Introduction
Pancreatic cancer is a lethal and intractable
malignancy (1,2). Substantial advances have been made in
understanding the biology of pancreatic cancer, as indicated by a
number of risk factors that have been identified, including tobacco
exposure, obesity, inherited susceptibility, persistent diabetes
and pancreatitis and heavy alcohol consumption (3). Despite noteworthy developments in the
treatment of pancreatic cancer, surgical resection is currently the
only possible strategy of cure. Findings of recent studies have
shown that 20% of the patients with pancreatic cancer is suitable
for surgery, but only 5% of those patients have a 5-year survival
(4,5). Pancreatic cancer is not sensitive to
treatment with most chemotherapeutic agents, although
chemoradiation and chemotherapy are the major therapies employed
for pancreatic cancer (2,6,7). Thus,
the poor prognosis of pancreatic cancer makes the identification of
novel chemotherapeutic drugs with high efficacy and minimal
side-effect imperative.
Histone deacetylase (HDAC) inhibitors are cytostatic
agents that induce differentiation and apoptosis of tumor cells and
have emerged as a promising new class of anticancer drugs.
Chidamide, with the brand name Epidaza, is a newly designed and
synthesized HDAC inhibitor, and currently studied in multiple
clinical trials as a single agent or in combination with other
agents for the treatment of various hematological and solid cancers
(8–10). Recent findings from preclinical
studies have identified that Chidamide was involved in the
inhibition of proliferation and progression of various types of
tumor (10–12). Recent investigations have focused on
the anti-cancer effect-related molecular mechanisms of Chidamide in
colon cancer, pancreatic cancer and leukaemia (8,13–15).
For instance, Liu et al showed that Chidamide promoted
apoptosis and led to cell arrest by increasing the acetylation
levels of histone H3 and suppressing the phosphoinositide
3-kinase/Akt and MAPK/Ras signaling pathways (8). Gong et al suggested that
Chidamide treatment resulted in G1 arrest at a low concentration
and induced differentiation at moderate concentrations in human
leukaemia cell lines (14).
Although in a recent study Qiao et al assessed the
anticancer activity of Chidamide in combination with gemcitabine in
pancreatic cancer cells (16),
in vivo studies are required to validate the result of the
in vitro study. Investigations of the underlying molecular
mechanism of the effect of Chidamide on pancreatic cancer should be
conducted.
In the present study, we examined the anticancer
effect of Chidamide in vitro and in vivo. The results
showed that Chidamide suppressed the proliferation of pancreatic
cancer cells and induced cell apoptosis in a dose-dependent manner.
The in vivo study confirmed the significant inhibitory
effect of Chidamide administration on pancreatic tumor growth. The
results also showed that Chidamide treatment markedly decreased the
expression of HDACs and p21 and promoted mitochondrial apoptosis
pathway-dependent cell apoptosis by regulating the ratio of
Bcl-2-like protein 4 (Bax), B-cell lymphoma 2 (Bcl-2) and uncleaved
Caspase-3.
Materials and methods
Cell line and cell culture
Chidamide was purchased from Chipscreen Biosciences
(Shenzhen, China) and was dissolved in dimethyl sulfoxide (DMSO) as
a stock solution. The human PaTu8988 pancreatic cancer cell line
was purchased from the cell bank of Shanghai, Chinese Academy of
Sciences. PaTu8988 cells were cultured in Dulbecco’s modified
Eagle’s medium [including 10% fetal bovine serum albumin,
penicillin (100 U/ml) and streptomycin (100 U/ml)]. The cell lines
were cultured in a 37°C incubator with 5% CO2.
Animals
Thirty BALB/c nude mice at the age of 5 weeks (18–22
g) were provided by the Animal Center of the Chinese Academy of
Sciences. The mice were housed in an animal facility under standard
laboratory conditions at a constant room temperature of 25±1°C with
a humidity of 40–60%. The animals were provided with free access to
food and water under a 12 h dark/light cycle. Experiments were
approved by the animal control committee of Shanghai Hospital,
Shanghai, China.
Apoptosis assay
Cell apoptosis was quantified using the Annexin
V-FITC/PI double staining kit according to the manufacturer’s
instructions (BioVision, Mountain View, CA, USA). PaTu8988 cells
were randomized into 4 groups and incubated in the absence or
presence of concentrations of 0, 1.25, 2.5 and 5 μM) of Chidamide
for 48 h. Following washing, the cells were consecutively stained
with Annexin-V-FITC and propidium iodide. Stained samples were
analyzed by flow cytometry (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA).
Cell proliferation assay
Proliferation of the PaTu8988 cells was evaluated
using CCK-8 (Biyuntian Biotechnology, Jiangsu, China) assay
according to the manufacturer’s instructions. PaTu8988 cells were
randomly into 4 groups and incubated in the absence or presence of
concentrations of 0, 1.25, 2.5 and 5 μM) of Chidamide for 48 h.
Subsequently, 10 μl CCK-8 was added in each well and incubated for
2 h. The optical density of each well was then measured with a
microplate reader (Bio-Tek Co., Bedfordshire, UK) at 450 nm. The
cell survival rate was calculated using the formula: Cell survival
rate (%) = 1 −
(ODctrl−ODsample)/ODctrl
×100%.
In vivo experiment
PaTu8988 cells (2×107) were suspended in
phosphate-buffered saline (PBS) and subcutaneously injected into
the right flank of each 5-week-old BALB/c nude mouse to establish
the pancreatic tumor murine model. Mice were selected for
subsequent experiments when the diameter of the pancreatic tumors
ranged from 0.5 to 1 cm. Twenty-four mice with approximately
uniform tumor size were randomly divided into the control
[intraperitoneal (i.p.) PBS], low Chidamide (i.p. 12.5 mg/kg/day
chidamide) and high chidamide (i.p. 50 mg/kg/day chidamide) groups.
PBS (vehicle) or Chidamide were administered consecutively for 21
days.
During the 21 days, mice body weight and tumor
volume were measured at intervals of 3 days. Tumor volume was
calculated using the formula: V=1/2 × ab2, where a is
the maximum and b the minimum length of tumor. The mice were
sacrificed by cervical dislocation after 21 days. Tumor was
isolated as a whole from each mouse to measure tumor weight. Tumor
sections were prepared and fixed in formalin for subsequent
experiments.
Hematoxylin and eosin (H&E)
staining
Tumor sections (5 μm) were stained with hematoxylin
for 10 min, followed by 1 sec differentiation in 1% hydrochloric
acid and 20–30 sec staining with eosin solution, sequentially. The
sections were mounted for pathological observations.
Quantitative polymerase chain reaction
(qPCR)
Total RNA of the PaTu8988 pancreatic cancer cell
line and mice tumor tissue was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). Total mRNA was
reverse transcribed using the reverse transcription kit (Promega,
Madison, WI, USA). Primers (Shenggong Bioengineering Co., Shanghai,
China) used for quantification measurements are shown in Table I. GAPDH was used as an internal
standard.
 | Table IPrimers used for quantification
measurements of mRNA expression. |
Table I
Primers used for quantification
measurements of mRNA expression.
| Gene | Primers |
|---|
| HDAC1 | F:
5′-ACCGGGCAACGTTACGAAT-3′
R: 5′-CTATCAAAGGACACGCCAAGTG-3′ |
| HDAC2 | F:
5′-TCATTGGAAAATTGACAGCATAGT-3′
R: 5′-CATGGTGATGGTGTTGAAGAAG-3′ |
| HDAC3 | F:
5′-TTGAGTTCTGCTCGCGTTACA-3′
R: 5′-CCCAGTTAATGGCAATATCACAGAT-3′ |
| GAPDH | F:
5′-GCTGGTCATCAACGGGAAA-3′
R: 5′-ACGCCAGTAGACTCCACGACA-3′ |
Total cDNA was used as a template for qPCR with the
standard protocol using 2 μl cDNA template, 12.5 μl SYBR-Green
Premix Ex Taq™, 1 μl forward primer, 1 μl reverse primer and 8.5 μl
dH2O. The thermal cycling conditions for PCR included 40
cycles of denaturation at 95°C for 15 sec, annealing at 55°C for 15
sec and extension at 72°C for 15 sec. The results were analyzed
using 2−ΔΔCt, in which ΔCt = Ct (target gene) − Ct
(internal reference), ΔΔCt = ΔCt (sample) − ΔCt (control).
Western blot analysis
The expression of Bax, Bcl-2, Caspase-3 and p21 was
examined by western blot analysis according to the standard
protocol. Primary antibodies used for Bcl-2 and Bax at a dilution
of 1:200 were purchased from Santa Cruz Biotechnology, Inc. (CA,
USA). Primary antibodies used for Caspase-3 and p21 at a dilution
of 1:200 were purchased from Cell Signaling Technology (Danvers,
MA, USA). Band intensities were measured using image analysis
software and expressed as ratios to β-actin (internal
reference).
Statistical analysis
The results were analyzed using one-way ANOVA with
post-test of the Bonferroni test in SPSS13.0 software. P<0.05
was considered to indicate a significant difference.
Results
Chidamide inhibits pancreatic tumor cell
proliferation
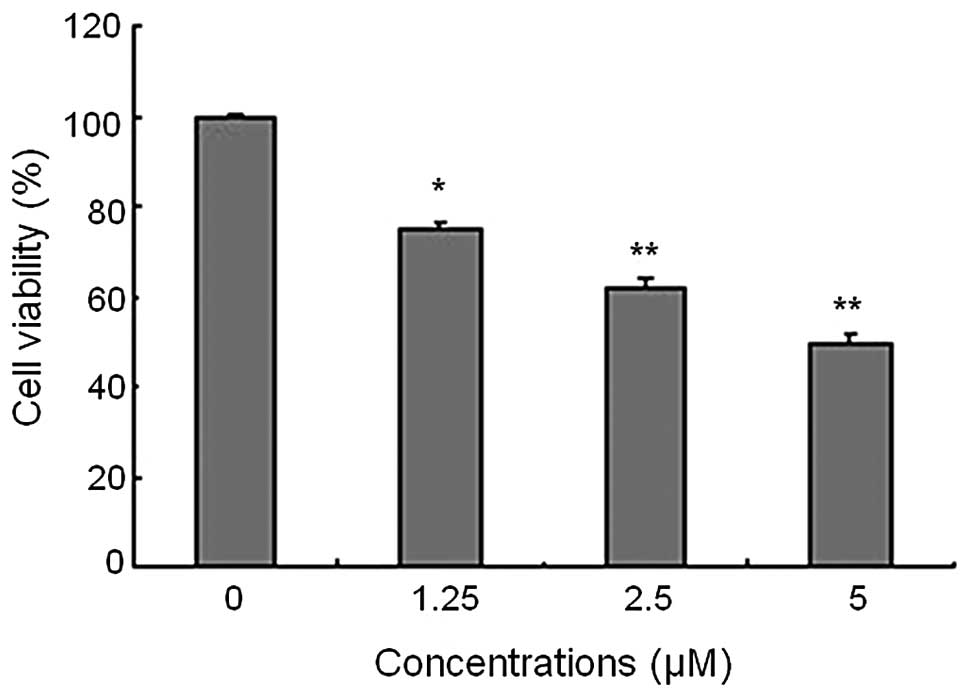
CCK-8 assay was used to examine the proliferation of
PaTu8988 cells in response to Chidamide treatment at concentrations
of 0, 1.25, 2.5 and 5 μM. As shown in Fig. 1, Chidamide caused a significant
concentration-dependent inhibitory effect on cell proliferation in
comparison to the vehicle-treated cells (P<0.05). The maximal
inhibitory effect was reached at 5 μM.
Chidamide induces cell apoptosis in vitro
and in vivo
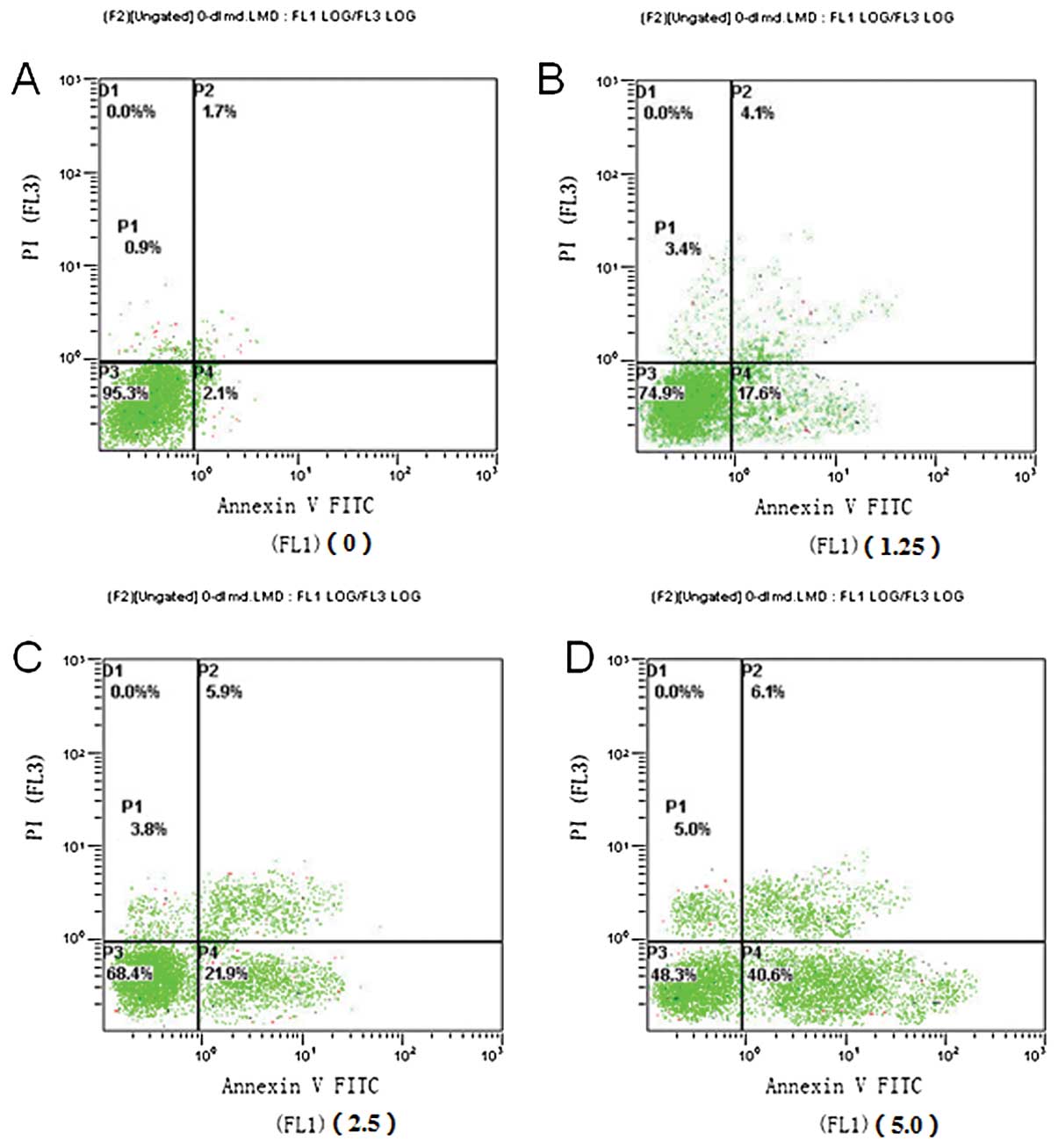
Flow cytometry was performed to examine the
apoptotic rate of PaTu8988 cells 48 h after chidamide
administration using the Annexin V-FITC/PI double staining method
(Fig. 2). Notably, cell exposure to
chidamide resulted in enhanced accumulation of autophagic, early
and late apoptotic cells in a dose-dependent manner, especially for
the early apoptotic cells.
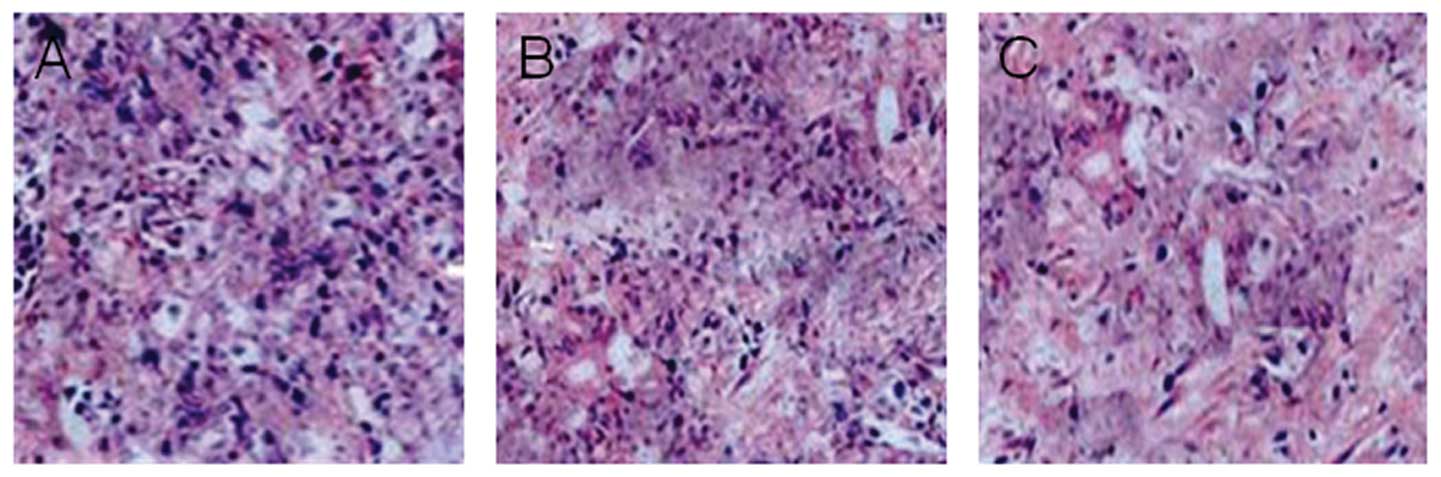
The histological morphology of the pancreatic tumors
in nude mice was examined using H&E staining. In the control
mice administered with vehicle, a compact mass of tumor cells was
observed with significant nuclear fission and mitosis. By contrast,
tumors treated with 12.5 or 50 mg/kg chidamide exhibited increased
cell apoptosis with less cell proliferation (Fig. 3A–C).
Chidamide inhibits the growth of
pancreatic tumors in nude mice
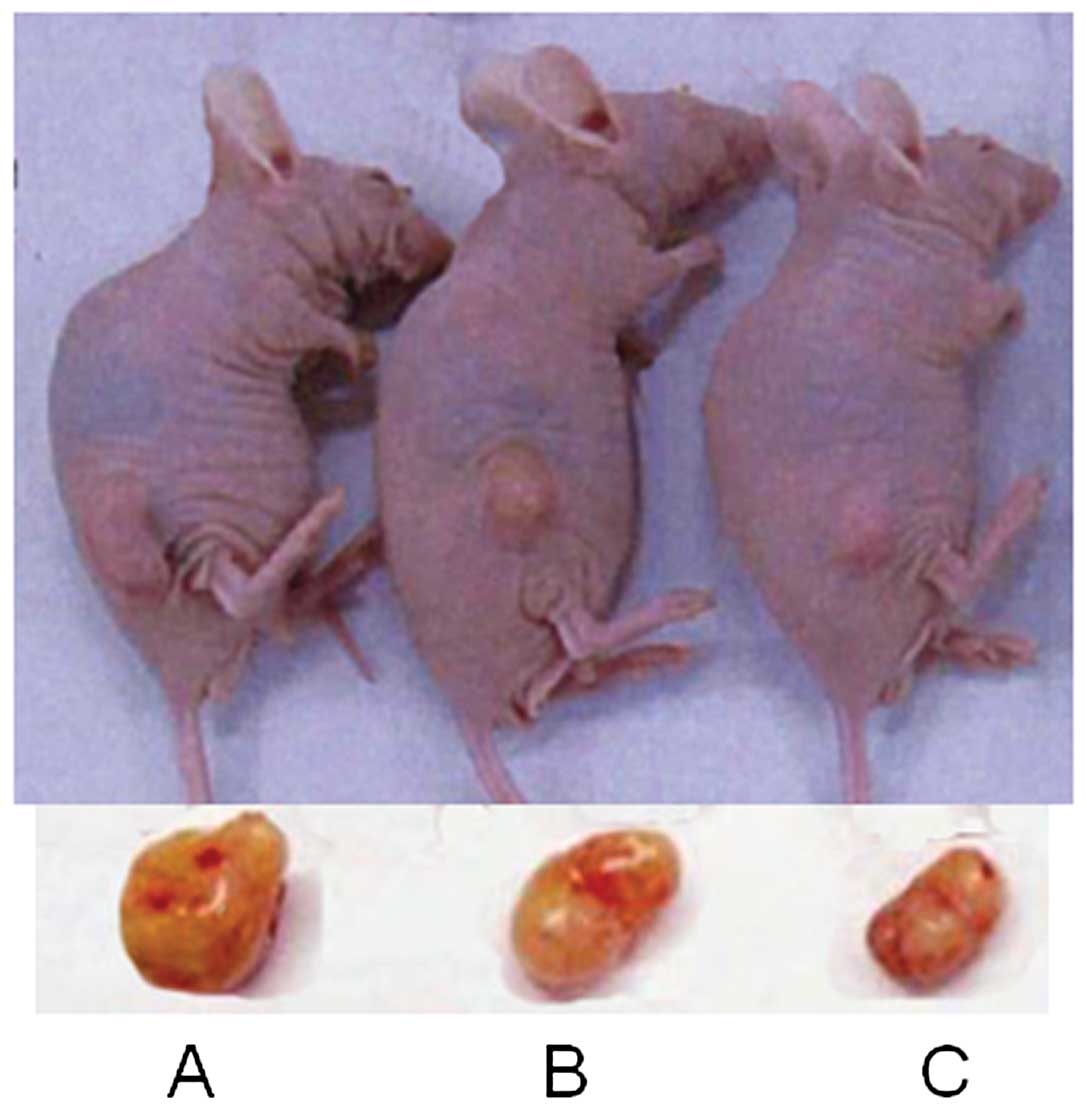
To confirm the effect of Chidamide in vivo,
pancreatic tumor nude murine models were established by
subcutaneous injection of PaTu8988 cells. On the second day after
injection, a rice grain-size tumor was palpable. On day 7, the
tumor size was ~100 mm3 and the tumor formation rate was
100%. Twenty-four mice with comparable-size tumors were selected
for subsequent experiments. The body weight of mice in the control
group was decreased, while the body weight of the mice in the
Chidamide-treated group was increased after 21 days of
administration. Increases in tumor volume and tumor weight were
also significantly arrested by Chidamide in a dose-dependent
manner. Representative examples of pancreatic tumor from mice are
shown in Fig. 4 for each group. The
tumors in Chidamide-treated mice grew at a significantly reduced
rate compared to those in the control group (P<0.05) (Table II).
 | Table IIMeasurement of variables relating to
pancreatic tumors in mice prior to and following Chidamide
treatment. |
Table II
Measurement of variables relating to
pancreatic tumors in mice prior to and following Chidamide
treatment.
| Body weight (g) | Tumor volume
(mm3) | | |
|---|
|
|
| | |
|---|
| Group | Before | After | Before | After | Tumor weight
(mg) | Suppression rate
(%) |
|---|
| Control (PBS) | 21.54±2.24 | 20.58±2.88 | 166.97±42.01 | 485.42±71.87b | 371.24±56.21 | 0 |
| Chidamide (12.5
mg/kg) | 20.85±2.73 | 24.03±3.14 | 155.98±63.27 | 213.88±49.21ac | 197.84±49.87e | 46.71±11.28 |
| Chidamide (50
mg/kg) | 22.05±225 | 22.37±3.01 | 168.09±52.84 |
187.83±31.56ad |
146.88±34.14e | 60.44±39.26 |
Effects of Chidamide treatment on the
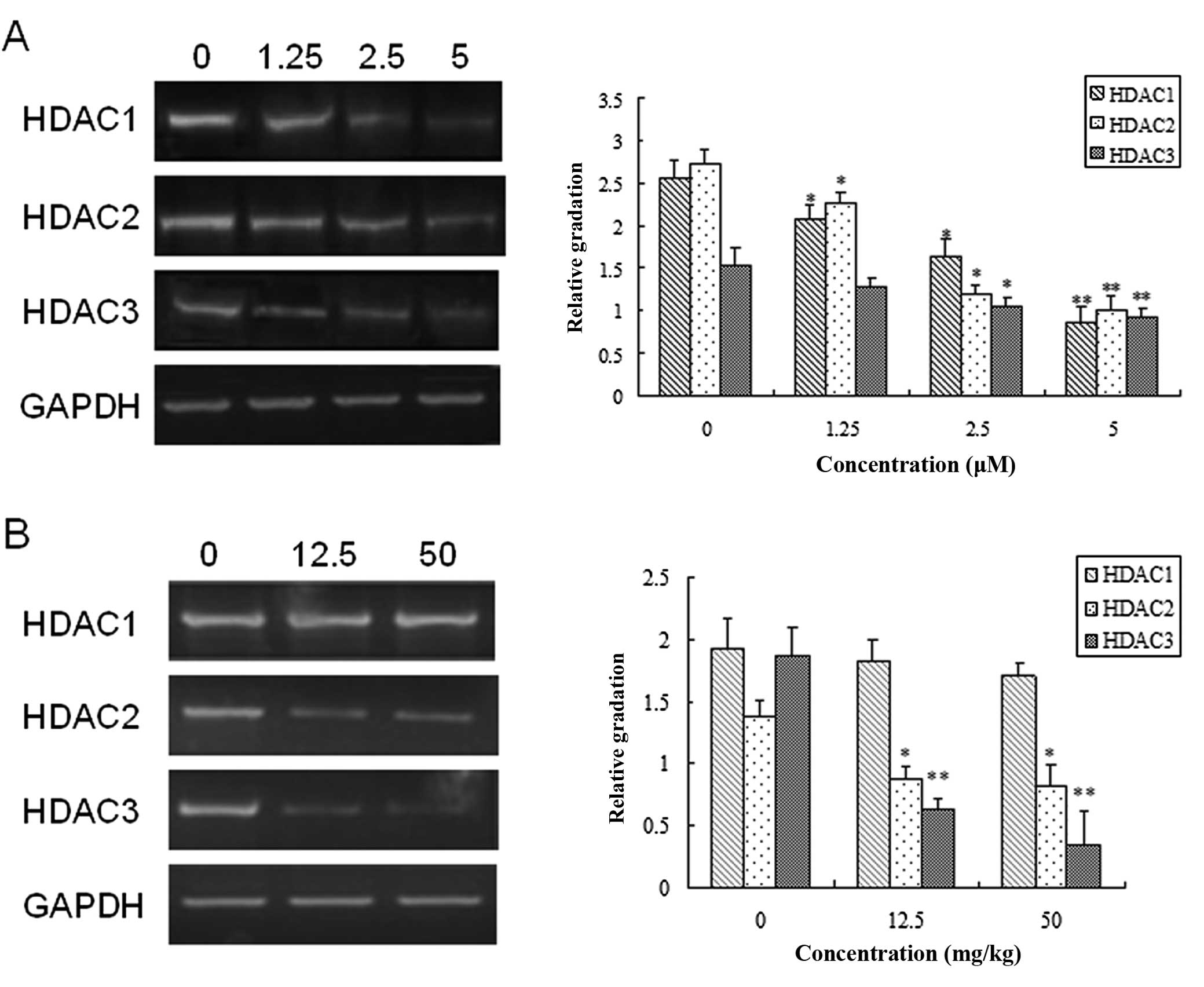
mRNA expression level of HDACs
The expression levels of different members of HDACs
class I (HDAC1, HDAC2 and HDAC3) were examined in Chidamide- and
vehicle-untreated PaTu8988 cells and pancreatic tumor mice.
Chidamide exposure significantly decreased the mRNA level of HDAC1,
HDAC2 and HDAC3 (Fig. 5A) in a
dose-dependent manner in PaTu8988 cells (P<0.05). Similarly, as
shown in Fig. 5B, in the pancreatic
tumor nude mice, Chidamide exerted a dose-dependent inhibitory
effect on the mRNA levels of HDAC2 and HDAC3 (P<0.05), but had
little impact on HDAC1 expression in pancreatic tumor nude mice at
different doses (12.5 and 50 mg/kg). Taken together, these results
suggested that Chidamide may arrest the growth of pancreatic tumor
through inhibition of the expression of HDACs, which may be
involved in the underlying mechanism for the therapeutic effect of
Chidamide on pancreatic cancer.
Chidamide decreases the expression level
of Bcl-2 and Caspase-3 and increases the expression level of Bax
and p21
To gain some insight into the molecular mechanism of
the tumor-inhibiting effect of chidamide, the protein expression
levels of Bcl-2, Bax, Caspase-3 and p21 were further investigated
in vitro and in vivo. In PaTu8988 cells, chidamide
treatment significantly enhanced the expression of Bax, but
suppressed the expression of Bcl-2 and uncleaved Caspase-3
(Fig. 6A) in a dose-dependent
manner (P<0.05). Since Caspase-3 is the downstream signal of the
mitochondrial apoptotic pathway (3), these data suggested that application
of Chidamide might promote cell apoptosis through the mitochondrial
apoptotic pathway.
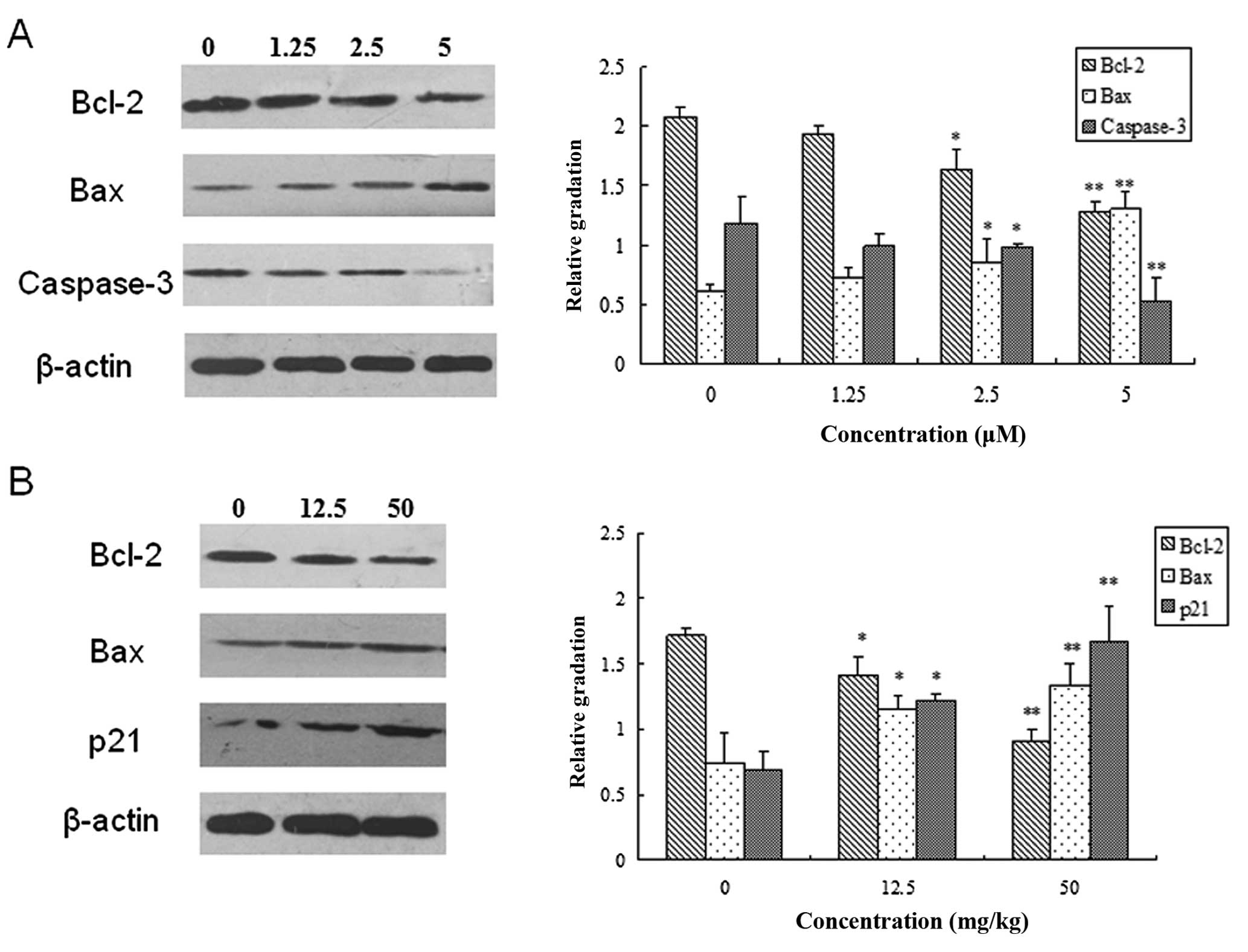 | Figure 6Chidamide modulated the expression of
B-cell lymphoma 2 (Bcl-2), Bax, Caspase-3 and p21 in pancreatic
cancer. (A) PaTu8988 cells were incubated in the absence or
presence of different concentrations (0, 1.25, 2.5 and 5 μM) of
Chidamide. Left panel, representative immunoblot of proteins; right
panel, quantitative analysis of expression of proteins as
indicated. (B) Murine tumor models were administered with PBS,
12.5mg/kg or 50 mg/kg Chidamide. Left panel, representative
immune-blot of proteins as indicated; Right, quantitative analysis
of expression of proteins as indicated. (*P<0.05;
**P<0.01, versus the control using one-way
ANOVA). |
Consistent with the result of the in vitro
experiments, in the pancreatic tumor nude mice Chidamide
administration led to a significant increase in the expression of
Bax and p21 and a decrease in the expression of Bcl-2 (Fig. 6B) (P<0.05). The effect of
Chidamide was dependent on the administration dose. The results
from the in vitro and in vivo studies provide
convincing evidence for the view that Chidamide may affect the
apoptosis and proliferation of pancreatic tumor cells by modulating
the mitochondrial apoptotic pathway and the expression of p21,
respectively.
Discussion
Pancreatic cancer is an excruciating
gastrointestinal tumor, characterized by poor prognosis and an
exceedingly high death rate (17).
Currently, there is a shortage of potent chemotherapeutic agents
with few side effects that may be used to treat the disease and
prolong patient survival. In the present study, we emphasized the
effect of Chidamide on inhibiting the proliferation of pancreatic
tumor cell in vitro and in vivo. We found that
administration of Chidamide suppressed the proliferation of
pancreatic tumor cells and induced cell apoptosis in a
dose-dependent manner.
Chidamide functions as a HDAC inhibitor which is
newly designed and synthesized in China (8) and may specifically suppress the level
of type I HDACs. Increasing findings, from in vitro studies
of tumor cell lines to in vitro studies of animal tumor
models, support that Chidamide is a potential therapeutic drug in
the treatment of a variety of cancers such as hepatocellular
carcinoma, lymphoma cancer and bladder tumor (12,18,19).
Despite substantial progress in understanding the effect of
Chidamide on cancer, the underlying molecular mechanism remains to
be determined. Therefore, we investigated the therapeutic effect of
chidamide on the treatment of pancreatic cancer and discussed
possible mechanism underlying this effect.
First, we found that Chidamide can suppress the
proliferation of pancreatic tumor cells in a dose-dependent manner
via CKK-8 assay. The homeostasis of cell proliferation is regulated
by cell apoptosis, thus it is of critical significance to target
apoptotic proteins for the treatment of pancreatic cancer. Two
major apoptosis pathways include intrinsic mitochondrial apoptosis
and extrinsic death receptor pathways or endoplasmic reticulum
stress pathways (20). The
mitochondrial apoptosis pathway is the most important pathway by
regulating the ratio of apoptotic proteins Bcl-2 and Bax.
Specifically, Bcl-2 suppresses apoptosis partly by blocking efflux
of cytochrome c which activates downstream caspase signals,
while Bax has an apoptosis-promoting effect by antagonizing Bcl-2
(12,21). Therefore, the ratio of Bcl-2/Bax
expression determines whether the apoptotic process occurs. An
increased Bax/Bcl-2 ratio can activate Caspase-3 and result in cell
death (22–24). In this study, the effect of
Chidamide on these apoptosis-related proteins was also
investigated. We found that in PaTu8988 cells, Chidamide treatment
promoted the expression of Bax, but inhibited the expression of
Bcl-2 and uncleaved Caspase-3 in a dose-dependent manner. The
results suggested that application of Chidamide significantly
enhanced the apoptotic process by activation of Caspase-3 which is
the downstream signal of the mitochondrial apoptotic pathway.
Chidamide treatment had a similar effect on the expression of Bax
and Bcl-2 in in vivo experiments of pancreatic tumor nude
mice. These findings suggest that the inhibitory effect of
Chidamide administration on pancreatic tumor cells may be partly
attributed to the activation of mitochondrial apoptosis
pathways.
It has been reported that HDAC inhibitors can induce
the activation of multiple signaling pathways, involving p53, p21
and Rb proteins that can result in cell cycle arrest and apoptosis
(25). P21, also known as
cyclin-dependent kinase inhibitor 1 has an important role in
modulating cell cycle and mediating cell senescence. P21 plays a
positive role in the suppression of tumor cell growth and
proliferation (26–28). Our study results revealed that
Chidamide administration inhibited pancreatic tumor growth by
promoting p21 expression. HDAC is an enzyme family in which type I
HDAC includes HDAC1, HDAC2, HDAC3 and HDAC8. The mRNA level of
HDAC2 and HDAC3 was significantly decreased in human pancreatic
cancer cells and pancreatic tumor nude mice in response to
Chidamide administration. The inhibitory effect of Chidamide was
positively correlated with its administration dose. Taken together,
these results suggest that HDACs may participate in the growth of
pancreatic tumor and Chidamide administration may suppress tumor
growth through inhibition of HDAC and promotion of p21 expression.
Thus, p21 is a potential candidate target for the therapeutic
treatment of pancreatic cancer.
In conclusion, this study have confirmed the
inhibitory effect of Chidamide on pancreatic cells by in
vitro and in vivo studies and suggested that Chidamide
may promote cell apoptosis via mitochondrial apoptosis pathway and
inhibit cell growth by downregulating the expression of p21, thus
suppressing the progression of pancreatic cancer. Our study
provides strong evidence for the clinical application of Chidamide
as a tumor-inhibiting agent and promoted the development of novel
therapeutic strategies with high efficacy that could lead to
improved treatment of the pancreatic cancer. Further studies on the
combined effect of Chidamide and other chemotherapeutic agents are
necessary for clinical application.
Acknowledgements
This study was sponsored by the Shanghai Pujiang
Program (13PJD001).
References
|
1
|
Lennon AM, Wolfgang CL, Canto MI, et al:
The early detection of pancreatic cancer: What will it take to
diagnose and treat curable pancreatic neoplasia? Cancer Res.
74:3381–3389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu L, Yuan H, Guo C, et al: Zearalenone
induces apoptosis and necrosis in porcine granulosa cells via a
caspase-3- and caspase-9-dependent mitochondrial signaling pathway.
J Cell Physiol. 227:1814–1820. 2012. View Article : Google Scholar
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaib Y, Davila J and El-Serag H: The
epidemiology of pancreatic cancer in the United States: changes
below the surface. Aliment Pharmacol Ther. 24:87–94. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sultana A, Smith CT, Cunningham D,
Starling N, Neoptolemos JP and Ghaneh P: Meta-analyses of
chemotherapy for locally advanced and metastatic pancreatic cancer.
J Clin Oncol. 25:2607–2615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burris HA III, Moore MJ, Andersen J, et
al: Improvements in survival and clinical benefit with gemcitabine
as first-line therapy for patients with advanced pancreas cancer: a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
8
|
Liu L, Chen B, Qin S, et al: A novel
histone deacetylase inhibitor Chidamide induces apoptosis of human
colon cancer cells. Biochem Biophys Res Commun. 392:190–195. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong M, Ning ZQ, Xing PY, et al: Phase I
study of chidamide (CS055/HBI-8000), a new histone deacetylase
inhibitor, in patients with advanced solid tumors and lymphomas.
Cancer Chemother Pharmacol. 69:1413–1422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Pan DS, Shan S, et al: Non-toxic
dose chidamide synergistically enhances platinum-induced DNA damage
responses and apoptosis in non-small-cell lung cancer cells. Biomed
Pharmacother. 68:483–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Chen B, Qin S, et al: A novel
histone deacetylase inhibitor Chidamide induces apoptosis of human
colon cancer cells. Biochem Biophys Res Commun. 392:190–195. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Guo Y, Fu M, et al: Antitumor
activity of Chidamide in hepatocellular carcinoma cell lines. Mol
Med Rep. 5:1503–1508. 2012.PubMed/NCBI
|
|
13
|
Ning ZQ, Li ZB, Newman MJ, et al:
Chidamide(CS055/HBI-8000): a new histone deacetylase inhibitor of
the benzamide class with antitumor activity and the ability to
enhance immune cell-mediated tumor cell cytotoxicity. Cancer
Chemother Pharmacol. 69:901–909. 2012. View Article : Google Scholar
|
|
14
|
Gong K, Xie J, Yi H and Li W: CS055
(Chidamide/HBI-8000), a novel histone deacetylase inhibitor,
induces G1 arrest, ROS-dependent apoptosis and differentiation in
human leukaemia cells. Biochem J. 443:735–746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiao Z, Ren S, Li W, et al: Chidamide, a
novel histone deacetylase inhibitor, synergistically enhances
gemcitabine cytotoxicity in pancreatic cancer cells. Biochem
Biophys Res Commun. 434:95–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao Z, Ren S, Li W, et al: Chidamide, a
novel histone deacetylase inhibitor, synergistically enhances
gemcitabine cytotoxicity in pancreatic cancer cells. Biochem
Biophys Res Commun. 434:95–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yen MC, Weng TY, Chen YL, et al: An HDAC
inhibitor enhances cancer therapeutic efficiency of RNA polymerase
III promoter-driven IDO shRNA. Cancer Gene Ther. 20:351–357. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ning Z-Q, Li Z-B, Newman MJ, et al:
Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of
the benzamide class with antitumor activity and the ability to
enhance immune cell-mediated tumor cell cytotoxicity. Cancer
Chemother Pharmacol. 69:901–909. 2012. View Article : Google Scholar
|
|
20
|
Fiandalo MV and Kyprianou N: Caspase
control: protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
21
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siu WP, Pun PB, Latchoumycandane C and
Boelsterli UA: Bax-mediated mitochondrial outer membrane
permeabilization (MOMP), distinct from the mitochondrial
permeability transition, is a key mechanism in diclofenac-induced
hepatocyte injury: Multiple protective roles of cyclosporin A.
Toxicol Appl Pharmacol. 227:451–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohtsuka T, Buchsbaum D, Oliver P, Makhija
S, Kimberly R and Zhou T: Synergistic induction of tumor cell
apoptosis by death receptor antibody and chemotherapy agent through
JNK/p38 and mitochondrial death pathway. Oncogene. 22:2034–2044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang WJ, Liang YC, Chuang SE, et al:
NBM-HD-1: A novel histone deacetylase inhibitor with anticancer
activity. Evid Based Complement Alternat Med. 2012:7814172012.
View Article : Google Scholar
|
|
26
|
Ohashi K, Nemoto T, Eishi Y, Matsuno A,
Nakamura K and Hirokawa K: Expression of the cyclin dependent
kinase inhibitor p21WAF1/CIP1 in oesophageal squamous cell
carcinomas. Virchows Arch. 430:389–395. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai L, Liu Y, Liu J, et al: A novel
cyclinE/cyclinA-CDK inhibitor targets p27Kip1
degradation, cell cycle progression and cell survival: implications
in cancer therapy. Cancer Lett. 333:103–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoefer J, Schäfer G, Klocker H, et al:
PIAS1 is increased in human prostate cancer and enhances
proliferation through inhibition of p21. Am J Pathol.
180:2097–2107. 2012. View Article : Google Scholar : PubMed/NCBI
|