Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of cancer worldwide and causes approximately one
million deaths each year (1–3). The
incidence of HCC in the USA has increased in the past two decades,
possibly due to increased incidence in cirrhosis and longstanding
hepatitis C infection (1). HCC is
considered a major health problem due to endemic hepatitis B and C
and regional exposure to environmental pathogens (3). HCC is invasive and resistance to
chemotherapeutic agents limits treatment options. At present, the
only curative treatment is surgery (4). Surgical resection offers the best
prognosis when the tumor is <5 cm in diameter and limited to one
lobe of the liver, has not invaded the liver vasculature, and liver
function is well preserved. However, long-term survival is rare,
mainly due to the recurrence and metastasis of HCC (5). Identification of target genes
associated with the progression of HCC and improved understanding
of the early molecular events in tumorigenesis may lead to
improvements in diagnostic efficiency and aid in the development of
new therapeutic strategies for HCC.
Apoptosis is a genetically determined process of
controlled cellular suicide (6).
Deregulation of apoptosis is involved in the pathophysiology of
liver disease including hepatocarcinogenesis (7). HCC cell resistance to apoptosis
impairs the efficacy of HCC therapeutic regimens (8). The oncogene DJ-1 encodes a
ubiquitously expressed 189-amino acid integral mitochondrial
protein reported to confer resistance to apoptosis (9–13).
DJ-1 has been reported to regulate cell death, and appears to play
a role in tumorigenesis, invasion and metastasis in breast cancer,
HCC, non-small cell lung carcinoma and prostate cancer (9–17). The
oncogenic effect of DJ-1 is mainly attributed to its anti-apoptotic
ability. A number of DJ-1 downstream effectors, such as PTEN, Akt,
IKK and NF-κB, are involved in regulating the progression and
invasion of tumors (16,18).
In the present study, we aimed to investigate the
role of DJ-1 in HCC tumorigenesis. HepG2 cells provide an in
vitro model system for the study of polarized human
hepatocytes, exhibiting robust morphological and functional
differentiation with apical and basolateral cell surface domains.
Selective shRNA expression vector-mediated RNAi is an effective
method for suppressing oncogene products in vitro (19). Therefore, shRNA can be
therapeutically used for the inhibition of aberrant mRNA expression
in cancer cells (20).
Using a shRNA expression vector, stable DJ-1
knockdown of HepG2 cells was obtained. We found that DJ-1
downregulation resulted in the growth inhibition of HepG2 cells
in vitro and in vivo. Increased PTEN expression and
decreased Akt phosphorylation were detected in DJ-1 knockdown HepG2
cells.
These findings suggested that DJ-1 knockdown altered
the malignant behavior of HepG2 cells, potentially through Akt
signaling, indicating a crucial role for DJ-1 in the oncogenesis of
HCC.
Materials and methods
Cell culture
he human HepG2, SMMC-7721 and Huh-7 HCC cell lines
were obtained from the China Center for Type Culture Collection
(Wuhan University, Wuhan, China). Human metastatic MHCC-97L and
MHCC-97H HCC cell lines were obtained from the Liver Cancer
Institute of Zhongshan Hospital (Fudan University, Shanghai, China)
(21). Cells were cultured at 37°C
with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM)
high-glucose medium containing 10% calf serum (both from Gibco-Life
Technologies, Carlsbad, CA, USA) and 1% penicillin and
streptomycin.
Construction of DJ-1 shRNA expression
plasmid
A candidate DJ-1 cDNA sequence (GenBank NM_007262:
AGTGTAGCCGTGATGTGGT) was selected for RNA interference (RNAi). The
oligonucleotide sequence used for DJ-1 silencing was BamHII
+ sense + loop + antisense + terminator + Sa1I +
HindII, with shRNA-DJ-1 (forward),
5′-CACCAGTGTAGCCGTGATGTGGTTTCAAGACGACCACATCACGGCTACACTTTTTTTG-3′
and (reverse), 5′-AGCTCAAAAAAAGTGTAGCCGTGATGTGGTCGTCTTGAAACCACATCACGGCTACACT-3′.
The negative control sequences were: shRNA-HK (forward),
5′-CACCTTTTTTCAAGACGGATGAACTTCAGGGTCAGCTTTTTTG-3′
and (reverse), 5′-AGCTCAAAAAAGCTGACCCTGAAGTTCATCCGTCTTGAAAAAA-3′. The
underlined sequences denote the loop. An oligonucleotide targeting
GAPDH served as a positive control while an irrelevant
oligonucleotide served as a negative control. Recombinant plasmid
synthesis and purification was performed by Wuhan Genesil
Biotechnology Co., Ltd. (Wuhan, China).
Stable transfection of HepG2 with
recombinant human DJ-1 shRNA plasmid
HepG2 cells at 60–80% confluency were transfected
with constructed plasmids using Lipofectamine 2000 (Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. At 48 h after transfection 450 μg/ml G418 was added
to the cell culture medium. The cells were cultured in medium
containing G418 for 8 weeks to select stable transfectants.
Individual clones were isolated and expanded. The green
fluorescence expressed by stable transfectant cells was observed by
fluorescence microscopy.
RNA extraction and semi-quantitative
PCR
Total RNA was isolated using the TRIzol reagent
(Invitrogen-Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions, 48–72 h after transfection. Total RNA
(2 μg) was used for semi-quantitative reverse transcription PCR on
a Bio-Rad S1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA)
(Promega, Madison, WI, USA). The primers used were: DJ-1,
5′-AGCAGAGGAAATGGAGACG-3′ (forward), and 5′-GCCAACAGAGCAGTAGGAC-3′
(reverse); internal control β-actin 5′-TCTACAATGAGCTGCGTGTG-3′
(forward), and 5′-ATCTCCTTCTGCATCCTGTC-3′ (reverse). PCR products
(5 μl) were analyzed by electrophoresis on 1.2% agarose gels.
Immunoblotting
Cells were collected, washed with phosphate-buffered
saline (PBS) three times, and lysed in cell lysis buffer containing
RIPA (ProMab, Changsha, China) and protease inhibitor (Promega).
The cell lysates were submitted to centrifugation at 12,000 × g for
30 min and the supernatants were collected as total protein, and
quantified by the bicinchoninic acid (BCA) method. Protein (50 μg)
was used for electrophoresis followed by transfer on PVDF
membranes, and probed with rabbit polyclonal antibodies against
human DJ-1 (Proteintech, China), PTEN (Epitomics, Inc., Burlingame,
CA, USA) or rabbit monoclonal antibodies against human β-actin
(Abzoom Biolabs, Dallas, TX, USA). Following incubation with
horseradish peroxidase-conjugated anti-rabbit secondary antibodies,
signals were detected with an enhanced chemiluminescence (ECL)
chromogenic substrate. Visualization and quantification were
carried out on a digital image analyzer.
Flow cytometric analysis
A single-cell suspension of HepG2 cells in the
logarithmic phase was seeded in new culture flasks and incubated
for 48 h followed by fixation with 75% ethanol at 4°C overnight.
The washed cells were resuspended in PBS containing RNase A, and
incubated at 37°C for 30 min prior to the addition of 50 μg/ml
propidium iodide (PI) for 30 min in the dark. After washing, the
cells were analyzed using a flow cytometer (Chemunex, Vernon Hills,
IL, USA).
Cell growth assay
Cell proliferation was assessed by a colorimetric
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The cells in the logarithmic phase were seeded at a density
of 1×102/μl in triplicate in 96-well plates (100
μl/well). After 24, 48, 79 and 96 h, 50 μl MTT (5 mg/ml) were added
to each well and incubated at 37°C. After 4 h, the supernatants
were carefully aspirated and the purple crystals dissolved with 150
μl DMSO. Absorbance was measured at 570 nm on a microtiter plate
reader.
Adhesion and invasion assays
Cellular adhesion to plastic and laminin- or
fibronectin-coated surfaces was evaluated in 96-well plates. The
plates were coated with 20 μl DMEM containing 2% BSA at 37°C for 1
h and rinsed three times with PBS. The cells (1×104)
were incubated in the plate for 1 h and non-adherent cells were
removed by washing with PBS. Cell viability was assessed by MTT
(0.5 μg) as described above.
The invasive activity of cells was determined using
transwell chambers. Matrigel (20 μl) (BD Biosciences, Bedford, MA,
USA) at 500 μg/ml in cold PBS was spread on the inner side of the
filter. The cells in the logarithmic phase were harvested and
suspended at 1×105/ml in DMEM. Then, 100 μl of cell
suspension was added to the upper compartment and incubated at 37°C
for 24 h. Filters were subsequently fixed with 10% neutral
formaldehyde for 30 min and stained with crystal violet for 5 min.
The cells on the upper side of the filter were removed with a
cotton swab and any cells that had migrated through the Matrigel to
reach the reverse side were counted under a microscope.
Animal experiments
Six- to eight-week male BALB/c-nu mice were obtained
from the Experimental Animal Center of the Huazhong University of
Science and Technology (Wuhan, China). Animal experiments were
conducted according to the guidelines of and approved by the
Committee on Animals of the Huazhong University of Science and
Technology. Animals were provided with food and water ad
libitum and allowed one week adaptation. The mice were housed
at 25±1°C under pathogen-free conditions with a 12-h light/dark
cycle and 40–60% relative humidity. Single-cell suspensions (HepG2,
RNAi-DJ-1 or RNAi-HK) were prepared and 5×106 cells in
0.2 ml Hanks solution injected subcutaneously into mice. Tumor size
was assessed every 4 days as ab2/2, where a and b are
the major and minor diameter, respectively. Four weeks after the
first measurement, the mice were sacrificed.
Statistical analysis
The results were expressed as means ± standard
deviation (SD). The SPSS 12.0 software (SPSS, Inc., Chicago, IL,
USA) was used to perform the analysis. One-way ANOVA was used for
comparisons among different groups. P<0.05 was considered to
indicate a statistically significant result.
Results
DJ-1 and PTEN expression in human HCC
cell lines
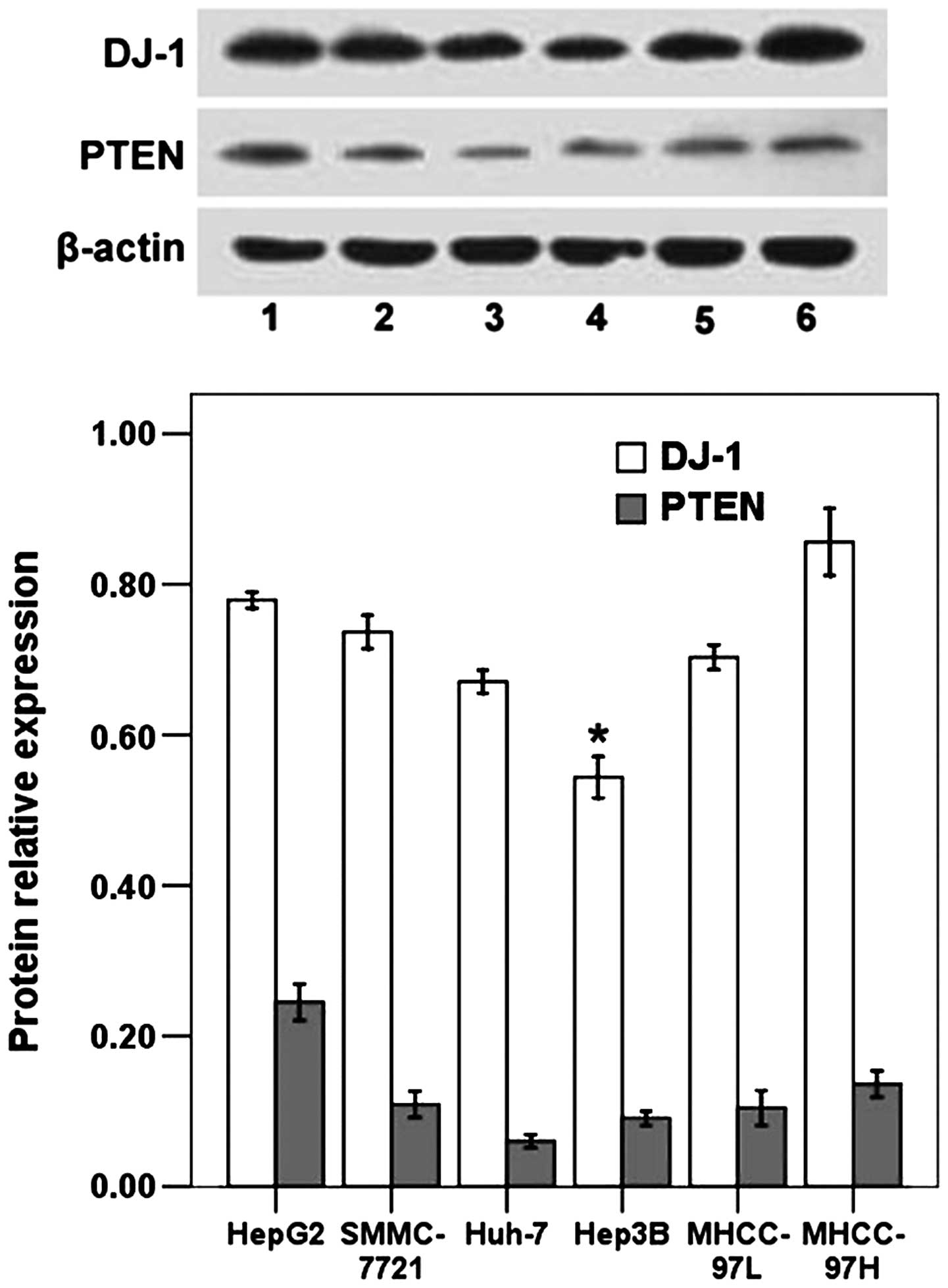
The expression levels of DJ-1 and PTEN were assessed
by immunoblot analysis in human HCC cell lines including HepG2,
SMMC-7721, Huh-7, Hep3B, MHCC-97L and MHCC-97H. As shown in
Fig. 1, DJ-1 expression varied
significantly between cell lines. Significantly lower DJ-1 levels
were observed for Hep3B cells, which possess low metastatic
potential, in comparison with the MHCC-97L cell line with low
metastatic potential and the MHCC-97H cell line with high
metastatic potential (P<0.05). The protein expression of DJ-1 in
MHCC-97L cells was significantly lower than that in MHCC-97H cells
(P<0.05; Fig. 1). The results
suggested that DJ-1 expression may correlate with the metastatic
potential of HCC cell lines. Concerning PTEN expression, no
significant differences were obtained when these cell lines were
compared (Fig. 1). HepG2 cells were
selected to examine the high level of DJ-1 expression in this cell
line. This cell line has been widely used for the in vitro
and in vivo studies of treatment of HCC and the mechanism
involved.
shRNA knockdown of DJ-1 expression
The DNA segment encoding a shRNA for DJ-1 was
inserted into a pGenesil-1 plasmid expression vector containing the
human U6 promoter, between the BamHII and SalII
restriction sites. The shRNA expression was directly driven by the
cytomegalovirus promoter. The correct inserts in the RNAi plasmids
shRNA-DJ-1 and negative control shRNA-HK were confirmed with DNA
sequencing (data not shown). The plasmids were transfected into
HepG2 cells, with a transfection efficiency of ~70% after 48 h
(data not shown). After 8 weeks of G418 selection stable sub-clones
expressing green fluorescent protein were isolated and expanded for
subsequent assays (data not shown).
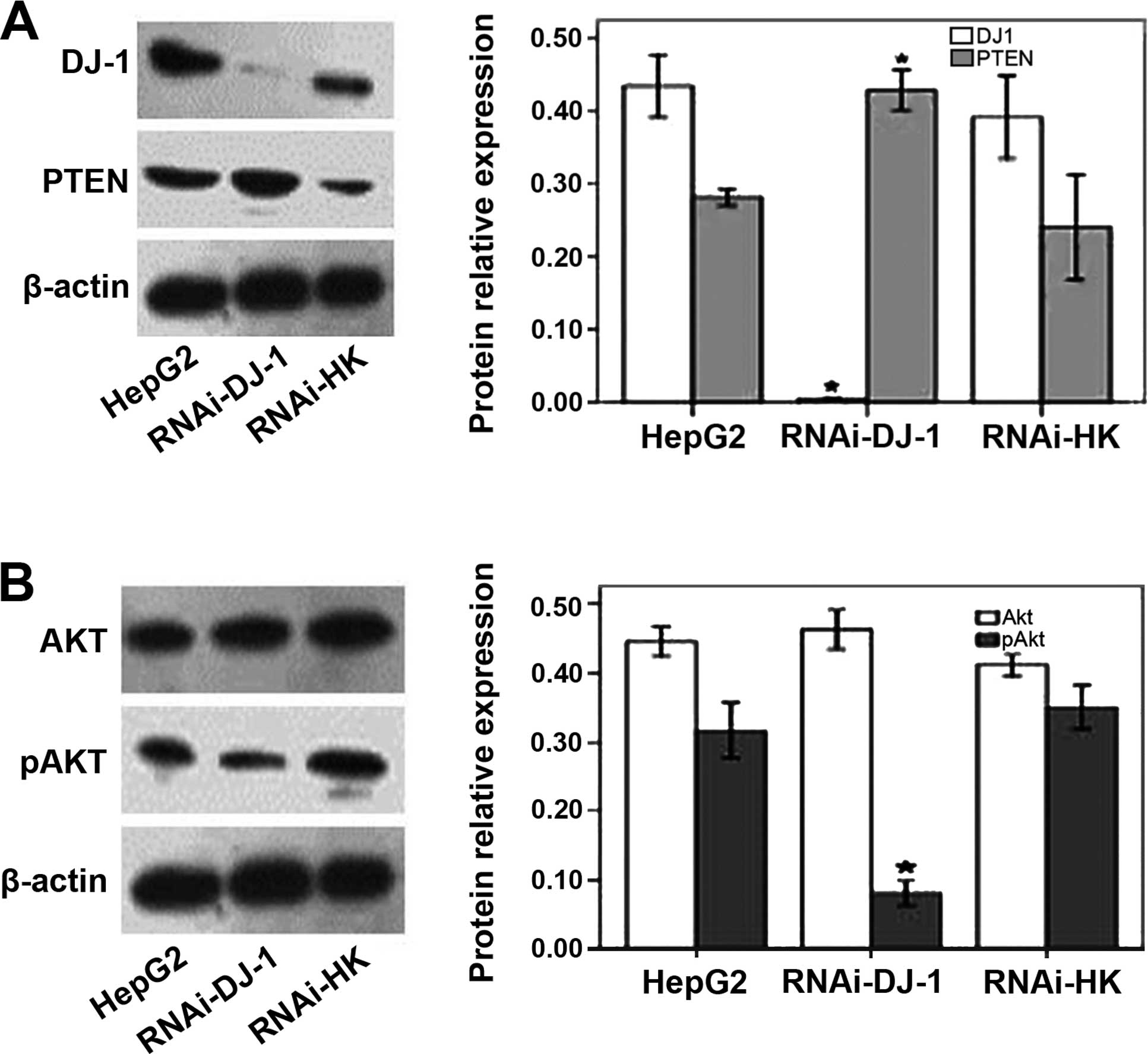
RT-PCR analysis confirmed that the DJ-1 shRNA
recombinant plasmid inhibited DJ-1 mRNA expression in HepG2 cells
(data not shown). DJ-1 protein expression levels were reduced by
98.78% after silencing (Fig.
2A).
DJ-1 shRNA inhibited Akt phosphorylation
in HepG2 cells
In cells treated with RNAi-DJ-1, PTEN protein levels
increased, compared to the untreated cells or cells transfected
with the control plasmid RNAi-HK (P<0.05; Fig. 2A). Akt phosphorylation (pAkt) levels
were significantly reduced in the RNAi-DJ-1-treated cells compared
to the control cells (p<0.05; Fig.
2B). By contrast, total Akt levels were not significantly
altered by RNAi-DJ-1 (P>0.05; Fig.
2B). These data indicated that the downregulation of DJ-1
resulted in an increase of PTEN protein levels as well as the
inhibition of Akt phosphorylation.
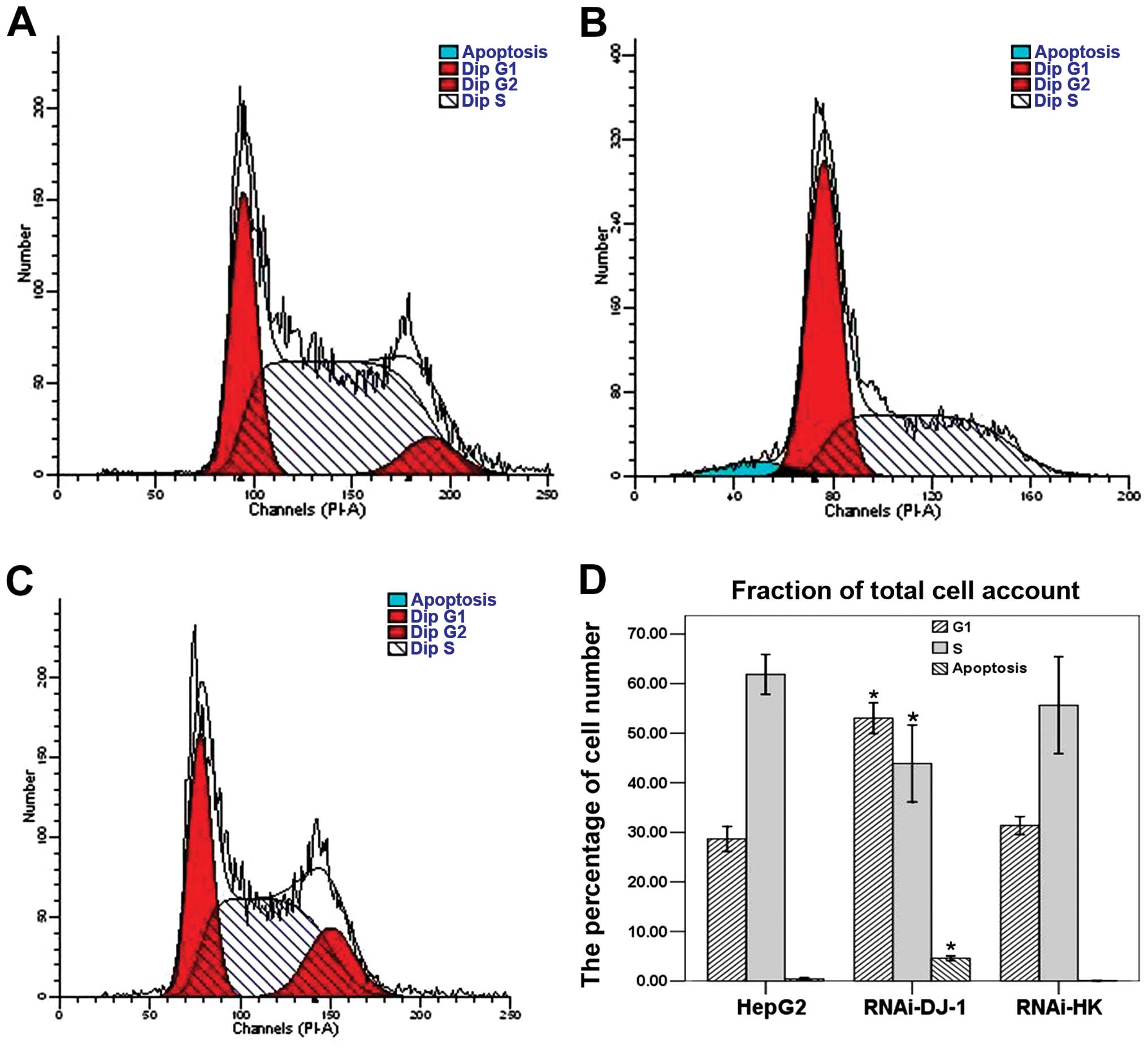
DJ-1 shRNA suppressed the proliferation,
adhesion and invasion of HepG2 cells
PI-stained cells were analyzed using flow cytometry.
In RNAi-DJ-1-transfected cells, we found a significant decrease in
cell populations with diploid DNA as well as an accumulation of
apoptotic cells (P<0.05; Fig.
3). These findings suggested that DJ-1 knockdown arrested the
cell cycle and induced apoptosis.
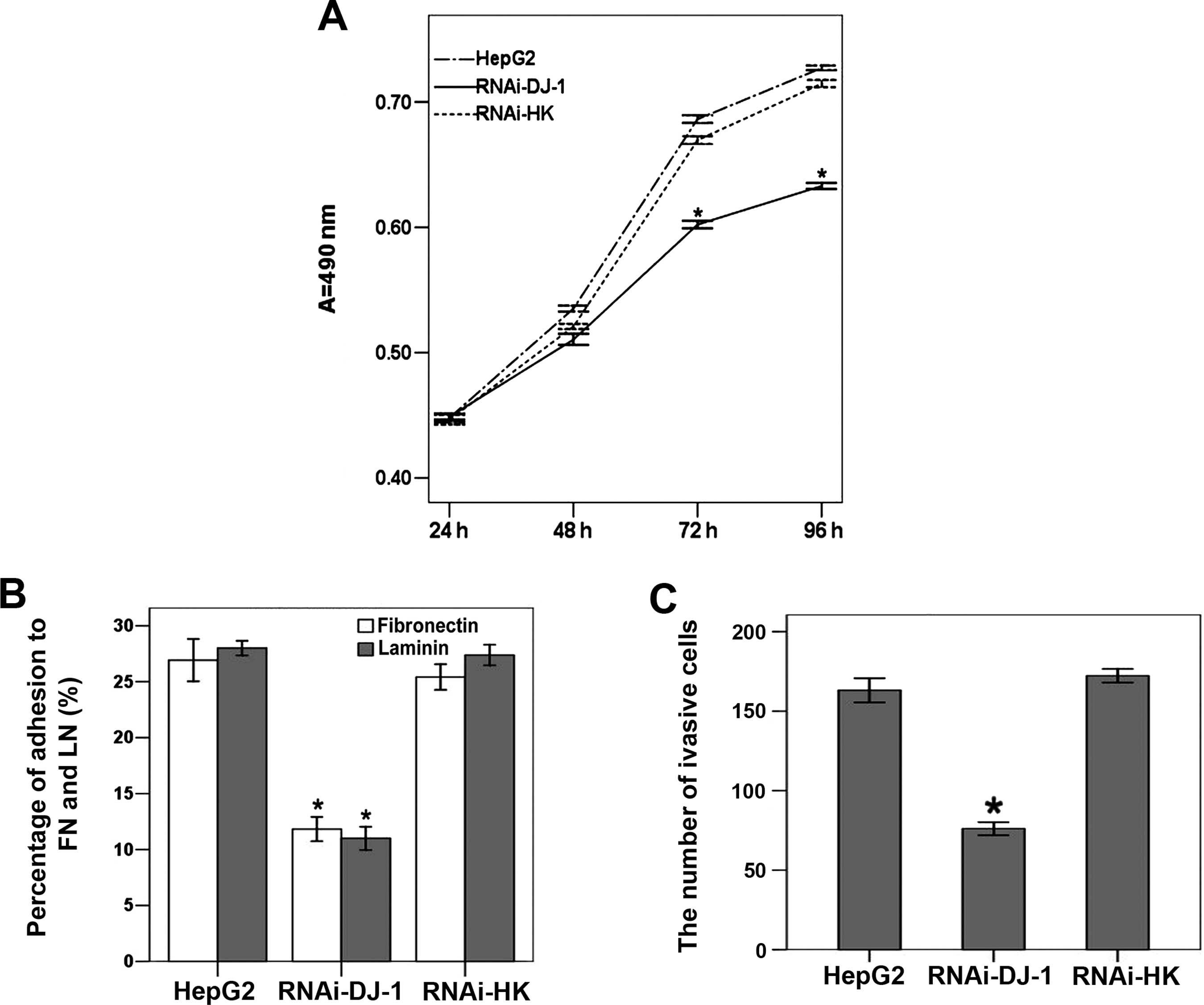
Using MTT assay (Fig.
4A), we detected that the proliferation of
RNAi-DJ-1-transfected cells was significantly reduced in comparison
with untreated cells and cells transfected with the control plasmid
(P>0.05; Fig. 4A).
Cell adhesion and invasion rates were also
significantly reduced in DJ-1 knockdown cells: binding to
fibronectin and laminin was reduced by 56.07 and 60.73% in
RNAi-DJ-1-transfected cells, respectively (P<0.05; Fig. 4B). Compared to untreated cells,
there were 53.34% fewer RNAi-DJ-1 cells that migrated through a
transwell filter after 24 h (P<0.05; Fig. 4C).
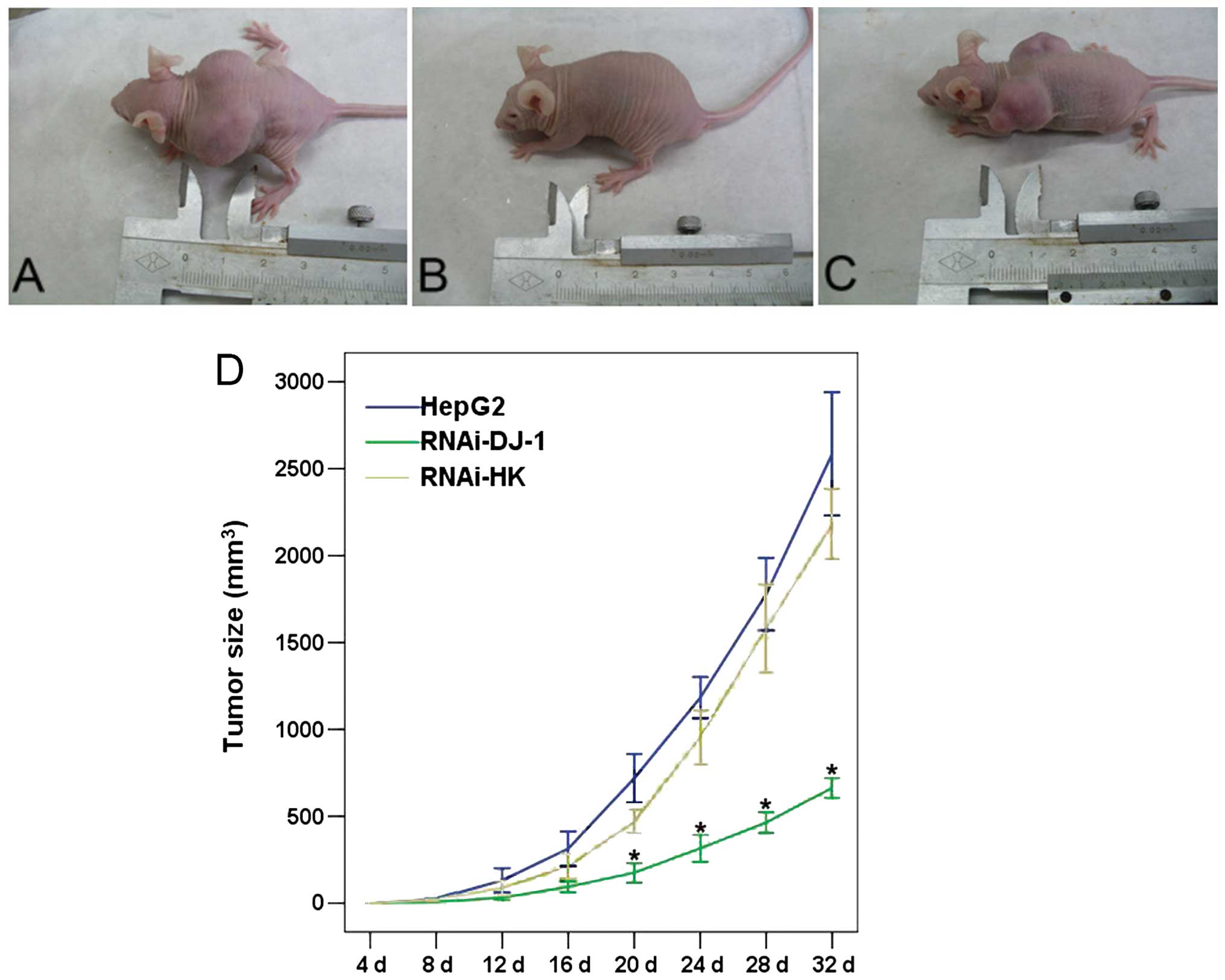
RNAi-DJ-1 suppression of xenograft tumor
formation in nude mice
Thirty nude mice received subcutaneous injections of
normal HepG2 cells with or without transfected shRNA plasmid. As
shown in Fig. 5, the xenograft of
untreated HepG2 induced tumor growth in transplanted animals in a
time-dependent manner. The RNAi-HK-transfected cells, when
transplanted, induced similar level of tumor growth in SD mice.
However, tumor growth in the RNAi-DJ-1 group was significantly
reduced as compared to that of normal HepG2 cells and cells
transfected with RNAi-HK (P<0.05). These data indicated that
inhibition of DJ-1 by shRNA inhibited the in vivo growth of
HepG2 cells in the xenograft transplant.
Discussion
Results of the present study have shown that DJ-1
was expressed in a variety of human HCC cell lines including HepG2,
SMMC-7721, Huh-7, Hep3B, MHCC-97L and MHCC-97H, and that DJ-1
expression correlated positively with the metastatic potential of
HCC cell lines (22). However,
Zhang et al recently described DJ-1 downregulation in the
early stages of HBV-infected HCC, suggesting an abnormal
pathophysiological state of the mitochondria (17). Authors of that study suggested that
mitochondrial disease occurs before tumorigenesis in HBV-infected
HCC (17). The conflicting findings
on DJ-1 levels may reflect the differences between tumorigenesis in
HBV-infected primary cells and HCC cell lines (17). Nevertheless, the observations
strongly suggest that DJ-1 is involved in HCC, although its precise
role in tumorigenesis remains to be determined.
The effects of DJ-1 on cell proliferation and
apoptosis have been reported in other studies. For examples, cells
harboring DJ-1 are more resistant to UV-induced apoptosis than DJ-1
knockdown cells (9). Overexpression
of DJ-1 in a prostatic benign hyperplasia cell line (BPH-1) or
prostate carcinoma cells (PC-3) results in resistance to the
apoptosis normally induced by cytotoxic agents (10). By contrast, knockdown of DJ-1
enhances apoptosis of prostate carcinoma cells (PC-3) when treated
with the same toxic reagents (11,12) as
well as TNF-related apoptosis-inducing ligand/Apo-2L
(TRAIL)-mediated apoptosis (13).
Overexpression of DJ-1 blocks neuronal apoptosis by inhibiting the
transcriptional silencing activity of the pyrimidine tract-binding
protein-associated splicing factor, or by preventing the
translocation of Daxx from the nucleus to the cytoplasm, where Daxx
activates the apoptotic signal-regulating kinase-1 death pathway
(10). In the present study, we
employed RNAi technology to construct a shRNA plasmid to silence
DJ-1 mRNA and demonstrated DJ-1 expression was markedly reduced in
HepG2 cells after knockdown. Notably, DJ-1 silencing resulted in
decreased proliferation, adhesion and invasion of HepG2 cells in
vitro and inhibited tumor formation in nude mice. These results
are consistent with a previous study (11,12)
suggesting DJ-1 in HepG2 cells plays a similar role in inhibiting
apoptosis and promoting cell proliferation, adhesion and
invasion.
DJ-1 was identified as a suppressor of the PTEN
function through a genetic screening in Drosophila for
gain-of-function mutants (16).
PTEN negatively regulates the Akt signaling pathway through the
dephosphorylation of PIP3 (23,24).
Activation of Akt is involved in the regulation of cell
proliferation, apoptosis and migration (25).
We found that DJ-1 knockdown in HepG2 cells resulted
in the upregulation of PTEN and decreased Akt phosphorylation.
These results suggest that the PI3P/Akt is the potential signaling
pathway in which DJ-1 functions.
In summary, the downregulation of DJ-1 increased
PTEN expression, and decreased phosphorylation of Akt in HepG2
cells. In addition, DJ-1 knockdown resulted in decreased
proliferation, adhesion and invasion of HepG2 cells in
vitro, and inhibited the growth of HepG2-induced tumor in
vivo. These findings suggest that DJ-1 plays a crucial role in
the oncogenesis of HCC, thereby providing a potential target for
drug development for HCC.
Acknowledgements
The present study was supported by the Ph.D.
Programs Foundation of the Ministry of Education of China (project
no. 20130142120043).
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giannelli G and Antonaci S: Novel concepts
in hepatocellular carcinoma: from molecular research to clinical
practice. J Clin Gastroenterol. 40:842–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sherman M: Hepatocellular carcinoma:
epidemiology, surveillance, and diagnosis. Semin Liver Dis.
30:3–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahbari NN, Mehrabi A, Mollberg NM, et al:
Hepatocellular carcinoma: current management and perspectives for
the future. Ann Surg. 253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adachi E, Maehara S, Tsujita E, et al:
Clinicopathologic risk factors for recurrence after a curative
hepatic resection for hepatocellular carcinoma. Surgery. 131(Suppl
1): S148–S152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diamantis A, Magiorkinis E, Sakorafas GH
and Androutsos G: A brief history of apoptosis: from ancient to
modern times. Onkologie. 31:702–706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabregat I: Dysregulation of apoptosis in
hepatocellular carcinoma cells. World J Gastroenterol. 15:513–520.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schulze-Bergkamen H and Krammer PH:
Apoptosis in cancer - implications for therapy. Semin Oncol.
31:90–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mo JS, Kim MY, Ann EJ, Hong JA and Park
HS: DJ-1 modulates UV-induced oxidative stress signaling through
the suppression of MEKK1 and cell death. Cell Death Differ.
15:1030–1041. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Junn E, Taniguchi H, Jeong BS, Zhao X,
Ichijo H and Mouradian MM: Interaction of DJ-1 with Daxx inhibits
apoptosis signal-regulating kinase 1 activity and cell death. Proc
Natl Acad Sci USA. 102:9691–9696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuen HF, Chan YP, Law S, et al: DJ-1 could
predict worse prognosis in esophageal squamous cell carcinoma.
Cancer Epidemiol Biomarkers Prev. 17:3593–3602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davidson B, Hadar R, Schlossberg A, et al:
Expression and clinical role of DJ-1, a negative regulator of PTEN,
in ovarian carcinoma. Hum Pathol. 39:87–95. 2008. View Article : Google Scholar
|
|
13
|
Zhang HY, Wang HQ, Liu HM, Guan Y and Du
ZX: Regulation of tumor necrosis factor-related apoptosis-inducing
ligand-induced apoptosis by DJ-1 in thyroid cancer cells. Endocr
Relat Cancer. 15:535–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hod Y: Differential control of apoptosis
by DJ-1 in prostate benign and cancer cells. J Cell Biochem.
92:1221–1233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
MacKeigan JP, Clements CM, Lich JD, Pope
RM, Hod Y and Ting JP: Proteomic profiling drug-induced apoptosis
in non-small cell lung carcinoma: identification of RS/DJ-1 and
RhoGDIα. Cancer Res. 63:6928–6934. 2003.PubMed/NCBI
|
|
16
|
Kim RH, Peters M, Jang Y, et al: DJ-1, a
novel regulator of the tumor suppressor PTEN. Cancer Cell.
7:263–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang D, Lim SG and Koay ES: Proteomic
identification of down-regulation of oncoprotein DJ-1 and
proteasome activator subunit 1 in hepatitis B virus-infected
well-differentiated hepatocellular carcinoma. Int J Oncol.
31:577–584. 2007.PubMed/NCBI
|
|
18
|
Das R, Mahabeleshwar GH and Kundu GC:
Osteopontin stimulates cell motility and nuclear factor κB-mediated
secretion of urokinase type plasminogen activator through
phosphatidylinositol 3-kinase/Akt signaling pathways in breast
cancer cells. J Biol Chem. 278:28593–28606. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McManus MT and Sharp PA: Gene silencing in
mammals by small interfering RNAs. Nat Rev Genet. 3:737–747. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang SL, Yao HH and Qin ZH: Strategies for
short hairpin RNA delivery in cancer gene therapy. Expert Opin Biol
Ther. 9:1357–1368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romashkova JA and Makarov SS: NF-κB is a
target of AKT in anti-apoptotic PDGF signalling. Nature. 401:86–90.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Tang ZY, Ye SL, et al: Establishment
of cell clones with different metastatic potential from the
metastatic hepatocellular carcinoma cell line MHCC97. World J
Gastroenterol. 7:630–636. 2001.
|
|
23
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
24
|
Sun H, Lesche R, Li DM, et al: PTEN
modulates cell cycle progression and cell survival by regulating
phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B
signaling pathway. Proc Natl Acad Sci USA. 96:6199–6204. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitsiades CS, Mitsiades N and Koutsilieris
M: The Akt pathway: molecular targets for anti-cancer drug
development. Curr Cancer Drug Targets. 4:235–256. 2004. View Article : Google Scholar : PubMed/NCBI
|