Introduction
Angiogenesis, the formation of new blood vessels
which includes activation of endothelial cells and recruitment of
pericytes, plays important roles in cancer growth and metastasis.
This response is controlled by changes in a delicate balance
between angiogenic and anti-angiogenic factors (1,2).
Vascular endothelial growth factor (VEGF) and the VEGF receptor
(VEGFR) have been well characterized as key factors that trigger
signaling events in angiogenesis. VEGF-A, one of the most common
members of the VEGF family, binds to its cognate receptor VEGFR-2
and activates signaling pathways, resulting in endothelial cell
proliferation, migration, survival and vascular permeability
associated with cancer progression. Thus, selective inhibition of
VEGF-A/VEGFR-2 activation or the downstream signaling pathways is
appreciated as a potent strategy, compared to conventional
chemotherapy. Many drugs that target VEGF, VEGFR and the downstream
signaling pathways are currently in clinical trials or use
(3–6).
Siegesbeckia glabrescens (SG) Makino
(Compositae) has been used as a traditional medicine for the
treatment of acute hepatitis, paralysis, hypertension, asthma and
rheumatoid arthritis. The extracts and bioactive components of SG
have anti-inflammatory, anti-allergic and anticancer activities
(7–10). We previously reported that the
anticancer activity of SG against ovarian cancer and non-small cell
lung cancer cells is mediated through downregulation of receptor
tyrosine kinases and their signaling pathways (11,12).
In addition, a recent study demonstrated that SG has
anti-angiogenic and anti-adipogenic activities (13). However, no detailed mechanisms of SG
responsible for the regulation of angiogenesis have been clearly
elucidated to date. In the present study, we evaluated the
regulatory effects and signaling pathways of SG on proliferation,
adhesion, migration and tube formation in human umbilical vein
endothelial cells (HUVECs). We showed for the first time that the
anti-angiogenic activity of SG in VEGF-A-treated HUVECs is mainly
mediated through inactivation of the VEGF-A/VEGFR-2 downstream
signaling pathways such as Akt and p70S6K.
Materials and methods
Cell culture conditions
Primary cultures of HUVECs were purchased from Lonza
Walkersville Inc. (Walkersville, MD, USA) and used between passages
4 and 6 for all experiments. Cells were cultured in
EGM-2® BulletKit media, according to the manufacturer’s
instructions (Lonza).
Reagents
The following pharmacological agents and antibodies
were purchased from commercial sources: VEGF-A and LY294002 [an
inhibitor of the phosphatidylinositol3-kinase (PI3-K)/Akt pathway;
Merck Millipore, Billerica, MA, USA]; rapamycin [an inhibitor of
the mammalian target of rapamycin (mTOR)/p70S6K pathway;
Sigma-Aldrich, St. Louis, MO, USA]; anti-phospho-extracellular
signal-regulated kinase (ERK) (T202/Y204), anti-phospho-Akt (S473),
anti-phospho-p70S6K (T421/S424) and anti-phospho-pRb
(S780) (Cell Signaling Technology Inc., Beverly, MA, USA);
anti-p27Kip1 (BD Biosciences, Bedford, MA, USA);
anti-ERK, anti-Akt, anti-p70S6K, anti-Cdk4, anti-Cdk2,
anti-cyclin D, anti-cyclin E, anti-p27WAF1/Cip1,
anti-actin antibodies, and mouse and rabbit IgG-horseradish
peroxidase conjugates (Santa Cruz Biotechnology, Santa Cruz, CA,
USA).
Preparation of the SG extract
SG was purchased from Dae Kwang Herb Medicine Co.
(Chuncheon, Gangwon-do, Korea) and deposited at the herbarium of
the Radiant Research Institute (Radiant Inc., Chuncheon,
Gangwon-do, Korea). One hundred grams of SG was extracted with 1
liter of ethanol and stirred for 90 min. The extract of SG was
obtained as previously reported (11,12).
Cell viability and proliferation
assay
Subconfluent cells, plated on 6-well plates
(1×105 cells/well; BD Biosciences), were serum-starved
for 14 h to synchronize cells in the G1/G0
phase of the cell cycle, pretreated with SG (0.01–1 μg/ml) for 30
min in the presence or absence of LY294002 (10 μM) or rapamycin (50
nM) as indicated and further incubated with VEGF-A (10 ng/ml) for
24 h. Following culture for 24 h, cell viability was determined by
a Muse™ cell analyzer using a cell count and viability assay kit
(Merck Millipore), and the cell proliferation was quantified as
previously described (14). The
results from triplicate determinations (mean ± standard deviation)
are presented as the fold-increase of the untreated controls or the
percentage of viable cells of the total cell count.
Cell cycle analysis
Serum-starved cells were pretreated with SG (1
μg/ml) for 30 min, followed by VEGF-A (10 ng/ml) for 24 h. Cells
were harvested with trypsin-EDTA, rinsed with phosphate-buffered
saline (PBS, pH 7.4) and then fixed with ice-cold 70% ethanol for 3
h. After washing with PBS, cells were stained with Muse™ cell cycle
reagent. The profile of cells in the G1/G0, S
and G2/M phases of the cell cycle was analyzed with a
Muse™ cell analyzer (Merck Millipore) (15).
Western blot analysis
Subconfluent cells in 100-mm dishes
(1×106 cells/dish; BD Biosciences) were serum-starved
for 14 h, pretreated with SG for 30 min followed by VEGF-A (10
ng/ml) for 15 min or 24 h, as indicated. Cells were rinsed twice
with ice-cold PBS and lysed by incubation in 50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EDTA, 100
μg/ml 4-(2-aminoethyl)benzenesulfonyl fluoride, 10 μg/ml aprotinin,
1 μg/ml pepstatin A, 0.5 μg/ml leupeptin, 80 mM β-glycerophosphate,
25 mM sodium fluoride and 1 mM sodium orthovanadate for 30 min at
4°C. Cell lysates were clarified at 12,500 × g for 20 min at 4°C,
and the supernatants were subjected to western blot analysis as
described previously (16,17). All western blot analyses were
performed at least in triplicate experiments and representative
gels are shown. Bands of interest were integrated and quantified by
the use of National Institutes of Health (NIH) ImageJ Version 1.34s
software.
Adhesion assay
Subconfluent cells were detached with trypsin-EDTA
and allowed to recover in EGM-2® BulletKit media for 1 h
at 37°C with gentle rocking. After recovery, the cells were
collected by low-speed centrifugation and resuspended in serum-free
EBM-2 media (Lonza). The cell suspension was pretreated with or
without SG (1 μg/ml) for 30 min followed by VEGF-A (10 ng/ml)
treatment. The cells were plated on 96-well plates
(1.5×104 cells/well) and further incubated for 2 h at
37°C. Following the incubation, the unattached cells were removed
by washing the wells 3 times with ice-cold PBS. Attached cells were
fixed with methanol and then stained with 0.04% Giemsa staining
solution (Sigma-Aldrich). The cells were photographed and counted.
The results (mean ± standard deviation) are presented as the
numbers of adherent cells (18).
Migration assay
Cell migration was quantified in the in vitro
wound-healing assay as described previously (14,19).
After cells were plated on 48-well plates and grown to confluence,
a single wound was created in the center of the cell monolayer by
the gentle removal of the attached cells with a sterile plastic
pipette tip. Following serum starvation with EBM-2 for 2 h, cells
were pretreated with SG (0.01–1 μg/ml) for 30 min in the presence
or absence of LY294002 (10 μM) or rapamycin (50 nM) as indicated,
followed by VEGF-A (10 ng/ml) stimulation for 15 h. Cells were
fixed with methanol and then stained with 0.04% Giemsa solution.
The migration of the cells into the wound was observed with still
images captured at the indicated time-point.
Tube formation assays
Matrigel® basement membrane matrix (10.4
mg/ml, BD Biosciences) was thawed overnight at 4°C, and each well
of pre-chilled 24-well plates was coated with 200 μl Matrigel and
then incubated at 37°C for 30 min. Following serum starvation with
EBM-2 for 2 h, cells (4×104 cells/ml) were added to the
Matrigel-coated plates and pretreated with SG (1 μg/ml) for 30 min,
followed by VEGF-A (10 ng/ml) for 6 h. Tube formation was observed
with an Olympus CKX41 inverted microscope (CAchN 10/0.25php
objective) and ToupTek Toupview software (version x86, 3.5.563,
Hangzhou ToupTek Photonics Co., Zhejiang, China).
Statistical analysis
Statistical analysis was performed using the
Student’s t-test and was based on at least 3 different experiments.
The results were considered to be statistically significant at
P<0.05.
Results
SG suppresses VEGF-A-stimulated
endothelial cell proliferation through regulating the expression of
cyclin D and cyclin-dependent kinase inhibitors
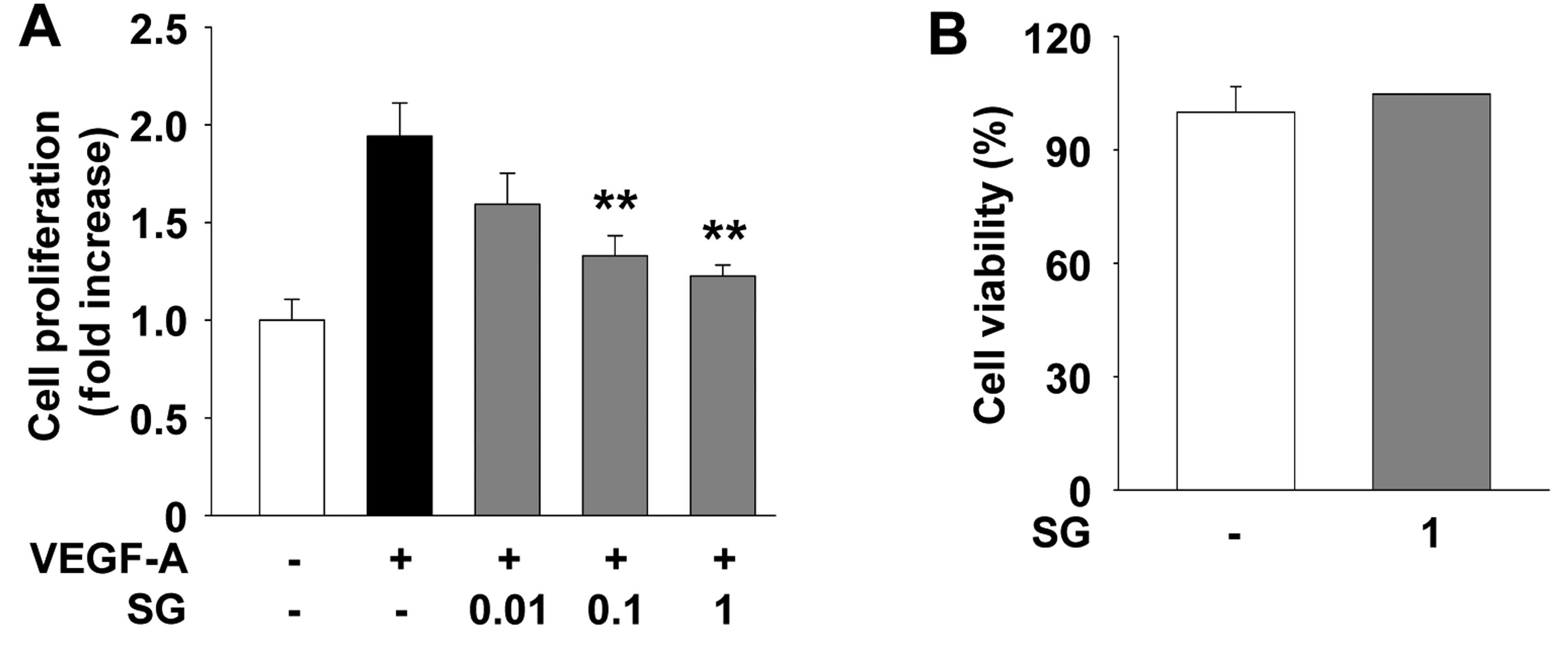
We first examined the effect of SG on cell
proliferation of HUVECs. SG treatment suppressed VEGF-A-stimulated
endothelial cell proliferation in a dose-dependent manner (Fig. 1A). In addition, treatment of
non-stimulated HUVECs with SG at the highest concentration used in
this study did not alter the viability or the proliferative
response of HUVECs (Fig. 1B),
indicating that SG inhibition of cell proliferation was not
mediated by induction of apoptosis or cytotoxicity. This finding is
similar to the patterns of SG in other cell types as previously
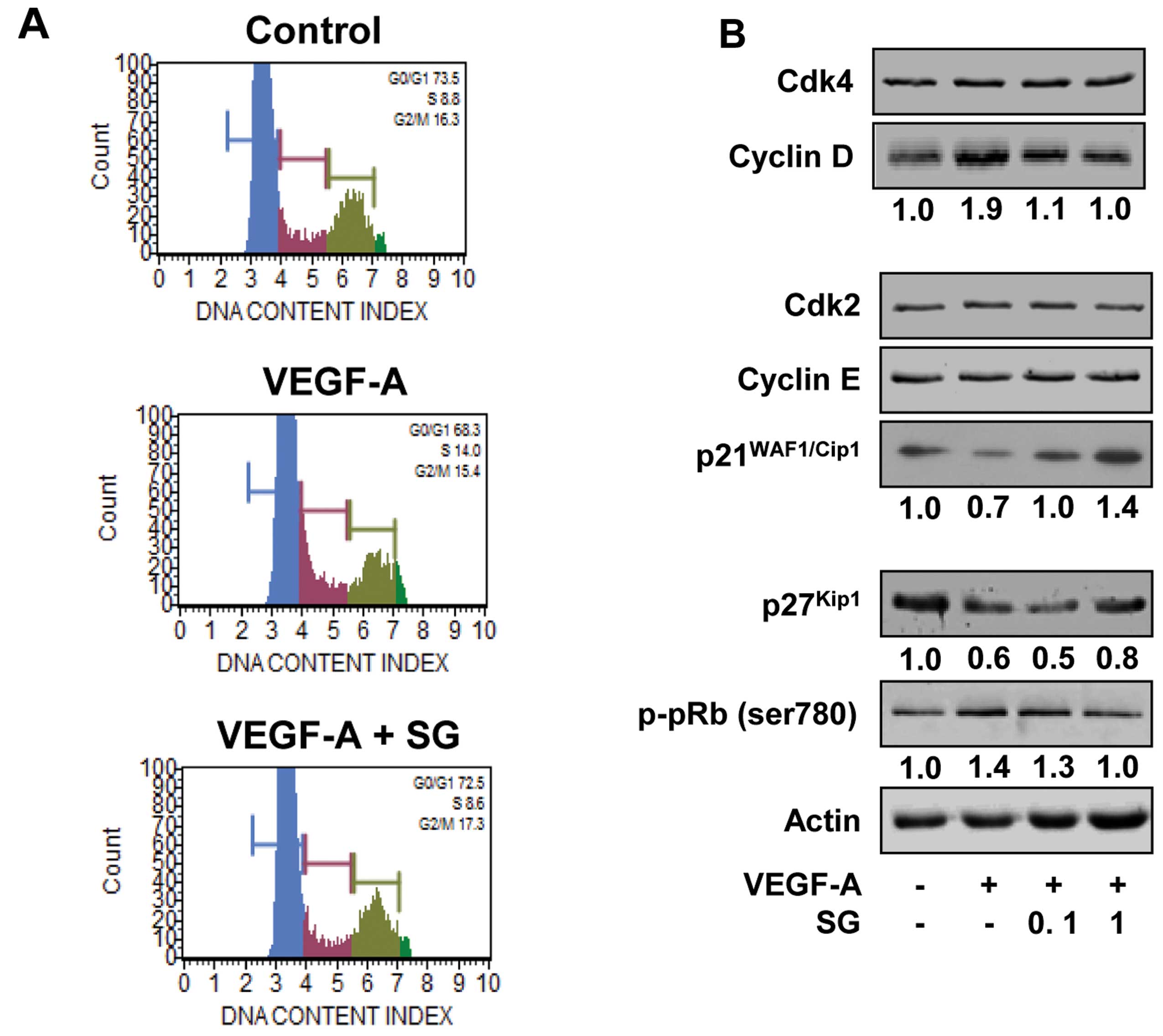
reported (11,12). We next investigated the effect of SG
on the cell cycle by DNA content analysis (Fig. 2A). VEGF-A stimulation for 24 h
increased the percentage of cells in the S phase, compared with the
untreated controls (8.8 vs. 14.0%) and resulted in the concomitant
decrease of cells in the G1 phase (73.5 vs. 68.3%). SG
treatment prevented the increase in the percentage of cells in the
S phase (14.0 vs. 8.6%) and the decrease in the percentage of cells
in the G1 phase (68.3 vs. 72.5%) associated with VEGF-A
stimulation, similar to those of the untreated controls. These
findings indicate that SG inhibits the transition from
G1 to S phase, leading to G1 arrest, which is
well correlated with inhibition of cell proliferation (Fig. 1A). SG has previously been reported
to inhibit proliferation by downregulation of cyclin-dependent
kinase 4 (Cdk4), cyclin D and cyclin E and upregulation of
p27Kip1 in SKOV-3 ovarian cancer cells (11). In addition, SG-mediated inhibition
of proliferation in A549 and H1299 non-small cell lung cancer cells
was mediated by suppression of Cdk4 and Cdk2 (12). Based on these observations, we
analyzed the changes in cell cycle-related proteins such as Cdks,
cyclins and Cdk inhibitors in the SG-treated HUVECs. As shown in
Fig. 2B, SG treatment markedly
suppressed the expression of cyclin D and induced the levels of Cdk
inhibitors such as p21WAF1/Cip1 and p27Kip1,
resulting in inhibition of pRb phosphorylation in response to
VEGF-A stimulation. Although the molecular mechanism of SG in
regulating cell cycle progression appears slightly different in
various cell types, these findings clearly demonstrate the
antiproliferative activity of SG against various types of
cells.
SG abrogates VEGF-A-stimulated
endothelial cell migration and tube formation
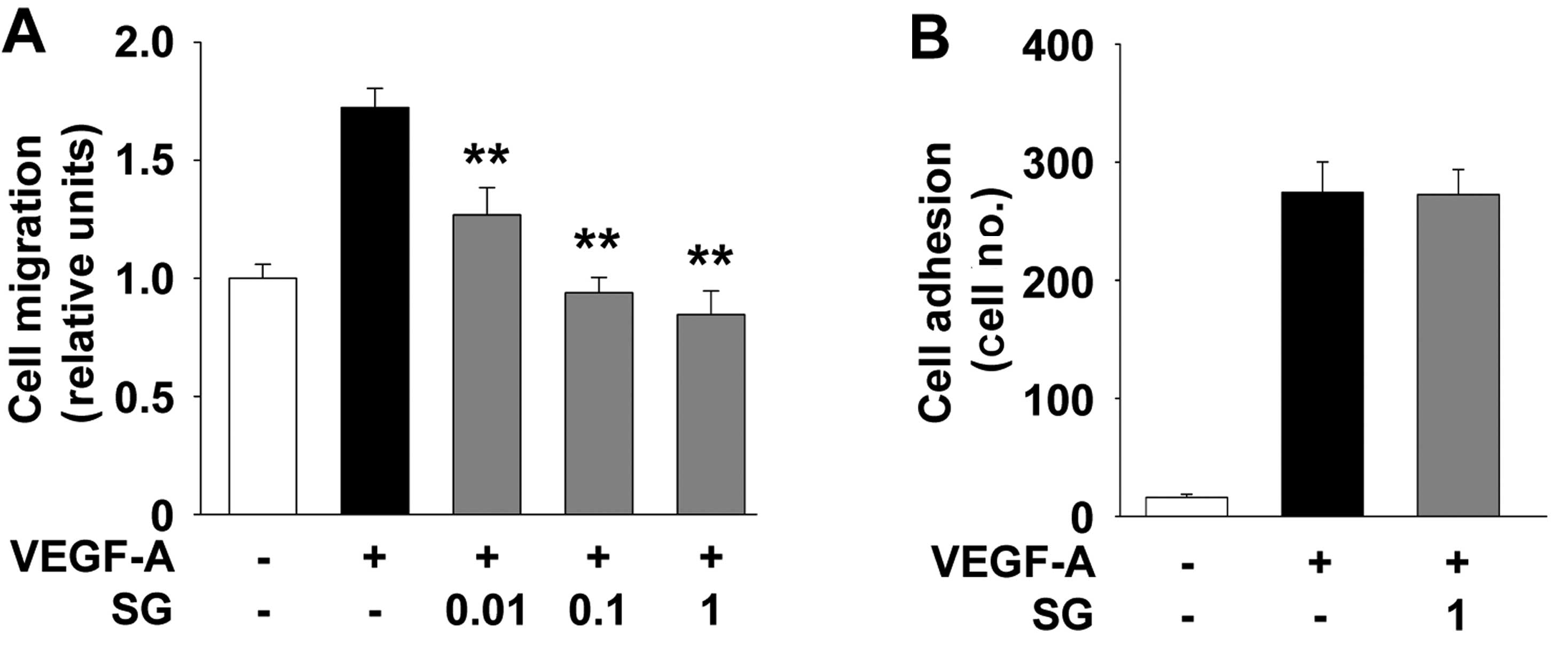
Interaction of endothelial cells with extracellular
matrix molecules plays important roles in cell migration, adhesion
and capillary-like structure formation which are associated with
cancer growth and progression (1,2). SG
treatment inhibited VEGF-A-stimulated cell migration in a
dose-dependent manner (Fig. 3A). In
contrast to cell migration, SG did not alter the VEGF-A-induced
endothelial cell adhesion (Fig.
3B), indicating that stable adhesiveness may contribute, at
least in part, to reduced cell migration as previously reported
(20,21). However, in SKOV-3 ovarian cancer
cells SG was found to markedly simultaneously block mitogen-induced
cell adhesion as well as migration (11). Collectively, these findings indicate
that SG may differentially regulate cell adhesion and migration,
depending on the type of cells or growth factor. We next
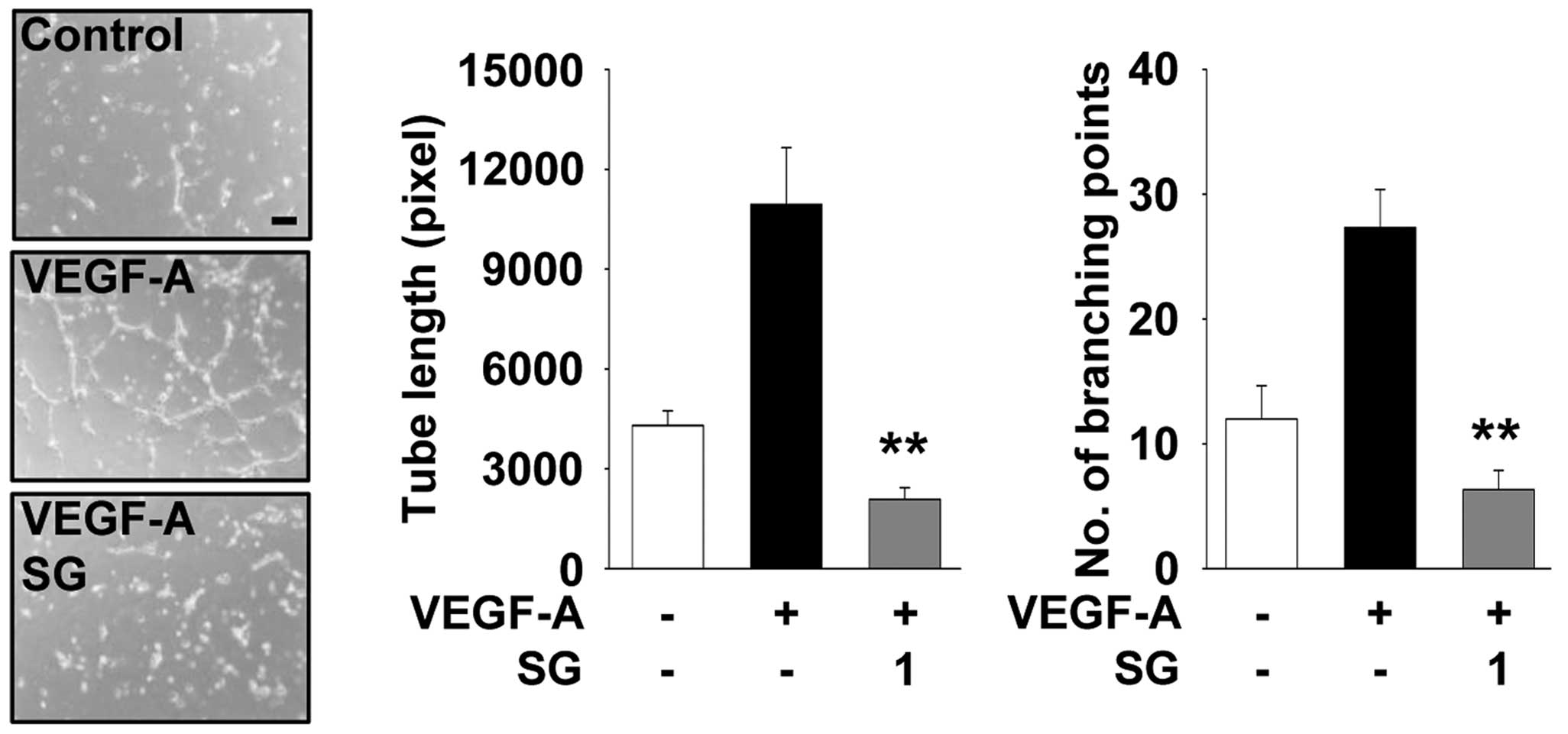
investigated the ability of SG to regulate the formation of
capillary-like structures in HUVECs. As shown in Fig. 4, SG completely inhibited
VEGF-A-stimulated tube formation to the levels observed in the
untreated controls, similar to inhibition of cell migration
(Fig. 3A). These observations
suggest that the ethanolic extract of SG possesses a variety of
biologically active components which act on multiple targets and
mechanisms involved in the regulation of cell proliferation,
migration and tube formation in HUVECs.
Anti-proliferative and anti-migratory
effects of SG are mediated through inhibition of the Akt- and
p70S6K-dependent signaling pathways
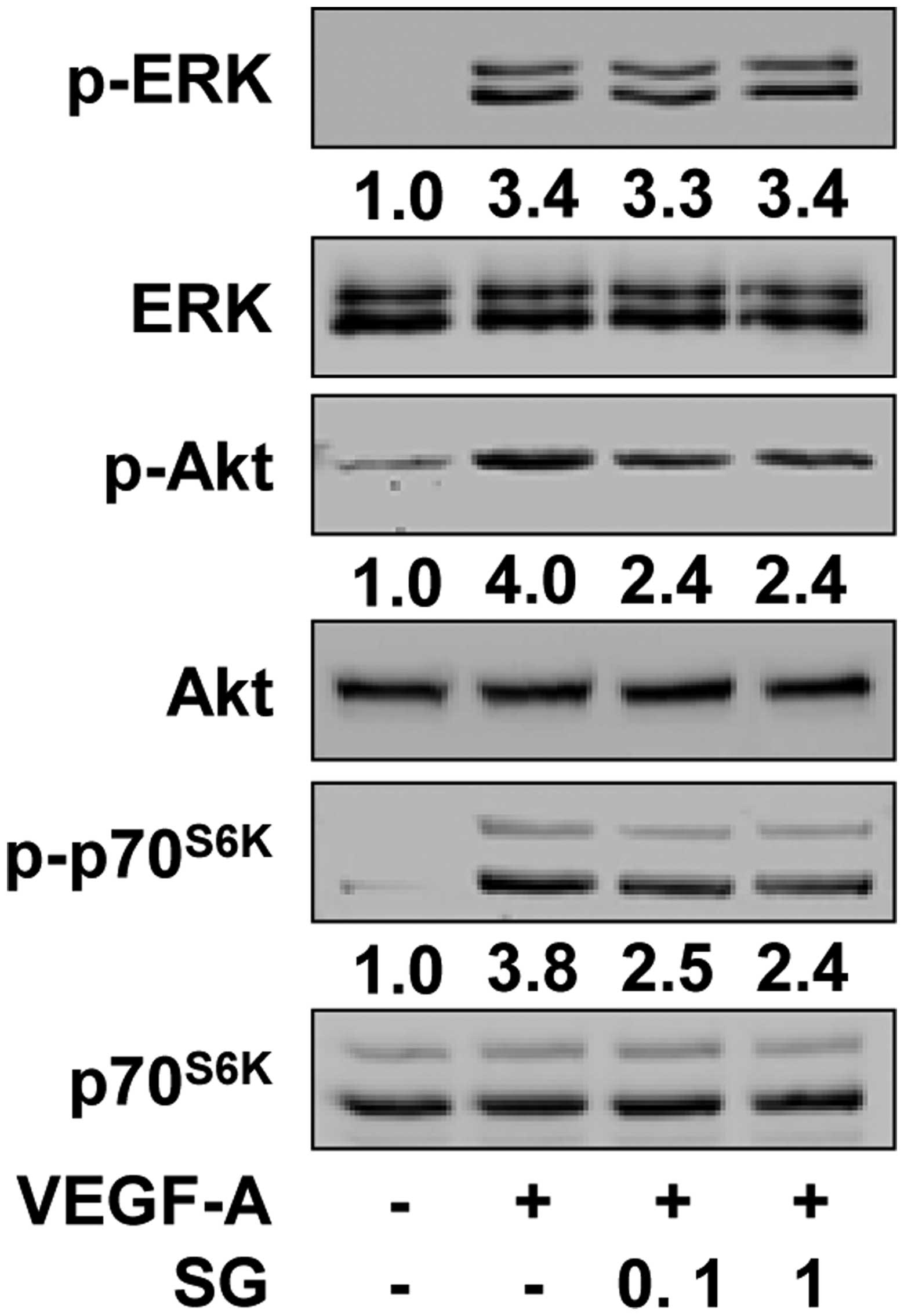
To investigate the molecular mechanisms by which SG
regulates VEGF-A-induced endothelial cell responses, we examined
the changes in the activation of VEGF-A/VEGFR-2 downstream
signaling pathways including ERK, PI3-K/Akt and
mTOR/p70S6K, which play important roles in cellular fate
(22). Compared with the
unstimulated controls, VEGF-A treatment markedly increased the
phosphorylation/activation of ERK, Akt and p70S6K in
HUVECs (Fig. 5). However, SG
treatment significantly inhibited VEGF-A-stimulated phosphorylation
of Akt and p70S6K, but not that of ERK. To directly
examine the contribution of inactivation of Akt and
p70S6K activity to the anti-angiogenic activity of SG,
we studied the changes in cell proliferation and migration in the
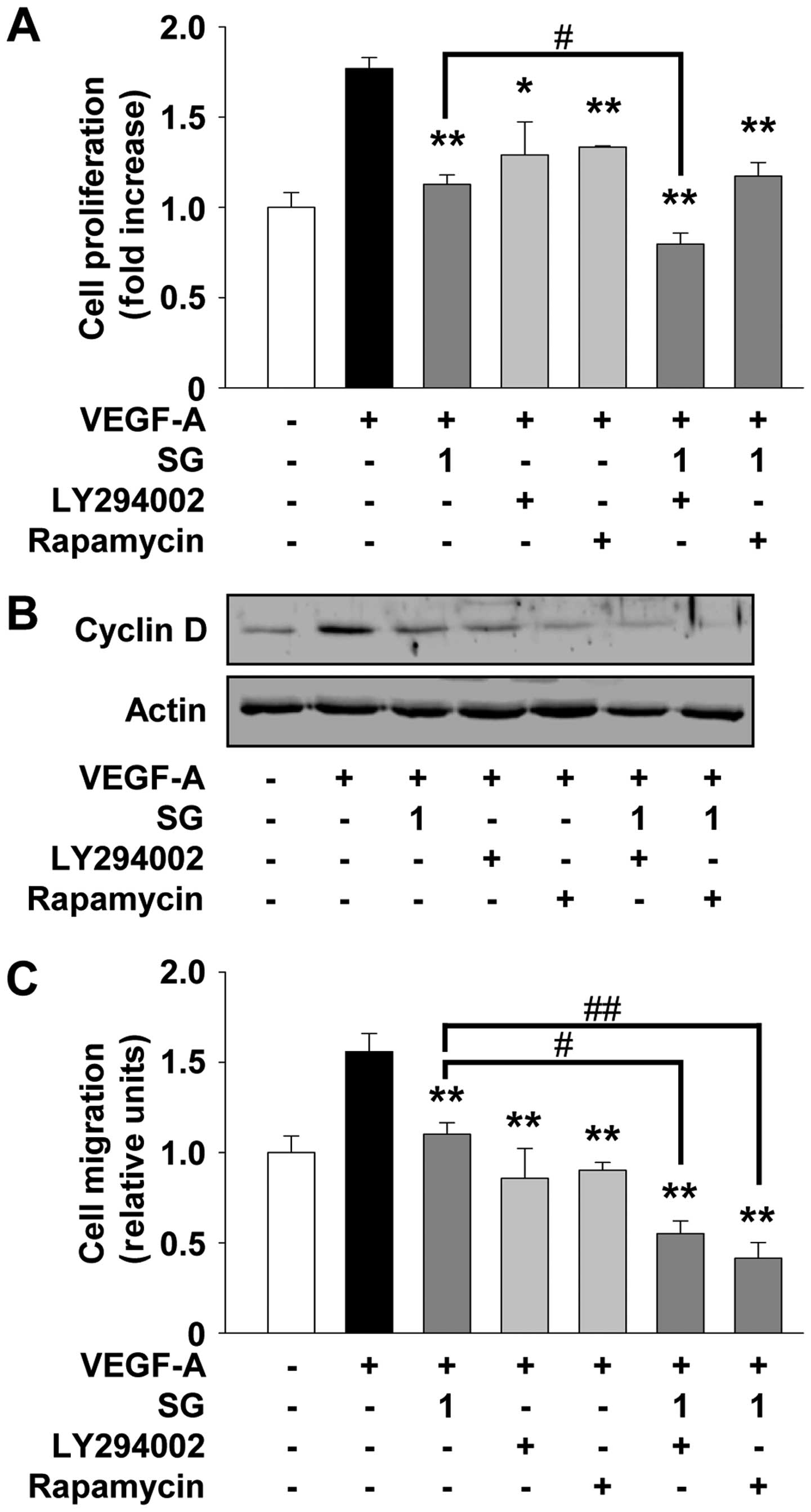
presence of LY294002 and rapamycin (Fig. 6). Pretreatment of cells with
LY294002 or rapamycin mimicked the suppressive effect of SG on
VEGF-A-stimulated cell proliferation and migration (Fig. 6A and C). As shown in Fig. 6B, both LY294002 and rapamycin also
mimicked the SG-mediated suppression of cyclin D expression, with a
high correlation with previous cell proliferation and cell
cycle-related protein expression experiments (Fig. 1A and 2B). The addition of LY294002 significantly
enhanced the ability of SG to inhibit cell proliferation and cyclin
D expression as well as migration (Fig.
6). Rapamycin treatment enhanced the inhibitory effect of SG on
cell migration, but not cell proliferation (Fig. 6A and C). Collectively, these
observations suggest that SG may contain pharmacologically
effective components similar to these inhibitors and share the
roles and mechanisms of action in regulating angiogenic responses
in vitro.
Discussion
Siegesbeckia glabrescens has been used as a
traditional medicine for the treatment of several diseases
including acute hepatitis, paralysis, hypertension, asthma and
rheumatoid arthritis. These applications are well supported by
previous investigations that SG possesses biologically active
components to reduce allergic and inflammatory responses (8,9,23). In
addition, SG has been reported to have anticancer activity against
several different cell lines including breast cancer, ovarian
cancer and non-small cell lung cancer (10–12). A
recent study demonstrated that SG exerts anti-angiogenic and
anti-adipogenic activities (13).
However, the effects and molecular mechanisms of SG in angiogenesis
have not yet been clearly identified. In the present study, we
demonstrated for the first time that the ethanol extract of SG
inhibited VEGF-A-stimulated endothelial cell proliferation,
migration and capillary-like structure formation. These
anti-angiogenic activities of SG in the VEGF-A-treated HUVECs were
found to be mediated through the inactivation of VEGF-A/VEGFR-2
downstream signaling pathways such as Akt and
p70S6K.
Angiogenic stimulation by the VEGF-A/VEGFR-2
signaling pathways includes the secretion and activation of matrix
metalloproteinases (MMPs), resulting in the degradation of the
extracellular matrix and remodeling of the tissue microenvironment
associated with cell growth, migration and invasion (20,24–28).
Based on the regulatory effects of SG on angiogenic responses in
vitro, we examined the ability of SG to alter the levels of
MMP-2 and tissue inhibitors of metalloproteinases-2 (TIMP-2), an
endogenous inhibitor of MMPs, in VEGF-A-treated HUVECs (29–31).
SG treatment showed little or no change of MMP-2 and TIMP-2
expression (data not shown), similar to previous studies in other
types of cells (11,12). These findings indicate that the
anti-angiogenic activity of SG may not require the regulation of
MMP-2 and TIMP-2, however, it does not rule out the possibility
that SG might modulate the expression and activity of other MMP and
TIMP family members.
In conclusion, this study describes the
pharmacological roles and mechanisms of SG in the regulation of
angiogenesis. Further investigation of SG is warranted in regards
to the prevention and treatment of a variety of diseases associated
with angiogenesis.
Acknowledgements
This study was supported by the research fund of
Dankook University in 2013.
References
|
1
|
Folkman J: Angiogenesis: an organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown DM and Regillo CD: Anti-VEGF agents
in the treatment of neovascular age-related macular degeneration:
applying clinical trial results to the treatment of everyday
patients. Am J Ophthalmol. 144:627–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cook KM and Figg WD: Angiogenesis
inhibitors: current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ng EW, Shima DT, Calias P, Cunningham ET
Jr, Guyer DR and Adamis AP: Pegaptanib, a targeted anti-VEGF
aptamer for ocular vascular disease. Nat Rev Drug Discov.
5:123–132. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Prac Oncol. 3:24–40. 2006. View Article : Google Scholar
|
|
7
|
Kang BK, Lee EH and Kim HM: Inhibitory
effects of Korean folk medicine ‘Hi-Chum’ on histamine release from
mast cells in vivo and in vitro. J Ethnopharmacol. 57:73–79. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JY, Lim HJ and Ryu JH: In vitro
anti-inflammatory activity of 3-O-methyl-flavones isolated from
Siegesbeckia glabrescens. Bioorg Med Chem Lett. 18:1511–1514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HM, Lee JH, Won JH, et al: Inhibitory
effect on immunoglobulin E production in vivo and in vitro by
Siegesbeckia glabrescens. Phytother Res. 15:572–576. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jun SY, Choi YH and Shin HM: Siegesbeckia
glabrescens induces apoptosis with different pathways in human
MCF-7 and MDA-MB-231 breast carcinoma cells. Oncol Rep.
15:1461–1467. 2006.PubMed/NCBI
|
|
11
|
Cho YR, Choi S and Seo DW: The in vitro
antitumor activity of Siegesbeckia glabrescens against ovarian
cancer through suppression of receptor tyrosine kinase expression
and the signaling pathways. Oncol Rep. 30:221–226. 2013.PubMed/NCBI
|
|
12
|
Lee HN, Joo JH, Oh JS, Choi SW and Seo DW:
Regulatory effects of Siegesbeckia glabrescens on non-small cell
lung cancer cell proliferation and invasion. Am J Chin Med.
42:453–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang JH and Kim JD: Inhibitory effects of
Siegesbeckiae herba extract on angiogenesis and adipogenesis.
Biotechnol Bioprocess Eng. 16:144–152. 2011. View Article : Google Scholar
|
|
14
|
Kim HJ, Cho YR, Kim SH and Seo DW:
TIMP-2-derived 18-mer peptide inhibits endothelial cell
proliferation and migration through cAMP/PKA-dependent mechanism.
Cancer Lett. 343:210–216. 2014. View Article : Google Scholar
|
|
15
|
Lee HN, Kim JK, Kim JH, et al: A
mechanistic study on the anti-cancer activity of ethyl caffeate in
human ovarian cancer SKOV-3 cells. Chem Biol Interact. 219:151–158.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seo DW, Kim SH, Eom SH, et al: TIMP-2
disrupts FGF-2-induced downstream signaling pathways. Microvasc
Res. 76:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo DW, Li H, Qu CK, et al: Shp-1 mediates
the antiproliferative activity of tissue inhibitor of
metalloproteinase-2 in human microvascular endothelial cells. J
Biol Chem. 281:3711–3721. 2006. View Article : Google Scholar :
|
|
18
|
Kim SH, Cho YR, Kim MD, Kim HJ, Choi SW
and Seo DW: Inhibitory effects of sepiapterin on vascular
endothelial growth factor-A-induced proliferation and adhesion in
human umbilical vein endothelial cells. Arch Pharm Res.
34:1571–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoon HJ, Cho YR, Joo JH and Seo DW:
Knockdown of integrin α3β1 expression induces proliferation and
migration of non-small cell lung cancer cells. Oncol Rep.
29:662–668. 2013.
|
|
20
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Kim JY, Hyeon J, Lee HJ and Ryu JH:
In vitro antiinflammatory activity of a new sesquiterpene lactone
isolated from Siegesbeckia glabrescens. Phytother Res.
25:1323–1327. 2011.PubMed/NCBI
|
|
24
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: mechanisms of anti-tumour activity. Nat Rev Cancer.
8:579–591. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SH, Cho YR, Kim HJ, et al: Antagonism
of VEGF-A-induced increase in vascular permeability by an integrin
α3β1-Shp-1-cAMP/PKA pathway. Blood. 120:4892–4902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: a moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stetler-Stevenson WG: Tissue inhibitors of
metalloproteinases in cell signaling: metalloproteinase-independent
biological activities. Sci Signal. 1:re62008.PubMed/NCBI
|
|
30
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): an ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seo DW, Li H, Guedez L, et al: TIMP-2
mediated inhibition of angiogenesis: an MMP-independent mechanism.
Cell. 114:171–180. 2003. View Article : Google Scholar : PubMed/NCBI
|