Introduction
Hepatocellular carcinoma (HCC), the most common type
of primary liver malignancy, is the fifth most common cancer in men
and the seventh most common cancer in women according to the
International Agency for Research on Cancer (IARC) (1,2). The
incidence of HCC has increased dramatically by 80% in the last two
decades in many of the developed and developing countries of the
world (3). Despite better imaging
studies and improvement of surgical techniques in this area, the
5-year survival rate for HCC still remains low and HCC is one of
the malignancies with a high mortality rate (4,5).
Accumulating evidence suggests that immunotherapy is an alternative
potent therapeutic strategy for patients with HCC (6,7).
Recent studies have shown that human cancer cells and immune cells
in the cancer microenvironment upregulate expression of inhibitory
B7 molecules and that these ectopic molecules contribute to tumor
immune evasion (8). Therefore, the
manipulation of the expression of and signaling through these
molecules may be a promising strategy with which to treat human
types of cancers.
In HCC, immunosuppressive ligands, including the
costimulatory molecule B7 homologue 1 (B7-H1) and B7 homologue 3
(B7-H3), are aberrantly expressed at the tumor cell surface and in
the cytoplasm (9–11). B7-H3, a newly identified member of
the B7/CD28 superfamily, was identified as an accessory
costimulatory molecule after initial antigen priming in cooperation
with a putative counter receptor (12,13).
Although the role of B7-H3 in adaptive immune responses still
remains controversial, overexpression of B7-H3 in HCC suggests that
it may play a significant role in the ‘immune escape’ theory of
tumors (9,14). However, the multiple changes trigged
by B7-H3 overexpression in HCC and how these overexpressed HCC
cells disturb the microenvironment balance to induce immune escape
remain to be clarified.
The tumor microenvironment is a complex system
composed of many cell types, among which macrophages are the most
abundant ones and its subpopulations can be recruited and polarized
according to tumor-secreted cytokines in the tumor milieu, which
have great potential for influencing tumor progression (15,16).
In general, the phenotype of these tumor-associated macrophages
(TAMs) can be categorized into two subpopulations and each of them
has diverse effects on the tumor: M1 macrophages are generally
antitumoral, and M2 macrophages exert pro-tumoral effects (17,18).
In HCC, the phenotype of TAMs are largely immunosuppressive, and
the degree of macrophage infiltration has been positively
associated with both poor cancer-free survival and overall survival
of patients (19).
B7-H3 is a multifunctional costimulatory molecule
that is also involved in non-immunological functional regulation,
such as cell growth, invasion and metastasis and drug resistance
(10,20,21).
Recent studies have shown that B7-H3 proteins promote the
progression of lung cancer by inducing the development of monocytes
into M2 macrophages (22). In
addition, B7-H3 augments proinflammatory cytokine release by
binding its putative receptor on monocytes/macrophages and
contributes positively to the development of sepsis (23). To date, substantial expression of
B7-H3 in monocytes and tumor-infiltrating macrophages in HCC
patients has not been documented; therefore, we aimed to denote the
functions of B7-H3 in the present study.
Materials and methods
Patients and clinical specimens
According to the inclusion and exclusion criteria,
116 HCC tumor tissues and corresponding adjacent non-cancerous
liver tissues used in immunohistochemical analysis were randomly
obtained from patients undergoing liver curative resection between
2004 and 2008 who were hospitalized in the Department of
Hepatobiliary Surgery, The Fourth Hospital of Hebei Medical
University. The inclusion and exclusion criteria included patients:
i) with distinctive pathologic diagnosis of HCC; ii) with no
anticancer treatment before liver resection; iii) who underwent
primary and curative resection for HCC between 2004 and 2008; and
iv) with complete clinicopathologic and follow-up data. All
specimens were collected in the operating theater immediately (≤15
min) after resection of the tumors and then were snap frozen in
liquid nitrogen or fixed in 10% buffered formalin solution and
embedded in paraffin for histological analysis. The histologic
grade of tumor differentiation was determined by the
Edmondson-Steiner grading system. Liver function was assessed by
the Child-Pugh scoring system. Tumors were classified according to
the WHO classification and the International Union against Cancer
tumor-node-metastasis (TNM) classification. If patients had
multiple lesions in the liver, we selected the main nodule for the
present study. All samples were obtained following informed consent
and their use was approved by the ethics committee of the
institution. The median follow-up period was 33.5 months [range,
9–62 months; standard deviation (SD), 11.6 months]. At the last
follow-up (December 31, 2012), 79 (68.1%) patients were deceased,
including 32 due to liver failure or bleeding from the
gastrointestinal tract and the remaining 47 cases due to tumor
recurrence.
Cell lines and culture conditions
The human monocytic cell line THP-1 and the HCC
HepG2 cell line were obtained from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China) and cultured according to the
instructions from the American Type Culture Collection (ATCC).
These cells were maintained in RPMI-1640 supplemented with 10%
fetal calf serum (Gibco) and incubated at 37°C in a humidified
chamber containing 5% CO2.
Immunohistochemistry for B7-H3 and
TAMs
Immunohistochemistry for B7-H3 was performed on a
5-μm thick section using a two-step method with a polyclonal goat
anti-B7-H3 antibody (1:200 dilution; R&D Systems, Inc.) as the
primary antibody and a rabbit anti-goat IgG antibody conjugated to
horseradish peroxidase (ZSGB-BIO, Beijing, China) as the secondary
antibody. Immunohistochemistry for TAMs was carried out on
consecutive section using a three-step protocol with a monoclonal
mouse anti-CD68 antibody (1:100 dilution; Abcam, UK) as the primary
antibody and a rabbit anti-mouse IgG antibody conjugated to
horseradish peroxidase (ZSGB-BIO) as the secondary antibody. In
brief, paraffin sections were dewaxed in xylene and rehydrated
through graded ethanol solutions. Antigens were retrieved by
heating the tissue sections at 100°C for 30 min in EDTA solution.
Sections were cooled down and immersed in 0.3%
H2O2 solution for 20 min to block endogenous
peroxidase activity, and then rinsed in phosphate-buffered saline
(PBS) for 5 min, blocked with 5% BSA at room temperature for 20
min, and incubated with primary antibodies at 4°C overnight.
Negative controls were performed by replacing the specific primary
antibody with PBS. After three PBS washes, sections were incubated
with secondary antibodies for 30 min at room temperature.
Diaminobenzene was used as the chromogen and hematoxylin as the
nuclear counterstain. Sections were dehydrated, cleared and
mounted.
Evaluation of B7-H3 and CD68 staining in cancer
cells and TAMs were evaluated by authorized pathologists who had no
knowledge of the patient clinical status and outcome. B7-H3
expression scores were given separately for the stained area and
for the intensity of staining. Quantification was conducted as
follows: ≤33% of the cancer cells, 1; >33 to ≤66% of the cancer
cells, 2; >66% of the cancer cells, 3; and intensity of
staining: absent/weak, 1; moderate, 2; and strong, 3. The intensity
of B7-H3 staining was considered weak when either cytoplasmic
expression or rare membranous condensation was present, moderate
when incomplete and discontinuous moderate membranous expression
was present, and strong when complete membranous expression of the
molecule was present. Each section had a final grade that was
derived from the multiplication of the area and intensity scores.
Sections with a final score of ≤3 were classified as tumors with
low B7-H3 expression, whereas sections with a final score of >3
were classified as tumors with high B7-H3 expression.
To determine the intratumoral TAM densities, 10
representative high-power fields (x400 magnification) per tissue
section were selected using a Leica DM2500 microscope. The number
of nucleated cells with positive staining for CD68 in each of the
examined cancer tissue areas was counted manually. The number of
TAMs in each sample was determined by averaging the number of TAMs
in at least 3 fields. According to the number of infiltration TAMs
in the tumor, the average of grade I group was 75, grade II group
was 175, and grade III group was 250, respectively.
THP-1 cell and HCC cell coculture
Phorbol 12-myristate 13-acetate (PMA) (Sigma, St.
Louis, MO, USA) was used to induce THP-1 cells to differentiate
into macrophages (24). THP-1 cells
(1×106) were seeded into the lower insert of a 6-well
Transwell apparatus with a 0.4-μm pore size and treated with PMA at
a concentration of 350 nM for 24 h. After a thorough wash to remove
all PMA, HepG2 cells were plated at 5×105 cells/well
into the upper insert and cocultured with PMA-treated THP-1
macrophage cells without direct contact. In the coculture system,
HepG2 cells were cocultured with THP-1-differentiated macrophages
for 24 and 48 h and were then harvested for use in the subsequent
experiments.
RT-PCR analysis
Total cellular RNA was extracted for RT-PCR as
previously described (25). Primers
included were the following: B7-H3 (sense,
5′-ctctccaaaggaaagcgaggtgg acat-3′ and antisense,
5′-agactgtacactgtaggtgctgaaatca-3′); HLA-DR (sense,
5′-tcacgtggcttcgaaatgga-3′ and antisense,
5′-tccaccctgcagtcgtaaac-3′); iNOS (sense, 5′-tccaaggtatcct
ggagcga-3′ and antisense, 5′-cagggacgggaactcctcta-3′); TNF-α
(sense, 5′-ctgggcaggtctactttggg-3′ and antisense, 5′-ctggaggccc
cagtttgaat-3′); arginase 1 (Arg1) (sense, 5′-acggaagaatcagcctg
gtg-3′ and antisense, 5′-gtccacgtctctcaagccaa-3′);
macrophage-derived chemokine (CCL22) (sense,
5′-gcctactctgatgaccgtgg-3′ and antisense,
5′-agagagttggcacaggcttc-3′); vascular endothelial cell growth
factor (VEGF) (sense, 5′-tgcggatcaaacctca ccaa-3′ and antisense,
5′-ctccagggcattagacagca-3′). The housekeeping gene GAPDH was used
as the PCR control. RT-PCR products were analyzed via 1.5% agarose
gel electrophoresis and stained with ethidium bromide for
visualization using ultraviolet light.
Western blot assay
THP-1 cells were washed with PBS twice and lysed
with 1 ml RIPA lysis buffer containing a protease and phosphatase
inhibitor for 30 min on ice. After removing the insoluble material
by 12,000 × g centrifugation for 30 min at 4°C, the supernatants
were collected. Cell lysate protein content was determined using a
bicinchoninic acid (BCA) protein assay kit. Equivalent amounts of
whole cell extracts were subjected to SDS-PAGE and transferred to
PVDF membranes. The membranes were blocked with 5% non-fat milk for
2 h and then incubated with the respective primary antibody
overnight at 4°C followed by incubation with the appropriate
HRP-conjugated secondary antibody for 2 h at room temperature.
Blots were visualized with an ECL detection kit (Pierce, USA) and
GAPDH was used as a loading control.
Short interfering RNA experiments
Short interfering RNA (siRNA) targeting human B7-H3
and the control scrambled siRNA were purchased from OriGene
Company. Transfection was carried out by Lipofectamine 2000
(Invitrogen). Twenty-four and 48 h after transfection, HepG2 cells
were cocultured with THP-1-differentiated macrophages for 12 h and
then harvested for later RT-PCR analysis.
Inhibition of mitogen-activated protein
kinase (MAPK) and signal transducer and activator of transcription
3 (STAT3) signaling pathways
For the inhibition of the MAPK pathway, HepG2 cells
were treated with SB203580, PD98059 and SP600125, which are
specific inhibitors of p38, ERK and JNK, respectively (Sigma) at 15
ng/ml for 2 h. After a thorough wash to remove all the inhibitors,
HepG2 cells were cocultured with THP-1-differentiated macrophages
for 12 h and then harvested for later use. For the inhibition of
the STAT3 pathway, HepG2 cells were treated with Tyrphostin AG490
(Sigma) at 20 ng/ ml for 24 h. After a thorough wash to remove all
the AG490, HepG2 cells were cocultured with THP-1-induced
macrophages for 12 h and then harvested for later use.
Results
B7-H3 overexpression is associated with
TAM infiltration in HCC tissues
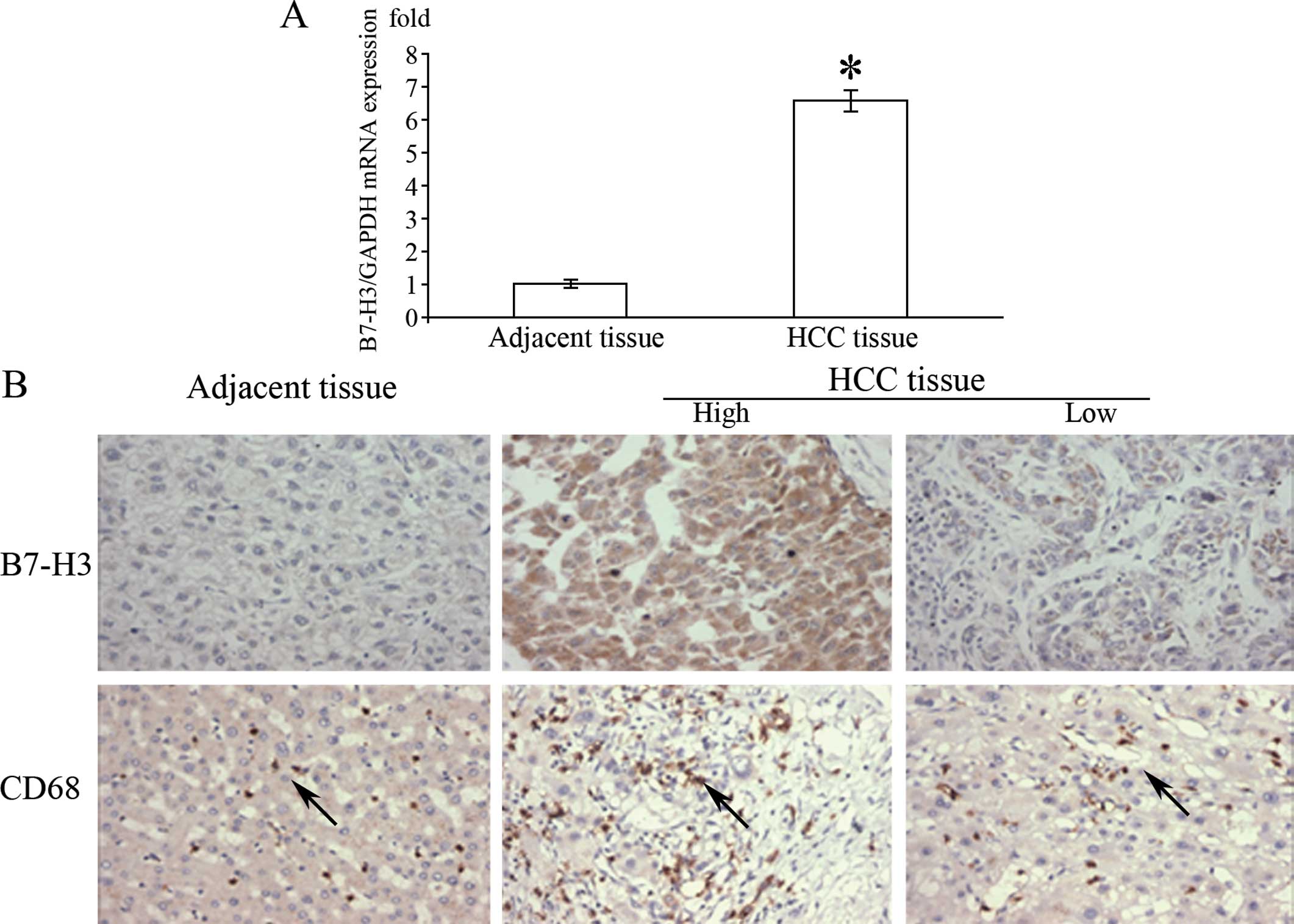
As illustrated in Fig.
1A, the mRNA level of B7-H3 in the HCC tissues was
significantly higher than that in the adjacent normal tissues of
the same patients. Immunohistochemical results showed that B7-H3
was overexpressed in the majority of cases in contrast to the
normal liver cells, which appeared to be extremely diffuse in the
tumor cell membrane, cytoplasm or both (Fig. 1B). Depending on the area of positive
immunoreactivity, a final overall score (high tumor B7-H3 or low
tumor B7-H3 expression) was established as described in Materials
and methods. Of the 116 cases, 79.3% showed high expression of
B7-H3 and 20.1% displayed a low degree of B7-H3 expression. In
order to explore the potential role of B7-H3 in the tumor
microenvironment, we analyzed the number of infiltrating TAMs.
Among all HCC specimens, the number of infiltrating TAMs was
significantly higher in the cancer than that in the normal tissue
samples (Table I). Moreover, the
number of infiltrating CD68+ TAMs was significantly
higher in the HCC tissues with high B7-H3 expression than in the
HCC tissues with weak expression; the mean number of infiltrating
macrophages being 243.1±12.8 and 87.3±5.9, respectively.
 | Table IRelationship between B7-H3 or TAM
infiltration and the clinicopathological parameters of the HCC
cases. |
Table I
Relationship between B7-H3 or TAM
infiltration and the clinicopathological parameters of the HCC
cases.
| Parameters | Total no. of
cases | B7-H3
expression | P-value | Total no. of
cases | TAM
infiltration | P-value |
|---|
|
|
|---|
| Low | High | I | II | III |
|---|
| Age (years) | | | | 0.571 | | | | | 0.285 |
| ≤50 | 43 | 9 | 34 | | 43 | 9 | 21 | 13 | |
| >50 | 73 | 15 | 58 | | 73 | 12 | 28 | 33 | |
| Gender | | | | 0.481 | | | | | 0.808 |
| Male | 84 | 16 | 68 | | 84 | 14 | 36 | 34 | |
| Female | 32 | 8 | 24 | | 32 | 7 | 13 | 12 | |
| Vascular
invasion | | | | 0.001 | | | | | 0.015 |
| + | 54 | 3 | 51 | | 54 | 5 | 21 | 28 | |
| − | 62 | 21 | 41 | | 62 | 16 | 28 | 18 | |
| TNM stage | | | | 0.038 | | | | | 0.036 |
| I–II | 77 | 20 | 57 | | 77 | 18 | 34 | 25 | |
| III–IV | 39 | 4 | 35 | | 39 | 3 | 15 | 21 | |
| Lymph
metastasis | | | | 0.041 | | | | | 0. 034 |
| + | 32 | 3 | 29 | | 32 | 2 | 12 | 18 | |
| − | 84 | 21 | 63 | | 84 | 19 | 37 | 28 | |
| Tumor size
(cm) | | | | 0.014 | | | | | 0.298 |
| ≤5 | 77 | 21 | 56 | | 77 | 17 | 31 | 29 | |
| >5 | 39 | 3 | 36 | | 39 | 4 | 18 | 17 | |
| Tumor
encapsulation | | | | 0.086 | | | | | 0.323 |
| + | 18 | 1 | 17 | | 18 | 2 | 9 | 7 | |
| − | 98 | 23 | 75 | | 98 | 19 | 40 | 39 | |
| Microsatellite
tumors | | | | 0.023 | | | | | 0.036 |
| Yes | 31 | 2 | 29 | | 31 | 2 | 13 | 16 | |
| No | 85 | 22 | 63 | | 85 | 19 | 36 | 30 | |
| Child-Pugh
classification | | | | 0.789 | | | | | 0.624 |
| A | 60 | 13 | 47 | | 60 | 11 | 27 | 22 | |
| B | 56 | 11 | 45 | | 56 | 10 | 22 | 24 | |
| Liver
cirrhosis | | | | 0.203 | | | | | 0.83 |
| Weak | 24 | 8 | 16 | | 24 | 4 | 9 | 11 | |
| Moderate | 58 | 10 | 48 | | 58 | 12 | 26 | 20 | |
| Strong | 34 | 6 | 28 | | 34 | 5 | 14 | 15 | |
| HBsAg | | | | 0.142 | | | | | 0.212 |
| + | 76 | 13 | 63 | | 76 | 11 | 36 | 29 | |
| − | 40 | 11 | 29 | | 40 | 10 | 13 | 17 | |
| AFP | | | | 0.242 | | | | | 0.147 |
| ≤200 | 48 | 8 | 40 | | 48 | 9 | 25 | 14 | |
| >200 | 67 | 16 | 51 | | 67 | 12 | 24 | 31 | |
| Transfusion | | | | 0.286 | | | | | 0.071 |
| + | 47 | 8 | 39 | | 47 | 5 | 18 | 24 | |
| − | 69 | 16 | 53 | | 69 | 16 | 31 | 22 | |
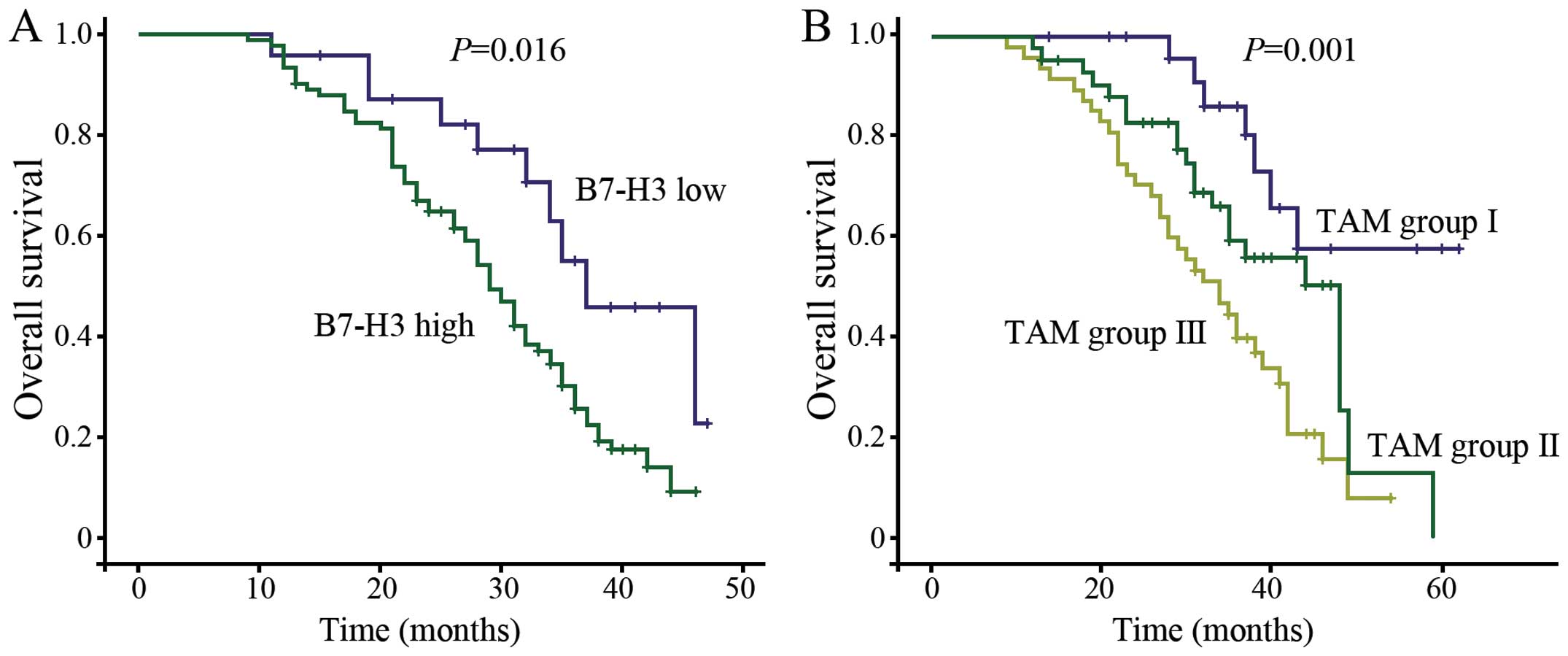
B7-H3 overexpression and infiltrating
TAMs predict poor outcome of HCC patients
Our previous results showed that the B7-H3
expression level was correlated with the number of TAMs in the
evaluated HCC tissues. We then ascertained whether these two
immunological factors could contribute to the outcome of the HCC
patients. Kaplan-Meier survival analysis was used to examine the
relationship between the B7-H3 expression level, the number of TAMs
labeled with CD68 and their correlation with patient survival. The
results showed that the overall survival time was significantly
shorter in the high B7-H3 expression group than the overall
survival time in the low B7-H3 expression group (risk ratio, 0.44;
95% CI, 0.225–0.858; p=0.016, Fig.
2A). A shorter overall survival time was also associated with a
higher number of infiltrating TAMs (Fig. 2B). Tumor cells that expressed higher
levels of B7-H3 exhibited higher levels of TAM infiltration
(p<0.001, Mann-Whitney test).
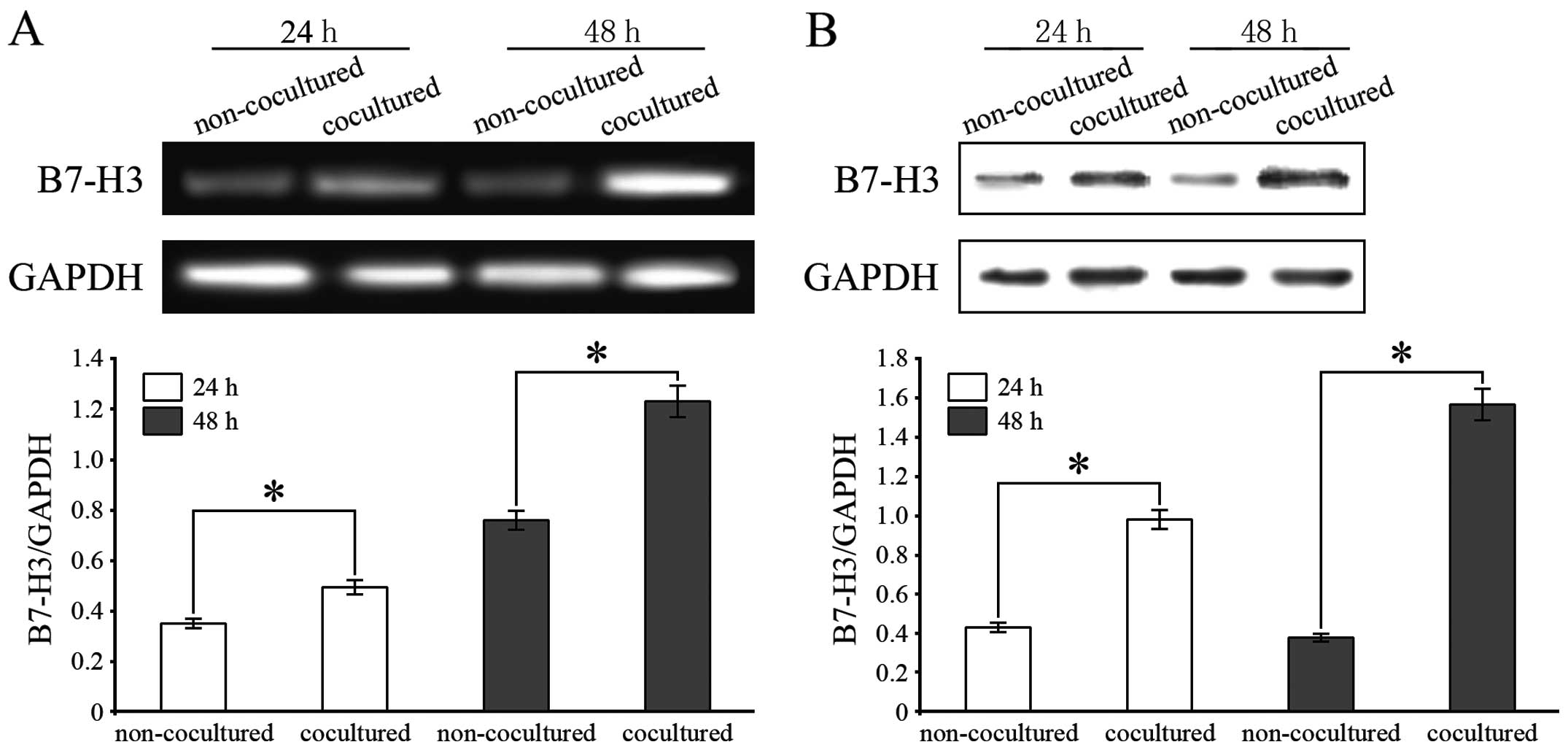
B7-H3 is upregulated on macrophages
induced by PMA after coculture with HCC cells
To determine whether human HCC cells could induce
the expression of B7-H3 on TAMs, we evaluated B7-H3 expression at
the mRNA and protein levels in PMA-treated THP-1 cells cocultured
with HepG2 cells by RT-PCR and western blot assays. B7-H3 mRNA
expression was significantly upregulated in the THP-1 cells
cocultured with HepG2 cells for 24 and 48 h (Fig. 3A). In addition, B7-H3 protein
expression was also increased in the TAMs after 24 and 48 h of
coculture with HCC cells (Fig. 3B).
In addition, monocytes were isolated from human peripheral blood
and induced to macrophages by PMA. The B7-H3 mRNA and its protein
expression in macrophages cocultured with HepG2 cell lines was
significantly upregulated (data not shown).
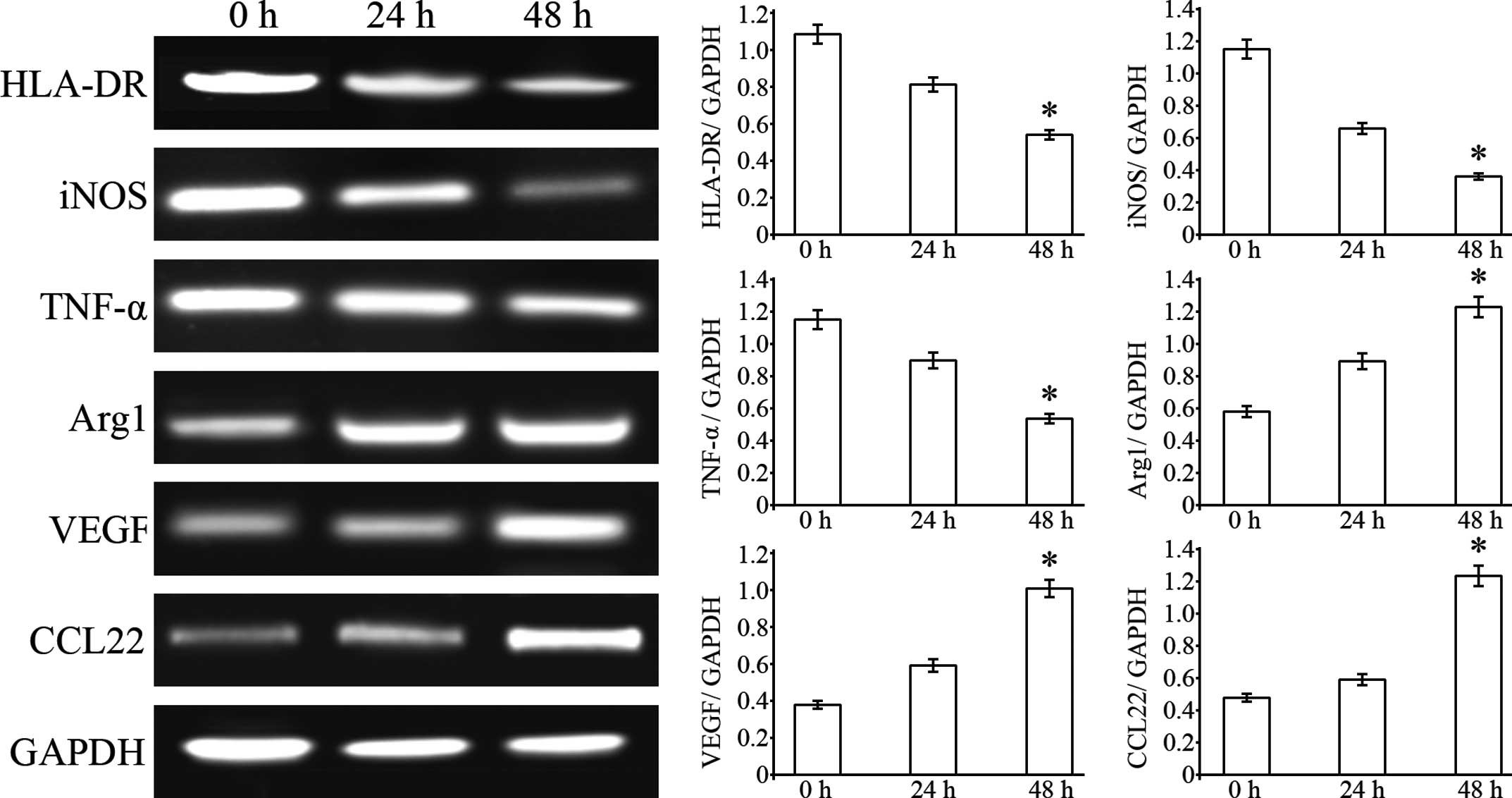
B7-H3 promotes M2 phenotype macrophage
polarization
PMA-induced THP-1 cells have been described as
‘innate’ macrophages that can differentiate into M1 or M2
macrophages (26). To determine
whether HepG2-secreted B7-H3 can influence THP-1 cell
differentiation into different macrophage subtypes, we examined the
expression of M1 and M2 macrophage markers by RT-PCR assays. The
results showed that HLA-DR, iNOS and TNF-α mRNA levels in the THP-1
cells were significantly reduced after 24 and 48 h of coculturing
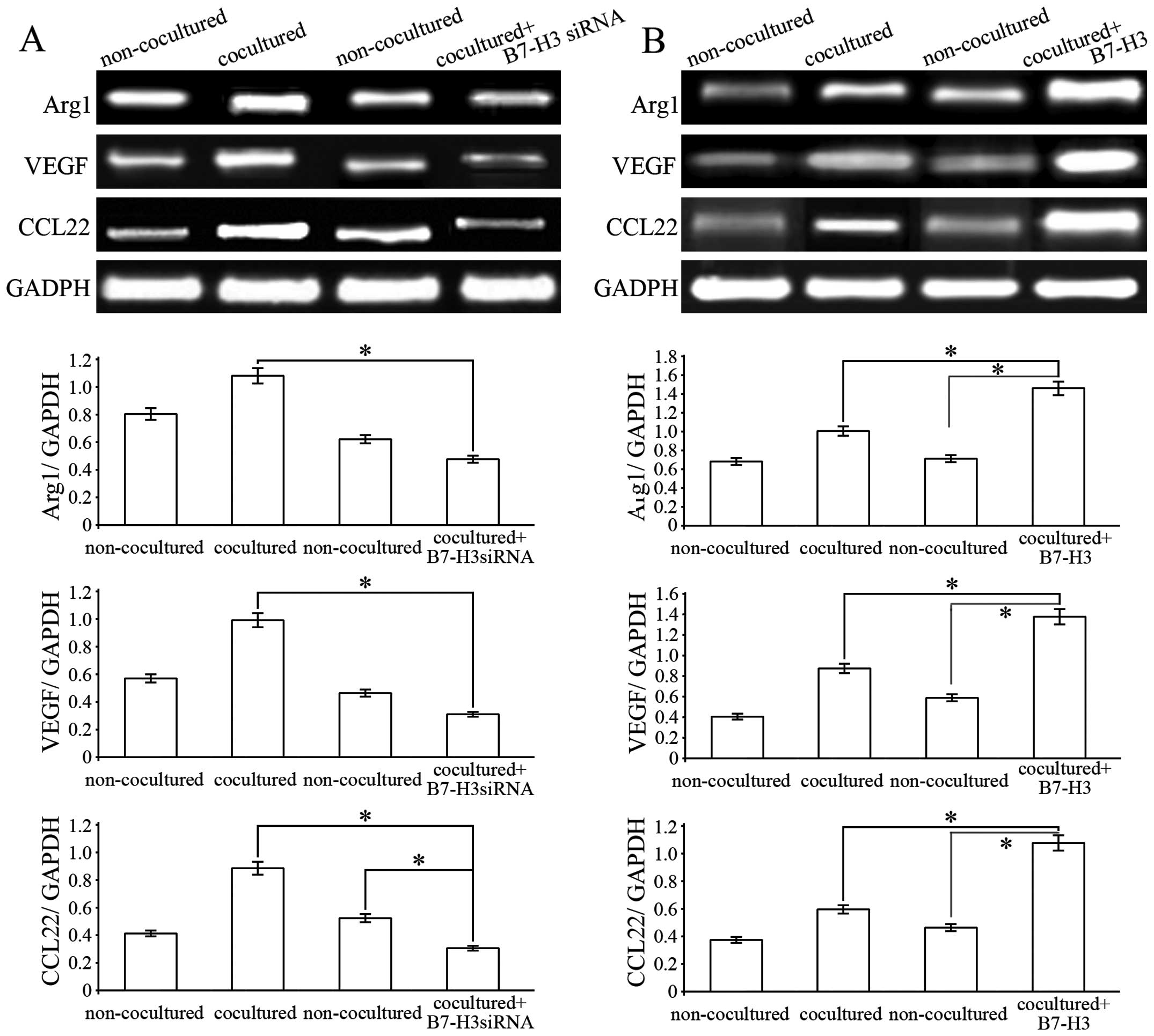
with HepG2 cells. However, Arg1, CCL22 and VEGF mRNA expression
levels in the THP-1 cells were significantly increased after 24 and
48 h of coculturing with the HepG2 cells (Fig. 4). The results suggest that hepatoma
cells can promote THP-1-differentiated macrophages towards M2
phenotype polarization.
Blocking of B7-H3 interferes with the
hepatoma cell-mediated M2 macrophage polarization
To determine whether B7-H3 is involved in promoting
THP-1-mediated macrophage differentiation into M2 macrophages, we
pretreated HepG2 cells with B7-H3 siRNA and then examined the
expression of M2 macrophage markers. The results showed that Arg1,
CCL22 and VEGF mRNA expression levels in the THP-1 cells were
significantly decreased after coculturing with B7-H3-knockdown
HepG2 cells, compared with the control group. This suggests that
pretreatment with B7-H3 siRNA abolished B7-H3-promoted M2 marker
expression in the THP-1 cells (Fig.
5A). In contrast, pretreatment with B7-H3 (30 ng/ml) markedly
increased the expression of M2 macrophage markers (Fig. 5B). Taken together, these results
showed that HepG2-secreted B7-H3 could polarize
THP-1-differentiated macrophages to an M2 macrophage phenotype.
This may lead to the alteration of proinflammatory cytokine
expression pattern, thus leading to suppression of the immune
response within the tumor microenvironment.
Upregulation of B7-H3 expression on THP-1
cells is inhibited by partial blocking of the STAT3 signaling
pathway
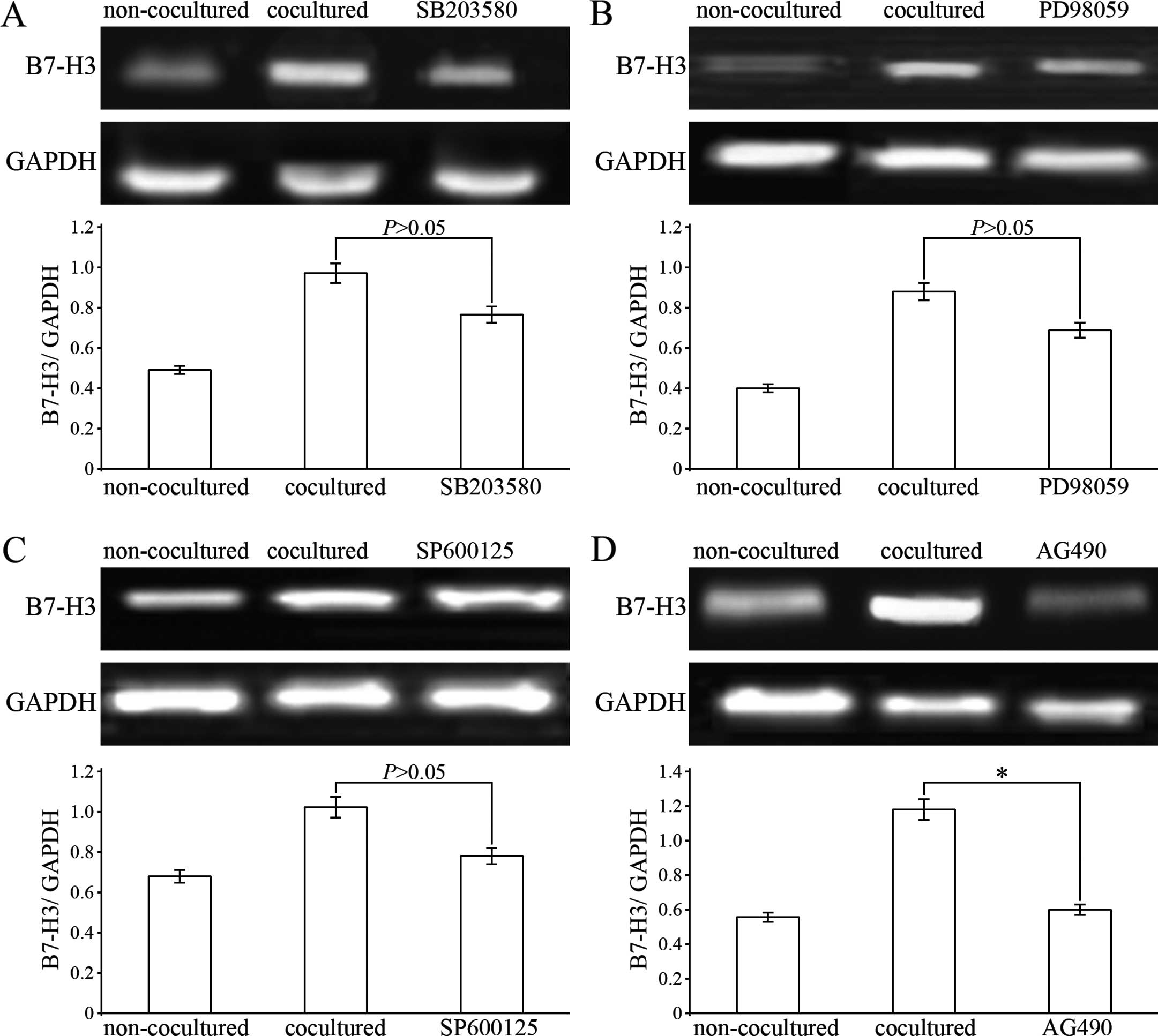
To further clarify the mechanism of upregulation of
B7-H3 expression on THP-1-differentiated macrophages cocultured
with HCC cells, experiments of the blocking of the MAPK (p38, ERK
and JNK) and STAT3 signaling pathways were performed. After
pretreatment with SB203580, PD98059 and SP600125, THP-1-induced
macrophages were cocultured with HepG2 cells for 6 h and then
harvested for detection of B7-H3 mRNA expression. The results
showed that SB203580, PD98059 and SP600125 had the tendency to
abrogate the upregulation of B7-H3 mRNA expression in THP-1-induced
macrophages, however, the data were not statistically significant
(Fig. 6A–C). As for the STAT3
pathway, we chose Tyrphostin AG490 as its specific inhibitor. AG490
partially inhibited the increase in B7-H3 mRNA expression in the
THP-1-induced macrophages cocultured with the HepG2 cells (Fig. 6D). These results suggest that
upregulation of B7-H3 expression on THP-1-differentiated
macrophages induced by the HCC cell microenvironment may be via the
STAT3 signaling pathway.
Discussion
As HCC has been shown to be immunogenic,
immunotherapy is an alternative promising therapeutic approach
(27). Immunotherapy aims to
provide a more efficient way to target tumor cells by inducing or
enhancing the existing tumor-specific immune response. However, HCC
demonstrated potential immunoresistance in the local tumor
microenvironment allowing the tumor to evade a cytotoxic response
(28). Aberrant regulation of
immune-stimulating antigens is one of the several complicated
mechanisms concerning tumor immunoescape (29,30).
In the present study, we showed that B7-H3 was uniformly
overexpressed in hepatoma cells. Increased B7-H3 expression was
detected in 79.3% of the 61 HCC specimens examined. Additionally,
B7-H3 expression was significantly correlated with the patient
overall survival time; that is lower levels of B7-H3 expression
were associated with a prolonged survival time. In light of
previous studies and the results of the present study (9,10,14),
it appears that B7-H3 plays a critical role in the pathogenesis and
development of HCC, however, its exact role remains unclear.
Several studies have demonstrated that TAMs play a
key role in the tumor progression of HCC (18,31).
TAMs are mainly polarized towards the M2 phenotype and favors tumor
formation and progression (32,33).
In order to explore the mechanisms underlying the overexpression of
B7-H3, we analyzed the relationship between B7-H3 expression and
TAMs in HCC. Immunohistochemical results showed that the B7-H3
expression in tumor cells was correlated with the infiltration of
TAMs in HCC tissues, and the number of TAMs had a negative
correlation with the patient survival time. Moreover, HCC cells
upregulated B7-H3 expression in PMA-induced THP-1 cells. These
findings suggest that the macrophages that infiltrate the HCC
tissues may be important for promoting tumor progression, and B7-H3
may be involved in this process. Our results are in line with
previous similar studies of other costimulatory molecules that
function in TAM regulation. For example, Chen et al showed
that TAMs could be induced to express the B7-H3 molecule in tumor
stroma when cultured with tumor cells in lung cancer. Upregulation
of B7-H3 on TAMs is a major pro-inflammatory resource and novel
immune escape mechanism in the tumor milieu (34).
TAMs are the prominent population of infiltrating
leukocytes and the major source of inflammatory cytokines in the
tumor milieu (35,36). Upon activation, macrophages can
release a vast diversity of cytokines, proteolytic enzymes, growth
factors and inflammatory mediators that may directly influence the
behavior of tumor cells (37).
Generally, the M1 phenotype could secrete reactive oxygen and
nitrogen intermediates to kill cancer cells, and immunomodulatory
factors including TNF-α and interleukin-1β (IL-1β) to recruit CTL
cells to attack cancer (38). The
M2 phenotype has the opposite effects. They release vascular
endothelial cell growth factor (VEGF), platelet-derived growth
factor (PDGF), tumor transforming growth factor (TGF)-β and IL-10
that promote cancer cell growth. Moreover, these M2-like TAMs can
produce a variety of matrix metalloproteinases and chemokines, such
as MMP-2, MMP-7, MMP-9, CCL18, CCL22 that facilitate cancer
micrometastasis (39,40). In the present study, the coculture
experiment revealed that M2 phenotype marker expression was
significantly increased on the PMA-treated THP-1 cells.
Accordingly, M1 phenotype marker expression was reduced vice versa.
Therefore, we concluded that the subtle cytokine changes in the HCC
microenvironment have the potential to promote TAMs to
differentiate into the M2 phenotype, and overexpression of B7-H3
may be involved in this.
Accumulating data revealed that B7 costimulatory
molecules were highly expressed on TAMs and these TAMs expressing
certain B7 molecules favored pro-inflammatory response to immune
tolerance in the tumor milieu. Chen et al demonstrated that
B7-H4-expressing macrophages were related to tumor size, lymph node
metastasis and TNM stage, which may promote tumor progression
(41). In addition, B7-H1 and B7-H4
have been found to be involved in the shift from inflammatory M1
macrophage to the anti-inflammatory M2-like macrophage
differentiation (42,43). In the present study, the macrophages
derived from the THP-1 cells cocultured with siB7-H3-treated HepG2
cells reduced expression levels of Arg1, CCL22 and VEGF, which are
distinctive of M2 macrophages, demonstrating that the B7-H3
signaling pathway significantly interfered with the switching of
the macrophage phenotype towards M2. Based on the results
collected, we can speculate that the complex of the HCC tumor
microenvironment resulted in the aberrant expression of
tumor-associated molecules including B7-H3, which further
accelerated the tumor progression and could be released into the
blood as its soluble form (sB7-H3). sB7-H3 with other
immunosuppressive cytokines, such as IL-10, TGF-β and IL-1β,
promote the polarization of TAMs towards the M2 phenotype.
Therefore, we hypothesized that B7-H3 may also function as a
chemotactic factor, attracting monocytes in the peripheral blood to
migrate to tumor tissues and induce the differentiation of
macrophages, thereby promoting tumor oncogenesis and
development.
Recent studies have demonstrated that the expression
of the B7-H3 gene is involved in activation of the STAT3 and MAPK
pathways (44,45). Moreover, several major signaling
pathways and their modulators/targets, including the MAPK and STAT
signaling pathways, are involved in directing the macrophage
plasticity and polarized function, and are associated with
reciprocal skewing of macrophage polarization between the M1 and M2
states (46,47). In the present study, the
upregulation of M2-phenotype marker expression in the
THP-1-differentiated macrophages was partially abrogated by
inhibitors specific for STAT3 signaling, while no obvious effects
were noted by the p38, JNK and ERK specific inhibitors. Therefore,
the M2-like TAMs in HCC tissues may be induced by the inflammatory
cytokines released from HCC tissues through activating the
B7-H3/STAT3 signaling pathway.
In summary, the present study revealed that
overexpression of B7-H3 in tumor cells was associated with TAM
infiltration in HCC tissues, and B7-H3 expression was induced on
the surface of PMA-induced macrophages facilitating M2-TAM
polarization in the HCC microenvironment. In support, inflammatory
cytokines released from M2-TAMs stimulated tumor growth and
metastasis. Furthermore, the B7-H3/STAT3 signaling pathway may be
involved in switching macrophages to the M2 phenotype and the
negative regulation of the T lymphocyte-mediated immune response.
Therefore, future studies to identify methods of inhibiting B7-H3
expression in HCC are warranted. TAM-tumor cell interaction-induced
B7-H3 represents a novel immune escape target in the HCC tumor
milieu.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81402228), HeBei Province Medical
Foundation (ZL20140334) and HeBei Province Education Foundation
(QN2014049).
References
|
1
|
Flores A and Marrero JA: Emerging trends
in hepatocellular carcinoma: focus on diagnosis and therapeutics.
Clin Med Insights Oncol. 8:71–76. 2014.PubMed/NCBI
|
|
2
|
Abdel-Rahman O: Systemic therapy for
hepatocellular carcinoma (HCC): from bench to bedside. J Egypt Natl
Canc Inst. 25:165–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salomao M, Remotti H, Vaughan R, Siegel
AB, Lefkowitch JH and Moreira RK: The steatohepatitic variant of
hepatocellular carcinoma and its association with underlying
steatohepatitis. Hum Pathol. 43:737–746. 2012. View Article : Google Scholar
|
|
4
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossi L, Zoratto F, Papa A, et al: Current
approach in the treatment of hepatocellular carcinoma. World J
Gastrointest Oncol. 2:348–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palmer DH, Midgley RS, Mirza N, et al: A
phase II study of adoptive immunotherapy using dendritic cells
pulsed with tumor lysate in patients with hepatocellular carcinoma.
Hepatology. 49:124–132. 2009. View Article : Google Scholar
|
|
7
|
Hernandez-Gea V, Alsinet C and Llovet JM:
Oncolytic immunotherapeutic virus in HCC: can it compete with
molecular therapies? J Hepatol. 59:882–884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ceeraz S, Nowak EC and Noelle RJ: B7
family checkpoint regulators in immune regulation and disease.
Trends Immunol. 34:556–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun TW, Gao Q, Qiu SJ, et al: B7-H3 is
expressed in human hepatocellular carcinoma and is associated with
tumor aggressiveness and postoperative recurrence. Cancer Immunol
Immunother. 61:2171–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang F, Wang G, Liu T, Yu G, Zhang G and
Luan X: B7-H3 was highly expressed in human primary hepatocellular
carcinoma and promoted tumor progression. Cancer Invest.
32:262–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geng L, Deng J, Jiang G, et al: B7-H1
up-regulated expression in human hepatocellular carcinoma tissue:
correlation with tumor interleukin-10 levels.
Hepatogastroenterology. 58:960–964. 2011.PubMed/NCBI
|
|
12
|
Wang L, Kang FB and Shan BE:
B7-H3-mediated tumor immunology: friend or foe? Int J Cancer.
134:2764–2771. 2014. View Article : Google Scholar
|
|
13
|
Chapoval AI, Ni J, Lau JS, et al: B7-H3: a
costimulatory molecule for T cell activation and IFN-γ production.
Nat Immunol. 2:269–274. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen W, Liu P, Wang Y, et al:
Characterization of a soluble B7-H3 (sB7-H3) spliced from the
intron and analysis of sB7-H3 in the sera of patients with
hepatocellular carcinoma. PLoS One. 8:e769652013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goubran HA, Kotb RR, Stakiw J, Emara ME
and Burnouf T: Regulation of tumor growth and metastasis: the role
of tumor microenvironment. Cancer Growth Metastasis. 7:9–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santoni M, Massari F, Amantini C, et al:
Emerging role of tumor-associated macrophages as therapeutic
targets in patients with metastatic renal cell carcinoma. Cancer
Immunol Immunother. 62:1757–1768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shirabe K, Mano Y, Muto J, et al: Role of
tumor-associated macrophages in the progression of hepatocellular
carcinoma. Surg Today. 42:1–7. 2012. View Article : Google Scholar
|
|
19
|
Fan QM, Jing YY, Yu GF, et al:
Tumor-associated macrophages promote cancer stem cell-like
properties via transforming growth factor-beta1-induced
epithelial-mesenchymal transition in hepatocellular carcinoma.
Cancer Lett. 352:160–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Zhang Q, Chen W, et al: B7-H3 is
overexpressed in patients suffering osteosarcoma and associated
with tumor aggressiveness and metastasis. PLoS One. 8:e706892013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H, Tekle C, Chen YW, et al: B7-H3
silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3
phosphorylation. Mol Cancer Ther. 10:960–971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun J, Mao Y, Zhang YQ, et al: Clinical
significance of the induction of macrophage differentiation by the
costimulatory molecule B7-H3 in human non-small cell lung cancer.
Oncol Lett. 6:1253–1260. 2013.PubMed/NCBI
|
|
23
|
Zhang G, Wang J, Kelly J, et al: B7-H3
augments the inflammatory response and is associated with human
sepsis. J Immunol. 185:3677–3684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tjiu JW, Chen JS, Shun CT, et al:
Tumor-associated macrophage-induced invasion and angiogenesis of
human basal cell carcinoma cells by cyclooxygenase-2 induction. J
Invest Dermatol. 129:1016–1025. 2009. View Article : Google Scholar
|
|
25
|
Wang L, Kang F, Li J, Zhang J and Shan B:
Overexpression of p65 attenuates celecoxib-induced cell death in
MDA-MB-231 human breast cancer cell line. Cancer Cell Int.
13:142013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu TH, Li YY, Wu TL, et al: Culture
supernatants of different colon cancer cell lines induce specific
phenotype switching and functional alteration of THP-1 cells. Cell
Immunol. 290:107–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kabilova TO, Kovtonyuk LV, Zonov EV, et
al: Immunotherapy of hepatocellular carcinoma with small
double-stranded RNA. BMC Cancer. 14:3382014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmidt N, Neumann-Haefelin C and Thimme
R: Cellular immune responses to hepatocellular carcinoma: lessons
for immunotherapy. Dig Dis. 30:483–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seliger B and Quandt D: The expression,
function, and clinical relevance of B7 family members in cancer.
Cancer Immunol Immunother. 61:1327–1341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bottino C, Dondero A, Bellora F, et al:
Natural killer cells and neuroblastoma: tumor recognition, escape
mechanisms, and possible novel immunotherapeutic approaches. Front
Immunol. 5:562014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Capece D, Fischietti M, Verzella D, et al:
The inflammatory microenvironment in hepatocellular carcinoma: a
pivotal role for tumor-associated macrophages. Biomed Res Int.
2013:1872042013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lievense LA, Bezemer K, Aerts JG and
Hegmans JP: Tumor-associated macrophages in thoracic malignancies.
Lung Cancer. 80:256–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Front Biosci.
13:453–461. 2008. View
Article : Google Scholar
|
|
34
|
Chen C, Shen Y, Qu QX, Chen XQ, Zhang XG
and Huang JA: Induced expression of B7-H3 on the lung cancer cells
and macrophages suppresses T-cell mediating anti-tumor immune
response. Exp Cell Res. 319:96–102. 2013. View Article : Google Scholar
|
|
35
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan A, Chen JJ and Yang PC:
Pathophysiology of tumor-associated macrophages. Adv Clin Chem.
45:199–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yue ZQ, Liu YP, Ruan JS, Zhou L and Lu Y:
Tumor-associated macrophages: a novel potential target for cancer
treatment. Chin Med J. 125:3305–3311. 2012.PubMed/NCBI
|
|
38
|
Wang H, Wang X, Li X, et al:
CD68+HLA-DR+ M1-like macrophages promote
motility of HCC cells via NF-κB/FAK pathway. Cancer Lett.
345:91–99. 2014. View Article : Google Scholar
|
|
39
|
Lee JH, Lee GT, Woo SH, et al: BMP-6 in
renal cell carcinoma promotes tumor proliferation through
IL-10-dependent M2 polarization of tumor-associated macrophages.
Cancer Res. 73:3604–3614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma YY, He XJ, Wang HJ, et al: Interaction
of coagulation factors and tumor-associated macrophages mediates
migration and invasion of gastric cancer. Cancer Sci. 102:336–342.
2011. View Article : Google Scholar
|
|
41
|
Chen C, Zhu YB, Shen Y, Zhu YH, Zhang XG
and Huang JA: Increase of circulating B7-H4-expressing
CD68+ macrophage correlated with clinical stage of lung
carcinomas. J Immunother. 35:354–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen J, Li G, Meng H, et al: Upregulation
of B7-H1 expression is associated with macrophage infiltration in
hepatocellular carcinomas. Cancer Immunol Immunother. 61:101–108.
2012. View Article : Google Scholar
|
|
43
|
Chen C, Qu QX, Shen Y, et al: Induced
expression of B7-H4 on the surface of lung cancer cells by the
tumor-associated macrophages: a potential mechanism of immune
escape. Cancer Lett. 317:99–105. 2012. View Article : Google Scholar
|
|
44
|
Tekle C, Nygren MK, Chen YW, et al: B7-H3
contributes to the metastatic capacity of melanoma cells by
modulation of known metastasis-associated genes. Int J Cancer.
130:2282–2290. 2012. View Article : Google Scholar
|
|
45
|
Chen X, Quinn EM, Ni H, et al: B7-H3
participates in the development of experimental pneumococcal
meningitis by augmentation of the inflammatory response via a
TLR2-dependent mechanism. J Immunol. 189:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang W, Xu W and Xiong S: Macrophage
differentiation and polarization via phosphatidylinositol
3-kinase/Akt-ERK signaling pathway conferred by serum amyloid P
component. J Immunol. 187:1764–1777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou D, Huang C, Lin Z, et al: Macrophage
polarization and function with emphasis on the evolving roles of
coordinated regulation of cellular signaling pathways. Cell Signal.
26:192–197. 2014. View Article : Google Scholar
|