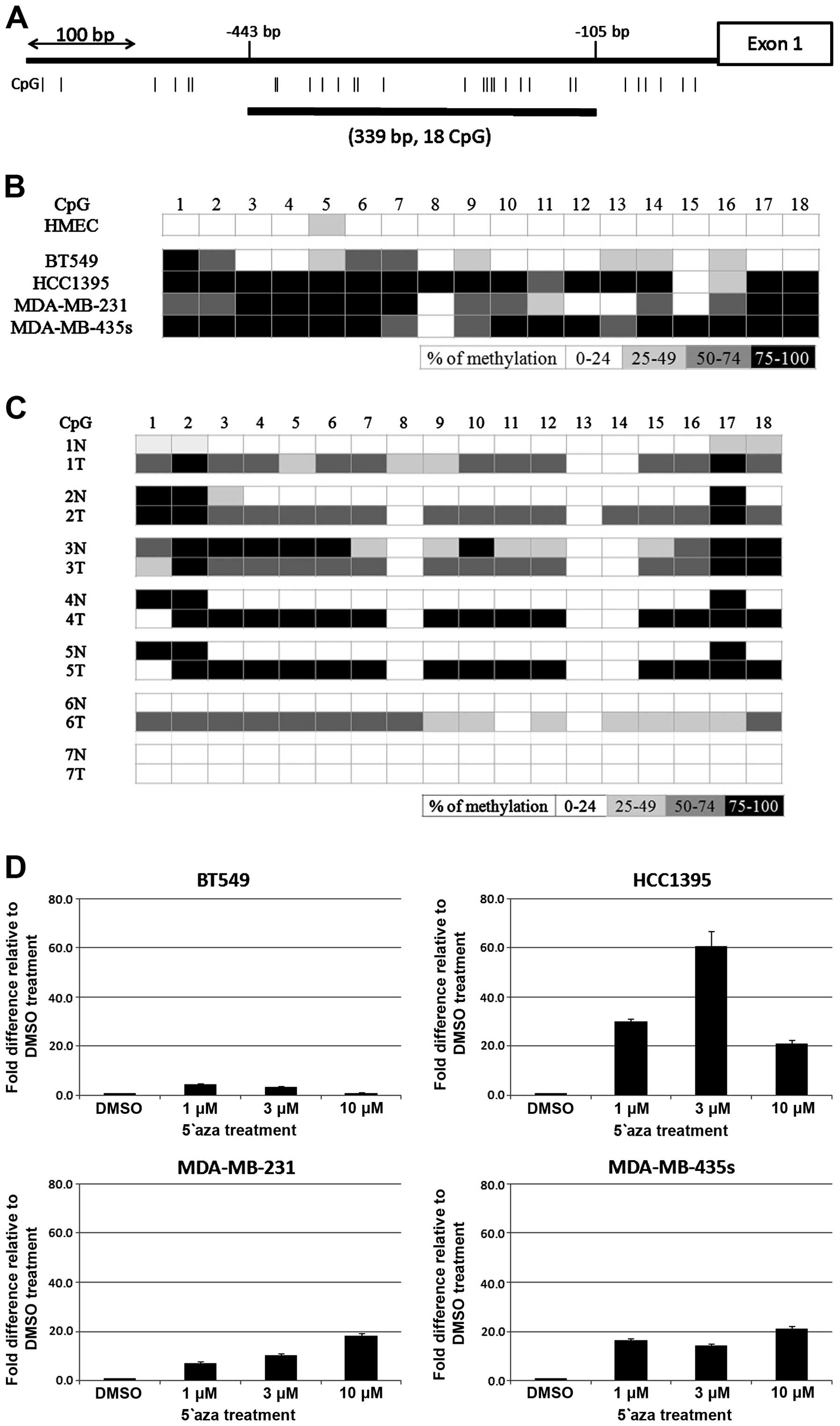
Promoter hypermethylation of PAMR1 in
breast cancer tissues and cell lines
To further investigate the molecular mechanism of
PAMR1 inactivation in breast cancer tissues, we sequenced
all exons of PAMR1 in 21 breast cancer cell lines. However,
we did not identify any mutations in our tested samples. We, then,
considered whether epigenetic inactivation could cause PAMR1
downregulation. Although we were not able to identify any CpG
island within the PAMR1 locus including a 10-kb region
encompassing its 5′ flanking region by in-silico analysis
(23), a CpG-rich region was found
within −443 to −105 bp of the PAMR1 promoter region
(Fig. 3A). From the result of the
bisulfite treated DNA sequencing analysis, hypermethylation was
found in 3 cancer cell lines, namely HCC1395, MDA-MB-231, and
MDA-MB-435s among the 4 cancer cell lines examined. Moreover, the
PAMR1 promoter was also found to be moderately methylated in
the BT549 cancer cells but not in normal HMECs (Fig. 3B). We, then, analyzed 7 pairs of
normal and tumor tissues from breast cancer patients and found
tumor-specific promoter hypermethylation in 5/7 tumor samples
(Fig. 3C).
We treated the breast cancer cell lines with
demethylating agent 5-aza-2′ deoxycytidine (5-aza-dC) and examined
PAMR1 expression by qPCR analysis. The expression of
PAMR1 was recovered after drug treatment by 4.2-, 62.7-,
18.1- and 20.8-fold in the BT549, HCC1395, MDA-MB-231 and
MDA-MB-435s cells, respectively (Fig.
3D). The expression of PAMR1 in the BT549 cells showed
the least degree of restoration compared to the other cell lines,
concordant with the low degree of DNA methylation in the BT549
cells (Fig. 3B). Taken together,
promoter hypermethylation is one of the mechanisms contributing to
the inactivation of PAMR1 in both breast cancer cell lines
and tumor tissues.
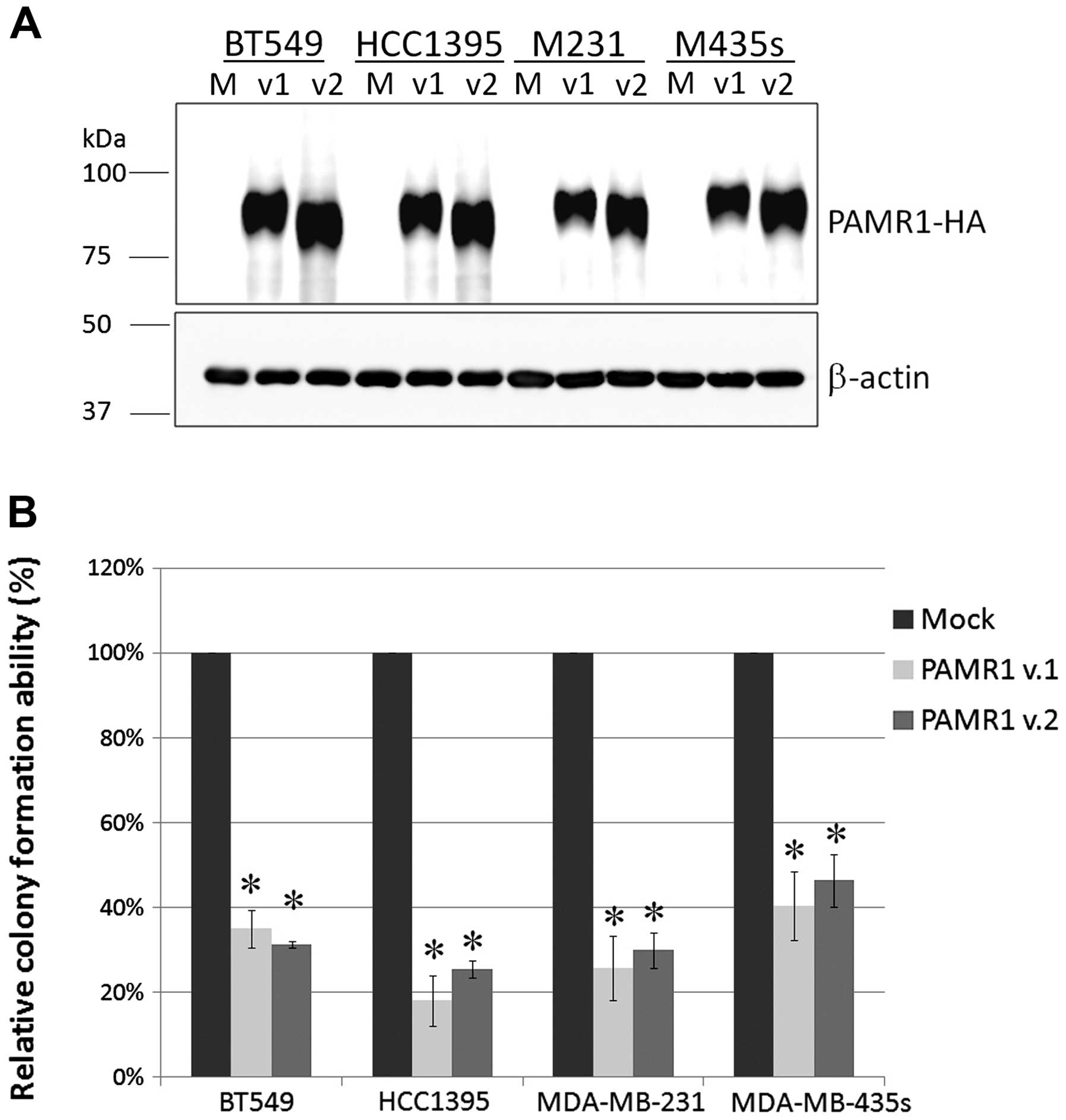
Suppression of tumor cell growth by
ectopic expression of PAMR1
To investigate the role of PAMR1 in breast
carcinogenesis, we constructed plasmids expressing variant 1 or
variant 2 of PAMR1. We confirmed the expression of PAMR1 protein in
all cancer cell lines examined (Fig.
4A). We next conducted colony formation assays and observed a
significant decrease in colony number (18–46%) for all
PAMR1-introduced cells (Fig.
4B), indicating the growth-suppressive function of PAMR1.
Discussion
In the present study, we identified PAMR1 as
a putative breast cancer tumor suppressor by a screening of the
gene expression profiling of 81 breast cancer tissues. Although we
did not find mutations of PAMR1 in 21 breast cancer cell
lines, promoter hypermethylation was frequently observed in both
breast cancer tissues and cell lines. The PAMR1 gene is
located at chromosome 11p13, which is frequently lost in breast
cancer samples (20.8–58.3%) (24–26).
Therefore, both genetic and epigenetic inactivation would
contribute to the downregulation of PAMR1 in breast
cancer.
PAMR1 was first identified as a gene which
was down-regulated in myoblastic cells isolated from DMD mice. The
expression of PAMR1 was induced in gastrocnemius muscle
cells after crush injury, reaching the highest expression on day 4
and was reduced to a normal level on day 14. PAMR1 induction
was only observed in the regenerating muscle fibers by in
situ hybridization but not in normal muscle cells. Thus, PAMR1
is considered to be involved in the regeneration of skeletal
muscles (22). PAMR1 was
expressed in various tissues such as skeletal muscle, brain, and
mammary gland. Moreover, our microarray analyses indicated that
PAMR1 expression was reduced in several types of cancers
including breast, bladder, liver cancers and osteosarcoma (data not
shown). Therefore, PAMR1 may have as yet unidentified roles
other than muscle regeneration.
Although the molecular mechanism whereby
PAMR1 suppresses tumor cell growth has not yet been
clarified, PAMR1 contains putative signal peptides at the
N-terminal, a CUB domain, two EGF domains, two Sushi domains and an
inactive trypsin-like serine protease domain. The secreted
signal peptide CUB-EGF domain-containing protein 2 (SCUBE2)
which contains CUB and EGF domains was shown to suppress breast
cancer cell growth (27).
Functional domain analysis revealed that the CUB domain bound to
bone morphogenetic protein (BMP) and antagonized BMP signaling to
suppress cell differentiation and proliferation. Moreover, the
EGF-like repeats of SCUBE2 interact with E-cadherin to inhibit the
β-catenin pathway (27–29). Since overexpression of PAMR1
in breast cancer cell lines significantly suppressed cancer cell
growth, secreted PAMR1 might exert a tumor-suppressive function by
antagonizing growth signals through the interaction with growth
factors or their receptors.
In conclusion, our study demonstrated that
PAMR1 may be a novel TSG for breast cancer. We provide
evidence that promoter hypermethylation plays an important role in
PAMR1 inactivation during breast carcinogenesis. Although
further functional studies and pathway analyses are necessary,
identification of its downstream pathway would lead to the
development of novel breast cancer therapy by using recombinant
soluble PAMR1 protein.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Campo E: Whole genome profiling and other
high throughput technologies in lymphoid neoplasms - current
contributions and future hopes. Mod Pathol. 26:S97–S110. 2013.
View Article : Google Scholar
|
|
3
|
Ajiro M, Katagiri T, Ueda K, et al:
Involvement of RQCD1 over-expression, a novel cancer-testis
antigen, in the Akt pathway in breast cancer cells. Int J Oncol.
35:673–681. 2009.PubMed/NCBI
|
|
4
|
Kim JW, Fukukawa C, Ueda K, Nishidate T,
Katagiri T and Nakamura Y: Involvement of C12orf32 overexpression
in breast carcinogenesis. Int J Oncol. 37:861–867. 2010.PubMed/NCBI
|
|
5
|
Shimo A, Nishidate T, Ohta T, Fukuda M,
Nakamura Y and Katagiri T: Elevated expression of protein regulator
of cytokinesis 1, involved in the growth of breast cancer cells.
Cancer Sci. 98:174–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimo A, Tanikawa C, Nishidate T, et al:
Involvement of kinesin family member 2C/mitotic
centromere-associated kinesin overexpression in mammary
carcinogenesis. Cancer Sci. 99:62–70. 2008.
|
|
7
|
Kim JW, Akiyama M, Park JH, et al:
Activation of an estrogen/estrogen receptor signaling by BIG3
through its inhibitory effect on nuclear transport of PHB2/REA in
breast cancer. Cancer Sci. 100:1468–1478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshimaru T, Komatsu M, Matsuo T, et al:
Targeting BIG3-PHB2 interaction to overcome tamoxifen resistance in
breast cancer cells. Nat Commun. 4:24432013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agrawal A, Murphy RF and Agrawal DK: DNA
methylation in breast and colorectal cancers. Mod Pathol.
20:711–721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu Z, Xiao Q, Zhao L, et al: DNA
methyltransferase 1/3a over-expression in sporadic breast cancer is
associated with reduced expression of estrogen
receptor-alpha/breast cancer susceptibility gene 1 and poor
prognosis. Mol Carcinog. Jan 25–2014.(Epub ahead of print).
View Article : Google Scholar
|
|
11
|
Radpour R, Kohler C, Haghighi MM, Fan AX,
Holzgreve W and Zhong XY: Methylation profiles of 22 candidate
genes in breast cancer using high-throughput MALDI-TOF mass array.
Oncogene. 28:2969–2978. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niwa Y, Oyama T and Nakajima T: BRCA1
expression status in relation to DNA methylation of the BRCA1
promoter region in sporadic breast cancers. Jpn J Cancer Res.
91:519–526. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barekati Z, Radpour R, Lu Q, et al:
Methylation signature of lymph node metastases in breast cancer
patients. BMC Cancer. 12:2442012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van De Voorde L, Speeckaert R, Van Gestel
D, et al: DNA methylation-based biomarkers in serum of patients
with breast cancer. Mutat Res. 751:304–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Medina-Franco JL and Caulfield T: Advances
in the computational development of DNA methyltransferase
inhibitors. Drug Discov Today. 16:418–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schrump DS, Fischette MR, Nguyen DM, et
al: Phase I study of decitabine-mediated gene expression in
patients with cancers involving the lungs, esophagus, or pleura.
Clin Cancer Res. 12:5777–5785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Issa JP, Kantarjian HM and Kirkpatrick P:
Azacitidine. Nature Rev Drug Discov. 4:275–276. 2005. View Article : Google Scholar
|
|
18
|
Lin J, Deng Z, Tanikawa C, et al:
Downregulation of the tumor suppressor HSPB7, involved in the p53
pathway, in renal cell carcinoma by hypermethylation. Int J Oncol.
44:1490–1498. 2014.PubMed/NCBI
|
|
19
|
Nishidate T, Katagiri T, Lin ML, et al:
Genome-wide gene-expression profiles of breast-cancer cells
purified with laser microbeam microdissection: identification of
genes associated with progression and metastasis. Int J Oncol.
25:797–819. 2004.PubMed/NCBI
|
|
20
|
Tanikawa C, Furukawa Y, Yoshida N, Arakawa
H, Nakamura Y and Matsuda K: XEDAR as a putative colorectal tumor
suppressor that mediates p53-regulated anoikis pathway. Oncogene.
28:3081–3092. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clark HF, Gurney AL, Abaya E, et al: The
secreted protein discovery initiative (SPDI), a large-scale effort
to identify novel human secreted and transmembrane proteins: a
bioinformatics assessment. Genome Res. 13:2265–2270. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakayama Y, Nara N, Kawakita Y, et al:
Cloning of cDNA encoding a regeneration-associated muscle protease
whose expression is attenuated in cell lines derived from Duchenne
muscular dystrophy patients. Am J Pathol. 164:1773–1782. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li LC and Dahiya R: MethPrimer: designing
primers for methylation PCRs. Bioinformatics. 18:1427–1431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Y, Niu Y, Wang X, et al: Chromosome
aberrations associated with centrosome defects: a study of
comparative genomic hybridization in breast cancer. Hum Pathol.
42:1693–1701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hawthorn L, Luce J, Stein L and Rothschild
J: Integration of transcript expression, copy number and LOH
analysis of infiltrating ductal carcinoma of the breast. BMC
Cancer. 10:4602010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Y, Bove B, Wu Y, et al:
Microsatellite instability during the immortalization and
transformation of human breast epithelial cells in vitro. Mol
Carcinog. 24:118–127. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng CJ, Lin YC, Tsai MT, et al: SCUBE2
suppresses breast tumor cell proliferation and confers a favorable
prognosis in invasive breast cancer. Cancer Res. 69:3634–3641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin YC, Lee YC, Li LH, Cheng CJ and Yang
RB: Tumor suppressor SCUBE2 inhibits breast-cancer cell migration
and invasion through the reversal of epithelial-mesenchymal
transition. J Cell Sci. 127:85–100. 2014. View Article : Google Scholar
|
|
29
|
Lin YC, Chen CC, Cheng CJ and Yang RB:
Domain and functional analysis of a novel breast tumor suppressor
protein, SCUBE2. J Biol Chem. 286:27039–27047. 2011. View Article : Google Scholar : PubMed/NCBI
|