Introduction
Despite a better understanding of tumor cell biology
(1), the treatment of most cancers
has not significantly changed in the past three decades, and drugs
that do not discriminate between tumor cells and normal tissues
remain the mainstay of anti-cancer therapy (2). Therefore, finding anticancer drugs
that specifically kill tumor cells with no obvious effects on
normal cells is the research initiative around the world. Notably,
in 1995, Jiang et al (3)
reported that melanoma differentiation associated
gene-7/interleukin-24 (mda-7/IL-24) inhibited the proliferation of
melanoma cells. IL-24 is an intriguing and exciting molecule due to
its ability to selectively kill and induce apoptosis in a wide
range of cancer cells, both in vitro and in vivo,
without harming the equivalent normal human cells, including
epithelial cells, fibroblasts, melanocytes and orastrocytes
(4,5). This characteristic has led to its
characterization as a ‘tumor suppressor’ (6). Of importance, IL-24 also exerts
immunomodulatory effects (7,8), and
combinatorial treatment with other conventional therapeutic
modalities or agents, such as radiotherapy (4,9,10),
chemotherapy (11,12), monoclonal antibody therapy (4,13),
sulindac (4),
antisense-oligonucleotides (14),
reactive oxygen species generators (15), and non-steroidal anti-inflammatory
drugs (NSAIDs) (16), can induce
dramatic enhancement in its apoptotic and tumor-suppressing
capacities compared to its use as a single agent (17,18).
Dr Paul B. Fisher, its discoverer, suggested that this novel
cytokine may be the long sought after and proverbial ‘magical
bullet’ for a diverse set of cancers (19).
Ectopic expression of IL-24 in cancer cells, either
by transfection of the tumor cells with a plasmid containing IL-24
cDNA (20) or by infecting the
tumor cells with a recombinant adenovirus (Ad.IL-24) (21) or adeno-associated virus (AAV-IL-24)
(22) to deliver the gene, rapidly
inhibits the growth of cells and induces apoptosis in a broad
spectrum of human and rodent tumors in vitro and in
vivo. In contrast, overexpression of IL-24 in normal primary or
immortalized cells does not affect their growth and viability. The
overexpressed IL-24 protein was shown to localize to the
endoplasmic reticulum (ER)/ Golgi compartments and was secreted
from infected tumor cells as soluble IL-24 (sIL-24), resulting in
‘toxic bystander’ effects on distant uninfected tumor cells
(5,23). This effect is expected for a
cytokine and could make IL-24 treatment an ideal and effective tool
for cancer gene therapy. The exogenous IL-24 protein can robustly
induce the expression of endogenous IL-24, which generates the
signaling events necessary for ‘toxic bystander’ killing and is
essential for recombinant IL-24-induced apoptotic effects (24).
The recombinant IL-24 protein can act on tumor cells
in different ways. Bacterially synthesized GST-IL-24 can be taken
up by cells (25), or its entry
into tumor cells is mediated in a receptor-independent manner. Yet,
some researchers did not observe any apoptosis-inducing effects of
GST-IL-24 or other bacterially expressed IL-24 on melanoma and lung
cancer cells (25). However, the
purified recombinant IL-24 protein, synthesized in mammalian cells
and secreted into the cell media, preferred to kill cancer cells in
a receptor-dependent fashion. IL-24 binds to the currently
recognized IL-20 receptor complexes; these complexes consist of two
sets of heterodimeric chains, IL-20R1/IL-20R2 or IL-22R1/IL-20R2
(25–27). Upon ligand binding, both receptors
induce the phosphorylation of STAT3, which then translocates into
the nucleus where it upregulates Bax and induces apoptosis in
melanoma cells (26). In contrast
to GST-IL-24 and Ad.IL-24, purified IL-24 synthesized in mammalian
cells does not appear to have any biological effect on cells
lacking expression of the IL-20 receptor complexes, such as the
human lung cancer A549 cell line in vitro (6).
Even though full-length IL-24 cDNA has been used in
most experiments, early results showed that a specific peptide of
IL-24 (M4, residues 104–206) mimics the biological properties of
the full-length protein, including its in vivo tumor
suppression properties. M4 interacted with the endoplasmic
reticulum chaperone BiP/GRP78 through the conserved sequences in
its α-helices C and F; M4 also maximally activated p38 MAPK and
promoted the expression of its downstream targets, such as GADD34
and GADD153 (28). At the same
time, bacterially synthesized GST-M4 has a higher expression level
than GST-IL-24 because of its smaller size, but GST-M4 is also
equally as potent as GST-IL-24 at inhibiting growth and inducing
apoptosis in four NSCLC cell lines. At low doses, GST-M4 was
relatively more potent than GST-IL-24 in exerting its killing
effect. Both GST-IL-24 and GST-M4 activated the p38 MAPK pathway,
which is involved in mediating the induction of apoptosis by IL-24
protein in several tumor models (29). Thus, small-molecule peptides that
mimic the IL-24 protein may lead to new therapeutics that can
selectively target and kill cancer cells based on their increased
level of stress compared with normal cells. At present, it is not
clear whether an IL-24 peptide smaller than M4 could have the same
activity as the full length IL-24.
The use of peptides in cancer therapeutics has
recently become popular because of their potency, specificity, low
toxicity, and the limitations of viral vector gene therapy
approaches (30,31). Small anticancer peptides can
specifically inhibit the proliferation of cancer cells during
cancer development and have beneficial effects on multidrug
resistant cancer cells due to their low molecular weight and high
efficiency. At the same time, small anticancer peptides can be
administered to patients in many forms and do not induce immune
reactions. Compared to large recombinant protein drugs, peptides
are easier to be restructured and synthesized at low cost. All of
these properties show that small anticancer peptides have the
potential to be widely applied as clinical anticancer
treatments.
Assuming that the M4 mutant has the same bioactivity
against cancer cells as the full-length protein, we hypothesized
that IL-24 peptides smaller than M4 may inhibit the proliferation
of cancer cell lines. To test this hypothesis, a small peptide, M1,
was designed using a computer-guided modeling method, and we
carefully assessed the effect of this peptide on the proliferation
of cancer cells in vitro. Our results revealed that the
chemically synthesized M1 peptide specifically inhibited the growth
of the esophageal squamous cell carcinoma (ESCC) cell line Eca-109
and the melanoma cell line A375, while having almost no effects on
the human embryo lung (HEL) fibroblast cell line. The aim of the
present study was to identify the therapeutic potential of a small
IL-24 peptide that can selectively target and kill cancer
cells.
Materials and methods
Cells and materials
The melanoma carcinoma cell line A375 and the lung
cancer cell line A549 were obtained from the Cell Center of Peking
Union Medical College. The ESCC cell line Eca-109 and the human
embryo lung fibroblast cell line HEL were kindly provided by Dr
Xiaofei Zheng at the Beijing Institute of Radiation Medicine. All
cells were checked by STR (short tandom repeat) analysis in Beijing
Microread Biotechnology Co. and maintained in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco, Inc., Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS; Life Technologies, Inc., Grand
Island, NY, USA), 100 units/ml penicillin, 100 μg/ml streptomycin,
2 mmol/l L-glutamine, and HEPES buffer (Life Technologies).
Anti-IL-24 antibodies (AF1965 and MAB1965) and anti-IL-20R1
(MAB11762), anti-IL-20R2 (AF1788) and anti-IL-22R1 (MAB2770)
antibodies were purchased from R&D Systems China Co., Ltd.
(Shanghai, China). All other primary and secondary antibodies were
purchased from Beijing Zhongshan Golden Bridge Biotechnology Co.
(Beijing, China).
Computer modeling design and chemical
synthesis of the IL-24 peptide
Theoretical simulation of the space
structure of IL-24
First, we retrieved the sequence information of
IL-24 from the UniProtKB/SwissProt database (http://www.ebi.ac.uk). Then, with the help of the
BlastP program (http://www.ncbi.nlm.nih.gov/BlastP), we made a similar
retrieval from the Protein Data Bank, where we obtained the protein
with the highest sequence similarity to IL-24. Finally, using
computer guided homologous modeling technology and with the aid of
the Homology module of the InsightII 2005 package, we modeled a
three-dimensional theoretical structure of hIL-24.
Analysis of the physical and chemical
properties and apparent characteristics of IL-24 based on its
spatial structure
We analyzed the solvent accessibility of IL-24 based
on its spatial modeling structure and the solvent accessibility
principle. We also analyzed the surface electrostatic potential
distribution of IL-24 using the Delphi program.
Prediction of potential active sites in
the IL-24 space structure
Using our reasonable IL-24 space structure, combined
with the predicted IL-24 apparent physical and chemical properties
(solvent accessibility and apparent electrostatic properties), we
predicted the most likely active position of IL-24 based on the
prediction method of the binding site.
Chemical synthesis of IL-24
The peptides used in the present study were
synthesized by Beijing B&M Biotech Co., Ltd. (Beijing, China).
All peptides were synthesized by solid-phase chemistry, purified to
homogeneity (>90% purity) by reversed-phase high-pressure liquid
chromatography (RP-HPLC), and assessed by mass spectrometry (MS).
The peptides were dissolved in DMEM.
Immunofluorescence staining and
fluorescence microscopy
Briefly, 5×104 cells were seeded in a
96-well plate, maintained in DMEM with 10% FBS and cultivated for
24 h to 60–70% confluency at 37°C. The cells were washed with PBS
three times, fixed with 4% formaldehyde, permeabilized with 0.5%
NP40 in PBS, and blocked with 1% BSA in PBS for 30 min at room
temperature. Then, the cells were incubated with anti-IL-20R1,
anti-IL-20R2 or anti-IL-22R1 (1:10) antibody in PBS overnight at
4°C. The cells were washed and incubated in TRITC-conjugated
secondary antibody (1:200) in PBS for 1 h at room temperature. The
nuclei were stained using DAPI and examined by fluorescence
microscopy (Nikon, Tokyo, Japan), and the resulting images were
merged using fluorescence microscopy (original magnification,
×200). Controls were incubated with only the secondary antibodies
under the same experimental conditions.
Cell proliferation assay
Cell viability was assessed by MTT assays as
previously described (15).
Briefly, cells were seeded in 96-well plates at a density of 2,500
cells/well for 24 h at 37°C. The next day, the cells were treated
with the peptide M1 at different concentrations of 0.5, 1, 2, 5,
10, 20, 50, 100 or 200 μg/ml. On day 4 after treatment, the medium
was removed, and MTT (final concentration of 0.5 mg/ml) was added
to each well. The cells were maintained at 37°C for 4 h, and then
150 μl DMSO was added to each well and mixed thoroughly. The
absorbance from the plates was read on a Bio-Rad microplate reader
Model 550 at 490 nm. MTT absorbance of the untreated control cells
was set to 1 to calculate the relative number of viable cells. The
experiments were repeated at least three times to ensure
reproducibility and statistical significance.
Statistical analysis
All of the experiments were performed at least three
times. Statistical comparisons between the groups were performed
using an unpaired Student’s t-test with the SPSS version 15
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant result, and P<0.01 was
considered to indicate a highly statistically significant
result.
Results
Theoretical simulation of the space
structure of IL-24
We used the UniProtKB/SwissProt (http://www.ebi.ac.uk) database to retrieve the protein
sequence of human IL-24 (UniProtKB code: Q13007). hIL-24 is
composed of 206 amino acids, where 1–51 represent the signal
peptide, and 52–206 represent the mature extracellular chain.
N-glycosylation occurs at sites 85, 99 and 126.
Using computer guided homologous modeling technology
and the InsightII 2005 package with the Homology module, we
constructed a three-dimensional structure of hIL-24. Selecting CVFF
(consistent valence force field), the CHARMM force field in turn,
the steepest descent (convergence criterion, 0.02 Kcal/mol;
convergence step: 200,000 steps), and the conjugate gradient
(convergence criterion, 0.01 Kcal/mol; convergence step, 250,000
steps) methods were used to optimize the initial spatial
conformation of hIL-24. The optimized conformation of hIL-24 is
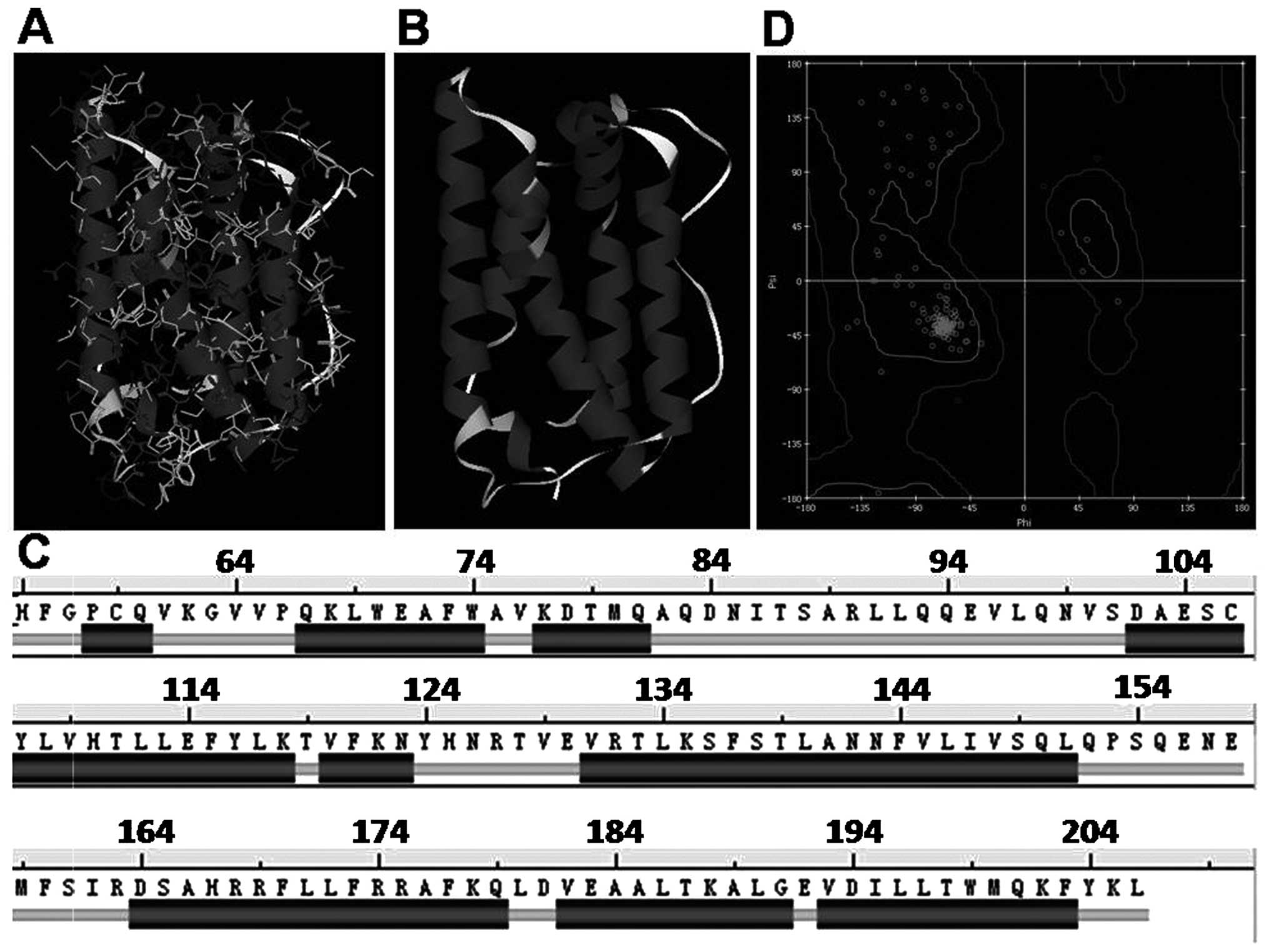
shown in Fig. 1A and B. The hIL-24
possesses structural properties that are found in the cytokine
superfamily, including four long anti-parallel helical structures
connected by a random coil (Fig.
1C, helix A, 67–82; helix B, 102–123; helix C, 131–151; helix
D, 164–203). The secondary structure of hIL-24 was analyzed based
on the K-S rules and the secondary structure of IL-24 is shown in
Fig. 1C. Making use of the spatial
modeling structure of hIL-24, we rationally assessed its structure
using R-diagram. As shown in Fig.
1D, the spatial conformation of the hIL-24 model is rational.
Therefore, the selected simulation optimization and the
conformation optimization force field and the methods we used were
all adequate.
Analysis of the physical and chemical
properties and apparent characteristics based on the spatial
structure of IL-24
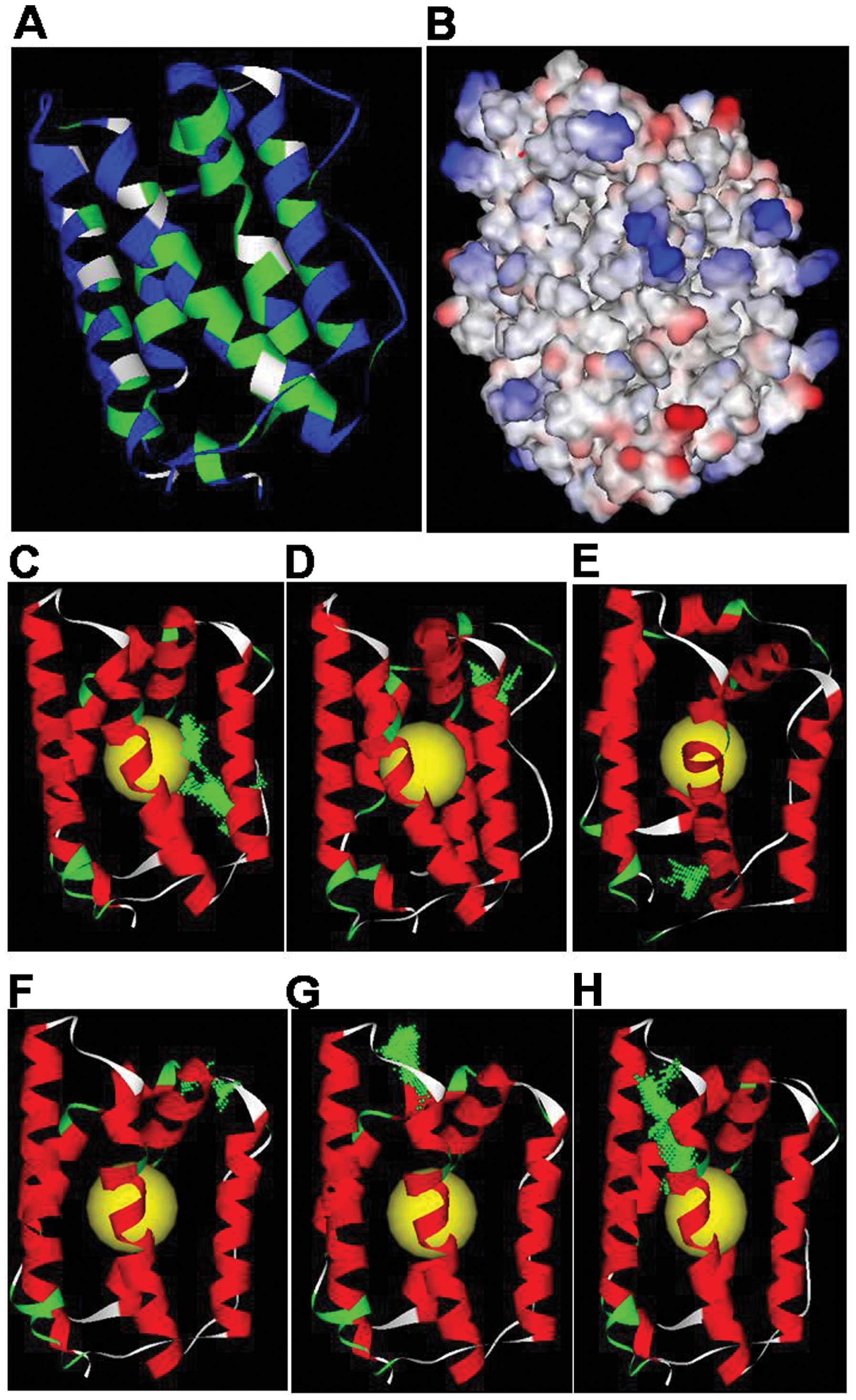
Space structure and the apparent characteristics of
a protein are related closely to its biological function. We
analyzed the solvent accessibility of IL-24 based on the spatial
structure of the protein and the solvent accessibility principle.
The solvent accessibility of IL-24 is shown in Fig. 2A; blue amino acid residues are fully
exposed to the solution, while green amino acid residues are buried
within the protein. Residues that are completely exposed to the
solvent, such as Loop AB (83–104) or Loop CD (154–164), may
constitute potential functional sites of IL-24.
We analyzed the surface electrostatic potential
distribution of IL-24 using the Delphi program. As shown in
Fig. 2B, the blue regions represent
positive electricity areas, the red regions represent negative
electricity areas, and the white regions are neutrally charged. The
N-terminus of Loop AB and the C-terminus of Loop CD have strong
negative electricity, while the C-terminus of Loop AB and the
N-terminus of Loop CD have strong positive electricity, suggesting
that these sites may be potential functional sites.
Prediction of potential active sites in
the IL-24 space structure
Based on the IL-24 space structure which was
reasonably simulated and combined with the predicted IL-24 physical
and chemical properties (solvent accessibility and apparent
electrostatic properties), we defined the globe core reasonably and
predicted the most likely active position using the prediction
method of the binding site. As shown in Fig. 2C–H, the yellow ball represents the
defining core position, while the green point represents a
potential active site. Within the IL-24 potential active sites,
sites G and H generally cannot become active since they include the
amino acid residues within the screw. The rest of the sites, such
as C, D, E and F, are residues in the Loop AB or Loop CD; in
addition, the N-terminus and C-terminus of the Loop AB and the
N-terminus of Loop CD comprise the active site with the greatest
potential. Thus, the peptide M1 we designed was 30 amino acid
residues from 76 to 105 and its sequence was
VKDTMQAQDNITSARLLQQEVLQNVSDAES. we also designed another peptide M2
of IL-24 (residues 146–174, sequence was
LIVSQLQPSQENEMFSIRDSAHRRFLLFR), which covered Loop CD, and had
nearly no function on proliferation of Eca-109 and A375 cells (data
not shown).
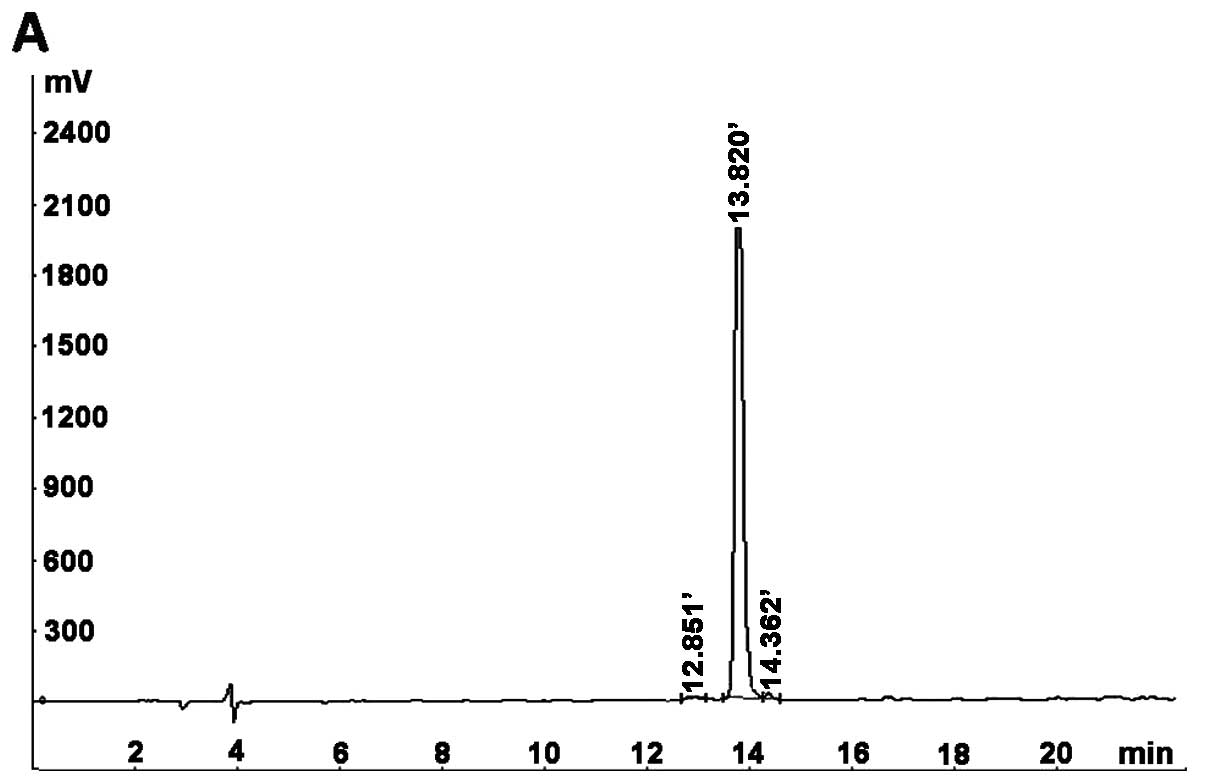
Chemical synthesis of IL-24
The M1 peptides used in the present study were
synthesized at the Beijing B&M Biotech Co. using solid-phase
chemistry and were purified to homogeneity (>95% purity) by
RP-HPLC; the molecular weight of the peptide was determined by MS.
As shown in Fig. 3, the purity of
the M1 peptide was 98.46%, while the molecular weight of M1 was
3333.4 Da, which is close to the theoretical value of 3332.68 Da.
The peptides were dissolved in DMEM supplemented with 10% FBS.
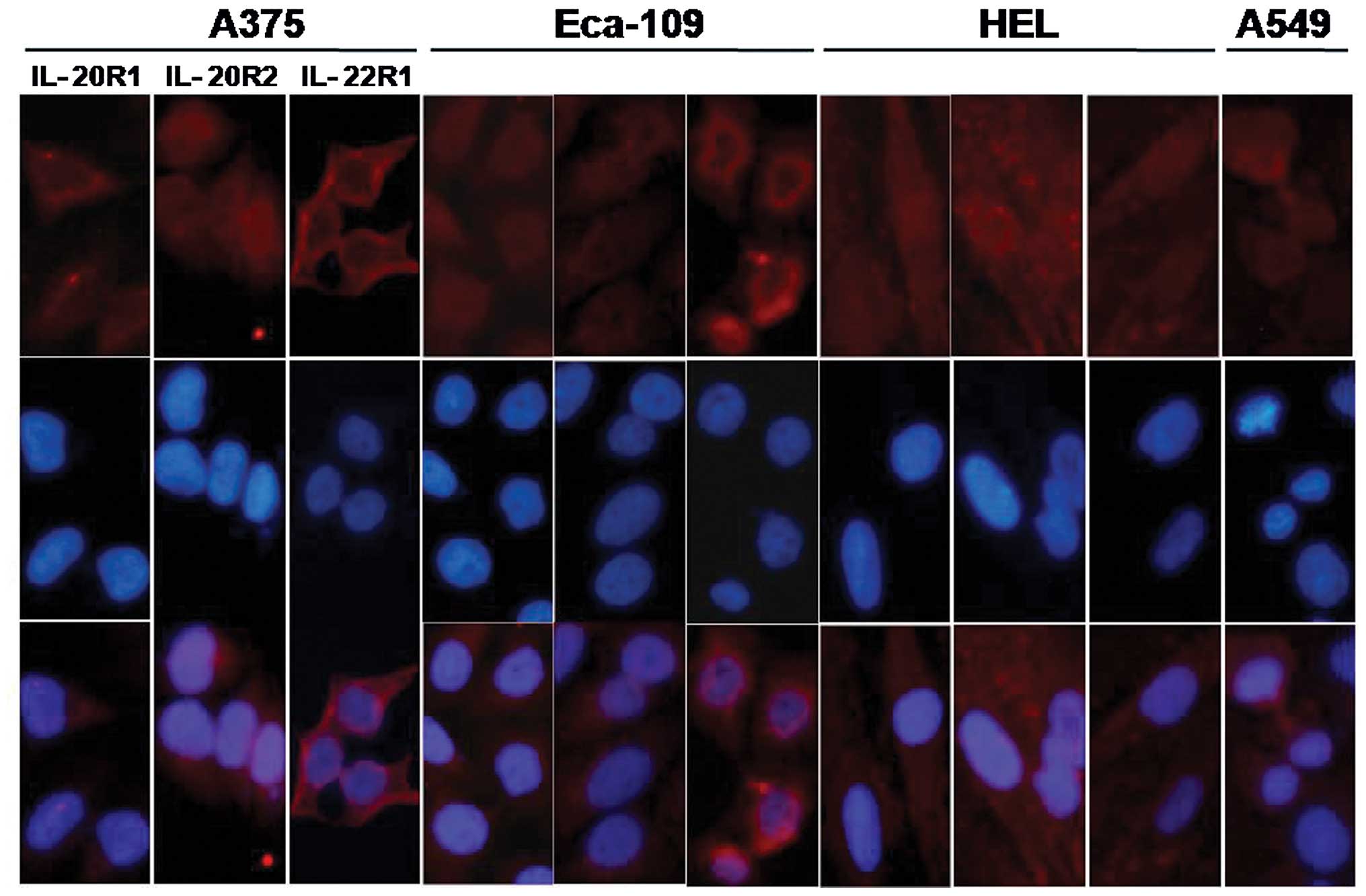
Expression of IL-20 receptor complexes in
the different cell lines
Classic cytokine signaling events require the
binding of a cytokine to its receptor(s). The expression patterns
of the IL-20 receptor complexes on the cell surface were identified
by immunofluorescence staining and fluorescence microscopy. As
showed in Fig. 4, the Eca-109, A375
and HEL cell lines expressed three types of subunits, IL-20R1,
IL-20R2 and IL-22R1. The A549 cell line was positively stained for
IL-20R2, and the two other receptor subunits were not stained.
Therefore, the A375 cells were used as a positive control for the
expression of IL-20 receptor complexes, which are the target
receptors of IL-24; A549 cells were used as the negative control
for receptor expression, and HEL as the control of the primary
normal cell line.
The M1 peptide selectively induces growth
inhibition in the cancer cell lines
The effects of the M1 peptide on the growth of cells
were analyzed 4 days post-treatment using the MTT assay. Different
concentrations of the peptide, ranging from 0 to 200 μg/ml, were
tested and PBS was used as a control. As shown in Fig. 5A, the M1 peptide induced a
significant decrease (p<0.05; p<0.01) in the cell viability
of the Eca-109 and A375 cells, compared with the PBS-treated cells.
In these two cell lines, a decrease in cell viability was evident
even after treatment with 10 or 0.5 μg/ml of the M1 peptide,
respectively and increasing concentrations of the peptide produced
a more pronounced effect. The highest inhibition rate (%) of the M1
peptide on the Eca-109 and A375 cell lines was 21% at 200 μg/ml and
27% at 2 μg/ml, respectively. However, treatment with the M1
peptide at the same concentrations, 0.5–100 μg/ml, did not produce
a significant decrease in the viability of the other two cell lines
(A549 and HEL); the M1 peptide had an effect on the HEL cell line
only when it was administered at a very high concentration (200
μg/ml). To confirm that the inhibitory effect was due to treatment
with the M1 peptide, the IL-24 antibodies (polyclonal antibody
AF1965 and monoclonal antibody MAB1965) and the IL-20R antibodies
(polyclonal antibodies IL-20R1, IL-20R2 and IL-22R1) were added
concurrently with the M1 peptide at a concentration of 100 μg/ml.
The inhibition of proliferation was significantly neutralized in
the Eca-109 and A375 cell lines, as shown in Fig. 5B and C. These MTT results indicated
that the M1 peptide can specifically inhibit the proliferation of
cancer cell lines that express the IL-20 receptor complexes.
Additionally, the M1 peptide had no biological effects on the
growth or viability of cancer cell lines, such as A549, which lack
expression of the IL-20 receptor complexes or primary normal cells
that express the IL-20 receptor complexes.
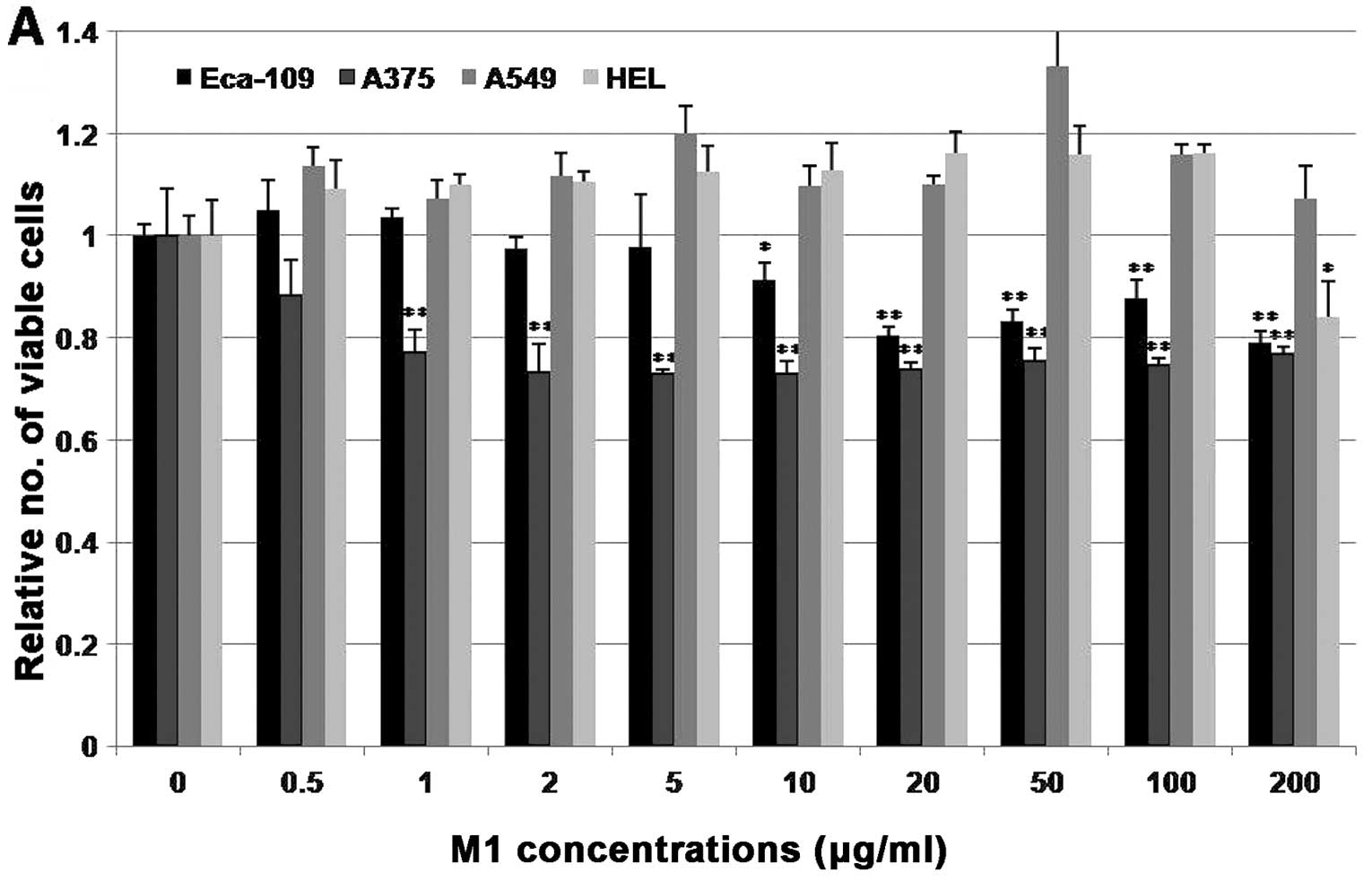 | Figure 5Comparative growth inhibition of the
M1 peptide on different cells. (A) The effect of the M1 peptide on
cell viability. Cells (Eca-109, A375, A549 and HEL) were treated
with the M1 peptide at different concentrations. A significant
decrease in cell viability was observed in the Eca-109 and A375
cells, but almost no effects were observed in the A549 and HEL
cells. (B and C) Inhibition of the proliferation of Eca-109 and
A375 cells was significantly neutralized by the addition of
anti-IL-24 antibodies (MAB, monoclonal antibody MAB1965; AF,
polyclonal antibody AF1965) or anti-IL-20 receptor complex
antibodies (20R1, anti-IL-20R1 antibody; 20R2, anti-IL-20R2
antibody; 22R1, anti-IL-22R1 antibody). Columns, the average of
three independent experiments; bars, ± SD. *p<0.05;
**p<0.01 |
Discussion
Since 1995, numerous studies have shown that IL-24
selectively kills a large variety of cancer cells, in vivo
and in vitro, and leaves healthy cells unharmed. In a
remarkably short time frame, IL-24 has changed from a laboratory
discovery into a clinical treatment. These remarkable findings have
led to the development of INGN 241, a replication-incompetent
IL-24-expressing adenovirus, which is currently being assessed in
clinical trials (Introgen Company homepage) (32,33).
However, IL-24 therapy in its current form is a type of gene
therapy, and based on simple mass action effects, it is not
possible to infect every tumor cell within a tumor using an
adenovirus even with intra-tumoral injection. This is one possible
reason why so many gene therapy approaches have failed in the
clinic (34).
To avoid the problems mentioned above, some
alternative strategies have been developed. To develop these
strategies, it is essential that the apoptosis-inducing properties
and the mechanisms by which different recombinant forms of IL-24
protein (bacterial GST-fusion protein, baculovirus-expressed IL-24,
secreted IL-24 from transfected mammalian cells) induce apoptosis
are determined. In the present study, we demonstrated that peptide
M1 created by computer-aided design can specifically inhibit the
growth of the ESCC cell line Eca-109 and the melanoma cell line
A375, but has almost no effects on human lung cancer A549 cells and
the human embryo lung fibroblast cell line HEL. This result was
identical to what we obtained with the full length IL-24
recombinant protein secreted from mammalian cells on these four
types of cells (unpublished data).
Concerning the structure of IL-24, some studies have
reported that IL-24 belongs to the 4-helix bundle family of
cytokine molecules, which are most closely related to the IL-10
subfamily. Tertiary structure predictions, which are based on
computer simulations, generated a compact globular structure
consisting of 4 helical regions interspersed by loops of
unpredicted structure (4,35). However, there is no detailed
information concerning these 4 helices. We created a
three-dimensional structure of human IL-24 with the help of
computer aided homologous modeling technology and the InsightII
2005 package with the Homology module. The IL-24 protein possesses
structural properties that are always found in the cell factor
superfamily, including four long antiparallel helical structures
connected by a random coil. The four helices A, B, C and D in IL-24
include the 67–82, 102–123, 131–151 and 164–203 amino acids,
respectively. On the other hand, some researchers have reported
that all monomeric members of the IL-10 family can adopt similar
patterns of tertiary folding, as predicted from the primary
sequence alignment and computer-based threading analyses. All IL-10
family members have a consistent pattern of predicted α-helical
structure that corresponds to the known six-helices (A to F), based
on the crystal structure of ebvIL-10, Epstein-Barr Virus-encoded
IL-10 homolog (36,37). In fact, the six-helices are the same
as the four-helices since helices C and D can each be divided into
two helixes.
Several experimental studies have shown that the
IL-24 protein can be secreted from the cell, and purified IL-24
protein can interact with two different heterodimeric receptor
complexes: type I (IL-20R1/IL-20R2) or type II (IL-22R1/ IL-20R2).
In receptor-expressing cells, IL-24 activates the JAK-STAT pathway,
as shown by phosphorylation of STAT3 (33,38).
The receptor chains IL-20R1 and IL-22R1 are widely expressed in
many tissues; nevertheless, the expression of a functional IL-24
receptor depends on the presence of IL-20R2, the common chain for
both receptor pairs, which is absolutely required for receptor
activation (33). However, most of
the IL-24 receptor expression studies relied on RT-PCR, which is
neither quantitative nor does it establish the presence of the
corresponding proteins and functional receptors. Therefore, we
assessed the expression of the receptors using immunofluorescence
staining. The Eca-109 and A375 cells expressed the complete set of
IL-20 receptor complexes (IL-20R1, IL-20R2 and IL-22R1) and were
efficiently treated by the M1 peptide. On the other hand, A549
cells lack a full complement of IL-20 receptor complexes, similar
to the results of Su et al (10), which make the A549 cells relatively
resistant to M1 peptide treatment. Our analysis revealed a variable
level of IL-20 receptor complex expression in different cancer cell
lines, indicating that an effective strategy needs to be utilized
to facilitate the efficient infection of all types of cancer cells
with the M1 peptide. Most studies show that A375 cells are positive
for the expression of the IL-20 receptor complexes (39), but a few do not. Kreis et al
(33) reported that A375 cells only
express IL-22R1 according to RT-PCR and express no receptors
according to western blot analysis; therefore, these cells did not
react to the IL-24 protein as they do not express sufficient
amounts of the specific receptor pairs. Many studies have reported
that A549 cells do not express the IL-20 receptor complexes, and
extracellular treatment with mammalian cell synthesized IL-24
resulted in no biological effect on cell growth or viability
(6). However, when A549 cells were
co-cultured with HEK293 cells producing sMDA-7/IL-24, the in
vivo tumor growth of nude mice was inhibited. Similarly,
systemic administration of sMDA-7/IL-24 also inhibited lung tumor
growth in a mouse xenograft model. In this context, one would
anticipate that IL-24 might exert its antitumor properties in
vivo by evoking multiple pathways, including direct induction
of cancer cell apoptosis, inhibition of angiogenesis and modulation
of immune responses (4).
The morbidity of ESCC differs between countries or
areas. China has the highest morbidity and the highest mortality of
this disease. Approximately 310,400 new cases are diagnosed around
the world each year, and ~167,200 of these cases are in China.
According to the American Cancer Society (www.cancer.gov), in 2013, nearly 18,000 people in the
United States were estimated to be diagnosed with ESCC, and 15,210
deaths were expected to occur from this disease. Pataer et
al (23) reported that the
adenoviral ER-targeted IL-24 vector (Ad-ER-IL-24) selectively and
effectively inhibited the growth and proliferation of ESCC cells
(Seg1 and Bic1) by enhancing cell death. Both Ad-IL-24 and
Ad-ER-IL-24 activated a novel pathway of ER stress-induced
apoptosis characterized by the unregulated expression of
phosphorylated JNK (p-JNK), phosphorylated cJun (p-cJun), and
phosphorylated RNA-dependent protein kinase (p-PKR). At the present
time, it is not clear whether the IL-24 protein or peptide has
biological functions in ESCC cells. Our previous results showed
that the purified IL-24 secreted from transfected mammalian cells
specifically inhibited proliferation in vivo and in
vitro and could induce apoptosis in the ESCC cell line Eca-109
(unpublished data). The M1 peptide also inhibited the growth of
Eca-109 cells. Our results indicate that Eca-109 cells are
sensitive to IL-24 protein and peptide, and the use of the IL-24
peptide may be a new strategy for the treatment of ESCC.
Further studies are required to determine the
effects of the M1 peptide on in vivo Eca-109 tumor models;
if successful, these models could serve as an entry point for the
translation of the treatment into the clinic setting by defining
the safety and efficacy of the treatment in patients with ESCC. The
antibodies against IL-24 (AF1965 and MAB1965) and IL-20 receptor
subunits (IL-20R1, IL-20R2 and IL-22R1) could neutralize the
inhibitive function of M1 on tumor cells, indicating that M1 may
bind to the receptor and activate signaling in a receptor-dependent
manner and that other cancer cells expressing IL-20 receptor
complexes may be its target cells. It is not clear whether the M1
peptide would activate the receptor in the same way as full length
IL-24 protein and whether some conventional therapeutic modalities
or agents may strengthen the activity of M1 against cancer cells,
as was shown for the full-length IL-24 protein and GST-M4. The
range of cancer cells that might be affected by the M1 peptide
remains to be identified. Although the M1 peptide can kill cancer
cells, the half-life of the peptide needs to be improved by
polyethyleneglycol (PEG) modification. Novel systemic delivery
approaches, such as microbubbles or ultrasound delivery, are needed
to enhance the administration of the M1 peptide, specifically to
the tumor site or its microenvironment. It is also important to
identify how the M1 peptide triggers the proliferation pathways in
tumor cells but not in normal cells. Based on the early success of
IL-24 as a potential anticancer therapy in patients (26), we are optimistic that, with the
appropriate modifications, this cytokine may become a frontline
therapeutic agent for the treatment of multiple human cancers.
Acknowledgements
The present study was supported by the Beijing
Natural Science Foundation (grant no. 7142117) to Q.M., the
National Basic Research Program of China (973 Program, grant no.
2009CB521704) to Z.C., the Person Project of Beijing Jiaotong
University (grant no. KSRC12003532) and the Beijing Cancer
Rehabilitation Association Foundation (grant no. KSM13001531) to
Z.W., the National Natural Science Foundation of China (grant no.
81071855) and the Person Project of Beijing Jiaotong University
(grant no. KSRC11004536) to H.J.
References
|
1
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janet P, Whitney S, Fang X, et al:
Rational design of shepherdin, a novel anticancer agent. Cancer
Cell. 7:457–468. 2005. View Article : Google Scholar
|
|
3
|
Jiang H, Lin JJ, Su ZZ, et al: Subtraction
hybridization identifies a novel melanoma differentiation
associated gene, mda-7, modulated during human melanoma
differentiation, growth and progression. Oncogene. 11:2477–2286.
1995.PubMed/NCBI
|
|
4
|
Gupta P, Su ZZ, Lebedeva IV, et al:
mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing
cytokine. Pharmacol Ther. 111:596–628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mhashilkar AM, Schrock RD, Hindi M, et al:
Melanoma differentiation associated gene-7 (mda-7): a novel
anti-tumor gene for cancer gene therapy. Mol Med. 7:271–282.
2001.PubMed/NCBI
|
|
6
|
Dent P, Yacoub A, Hamed HA, et al:
MDA-7/IL-24 as a cancer therapeutic: from bench to bedside.
Anticancer Drugs. 21:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caudell EG, Mumm JB, Poindexter N, et al:
The protein product of the tumor suppressor gene, melanoma
differentiation-associated gene 7, exhibits immunostimulatory
activity and is designated IL-24. J Immunol. 168:6041–6046. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mumm JB, Ekmekcioglu S, Poindexter NJ, et
al: Soluble human MDA-7/IL-24: characterization of the molecular
form(s) inhibiting tumor growth and stimulating monocytes. J
Interferon Cytokine Res. 26:877–886. 2006. View Article : Google Scholar
|
|
9
|
Su ZZ, Lebedeva IV, Sarkar D, et al:
Melanoma differentiation associated gene-7, mda-7/IL-24,
selectively induces growth suppression, apoptosis and
radiosensitization in malignant gliomas in a p53-independent
manner. Oncogene. 22:1164–1180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su Z, Emdad L, Sauane M, et al: Unique
aspects of mda-7/IL-24 antitumor bystander activity: establishing a
role for secretion of MDA-7/IL-24 protein by normal cells.
Oncogene. 24:7552–7566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yacoub A, Liu R, Park MA, et al: Cisplatin
enhances protein kinase R-like endoplasmic reticulum kinase- and
CD95-dependent melanoma differentiation-associated
gene-7/interleukin-24-induced killing in ovarian carcinoma cells.
Mol Pharmacol. 77:298–310. 2010. View Article : Google Scholar :
|
|
12
|
Lebedeva IV, Washington I, Sarkar D, et
al: Strategy for reversing resistance to a single anticancer agent
in human prostate and pancreatic carcinomas. Proc Natl Acad Sci
USA. 104:3484–3489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bocangel D, Zheng M, Mhashilkar A, et al:
Combinatorial synergy induced by adenoviral-mediated mda-7 and
Herceptin in Her-2+ breast cancer cells. Cancer Gene
Ther. 13:958–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su Z, Lebedeva IV, Gopalkrishnan RV, et
al: A combinatorial approach for selectively inducing programmed
cell death in human pancreatic cancer cells. Proc Natl Acad Sci
USA. 98:10332–10337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lebedeva IV, Su ZZ, Sarkar D, et al:
Melanoma differentiation associated gene-7, mda-7/interleukin-24,
induces apoptosis in prostate cancer cells by promoting
mitochondrial dysfunction and inducing reactive oxygen species.
Cancer Res. 63:8138–8144. 2003.PubMed/NCBI
|
|
16
|
Zerbini LF, Czibere A, Wang Y, et al: A
novel pathway involving melanoma differentiation associated
gene-7/interleukin-24 mediates nonsteroidal anti-inflammatory
drug-induced apoptosis and growth arrest of cancer cells. Cancer
Res. 66:11922–11931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chada S, Mhashilkar AM, Liu Y, et al:
mda-7 gene transfer sensitizes breast carcinoma cells to
chemotherapy, biologic therapies and radiotherapy: correlation with
expression of bcl-2 family members. Cancer Gene Ther. 13:490–502.
2006. View Article : Google Scholar
|
|
18
|
Zerbini LF, Tamura RE, Correa RG, et al:
Combinatorial effect of non-steroidal anti-inflammatory drugs and
NF-κB inhibitors in ovarian cancer therapy. PLoS One. 6:e242852011.
View Article : Google Scholar
|
|
19
|
Fisher PB: Is mda-7/IL-24 a ‘magic bullet’
for cancer? Cancer Res. 65:10128–10138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong CY, Zhang F, Duan YJ, et al:
mda-7/IL-24 inhibits the proliferation of hematopoietic
malignancies in vitro and in vivo. Exp Hematol. 36:938–946. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saeki T, Mhashilkar A, Swanson X, et al:
Inhibition of human lung cancer growth following
adenovirus-mediated mda-7 gene expression in vivo. Oncogene.
21:4558–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tamai H, Miyake K, Yamaguchi H, et al:
AAV8 vector expressing IL24 efficiently suppresses tumor growth
mediated by specific mechanisms in MLL/AF4-positive ALL model mice.
Blood. 11:64–71. 2012. View Article : Google Scholar
|
|
23
|
Pataer A, Hu W, Xiaolin L, et al:
Adenoviral endoplasmic reticulum-targeted mda-7/interleukin-24
vector enhances human cancer cell killing. Mol Cancer Ther.
7:2528–2535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sauane M, Su ZZ, Gupta P, et al: Autocrine
regulation of mda-7/ IL-24 mediates cancer-specific apoptosis. Proc
Natl Acad Sci USA. 105:9763–9768. 2008. View Article : Google Scholar
|
|
25
|
Margue C and Kreis S: IL-24: physiological
and supraphysiological effects on normal and malignant cells. Curr
Med Chem. 17:3318–3326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dash R, Bhutia SK, Azab B, et al:
mda-7/IL-24: a unique member of the IL-10 gene family promoting
cancer-targeted toxicity. Cytokine Growth Factor Rev. 21:381–391.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng M, Bocangel D, Ramesh R, et al:
Interleukin-24 overcomes temozolomide resistance and enhances cell
death by down-regulation of O6-methylguanine-DNA
methyltransferase in human melanoma cells. Mol Cancer Ther.
7:3842–3851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta P, Walter MR, Su ZZ, et al:
BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of
cancer-specific apoptosis. Cancer Res. 66:8182–8191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta P, Emdad L, Lebedeva IV, et al:
Targeted combinatorial therapy of non-small cell lung carcinoma
using a GST-fusion protein of full-length or truncated MDA-7/IL-24
with Tarceva. J Cell Physiol. 215:827–836. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rosal R, Brandt-Rauf P, Pincus MR, et al:
The role of alpha-helical structure in p53 peptides as a
determinant for their mechanism of cell death: necrosis versus
apoptosis. Adv Drug Deliv Rev. 57:653–660. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Böttger A, Böttger V, Sparks A, et al:
Design of a synthetic Mdm2-binding mini protein that activates the
p53 response in vivo. Curr Biol. 7:860–869. 1997. View Article : Google Scholar
|
|
32
|
Tong AW, Nemunaitis J, Su D, et al:
Intratumoral injection of INGN 241, a nonreplicating adenovector
expressing the melanoma-differentiation associated gene-7
(mda-7/IL24): biologic outcome in advanced cancer patients. Mol
Ther. 11:160–172. 2005. View Article : Google Scholar
|
|
33
|
Cunningham CC, Chada S, Merritt JA, et al:
Clinical and local biological effects of an intratumoral injection
of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a
phase I study. Mol Ther. 11:149–159. 2005. View Article : Google Scholar
|
|
34
|
Dent P, Yacoub A, Hamed HA, et al: The
development of MDA-7/ IL-24 as a cancer therapeutic. Pharmacol
Ther. 128:375–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sauane M, Gopalkrishnan RV, Sarkar D, et
al: MDA-7/IL-24: novel cancer growth suppressing and apoptosis
inducing cytokine. Cytokine Growth Factor Rev. 14:35–51. 2003.
View Article : Google Scholar
|
|
36
|
Chada S, Sutton RB, Ekmekcioglu S, et al:
MDA-7/IL-24 is a unique cytokine - tumor suppressor in the IL-10
family. Int Immunopharmacol. 4:649–667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kotenko SV: The family of IL-10-related
cytokines and their receptors: related, but to what extent?
Cytokine Growth Factor Rev. 13:223–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lebedeva IV, Emdad L, Su ZZ, et al:
mda-7/IL-24, novel anticancer cytokine: Focus on bystander
antitumor, radiosensitization and antiangiogenic properties and
overview of the phase I clinical experience (Review). Int J Oncol.
31:985–1007. 2007.PubMed/NCBI
|
|
39
|
Chada S, Mhashilkar AM, Ramesh R, et al:
Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma
cells via an IL-20 receptor-dependent but STAT3-independent
mechanism. Mol Ther. 10:1085–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|