Introduction
Danshen (Salvia miltiorrhiza Bunge) has been
used extensively and historically in China to treat various
diseases, including cardiovascular diseases, cerebrovascular
diseases and cancer (1). Tanshinone
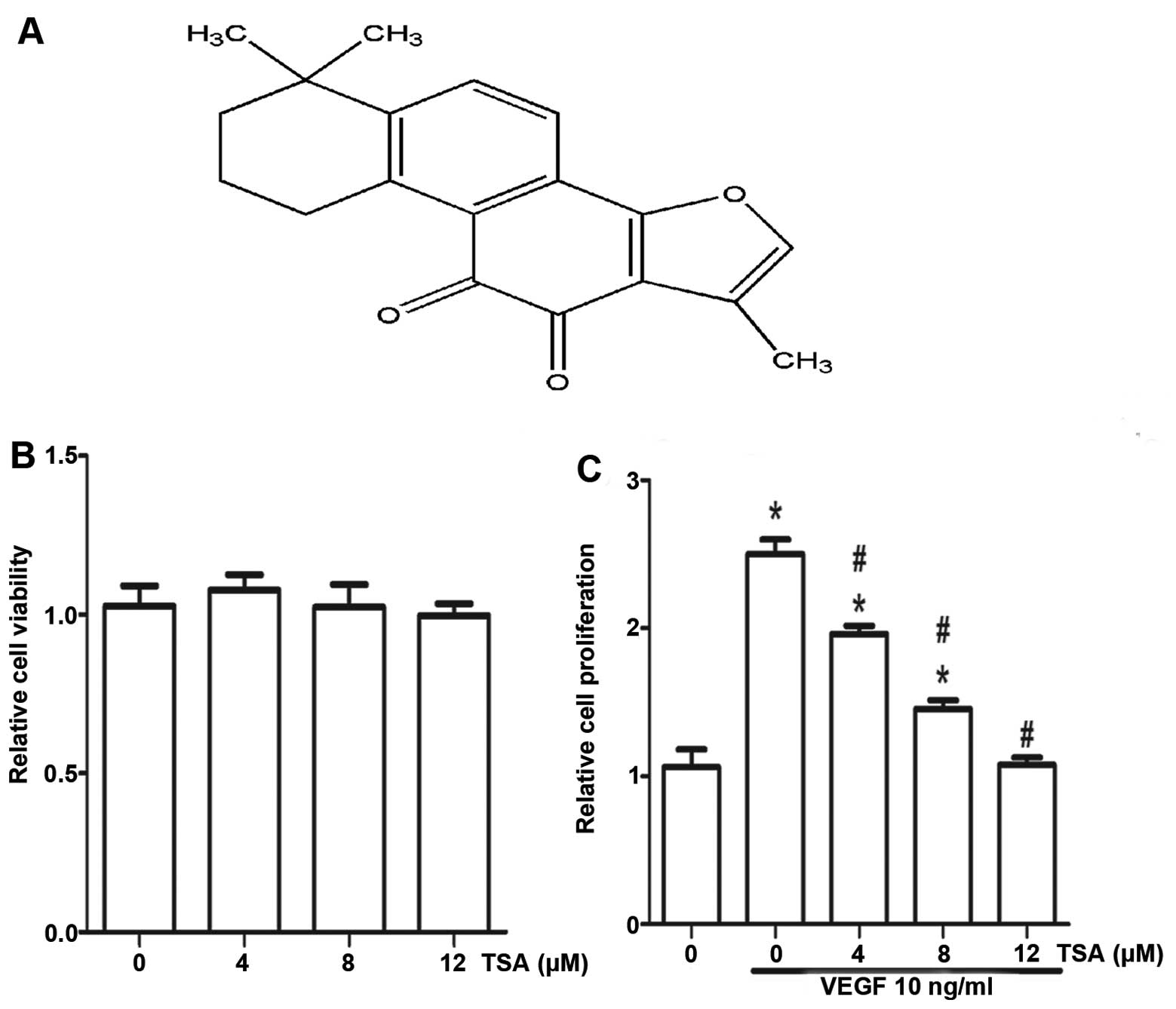
IIA (TSA) (Fig. 1A) is a major
monomer of phenanthrenequinones extracted from the root of
Salvia miltiorrhiza, which has many activities. The most
intensively investigated are anti-oxidant properties and
anti-inflammatory activities (2,3). Many
studies indicate that TSA is linked to the prevention and therapy
of various types of cancer cells such as colon cancer cells
(4,5) and human lung cancer cells (6). However, few studies have reported the
anti-angiogenic activity and hence the underlying molecular
mechanism of TSA.
Angiogenesis is the formation of new blood vessels,
which generally occurs during various physiological and
pathological processes (7), such as
embryonic development, tumor progression and metastasis. It is now
widely accepted that the growth and development of malignant tumors
require angiogenesis (8). Since the
inhibition of angiogenesis could also result in the suppression of
tumor growth, we investigated the potential mechanism concerning
the anti-angiogenic effect of TSA on human umbilical vascular
endothelial cells (HUVECs).
Numerous molecular mechanisms are responsible for
mediating the changes in the microenvironment during angiogenic
cascades. Vascular endothelial growth factor (VEGF) is one of the
most significant and specific angiogenesis factors (9), and is a potent angiogenic catalyst
secreted by many types of tumor cells. It plays an important part
in tumor progression by promoting neovascularization (10). In addition, the CD146 molecule is a
biomarker on the vascular endothelium (11). Evidence shows that CD146 is a
co-receptor for kinase insert domain receptor (KDR; a type III
receptor tyrosine kinase) also known as vascular endothelial growth
factor receptor 2 (VEGFR2) and plays a key role in the VEGF/VEGFR2
pathway in regards to angiogenesis and tumor growth (12,13).
What is more, matrix metalloproteinases (MMPs) are key players in
the remodeling and degradation of the extracellular matrix (ECM)
and components of the basement membrane (14). MMPs can promote tumor angiogenesis
through their proteolytic action on growth factors, cell adhesion
molecules and other bioactive enzymes (15). A previous study showed that the
anti-angiogenic effect of TSA involves inhibition of modification
of MMP-2/ tissue inhibitor of metalloproteinase-2 (TIMP-2)
secretion in vascular endothelial cells (16). Yet, a particular MMP has the
capacity to cleave a given matrix component at certain sites to
create fragments that can be recognized by specific receptors. It
is therefore not unexpected that a certain MMP may act in either a
pro-angiogenic or anti-angiogenic capacity in different
environments (17). Notably, we
noted that there are many studies related to downstream targets of
VEGFR2, such as the PI3K/Akt pathway (18) and p38 (19). They all can be regulated by TSA.
These observations suggest that TSA is likely a natural inhibitor
of KDR and CD146. In the present study, we aimed to demonstrate
that TSA has potent anti-angiogenic activity in vitro and
ex vivo models. Our results showed that TSA inhibits
endothelial cell proliferation, migration and tube formation by
targeting CD146 and the VEGF/VEGFR2 pathway in vitro.
Moreover, the expression of MMP-2/-9 was examined.
Materials and methods
Cell culture and reagents
Primary HUVECs were isolated from the umbilical vein
vascular wall based on a previous method (20). HUVECs were routinely passage in ECM
(Life Technologies, Carlsbad, CA, USA) with 5% CO2 at
37°C. Only those cells from passage 3 to 8 were used for
experiments.
Cell viability and proliferation
assay
HUVECs (5×103/well) were plated onto a
96-well culture plate and incubated for 24 h. Different dilutions
of TSA were added and further incubated for 48 h. Viable cells were
determined by MTT assay using the MTT Cell Proliferation Assay kit
(Promega, Madison, WI, USA) according to the manufacturer’s
instructions. The number of viable cells was presented relative to
the untreated control. Meanwhile, HUVECs were seeded (5,000
cells/well) in a 96-well culture plate and incubated for 24 h. The
medium was replaced with ECM, containing 1% fetal bovine serum
(FBS), 10 ng/ml VEGF and various concentrations of TSA (4, 8 and 12
μM). After 48 h, relative cell proliferation was determined by the
MTT assay. All assays were performed in triplicate wells.
Transwell assay
Endothelial cell migration was assessed using a
modified Boyden chamber assay. HUVECs (1×105) were
plated in Endothelial cell medium containing 1% FBS in the upper
chamber of the Transwell (8-μm PET; Millipore, Germany). Cells were
then treated for 30 min at 37°C. Endothelial cell medium containing
1% FBS, 10 ng/ml VEGF and various concentrations of TSA (4, 8 and
12 μM) were added to the lower chamber. Endothelial cell medium
containing 1% FBS was a negative control. After 8 h, the
non-migrated cells were removed by a cotton swap, and the migrated
cells were stained with crystal violet and examined under a
microscope. The number of migrated cells was quantified by counting
the cells with ×40 objective. Migration was normalized to the
percentage of migration.
Tube formation assay
The ECM gel-induced capillary tube formation assay
was used as an in vitro measurement of angiogenesis.
Briefly, a 24-well culture plate was coated with 50 μM/well ECM gel
(Sigma, Japan) and allowed to stand for 30 min at 37°C to form a
gel layer. After gel formation, 1×105 HUVECs in 0.5 ml
of growth medium were seeded to each well along with 10 ng/ml VEGF.
Various dilutions of TSA were added into the wells and incubated
for 6 h at 37°C in a humidified atmosphere with 5% CO2,
and the formation of capillary tubes was photographed using an
inverted microscope.
Chorioallantoic membrane (CAM) assay
The effect of TSA on ex vivo angiogenesis was
determined by the CAM assay. Briefly, fertilized chick eggs which
were incubated for 6 days were used for the experiment and were
incubated at 70% humidity and 37°C. A window was opened on the top
of each egg after 1 day of incubation. The windows were covered
with sterile tape and the eggs were returned to the incubator.
After an additional 1 day, the transparent tape was cut open and a
drug carrier plate with a 15-mm diameter was placed in the chamber.
Twenty microliters of each test reagent was added onto the plate.
Test substances including TSA (the dose of TSA was 4, 8 and 12 μM,
respectively). The eggs were incubated for 48 h and photographed.
Blood vessel density was quantified by counting the number of
branching blood vessels. Each experiment was performed three times
and represented as a bar diagram.
Rat aortic ring assay
The rat aortic ring assay was used as the ex
vitro angiogenesis study model (21). Dorsal aortas from freshly sacrificed
Sprague-Dawley rats were extracted in a sterile manner and rinsed
in ice cold phosphate-buffered saline (PBS). They were then cut
into 1-mm long rings using a surgical blade. Each ring was placed
in a collagen pre-coated 96-well plate. Different dilutions of TSA
were added to the wells. On day 7, the rings were analyzed by
phase-contrast microscopy, and microvessel outgrowths were
quantified (22) and photographed.
All experimental protocols were approved by the Ethics Committee
for Animal Experimentation of China Pharmaceutical University.
ELISA
A human MMP-2 and MMP-9 ELISA kit (R&D Systems,
Shanghai, China) was used to determine the levels of MMP-2 and
MMP-9 in conditioned media collected from HUVECs with or without a
24-h treatment with TSA. The experimental steps were followed as
described in the protocol provided by the manufacturer.
Transfection
The VEGF165 expression plasmid pcVEGF165 was
constructed in our laboratory. Cells were transfected with the
pcVEGF165 using Lipofectamine 2000 (Life Technology) according to
the manufacturer’s protocol. After 6 h, the medium was replaced
with ECM containing 1% FBS, 100 ng/ml endothelial cell growth
supplement (ECGS) and various concentrations of TSA (0, 4, 8 and 12
μM). After 24 and 48 h, total cellular RNA and protein were
respectively extracted for the next detection.
qRT-PCR
Total cellular RNA was extracted using TRIzol
reagent (Gibco-BRL, Gaithersburg, MD, USA), according to the
manufacturer’s protocol. Total RNA was subjected to cDNA synthesis
using M-MLV reverse transcriptase. The process was performed at
30°C for 10 min, 42°C for 1 h followed by denaturation at 95°C for
2 min. To determine mRNA expression levels, qRT-PCR was performed
using the ABI Prism 7500 Sequence Detector (Applied Biosystems,
Life Technologies). cDNA templates (2 μl) were amplified in a final
volume of 20 μl containing the SYBR-Green PCR Master Mix and
primer. Melt-curve analysis and agarose gel electrophoresis were
used to confirm amplicon specificity. The length of the amplified
product was confirmed using 2% agarose gel electrophoresis.
Relative quantification was performed using the 2−ΔΔCt
method. GAPDH served as an appropriate reference gene in this
experiment. qRT-PCR primers for KDR generated a 371-bp product and
were forward, 5′-CTGGCATGGTCTTCTGTGAAGCA-3′ and reverse,
5′-CCGCATCACATCCACTGGTATT-3′; primers for CD146 were forward,
5′-TGGTCATCGTGGCTGTGATTGTG-3′ and reverse,
5′-CCTTTGGAGGCTTTGGCTGAGAGAA-3′. An alternative set of MMP-2
primers generated a 754-bp product and were forward,
5′-TGACGGTAAGGACGGACTC-3′ and reverse, 5′-AGTCCGCCAAATGAACCG-3′;
primers for MMP-9 were forward, 5′-TGGGGGGCAACTCGGC-3′ and reverse,
5′-GGAATGATCTAAGCCCAG-3′; PCR primers for GAPDH were as described
and generated a 450-bp product forward, 5′-AAGGTCGGAGTCACCGGATT-3′
and reverse, 5′-CTGGAAGATGGTGATGGGATT-3′.
Western blotting
Cells were washed twice with 1X PBS and then lysed
in lysis buffer (20 mM Tris, 150 mM NaCl, 0.25% NP-40, 1 mM
phenylmethylsulfonyl fluoride and 1X protease inhibitors). Fifty
micrograms of protein was analyzed on 8% SDS-PAGE under denaturing
conditions and electro-transferred to PVDF membranes (Millipore,
Bedford, MA, USA). Non-specific protein binding was blocked by
incubating the membranes with blocking solution (TBST and 10%
non-fat dried milk) for 60 min at room temperature. Polyclonal
antibodies specific for KDR (1:250 in TBST containing 5% BSA) and
CD146 (1:500 in TBST containing 5% BSA) were applied to the
membrane and incubated overnight at 4°C. Membranes were washed in
TBST and then incubated in 1:5,000 diluted goat anti-rabbit IgG-HRP
secondary antibody (Bio-Rad, Hercules, CA, USA) for 1 h at room
temperature. The detection of specific signals was performed using
the ECL detection system (Amersham Pharmacia Biotech, Piscataway,
NJ, USA). Protein concentrations were measured using the method of
Bradford (Bio-Rad).
Statistical analysis
All data were obtained from at least three
independent experiments and expressed as mean ± SD unless stated
otherwise. Statistical comparisons were made relative to the
negative controls, and the significance was assessed using the
Student’s t-test and was indicated as P<0.05 or P<0.01 using
relevant symbols in the figures and legends. A P-value of <0.05
was considered to be statistically significant.
Results
Effect of tanshinone IIA on the viability
and proliferation of HUVECs
Cell viabilty was determined by the MTT assay.
Effect of TSA on HUVEC viability in culture is shown in Fig. 1B. TSA was found to be non-toxic to
HUVECs at concentrations of 4–12 μM and these concentrations were
used for further in vitro study. The proliferation of
endothelial cells is important for the formation of new blood
vessels. To investigate the mechanism of the anti-angiogenic
function of TSA, we performed an MTT assay to explore the effect of
TSA on proliferation of endothelial cells. As shown in Fig. 1C, HUVECs showed a very high rate of
proliferation when stimulated with VEGF. The endothelial cells were
then exposed to TSA of different doses. At 48 h, treatment with TSA
(12 μM) significantly inhibited the proliferation of HUVECs by ~60%
compared to the untreated cells. This suggests that reduced
proliferation contributes to the anti-angiogenic effect of TSA.
Effect of tanshinone IIA on cell
migration of HUVECs in vitro
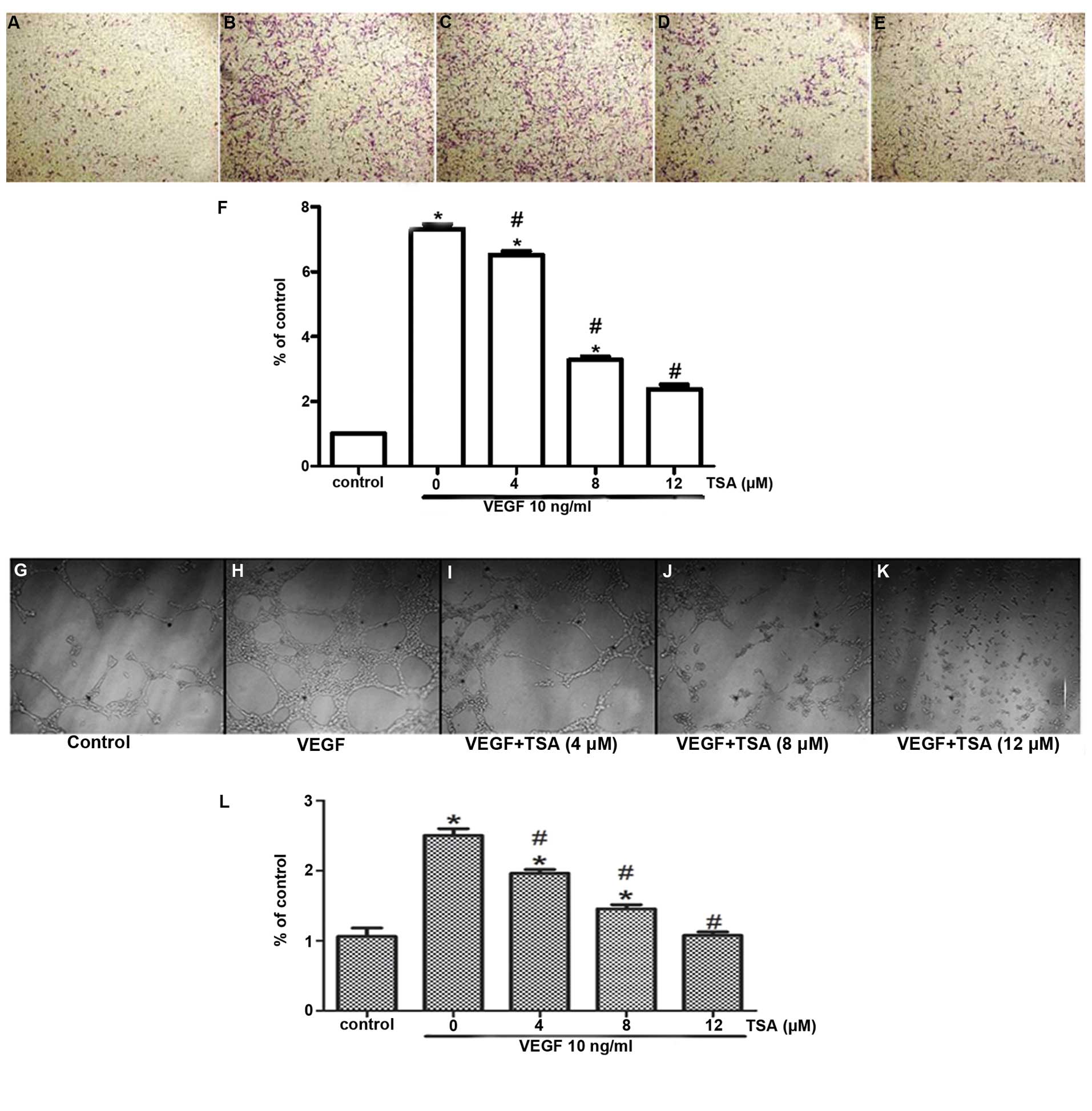
To further understand the anti-angiogenic function
of TSA, we examined whether TSA could exert any effect on the
migration of HUVECs by Transwell migration assay. The
dose-dependent inhibitory effect of TSA on cell migration was
demonstrated (Fig. 2A–F). The
migration rates of the cells following treatment with 4, 8 and 12
μM TSA were inhibited by 28, 49 and 59%, respectively demonstrating
that TSA also dose-dependently inhibits the migration of
HUVECs.
Tanshinone IIA inhibits the tube
formation of HUVECs in vitro
In order to study the anti-angiogenic function of
tanshinone IIA in vitro, we carried out an angiogenesis test
by examining the VEGF-induced tube formation of HUVECs. Treatments
with TSA (4, 8 and 12 μM) inhibited the VEGF-induced tube formation
of HUVECs in a dose-dependent manner, which indicates that TSA
inhibits the angiogenesis of HUVECs in vitro (Fig. 2G–L).
Tanshinone IIA inhibits the angiogenesis
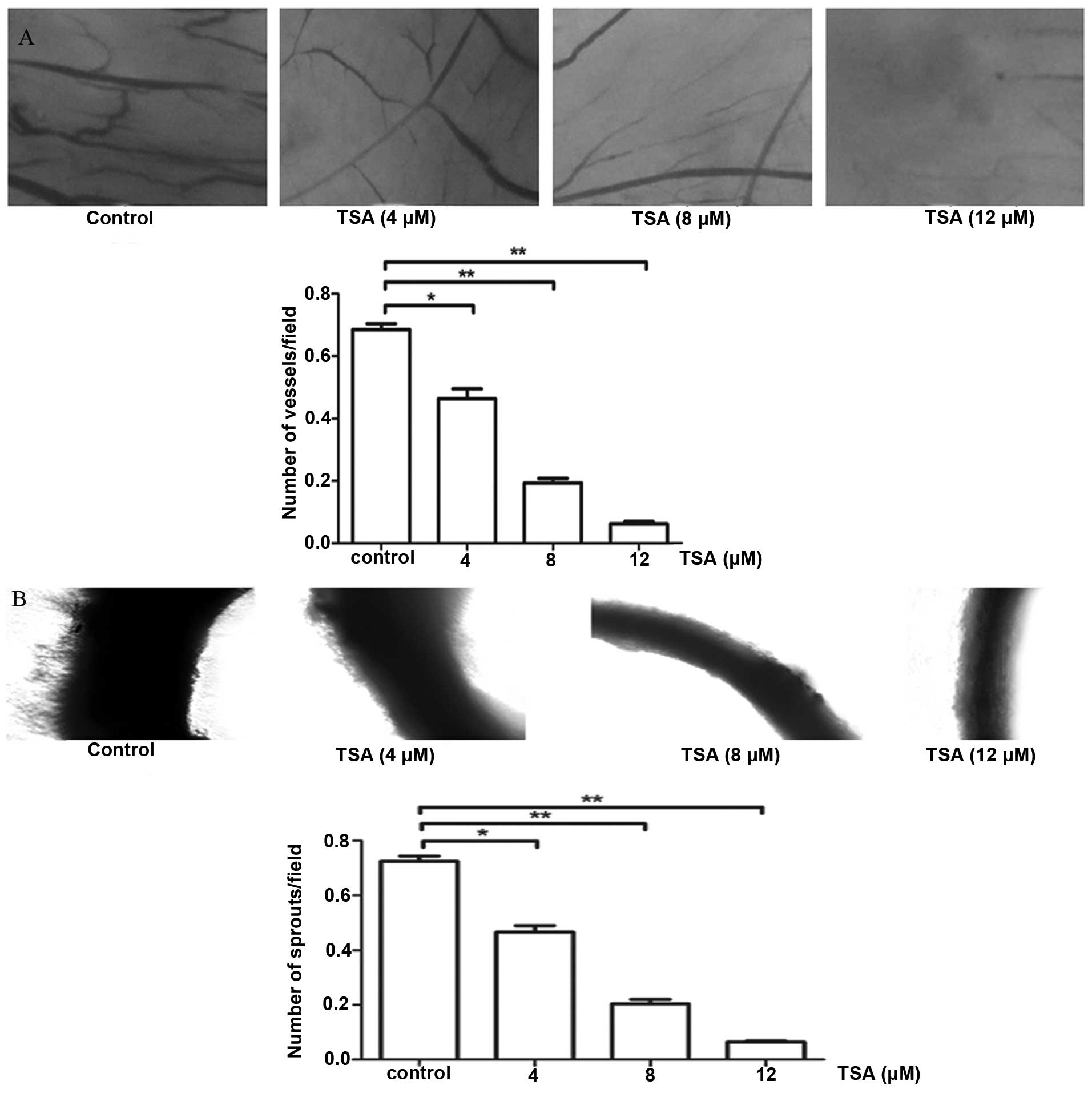
in a chick embryo CAM assay ex vivo
CAM is another ex vivo test for angiogenesis
and it is more convenient to quantify vascular development with
this method. We placed TSA-impregnated filter disks on blood
vessels in avascular sections of CAM (day 10) for 48 h. The disks
and underlying CAM tissues (day 12) were then harvested. We scored
angiogenesis by counting the vessel branches present in the CAM
tissue below the filter by digital images. Consistent with the data
above, upregulation of TSA led to a significant reduction in
angiogenesis, while the control CAM exhibited more
neovascularization (Fig. 3A), which
confirmed the anti-angiogenic potential of TSA through the ex
vivo assay.
Tanshinone IIA suppresses the
angiogenesis in a rat aortic ring assay ex vitro
We futher explored the anti-angiogenic activity of
TSA using ex vitro angiogenesis models. An isolated rat
aortic ring was embedded in Matrigel in ECM containing different
concentrations of TSA cultured for 7 days. TSA markedly suppressed
the outgrowth of cells from the aortic arch in a dose-dependent
manner, indicating that TSA inhibits angiogenesis in vitro
(Fig. 3B).
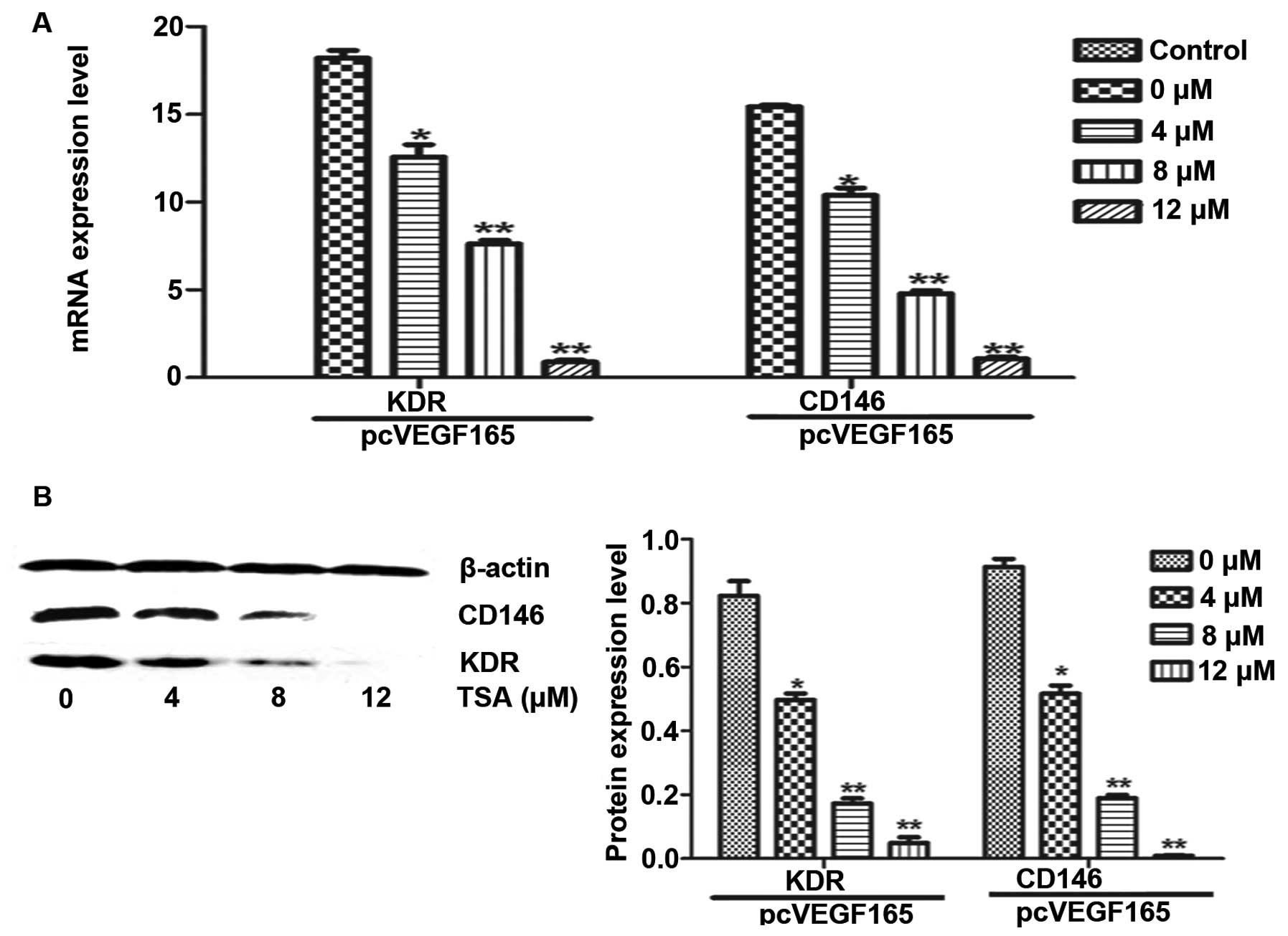
Tanshinone IIA inhibits the mRNA and
protein expression of KDR and CD146 in HUVECs
VEGF is a critical factor in the process of
angiogenesis, and activates the angiogenesis of endothelial cells
via integration with its receptor (VEGFR) (23,24).
Activation of the VEGF/VEGFR signaling pathway can trigger a
network signal cascade, consequently contributing to proliferation,
invasion and migration of vascular endothelial cells. Meanwhile,
human soluble CD146, a coreceptor for KDR, represents both an
attractive biomarker of placental vascular development and a
therapeutical target in pregnancy complications associated with
pathological angiogenesis (25). In
order to further investigate the mechanism underlying the
inhibitory effect of TSA on the invasion of human vascular
endothelial cells, we analyzed the effect of TSA on the mRNA and
protein expression of KDR and CD146 by RT-PCR and western blot
analysis. HUVECs were transfected with the pcVEGF165 plasmid to
induce the expression of KDR. Our results demonstrated the
inhibitory effect of TSA on mRNA and protein levels of KDR and
CD146 (Fig. 4) in a dose-dependent
manner, which definitely indicates that TSA inhibits angiogenesis
via repression of the VEGF/VEGFR2 pathway.
Tanshinone IIA reduces MMP-2 and -9
expression but not via mRNA
ECM degradation is also important for angiogenesis,
and is mainly regulated by a balance between different groups of
MMPs (26). MMPs and VEGF/KDR are
two foremost factors in angiogenesis (27). To understand the potential mechanism
of the inhibitory effect of TSA on the invasion of human vascular
endothelial cells through matrix, we analyzed the effect of TSA on
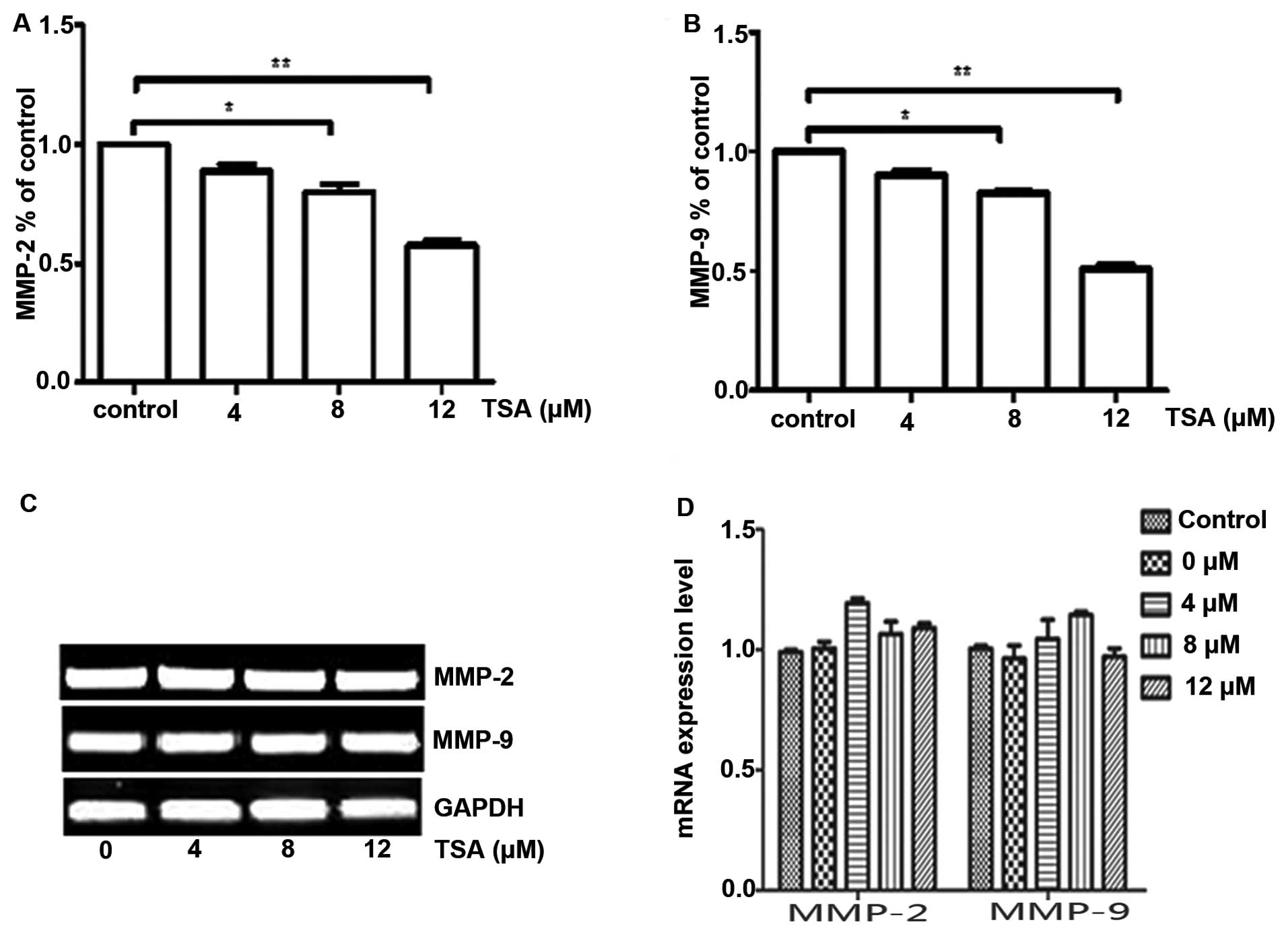
the production of MMP-2 and MMP-9 by ELISA method. Our results
demonstrated a dose-dependent inhibitory effect of TSA on MMP-2 and
MMP-9 (Fig. 5A and B). We further
examined whether TSA affects the gene expression of MMP-2 and MMP-9
at the mRNA level. The mRNA levels of MMP-2 and MMP-9 remained
unchanged after TSA treatment, which indicates that the
anti-angiogenic function of TSA on MMP-2 and MMP-9 in HUVECs is
likely mediated by a post-translational mechanism (Fig. 5C and D).
Discussion
Angiogenesis is critical for the growth and
progression of tumors. The importance of angiogenesis to tumor
survival offers the possibility of anti-angiogenic therapies in the
treatment of cancer (28). TSA has
been previously shown to inhibit migration, invasion and metastasis
of tumors (29,30). We investigated the molecular
mechanism of TSA on angiogenesis in HUVECs. In the present study,
we confirmed the inhibitory activity of TSA on angiogenesis. Our
results showed that TSA could inhibit various aspects of
angiogenesis, including endothelial cell proliferation, migration
and the tube formation of HUVECs. Meanwhile, TSA also attenuated
ex vivo angiogenesis in a CAM model and microvessel
outgrowth in a rat aortic ring assay. These results indicate that
TSA has capacity as an anti-angiogenic agent.
Anti-angiogenic drugs have been developed in recent
years. Among them the most promising drug for anti-angiogenesis is
targeting of the VEGF/VEGFR signaling pathway (31). Since VEGF and its receptors play a
critical role in angiogenesis and tumor progression, many
approaches have been developed to inhibit this pathway, such as
neutralizing antibodies and soluble receptors that inhibit the
binding of VEGF to its receptors, antisense constructs against VEGF
mRNA, and tyrosine kinase inhibitors that block downstream
signaling from membrane-bound VEGF receptors. Although various KDR
inhibitors based on this concepts, such as YN968D1, have recently
been developed (32), some are
still in the clinical stage and have various side effects. Many
antibodies and chemical agents that inhibit KDR signaling have been
developed and are being tested at the different stages in clinical
trials. However, much less is known concerning the inhibitory
activity of natural products. Our results revealed a novel activity
of TSA inhibition involving the VEGF/VEGFR2 pathway. The next issue
that challenged us was how TSA mediated the inhibition of HUVEC
proliferation. Physiologically, VEGFR2 (KDR), which is activated by
VEGF, undergoes dimerization and ligand-dependent tyrosine
phosphorylation in intact cells and results in a mitogenic,
chemotactic and pro-survival signal (33). Meanwhile, CD146 is required for
VEGF-induced KDR phosphorylation, and AKT/p38 MAPKs/NF-κB
activation. Therefore, we examined changes in KDR and CD146
expression levels, which are the starting point of VEGF signaling.
Both transcription and translation of KDR and CD146 were
downregulated by TSA. This reduction in KDR expression may have
been caused by the inhibition of VEGF signaling and the suppression
of CD146 by TSA. Evidence suggests that blocking KDR or CD146
limits the ability of most tumors to stimulate the formation of
blood vessels (34,35) and the data corroborate the fact that
inhibition of KDR and CD146 could suppress angiogenesis.
We attempted to further clarify the potential
mechanisms underlying the anti-angiogenic effect of TSA in tumor
progression, which have never been studied in detail. It is well
known that the angiogenic cascade relies on the degradation of the
basement membrane allowing invasion of endothelial cells into the
tissue, further removal of obstructing matrix proteins, the
migration of endothelial cells and the formation of tubules by
endothelial cells to contribute to the chaotic tumor vasculature
(36). MMPs, the main group of
proteolytic enzymes that degrade and remodel ECM, play a key role
in the angiogenic process (37).
Therefore, inhibition of the early degradation of ECM proteins
predominantly by MMPs is considered to be an important strategy by
which to inhibit angiogenesis. In the present study, we found that
TSA inhibits the production of MMP-2 and MMP-9. The mRNA levels of
MMP-2 and MMP-9 were not altered following TSA treatment, which is
in accordance with a previous study (16). Therefore, the anti-angiogenic effect
of TSA in vascular endothelial cells is also mediated by MMPs.
In summary, TSA was demonstrated to exert in
vitro anti-angiogenic effects. For the first time, inhibition
of cell migration and invasion was associated with the suppression
of the VEGF/VEGFR2 pathway and regulation of MMP-2/-9 secretion in
vascular endothelial cells by TSA. The dual inhibitory mechanisms
of TSA are thought to contribute to its more effective
anti-angiogenic effect. Together with the anti-tumor effects as
identified in previous studies, TSA could be a highly prospective
agent with which to inhibit cancer development and tumor
angiogenesis. Further studies are needed to evaluate the potential
of the anti-angiogenic activity of TSA in tumorigenesis and tumor
progression using a carcinogen-induced or genetically engineered
tumor model. Collectively, our data indicate that TSA may be a
potent inhibitor, antagonizing tumor angiogenesis via the
VEGF/VEGFR2 pathway and MMP-2/-9 regulation, and may be a useful
agent for the treatment of angiogenesis-related diseases.
In conclusion, our results indicate that TSA
inhibits various aspects of angiogenesis, including endothelial
cell proliferation, migration and the tube formation of HUVECs. In
addition, we demonstrated that TSA inhibits endothelial cell
function, at least in part, via the simultaneous inhibition of the
VEGF/VEGFR2 signaling pathway and regulation of MMP-2/-9
production.
References
|
1
|
Bussolino F, Mantovani A and Persico G:
Molecular mechanisms of blood vessel formation. Trends Biochem Sci.
22:251–256. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li W, Li J, Ashok M, et al: A
cardiovascular drug rescues mice from lethal sepsis by selectively
attenuating a late-acting proinflammatory mediator, high mobility
group box 1. J Immunol. 178:3856–3864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang AM, Sha SH, Lesniak W and Schacht J:
Tanshinone (Salviae miltiorrhizae extract) preparations attenuate
aminoglycoside-induced free radical formation in vitro and
ototoxicity in vivo. Antimicrob Agents Chemother. 47:1836–1841.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su CC and Lin YH: Tanshinone IIA
downregulates the protein expression of ErbB-2 and upregulates
TNF-α in colon cancer cells in vitro and in vivo. Int J Mol Med.
22:847–851. 2008.PubMed/NCBI
|
|
5
|
Su CC: Tanshinone IIA potentiates the
efficacy of 5-FU in Colo205 colon cancer cells in vivo through
downregulation of P-gp and LC3-II. Exp Ther Med. 3:555–559.
2012.PubMed/NCBI
|
|
6
|
Liu F, Yu G, Wang G, et al: An
NQO1-initiated and p53-independent apoptotic pathway determines the
anti-tumor effect of tanshinone IIA against non-small cell lung
cancer. PLoS One. 7:e421382012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somani RR and Bhanushali UV: Targeting
angiogenesis for treatment of human cancer. Indian J Pharm Sci.
75:3–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim KJ, Li B, Winer J, et al: Inhibition
of vascular endothelial growth factor-induced angiogenesis
suppresses tumour growth in vivo. Nature. 362:841–844. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Plate KH, Breier G, Weich HA and Risau W:
Vascular endothelial growth factor is a potential tumour
angiogenesis factor in human gliomas in vivo. Nature. 359:845–848.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang Y, Wang F, Feng J, Yang D, Yang X and
Yan X: Knockdown of CD146 reduces the migration and proliferation
of human endothelial cells. Cell Res. 16:313–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang T, Zhuang J, Duan H, et al: CD146 is
a coreceptor for VEGFR-2 in tumor angiogenesis. Blood.
120:2330–2339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang P, Luo Y, Duan H, et al: MicroRNA 329
suppresses angiogenesis by targeting CD146. Mol Cell Biol.
33:3689–3699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fanjul-Fernández M, Folgueras AR, Cabrera
S and López-Otín C: Matrix metalloproteinases: evolution, gene
regulation and functional analysis in mouse models. Biochim Biophys
Acta. 1803:3–19. 2010. View Article : Google Scholar
|
|
15
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
16
|
Tsai MY, Yang RC, Wu HT, Pang JH and Huang
ST: Anti-angiogenic effect of tanshinone IIA involves inhibition of
matrix invasion and modification of MMP-2/TIMP-2 secretion in
vascular endothelial cells. Cancer Lett. 310:198–206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deryugina EI and Quigley JP: Pleiotropic
roles of matrix metalloproteinases in tumor angiogenesis:
contrasting, overlapping and compensatory functions. Biochim
Biophys Acta. 1803:103–120. 2010. View Article : Google Scholar :
|
|
18
|
Won SH, Lee HJ, Jeong SJ, et al:
Tanshinone IIA induces mitochondria dependent apoptosis in prostate
cancer cells in association with an inhibition of phosphoinositide
3-kinase/AKT pathway. Biol Pharm Bull. 33:1828–1834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiao JW and Wen F: Tanshinone IIA acts via
p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and
lung-resistance protein in cisplatin-resistant ovarian cancer
cells. Oncol Rep. 25:781–788. 2011.
|
|
20
|
Baudin B, Bruneel A, Bosselut N and
Vaubourdolle M: A protocol for isolation and culture of human
umbilical vein endothelial cells. Nat Protoc. 2:481–485. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burbridge MF and West DC: Rat aortic ring:
3D model of angiogenesis in vitro. Methods Mol Med. 46:185–204.
2001.
|
|
22
|
Yi T, Cho SG, Yi Z, et al: Thymoquinone
inhibits tumor angiogenesis and tumor growth through suppressing
AKT and extracellular signal-regulated kinase signaling pathways.
Mol Cancer Ther. 7:1789–1796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cross MJ, Dixelius J, Matsumoto T and
Claesson-Welsh L: VEGF-receptor signal transduction. Trends Biochem
Sci. 28:488–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo ZY and Cao BL: Advances of VEGF
related molecular promoting tumor angiogenesis and targeting
therapy. Zhonghua Bing Li Xue Za Zhi. 39:282–284. 2010.(In
Chinese). PubMed/NCBI
|
|
25
|
Kaspi E, Guillet B, Piercecchi-Marti MD,
et al: Identification of soluble CD146 as a regulator of
trophoblast migration: potential role in placental vascular
development. Angiogenesis. 16:329–342. 2013. View Article : Google Scholar
|
|
26
|
Hua H, Li M, Luo T, Yin Y and Jiang Y:
Matrix metalloproteinases in tumorigenesis: an evolving paradigm.
Cell Mol Life Sci. 68:3853–3868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang JD and Zhang Y: Interregulation of
VEGF/R endostatin and MMP in the angiogenesis of hematologic
malignancies--review. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
13:1145–1150. 2005.(In Chinese).
|
|
28
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shan YF, Shen X, Xie YK, et al: Inhibitory
effects of tanshinone II-A on invasion and metastasis of human
colon carcinoma cells. Acta Pharmacol Sin. 30:1537–1542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuxian X, Feng T, Ren L and Zhengcai L:
Tanshinone II-A inhibits invasion and metastasis of human
hepatocellular carcinoma cells in vitro and in vivo. Tumori.
95:789–795. 2009.
|
|
31
|
Los M, Roodhart JM and Voest EE: Target
practice: lessons from phase III trials with bevacizumab and
vatalanib in the treatment of advanced colorectal cancer.
Oncologist. 12:443–450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian S, Quan H, Xie C, et al: YN968D1 is a
novel and selective inhibitor of vascular endothelial growth factor
receptor-2 tyrosine kinase with potent activity in vitro and in
vivo. Cancer Sci. 102:1374–1380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Claesson-Welsh L and Shibuya M: VEGF
receptor signal transduction. Methods Enzymol. 443:261–284. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan X, Lin Y, Yang D, et al: A novel
anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and
tumor growth. Blood. 102:184–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shaheen RM, Davis DW, Liu W, et al:
Antiangiogenic therapy targeting the tyrosine kinase receptor for
vascular endothelial growth factor receptor inhibits the growth of
colon cancer liver metastasis and induces tumor and endothelial
cell apoptosis. Cancer Res. 59:5412–5416. 1999.PubMed/NCBI
|
|
36
|
Eguchi M, Masuda H and Asahara T:
Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp
Nephrol. 11:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Packard BZ, Artym VV, Komoriya A and
Yamada KM: Direct visualization of protease activity on cells
migrating in three-dimensions. Matrix Biol. 28:3–10. 2009.
View Article : Google Scholar :
|