Introduction
Colorectal cancer (CRC) is a common gastrointestinal
malignancy worldwide and is a lethal and aggressive malignancy with
a dismal prognosis (1,2). It is the third most common malignant
tumor and is one of the most common cancers in China (3). With the recent development of
molecular biology, the treatment of CRC has made great progress but
it is still difficult to treat advanced CRC, which has a poor
prognosis and a 40% overall mortality rate (4–6). The
recurrence rates for this type of cancer remain high and new
therapeutic strategies are crucially needed. Therefore, it is of
great importance to understand the molecular mechanisms of CRC
progression as a prerequisite for developing more effective
therapeutic strategies.
The Eph family, the largest group among the tyrosine
kinase receptor families, is comprised of the EphA (EphA1-10) and
EphB (EphB1-6) subclasses of receptors. They are classified
according to their sequence homologies and their binding affinities
for their ligands, ephrins (Eph receptor interacting protein)
(7–9). The first member of the Eph family was
cloned from an erythropoietin-producing hepatocellular cancer cell
line in 1987 and was named EphA1. The ligands for the Eph
receptors, ephrins, are divided into two subclasses, ephrinA
(ephrinA1-6) and ephrinB (ephrinB1-3) (10–12).
The role of Eph-ephrin signaling has been studied in
great detail in reference to the development of the nervous system
and spans a wide range of functions, such as the development of
neuronal networks, axon guidance, formation and remodeling of
synaptic connections and nervous system repair (13). The interaction of Eph receptors with
ephrins is known to mediate cell-cell repulsion, regulate axon
outgrowth, restrict cell migration and maintain well-defined
boundaries between different anatomical components of the
developing brain (10,11,14).
The Eph-ephrin interaction also regulates the remodeling of
vascular network formation during embryonic development (15,16). A
detailed review of research on Eph receptors and ephrin ligands in
embryonic development and carcinogenesis has been reported in a
number of review articles (17–19).
An understanding of EphA2 and ephrinA1 expression, signaling and
deregulation are important in developing strategies for cancer
therapies.
EphrinB2 is a membrane-bound ligand and has an
intracellular domain that also possesses an intrinsic signaling
capacity called ‘reverse signaling’ (20). The complete deletion of the
intracellular domain of ephrinB2 results in a severe defect of
angiogenesis and embryonic lethality, which indicates that ephrinB2
plays a critical role in developmental blood vessel formation
(21). EphrinB2 reverse signaling
has been shown to regulate both developmental and tumor
angiogenesis by activating vascular endothelial growth factor
receptor 2 (VEGFR-2) (22,23). In a separate study, an analysis of
pericyte-specific ephrinB2 deficiency indicated that ephrinB2 was
essential for normal coverage of the microvasculature by pericytes
(24).
More recently, RNA interference (RNAi) has been used
to regulate gene expression. RNAi provides a simple, effective,
specific and important mechanism to silence gene expression
(25). RNAi uses a nuclease to cut
double-stranded RNA into small interfering RNA (siRNA) fragments
from 21 to 25 nucleotides. Based on the mechanism of base pairing,
these RNA fragments recognize and cleave their homogenous target
messenger RNA (mRNA) molecule, which silences the sequence-specific
mRNA (26). This mechanism is
sequence-specific and causes downregulation of specific protein
expression (27–29). RNAi technology is currently
considered an important tool for functional genomic analyses and is
also an important mechanism for specific gene-silencing therapies
(28,30,31).
In the present study, the siRNA technique was used to silence the
expression of ephrinB2 in human colon carcinoma cell line SW480 and
to evaluate the biological behavior of the cells to determine the
role of ephrinB2 expression in tumor proliferation, adhesion,
migration and invasion.
Many scholars have conducted extensive research into
gene therapy for CRC (32,33). However, several factors influence
the effectiveness of these therapies, resulting in unsatisfactory
effects. Recently, researchers have become aware that gene
silencing can be used as an effective regulation method for gene
expression because it enables the specific blocking of target gene
expression. Thus, gene silencing has a key role in research fields
such as gene functioning and targeted therapy (34). RNA-induced gene silencing occurs at
the transcriptional and posttranscriptional levels. At the
posttranscriptional level, it may be attributed to mRNA degradation
and is also known as RNAi.
We designed and synthesized the first specific siRNA
for ephrinB2 and evaluated its effects using experimental methods.
Furthermore, we analyzed the antitumor mechanisms of ephrinB2
silencing using ephrinB2 siRNA in a human CRC cell line (SW480). We
located a CRC cell line with high expression of ephrinB2. We used
RNAi to target ephrinB2 gene expression and detected interference
of ephrinB2, VEGF, CD105 and matrix metalloproteinase (MMP9). This
enabled us to study various biological characteristics in SW480
cells after disturbance, to investigate the effects of ephrinB2’s
biological role in CRC and its possible mechanisms. We provide a
theoretical and experimental basis for the ephrinB2 gene to become
a new target for CRC molecular diagnostics and gene therapy.
Materials and methods
Cell culture
The human CRC cell lines SW480, SW620 and HT29 used
in this study were provided by Professor Kun-Ming Wen from the
First Affiliated Hospital of Chongqing Medical University, China.
The human CRC cell line LOVO and 293T cells were purchased from the
Shanghai Cell Biology Research Institute of the Chinese Academy of
Sciences. All of the human CRC cell lines used in this study were
screened for ephrinB2 protein and mRNA expression, detected by
western blotting and real-time PCR, respectively (data not shown).
SW480 cells, which expressed the highest ephrinB2 levels among the
CRC cell lines examined, were used in the study. The cells were
grown and maintained at 37°C in a humidified incubator containing a
5% CO2 atmosphere. All of the experiments were performed
with cells in the logarithmic phase of growth.
Main reagents and equipment
The RNA extract reagent TRIzol and the PCR kits used
in this study were purchased from Takara Biotechnology Co. Ltd.
(Dalian, China). EphrinB2, VEGF, CD105 and MMP9 primers were also
synthesized by Takara Biotechnology. Anti-GAPDH antibody and
horseradish peroxidase (HRP)-conjugated goat anti-rabbit
immunoglobulin G were obtained from Beijing Zhongshan Jinqiao
Biotechnology Co. Ltd. (Beijing, China). EphrinB2, VEGF, CD105 and
MMP9 antibodies were purchased from Epitomics Inc. (Cambridge, MA,
USA), and Transwell plates were purchased from Millipore Inc.
(Billerica, MA, USA). Quantitative real-time PCR was performed in a
Rotor-Gene 6000 thermal cycler (Sydney, Australia).
Construction and screening of a high
efficiency interference plasmid
The coding sequence of the ephrinB2 gene in the
SW480 genome was submitted to Ambion siRNA Target Finder
(http://www.ambion.com/techlib/misc/siRNA_finder.html).
Three 19-nucleotide siRNA target sequences corresponding to
nucleotides E65 (CTGCGATTTCCAAATCGAT), E376 (CTGTGCCAAACCAGACCAA)
and E730 (CAACATCCTCGGTTCCGAA) of the ephrinB2 gene were
identified. The corresponding double-stranded siRNAs were
synthesized in vitro using a Silencer™ siRNA Construction
kit from Ambion (Austin, TX, USA) following the manufacturer’s
protocols. The annealed siRNA pSilencer-ephrinB2-siRNA65 (pE65),
pSilencer-ephrinB2-siRNA730 (pE730) and pSilencer-ephrinB2-siRNA376
(pE376) were transiently transfected into 293T cells using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to
the manufacturer’s instructions. As a control, siRNA targeting a
scrambled sequence with no known consensus to the human genome was
also introduced under identical conditions. After 48 h, the cells
were harvested and their mRNA and proteins were extracted. Using
quantitative real-time PCR and western blotting, the siRNA targeted
nucleotide E376 showed decreased ephrinB2 mRNA levels of 20% or
less. The siRNA targeted nucleotide E376 was the most effective of
the three siRNAs tested (data not shown). Mock transfection and the
use of the control siRNA did not affect levels of ephrinB2,
consistent with the specificity of the siRNA approach.
siRNA transfection and cell groups
Experimental SW480 cells were divided into several
groups: transfected pSilencer2.1-ephrinB2-shRNA376 cells (RNAi),
transfected siRNA negative control cells (NC) and normal SW480
cells (CON). These cells were transfection using the same methods
as described above. Briefly, 2×105 tumor cells were
seeded in 6-well culture plates in 2 ml of antibiotic-free growth
medium supplemented with FBS for 24 h. The supernatant was removed
when the cells were transfected. A total of 100 pM siRNA, 5 μl
transfection reagent and 500 μl serum-free DMEM were mixed
thoroughly and incubated at room temperature for 20 min. This
mixture was added to the cells and incubated for another 6 h. The
supernatant was then removed and replaced with fresh media
containing 10% FBS. After 48 h of incubation, the cells were
harvested for further analysis. Three wells were left untransfected
to serve as a negative control. The efficiency of the transfection
was assessed by fluorescence microscopy.
Detection of mRNA levels of ephrinB2,
VEGF, CD105 and MMP9 using real-time PCR
Real-time PCR was performed to analyze the mRNA
expression levels of ephrinB2, VEGF, CD105 and MMP9 in the SW480
cells. The total RNA was extracted from the SW480 cells using a
commercial extract reagent RNAiso Plus obtained from Takara
Biotechnology. First-strand complementary DNA was synthesized using
a PrimeScript® Real-Time Master Mix kit (obtained from
Takara Biotechnology). Quantitative real-time PCR was performed
using a SYBR® Premix Ex Taq™ II kit (Takara
Biotechnology) in a Rotor-Gene 6000 thermal cycler with continuous
monitoring of fluorescence. The reactions were carried out in a
25-μl reaction volume. The primer sequences used to amplify the
individual genes are shown in Table
I. The real-time PCR was performed with the following program:
95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for
5 sec and annealing at 60°C for 30 sec. All of the reactions were
performed in triplicate. A melting curve analysis was performed to
ensure the specificity of the quantitative PCR. The data were
analyzed using the 2−ΔΔCT method, in which GAPDH was
used as an internal standard in each experiment for the
quantitative evaluation of a gene’s mRNA expression.
 | Table IPrimer sequences used for the
real-time PCR. |
Table I
Primer sequences used for the
real-time PCR.
| Gene name
(accession no.) | Primer name | Primer
sequences | Primer Tm | Product size
(bp) |
|---|
| EphrinB2
(NM_004093.3) | Forward |
5′-CGATTGAGCCTTACGACAC-3′ | 56 | 256 |
| Reverse |
5′-TTTTAAGCGCTGAGCATTG-3′ | | |
| VEGF
(NM_001171623) | Forward |
5′-CTGCTGTCTTGGGTGCATTG-3′ | 60 | 107 |
| Reverse |
5′-TCGTGATGATTCTGCCCTCC-3′ | | |
| CD105
(NM_000118.2) | Forward |
5′-TACCCATACCCAAAACCG-3′ | 56 | 138 |
| Reverse |
5′-ATGAGGAAGGCACCAAAG-3′ | | |
| MMP9
(NM_004994.2) | Forward |
5′-AGCACGGAGACGGGTATC-3′ | 60 | 214 |
| Reverse |
5′-CAGGCGGAGTAGGATTGG-3′ | | |
| GAPDH | Forward |
5′-TGACTTCAACAGCGACACCCA-3′ | 55 | 121 |
| Reverse |
5′-CACCCTGTTGCTGTAGCCAAA-3′ | | |
Detection of the protein levels of
ephrinB2, VEGF, CD105 and MMP9 using western blot analysis
The cells were harvested and lysed in RIPA buffer
(Beyotime, Shanghai, China) with protease inhibitors
(Sigma-Aldrich, St. Louis, MO, USA) for 30 min while on ice. The
cell lysates were centrifuged and the protein concentration was
determined using a BCA protein assay kit (Solarbio, Beijing,
China). The protein (50 μg) in the cell lysates was boiled for 10
min in a quarter volume of loading buffer and then separated using
12% sodium dodecyl sulfate polyacrylamide gel electrophoresis at a
30 mA constant current until the bromophenol blue shifted out of
the bottom of the separating gel. The separated proteins were
electroblotted onto a polyvinylidene difluoride membrane using a
transfer buffer at 100 V for 60 min. The membranes were blocked
with 5% nonfat dry milk in Tris-buffered saline (TBS)-0.1% Tween
(TBST) for 2 h at room temperature, and then incubated with primary
antibody/rabbit anti-ephrinB2 (1:2,000 dilution), rabbit anti-VEGF
(1:2,000 dilution), rabbit anti-CD105 (1:2,000 dilution), rabbit
anti-MMP9 (1:2,000 dilution) and rabbit anti-GAPDH (1:5,000
dilution) in TBST overnight at 4°C. After washing 5 times in TBST,
the membranes were incubated with HRP-conjugated goat anti-rabbit
immunoglobulin G (1:5,000 dilution) for 2 h at room temperature.
After washing 5 times in TBST, the membranes were analyzed with an
enhanced chemiluminescence substrate and used to expose an X-ray
film according to the manufacturer’s protocol. The relative
expression of the protein bands of ephrinB2 (37 kDa), VEGF (22
kDa), CD105 (37 kDa) and MMP9 (92 kDa) were quantified using
densitometric scanning of the radiographic films with a GS-700
imaging densitometer and then analyzed with a computer program. The
results from each experimental group were expressed as the relative
integrated intensity compared to the GAPDH (35 kDa) band densities
measured in the same batch.
Detection of cell apoptosis and cell
cycle by flow cytometry
To assess cell apoptosis, the cells were harvested
48 h after transfection; washed in cold PBS twice; stained using
propidium iodide (PI); and then detected using an ApoScreen Annexin
V apoptosis detection kit (Southern Biotech, Birmingham, AL, USA),
according to the manufacturer’s protocol. Both the experiments and
apoptosis rates were conducted using BD FACSVerse (BD Biosciences,
Franklin Lakes, NJ, USA). To detect the cell cycle, the cells were
harvested and incubated overnight in 75% cold alcohol. After
washing with PBS, the cell suspensions were added to RNaseA at a
concentration of 0.5 mg/ml, stained with PI for 30 min in a dark
room and then analyzed using a cell cycle analysis kit (Beyotime,
Shanghai, China). Quantitative DNA fragments from the different
cell cycles were analyzed by flow cytometry.
Measurement of cell growth
Cell proliferation was measured by a methyl thiazole
tetrazolium (MTT) assay (18). The
cells were seeded onto 96-well plates at a density of
2×104 cells/well and incubated in a corresponding medium
containing 10% serum. Cell growth was analyzed after 1, 2, 3, 4 and
5 days. Six duplicate wells were established for each group. At
each time-point, 20 μl MTT (5 mg/ml, Sigma-Aldrich) was added to
each well and allowed to incubate for 4 h, then 150 μl DMSO was
added into each well. After complete solubilization of the dye, the
absorbance of the wells was measured using a microplate
spectrophotometer (from Bio-Rad) at 490 nm. Cell growth curves were
drawn using SigmaPlot 12.0 software.
Detection of cell migration by scratch
test
Cell migration was determined using a scratch assay
(35,36). The transfected cell lines were
seeded on a 24-well plate and allowed to reach confluency. After
scratching the bottom of the well with a pipette tip, the monolayer
of cells was washed 3 times with PBS to remove the detached cells.
The remaining adherent cells were incubated in a medium (either
DMEM/F12 or DMEM) containing 1% FBS for 24 h. This medium was then
replaced with a medium containing 10% FBS. Space filling by cell
migration was evaluated 24 h later using bright-field microscopy.
This experiment was performed in triplicate.
Detection of cell migration by a
Transwell assay
The Boyden chamber technique (also called Transwell
analysis) was also used to analyze cell migration. Filters with
8-μm pores were coated with 100 μl of 1 mg/ml Matrigel (dissolved
in serum-free RPMI-1640 medium). Then, 600 μl RPMI-1640 medium
containing 10% FBS was added to the lower chambers. Forty-eight
hour post-transfection, homogenous single-cell suspensions
(1×105 cells/well) were added to the upper chambers and
allowed to invade for 24 h at 37°C in a CO2 incubator.
The cells that remained attached to the upper surface of the
filters were carefully removed with cotton swabs. The migrated
cells were stained with 0.1% crystal violet for 10 min at room
temperature and then examined using light microscopy. A
quantification of the migrated cells was performed according to
established criteria (37).
Statistical analysis
The data were analyzed using SPSS 18.0 software. The
average values are presented as mean ± standard deviation (SD). The
statistical differences between the groups were analyzed using a
one-way ANOVA. Differences were considered significant when the
P-value was <0.05.
Results
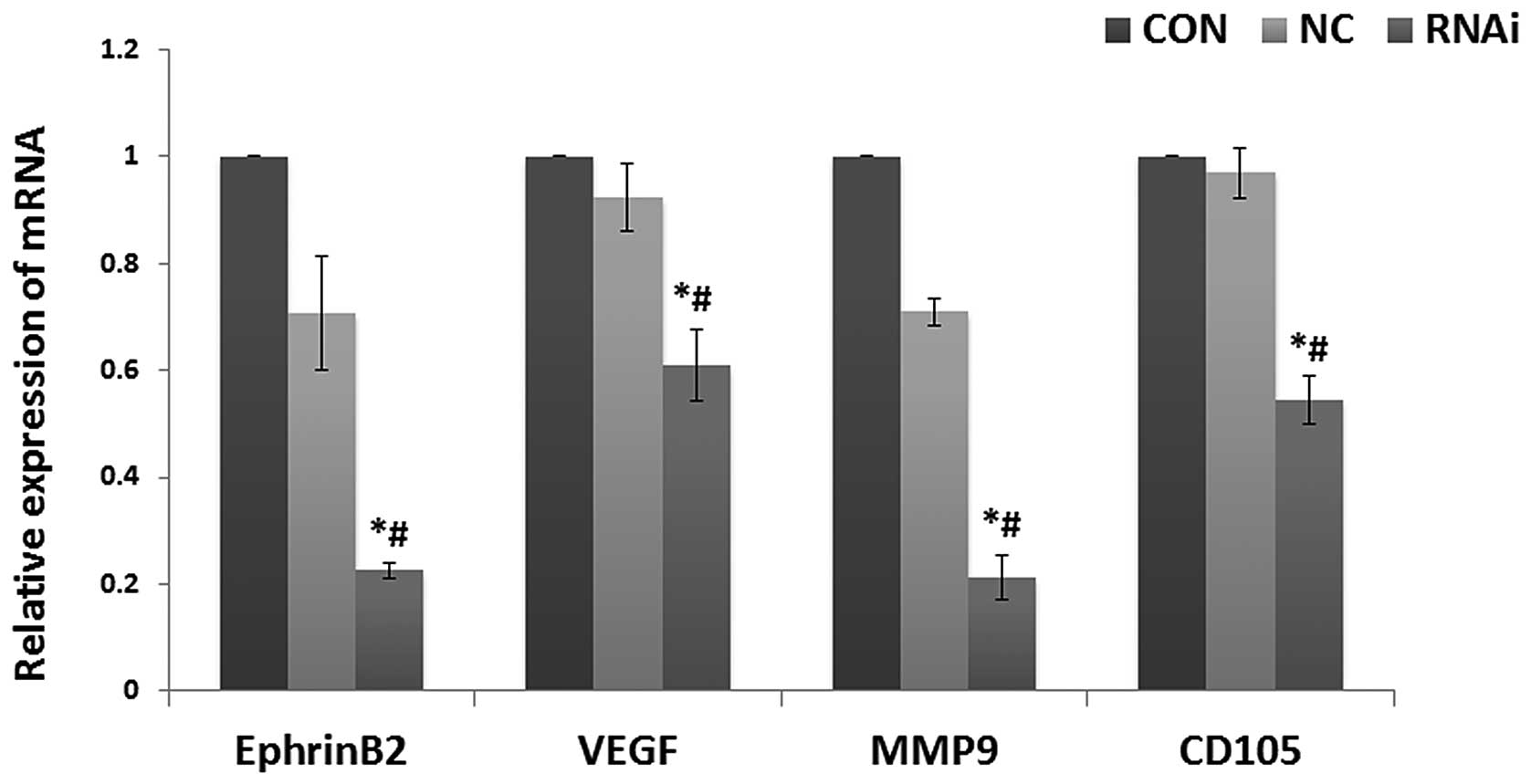
Effects of ephrinB2 siRNA on ephrinB2,
VEGF, CD105 and MMP9 mRNA levels in SW480 cells
VEGF, CD105, MMP9 and ephrinB2 mRNA was chosen for
analysis based on their key roles in tumor angiogenesis. Real-time
PCR was used to determine whether ephrinB2 siRNA could reduce the
expression level of each of these four key substances. As shown in
Fig. 1, the expression levels of
VEGF, CD105, MMP9 and ephrinB2 mRNA were all significantly
decreased in the siRNA group cells compared to the NC and CON cells
(all P<0.05). However, there was no significant difference
between the levels of expression of VEGF, CD105, MMP9 and ephrinB2
mRNA in the normal SW480 cells and the NC cells (P>0.05). These
results indicate that targeted ephrinB2 gene silencing not only
effectively silenced ephrinB2 expression, but also reduced VEGF,
CD105 and MMP9 mRNA expression levels.
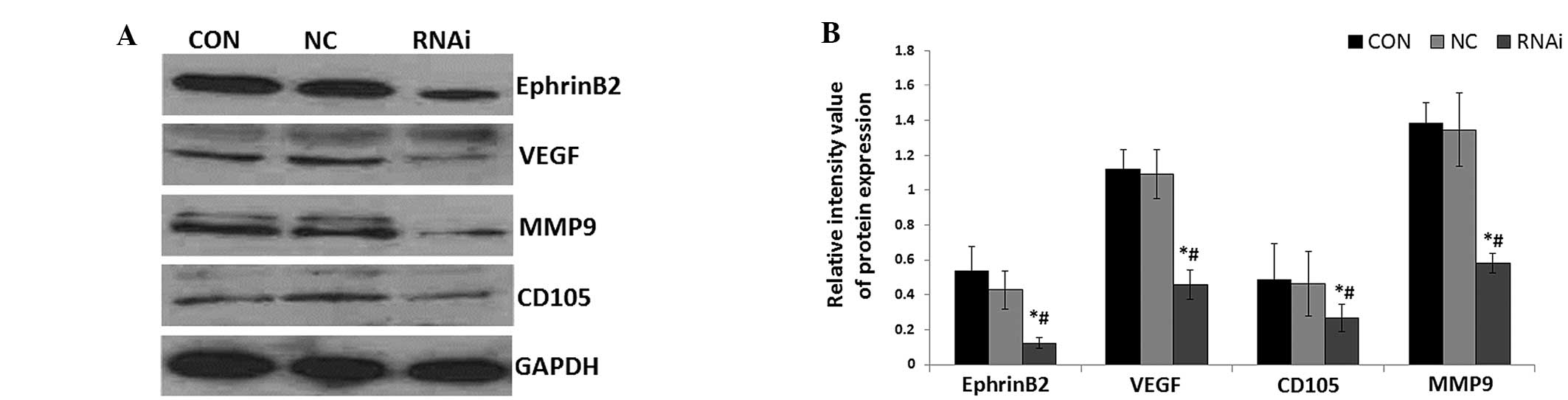
Effects of ephrinB2 siRNA on ephrinB2,
VEGF, CD105 and MMP9 protein levels in SW480 cells
To investigate the effect of ephrinB2 gene silencing
on colon carcinoma angiogenesis-related genes, VEGF, CD105, MMP9
and ephrinB2 protein levels were measured using western blot
analysis. As shown in Fig. 2, the
expression levels of VEGF, CD105, MMP9 and ephrinB2 protein were
all significantly decreased in the siRNA group cells compared with
the CON and NC group cells (all P<0.05). However, there were no
significant differences between the CON and NC group cells.
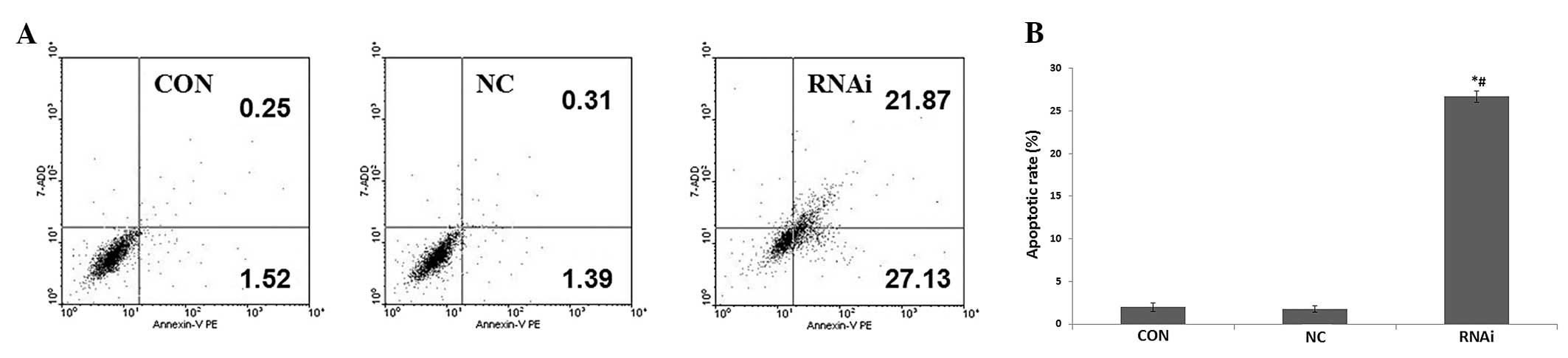
Effects of ephrinB2 siRNA on SW480 cell
apoptosis
Correlations between ephrinB2 silencing and
apoptotic ratios in SW480 cells were evaluated using flow
cytometry. These results showed that the apoptosis ratio in the
siRNA group cells (26.69±0.67) was significantly higher (P<0.05)
than this ration in both the CON group (1.97±0.47) and the NC group
(1.74±0.36) (Fig. 3). The results
of the apoptosis assay indicate that ephrinB2 siRNA promotes SW480
cell apoptosis.
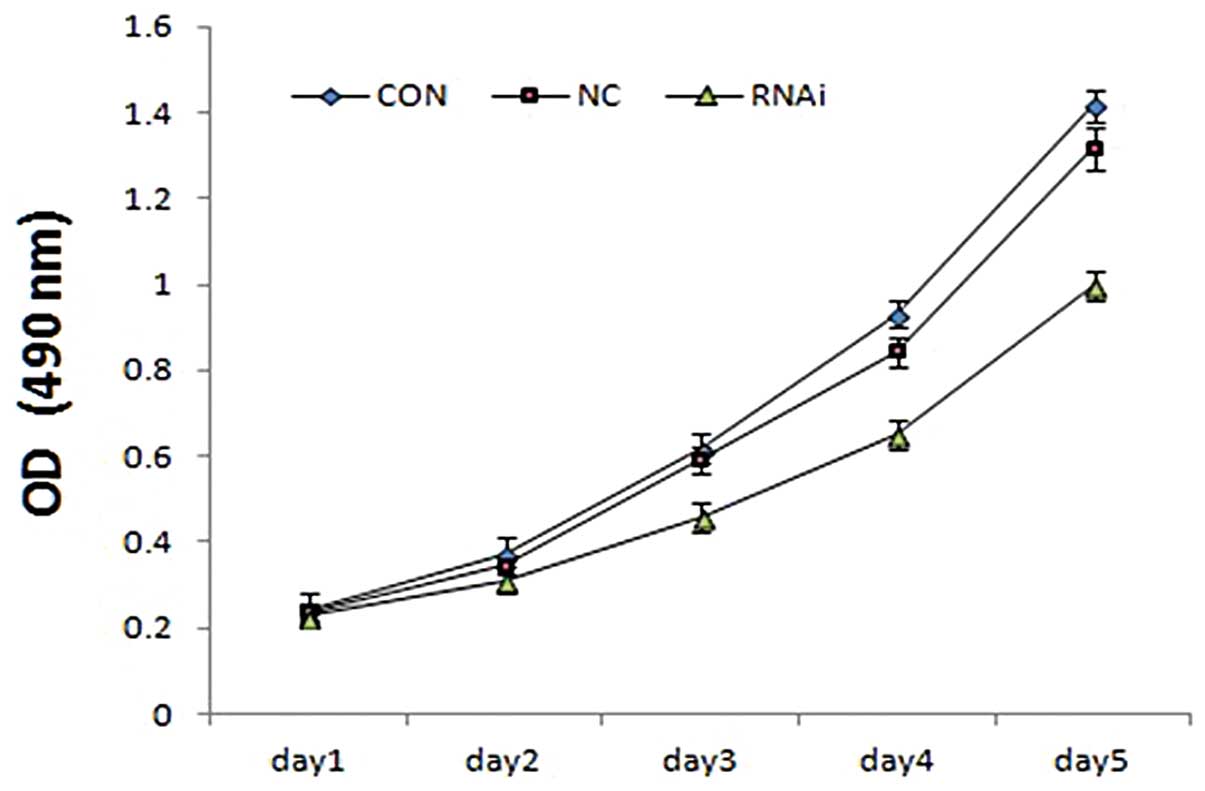
Effects of ephrinB2 siRNA on SW480 cell
proliferation
To determine the effects of ephrinB2 siRNA on cell
viability, the biological effects of ephrinB2 siRNA on the
proliferation of SW480 cells were monitored using MTT assays. The
MTT assays showed that the proliferation of SW480 cells in the
siRNA group cells was inhibited in a time-dependent manner,
compared with the control cells. There were no obvious differences
between the CON and NC group cells (Fig. 4). These results showed that the
knockdown of ephrinB2 by siRNA can inhibit the growth of SW480
cells.
Effects of ephrinB2 siRNA on the cell
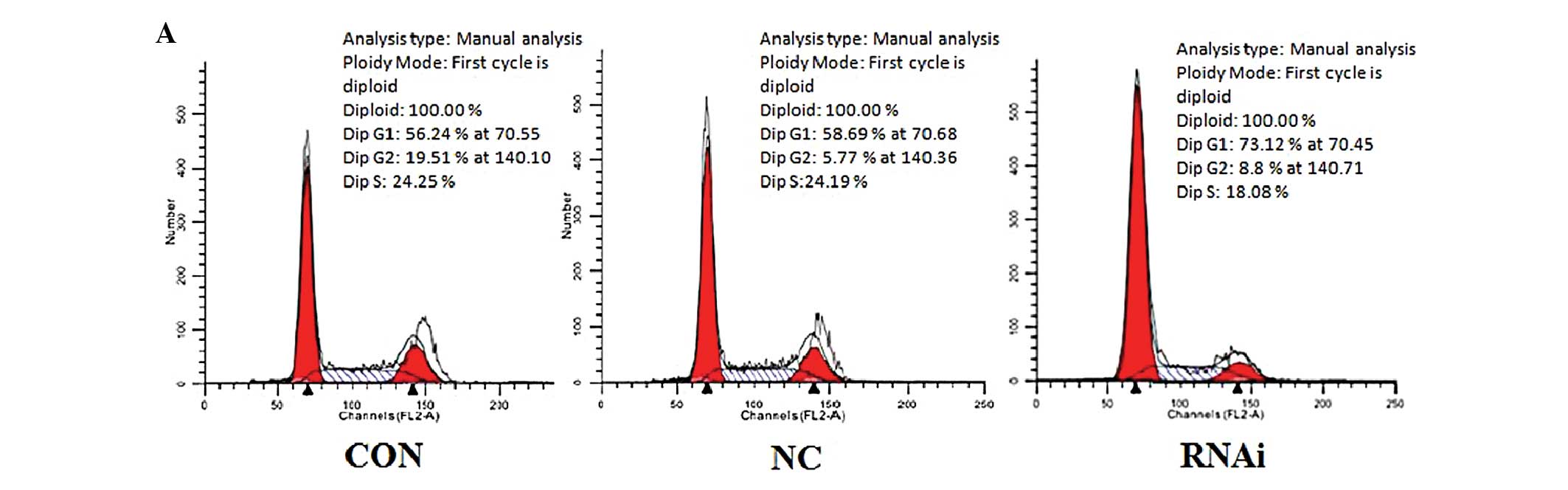
cycle distribution of SW480 cells
As shown in Fig. 5,
ephrinB2 siRNA was also found to have an effect on the cell cycle.
The percentage of cells in the S phase in the siRNA group
(18.32±0.38%) was significantly lower than that of the CON group
(25.22±0.89%) and the NC group (24.34±1.26%). However, the
percentage of cells in the G1 phase in the siRNA group
(74.49±1.55%) was significantly higher than that in the CON group
(55.98±1.66%) and the NC group (57.78±2.22%). These results suggest
that ephrinB2 siRNA may interfere with cell mitosis and cell cycle
progression, arresting cells in the G1 phase and inhibiting SW480
cell proliferation.
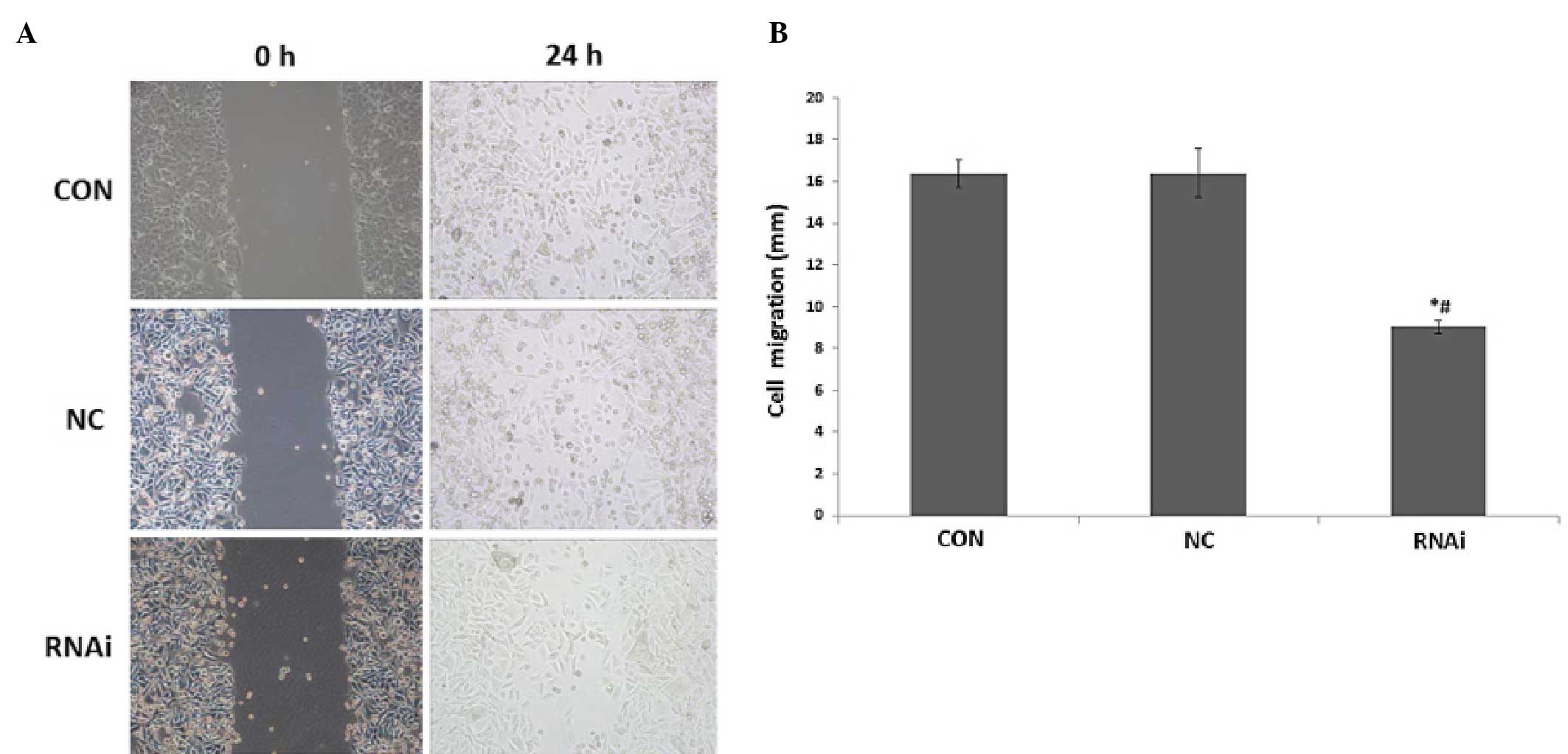
Effects of ephrinB2 siRNA on SW480 cell
migration
Scratch experiments were carried out to study the
effects of ephrinB2 siRNA on the migratory ability of SW480 cells.
As shown in Fig. 6, the cell
migration distance (mm) in the siRNA group (18.33+0.71) was
significantly shorter (P<0.05) than that in the CON (32.7±1.25)
and NC groups (30.57±1.36). There was no significant difference
between the cell migration distances in the CON and NC groups
(P>0.05).
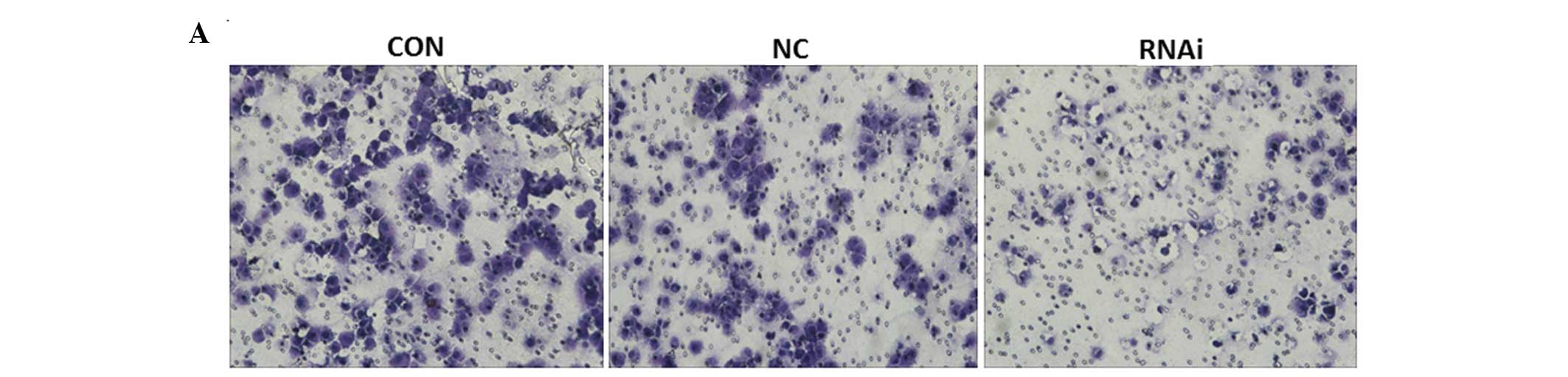
Effects of ephrinB2 siRNA on SW480 cell
invasion
As shown in Fig. 7,
the number of invasive cells in the siRNA group (37.33±5.51) was
significantly decreased (P<0.05) compared with those in the CON
(109±4.36) and NC groups (102±4.58). There was no significant
difference between the cell migration distances in the CON and NC
groups (P>0.05). The results of the Transwell analysis showed
that ephrinB2 siRNA suppressed the invasive capabilities of the
SW480 cells for 48 h compared with the CON and NC groups. These
results suggest that ephrinB2 siRNA suppressed the invasion of
SW480 cells in vitro.
Discussion
Previous studies have shown that tumor angiogenesis
may provide sufficient nutrients for the growth of a tumor and also
provide an effective channel for tumor invasion and metastasis.
Therefore, the roles and mechanisms of tumor angiogenesis and
anti-angiogenesis in tumor growth, invasion and migration have been
given a great deal of attention. The inhibition of tumor
angiogenesis is expected to become an important and effective means
of targeted tumor therapy. Recent studies have also found that the
ephrinB2/EphB4 system and its specific bidirectional signal
transduction plays a particularly important role in tumor
angiogenesis and is associated with the occurrence, development and
prognosis of tumors (38,39). EphrinB2 is part of the Eph family,
which is the largest subfamily of receptor tyrosine kinases. The
Eph family includes the Eph receptor and its corresponding ligand
ephrin. The Eph receptors are divided into two subfamilies, EphA
(A1-A8) and EphB (B1-B6) receptors (40,41).
The ligands can also be divided into the ephrinA (ephrinA1-6) and
ephrinB (ephrinB1-B6) categories (42). EphrinB2 can activate downstream
pathways through the combination of its extracellular portion and
the Eph receptor, which is phosphorylated at the intracellular
tyrosine kinase binding sites in Eph. EphrinB2 also has an
intracellular domain that possesses an intrinsic signaling capacity
called ‘reverse signaling’ (20).
The complete deletion of the intracellular domain of ephrinB2
results in a severe defect of angiogenesis and embryonic lethality,
indicating a critical role for ephrinB2 reverse signaling in
developmental blood vessel formation (21). This bi-directional signaling pathway
has an important function in angiogenesis (13). Numerous studies have shown that
ephrinB2 is mainly expressed in the small arteries, especially in
the neovessels in a proliferative state (43–47).
EphrinB2 can also increase the migratory capability of endothelial
cells and promote the formation of the vascular lumen by
facilitating the formation of endothelial cell filopodia (48). Functional defects or a lower
expression of ephrinB2 may decrease the extent of vascular
proliferation and vascular lumen formation; Erk and Akt in the VEGF
receptor signaling pathway are also affected (22,23).
EphrinB2 phosphorylation levels may also decrease, accompanied by a
decline in the vascular proliferation levels (47).
Although there is some controversy concerning the
role of ephrinB2 in tumor angiogenesis, most studies have held the
view that the reverse signaling signal transduction pathway,
mediated by ephrinB2, can promote endothelial cell invasion,
migration and tube formation; plays a catalytic effect on
angiogenesis; and is closely associated with tumor invasion and
metastasis (49–52). However, the effects of the reverse
signaling signal transduction pathway mediated by ephrinB2 on CRC
angiogenesis, invasion and metastasis and the specific molecular
mechanism by which this may occur remain unknown.
Angiogenesis is a highly varied process (53–56)
and the mechanism by which it progresses is extremely complex.
Multiple bioactive factors are involved, including tumor
angiogenesis promoting factors VEGF, MMP9, βGF, EGFR, TGF-β and
CD105; tumor angiogenesis inhibiting factors, such as MMP
inhibitors, endostatin and vascular endothelial growth factor
inhibitors (57,58); and multiple signaling pathways, such
as p38MAPK, Akt/Pkb, PI3K, receptor tyrosine kinases, Dll4-Notch
and VEGF-VEGFR. Studies have shown that the microvessel density is
a good indicator of tumor angiogenesis and has a close relationship
with tumor invasion, migration and prognosis (59). The level of CD105 (Enderlin) can
also reflect the microvessel density because CD105 is only
expressed in proliferating vascular cells. In addition to being a
marker of microvessel density, CD105 can also affect tumor
angiogenesis by other factors, such as through TGF-β (56).
Research has shown that endothelial growth factor
VEGF and MMP9 also play an important role in the process of tumor
angiogenesis and may have a synergistic effect (60–62).
VEGF is a member of the platelet derived growth factor family and
several subfamilies have been found, such as VEGF-A, VEGF-B, VEGF-C
and VEGF-D. VEGF-A plays a particularly important role in
angiogenesis. MMP9 is a member of the MMP family and is considered
the most important substance in dissolving tumor cells, endothelial
extracellular matrices and the basilar membrane (63). Many authors have demonstrated that
MMP expression patterns have a positive correlation with tumor
invasion and metastasis (64) and
that it plays an important role in the process of tumor invasion,
metastasis and angiogenesis (65,66).
High expression of MMP9 in gastrointestinal tumors is closely
related with tumor invasion and migration and can be used as an
independent risk factor affecting prognosis (67,68).
Recently, siRNA has been shown to inhibit the
expression of the corresponding target gene in mammals (69). In this process, siRNA molecules are
separated into single strands and incorporated into the RNA-induced
silencing complex, which then cleaves the corresponding cellular
mRNA. RNAi serves as a powerful technology to block the expression
of a specific target gene (70,71).
Yang et al (72) found that
RNAi against α-fetoprotein could silence the α-fetoprotein gene
effectively and thus inhibit cell growth and induce apoptosis in
Huh7 cells. Other studies have found that MACC1 siRNA transfection
affected HeLa cell biological behavior, caused a significant
decrease in cell proliferation and migration and increased the cell
apoptosis rate (73). Lui et
al (74) also showed that the
downregulation of VEGFR3 expression by siRNA provided a therapeutic
strategy for inhibiting tumor growth and metastasis.
Recently, RNAi technology has become more capable at
specifically silencing particular genes. It can be used as a
powerful tool for investigating the functions of genes and for
genetic therapy for carcinoma. To explore the role of ephrinB2 in
tumorigenesis and tumor progression, we silenced the expression of
ephrinB2 in the CRC cell line SW480 by using RNAi. The results
showed that stealth RNAi against ephrinB2 could not only silence
ephrinB2 expression effectively at both the mRNA and protein levels
in SW480 cells after transfection but also decreased the expression
of VEGF, CD105 and MMP9 compared with the CON and NC cells. Other
studies have shown that CD105 acts as a tumor marker for
angiogenesis and correlates well with the tumor microvessel
density. Our results found that the use of stealth RNAi against
ephrinB2 inhibited tumor angiogenesis. This effect may be
attributed to the regulation of VEGF and MMP9.
In addition, an MTT assay confirmed that the
proliferation of SW480 cells was reduced significantly after the
ephrinB2 gene was silenced by RNAi. This also illustrated,
indirectly, that the ephrinB2 gene may promote tumor cell
proliferation. The state of tumor cell proliferation is closely
related to the cell cycle and apoptosis. Given this fact, we used
flow cytometry to measure the cell cycle and apoptosis. The results
showed that the percentage of cells in the S phase in the siRNA
group was significantly decreased. However, the percentage of cells
in the G1 phase was significantly increased compared with those in
the CON and NC groups. This suggested that ephrinB2 siRNA may have
inhibited the SW480 cell proliferation by interfering with cell
mitosis and cell cycle progression. The results of the apoptosis
assay indicated that ephrinB2 siRNA promoted SW480 cell
apoptosis.
A significant feature of malignant tumors that is
different from carcinoid tumors is that the ability to invade and
metastasize is significantly stronger in malignant tumors. This is
one of the main reasons for differences in treatment and long-term
survival rates (68). To
investigate the effect of ephrinB2 RNAi on SW480 cell migration and
invasion capacity, a scratch test and Transwell experiments were
used. The results revealed that ephrinB2 siRNA suppressed the
migration and invasion of SW480 cells in vitro.
Taken as a whole, our study provide three important
findings. Stealth RNAi against ephrinB2 can silence the ephrinB2
gene effectively at both the mRNA and protein levels in SW480
cells. Silencing the expression of ephrinB2 inhibits tumor
angiogenesis, cell proliferation, invasion and migration and
induces apoptosis in SW480 cells. These effects may be attributed
to the regulation of VEGF and MMP9 in SW480 cells. EphrinB2 may
also play a role in tumor angiogenesis, invasion and metastasis in
CRC. However, more research is needed to confirm this finding.
These conclusions suggest that ephrinB2 may function as a CRC
growth stimulator. Thus, interference with ephrinB2 expression may
be an option for suppressing tumor growth and improving the
prognosis of CRC patients with high ephrinB2 levels, although
further in vivo studies are necessary to confirm and extend
these findings.
Acknowledgements
We thank Dr Li Ma for her critical review of the
manuscript.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar
|
|
3
|
Guo P, Huang ZL, Yu P and Li K: Trends in
cancer mortality in China: an update. Ann Oncol. 23:2755–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burotto M, Hartley ML, Marshall JL and
Pishvaian MJ: Future of targeted agents in metastatic colorectal
cancer. Colorectal Cancer. 5:433–443. 2012. View Article : Google Scholar
|
|
5
|
Wan D, He S, Xie B, et al: Aberrant
expression of miR-199a-3p and its clinical significance in
colorectal cancers. Med Oncol. 30:3782013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou J, Li P, Xue X, et al: Salinomycin
induces apoptosis in cisplatin-resistant colorectal cancer cells by
accumulation of reactive oxygen species. Toxicol Lett. 222:139–145.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gale NW and Yancopoulos GD: Ephrins and
their receptors: a repulsive topic? Cell Tissue Res. 290:227–241.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kullander K and Klein R: Mechanisms and
functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol.
3:475–486. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murai KK and Pasquale EB: ‘Eph’ective
signaling: forward, reverse and crosstalk. J Cell Sci.
116:2823–2832. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pasquale EB: The Eph family of receptors.
Curr Opin Cell Biol. 9:608–615. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zozulya SA and Udovichenko IP: Eph family
receptors as therapeutic targets. Bioorg Khim. 38:267–279. 2012.(In
Russian). PubMed/NCBI
|
|
12
|
Pasquale EB: Eph receptor signalling casts
a wide net on cell behaviour. Nat Rev Mol Cell Biol. 6:462–475.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pasquale EB: Eph-ephrin bidirectional
signaling in physiology and disease. Cell. 133:38–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zisch AH and Pasquale EB: The Eph family:
a multitude of receptors that mediate cell recognition signals.
Cell Tissue Res. 290:217–226. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heroult M, Schaffner F and Augustin HG:
Eph receptor and ephrin ligand-mediated interactions during
angiogenesis and tumor progression. Exp Cell Res. 312:642–650.
2006. View Article : Google Scholar
|
|
16
|
Cheng N, Brantley DM and Chen J: The
ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor
Rev. 13:75–85. 2002. View Article : Google Scholar
|
|
17
|
Surawska H, Ma PC and Salgia R: The role
of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev.
15:419–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vaught D, Brantley-Sieders DM and Chen J:
Eph receptors in breast cancer: roles in tumor promotion and tumor
suppression. Breast Cancer Res. 10:2172008. View Article : Google Scholar
|
|
19
|
Kinch MS and Carles-Kinch K:
Overexpression and functional alterations of the EphA2 tyrosine
kinase in cancer. Clin Exp Metastasis. 20:59–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuijper S, Turner CJ and Adams RH:
Regulation of angiogenesis by Eph-ephrin interactions. Trends
Cardiovasc Med. 17:145–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adams RH, Diella F, Hennig S, et al: The
cytoplasmic domain of the ligand ephrinB2 is required for vascular
morphogenesis but not cranial neural crest migration. Cell.
104:57–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sawamiphak S, Seidel S, Essmann CL, et al:
Ephrin-B2 regulates VEGFR2 function in developmental and tumour
angiogenesis. Nature. 465:487–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Nakayama M, Pitulescu ME, et al:
Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis.
Nature. 465:483–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Foo SS, Turner CJ, Adams S, et al:
Ephrin-B2 controls cell motility and adhesion during
blood-vessel-wall assembly. Cell. 124:161–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fire A, Xu S, Montgomery MK, et al: Potent
and specific genetic interference by double-stranded RNA in
Caenorhabditis elegans. Nature. 391:806–811. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martinez J, Patkaniowska A, Urlaub H, et
al: Single-stranded antisense siRNAs guide target RNA cleavage in
RNAi. Cell. 110:563–574. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi Y: Mammalian RNAi for the masses.
Trends Genet. 19:9–12. 2003. View Article : Google Scholar
|
|
28
|
Grimm D and Kay MA: Therapeutic
application of RNAi: is mRNA targeting finally ready for prime
time? J Clin Invest. 117:3633–3641. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McManus MT and Sharp PA: Gene silencing in
mammals by small interfering RNAs. Nat Rev Genet. 3:737–747. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aagaard L and Rossi JJ: RNAi therapeutics:
principles, prospects and challenges. Adv Drug Deliv Rev. 59:75–86.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee WR, Chung CL, Hsiao CJ, et al:
Suppression of matrix metalloproteinase-9 expression by
andrographolide in human monocytic THP-1 cells via inhibition of
NF-κB activation. Phytomedicine. 19:270–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Laar RK: An online gene expression
assay for determining adjuvant therapy eligibility in patients with
stage 2 or 3 colon cancer. Br J Cancer. 103:1852–1857. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Gramont A, de Gramont A, Chibaudel B,
et al: From chemotherapy to targeted therapy in adjuvant treatment
for stage III colon cancer. Semin Oncol. 38:521–532. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dykxhoorn DM and Lieberman J: The silent
revolution: RNA interference as basic biology, research tool and
therapeutic. Annu Rev Med. 56:401–423. 2005. View Article : Google Scholar
|
|
35
|
Wu B, Hu K, Li S, et al:
Dihydroartiminisin inhibits the growth and metastasis of epithelial
ovarian cancer. Oncol Rep. 27:101–108. 2012.
|
|
36
|
Wu B, Li S, Sheng L, et al: Metformin
inhibits the development and metastasis of ovarian cancer. Oncol
Rep. 28:903–908. 2012.PubMed/NCBI
|
|
37
|
Nicosia RF and Ottinetti A: Growth of
microvessels in serum-free matrix culture of rat aorta. A
quantitative assay of angiogenesis in vitro. Lab Invest.
63:115–122. 1990.PubMed/NCBI
|
|
38
|
Djokovic D, Trindade A, Gigante J, et al:
Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is
highly effective in disrupting tumor angiogenesis. BMC Cancer.
10:6412010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alam SM, Fujimoto J, Jahan I, et al:
Overexpression of ephrinB2 and EphB4 in tumor advancement of
uterine endometrial cancers. Ann Oncol. 18:485–490. 2007.
View Article : Google Scholar
|
|
40
|
Coulthard MG, Lickliter JD, Subanesan N,
et al: Characterization of the Epha1 receptor tyrosine kinase:
expression in epithelial tissues. Growth Factors. 18:303–317. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boyd AW, Ward LD, Wicks IP, et al:
Isolation and characterization of a novel receptor-type protein
tyrosine kinase (hek) from a human pre-B cell line. J Biol Chem.
267:3262–3267. 1992.PubMed/NCBI
|
|
42
|
Tandon M, Vemula SV and Mittal SK:
Emerging strategies for EphA2 receptor targeting for cancer
therapeutics. Expert Opin Ther Targets. 15:31–51. 2011. View Article : Google Scholar :
|
|
43
|
Arvanitis D and Davy A: Eph/ephrin
signaling: networks. Genes Dev. 22:416–429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gale NW, Baluk P, Pan L, et al: Ephrin-B2
selectively marks arterial vessels and neovascularization sites in
the adult, with expression in both endothelial and smooth-muscle
cells. Dev Biol. 230:151–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Korff T, Braun J, Pfaff D, et al: Role of
ephrinB2 expression in endothelial cells during arteriogenesis:
impact on smooth muscle cell migration and monocyte recruitment.
Blood. 112:73–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shin D, Garcia-Cardena G, Hayashi S, et
al: Expression of ephrinB2 identifies a stable genetic difference
between arterial and venous vascular smooth muscle as well as
endothelial cells and marks subsets of microvessels at sites of
adult neovascularization. Dev Biol. 230:139–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Salvucci O, Maric D, Economopoulou M, et
al: EphrinB reverse signaling contributes to endothelial and mural
cell assembly into vascular structures. Blood. 114:1707–1716. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bentley K, Mariggi G, Gerhardt H and Bates
PA: Tipping the balance: robustness of tip cell selection,
migration and fusion in angiogenesis. PLoS Comput Biol.
5:e10005492009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xia G, Kumar SR, Masood R, et al: EphB4
expression and biological significance in prostate cancer. Cancer
Res. 65:4623–4632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alam SM, Fujimoto J, Jahan I, et al:
Coexpression of EphB4 and ephrinB2 in tumour advancement of ovarian
cancers. Br J Cancer. 98:845–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Brantley-Sieders DM: Clinical relevance of
Ephs and ephrins in cancer: lessons from breast, colorectal and
lung cancer profiling. Semin Cell Dev Biol. 23:102–108. 2012.
View Article : Google Scholar :
|
|
52
|
Yancopoulos GD, Davis S, Gale NW, et al:
Vascular-specific growth factors and blood vessel formation.
Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun B, Zhang D, Zhang S, et al: Hypoxia
influences vasculogenic mimicry channel formation and tumor
invasion-related protein expression in melanoma. Cancer Lett.
249:188–197. 2007. View Article : Google Scholar
|
|
54
|
Cao Y: Tumor angiogenesis and therapy.
Biomed Pharmacother. 59:S340–S343. 2005. View Article : Google Scholar
|
|
55
|
Mahabeleshwar GH and Byzova TV:
Angiogenesis in melanoma. Semin Oncol. 34:555–565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nyberg P, Salo T and Kalluri R: Tumor
microenvironment and angiogenesis. Front Biosci. 13:6537–6553.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Perez-Gomez E, Del Castillo G, Juan
Francisco S, et al: The role of the TGF-β coreceptor endoglin in
cancer. Sci World J. 10:2367–2384. 2010. View Article : Google Scholar
|
|
59
|
Medetoglu B, Gunluoglu MZ, Demir A, et al:
Tumor angiogenesis in predicting the survival of patients with
stage I lung cancer. J Thorac Cardiovasc Surg. 140:996–1000. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kiselyov A, Balakin KV and Tkachenko SE:
VEGF/VEGFR signalling as a target for inhibiting angiogenesis.
Expert Opin Investig Drugs. 16:83–107. 2007. View Article : Google Scholar
|
|
61
|
Kubota Y: Tumor angiogenesis and
anti-angiogenic therapy. Keio J Med. 61:47–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bergers G, Brekken R, McMahon G, et al:
Matrix metalloproteinase-9 triggers the angiogenic switch during
carcinogenesis. Nat Cell Biol. 2:737–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bellon G, Martiny L and Robinet A: Matrix
metalloproteinases and matrikines in angiogenesis. Crit Rev Oncol
Hematol. 49:203–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Velinov N, Poptodorov G, Gabrovski N and
Gabrovksi S: The role of matrix metalloproteinases in the tumor
growth and metastasis. Khirurgiia (Sofiia). 1:44–49. 2010.(In
Bulgarian).
|
|
65
|
Groblewska M, Siewko M, Mroczko B and
Szmitkowski M: The role of matrix metalloproteinases (MMPs) and
their inhibitors (TIMPs) in the development of esophageal cancer.
Folia Histochem Cytobiol. 50:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim TD, Song KS, Li G, et al: Activity and
expression of urokinase-type plasminogen activator and matrix
metalloproteinases in human colorectal cancer. BMC Cancer.
6:2112006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lukaszewicz-Zajac M, Mroczko B and
Szmitkowski M: Gastric cancer - The role of matrix
metalloproteinases in tumor progression. Clin Chim Acta.
412:1725–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Grishok A, Tabara H and Mello CC: Genetic
requirements for inheritance of RNAi in C. elegans. Science.
287:2494–2497. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Harborth J, Elbashir SM, Bechert K, et al:
Identification of essential genes in cultured mammalian cells using
small interfering RNAs. J Cell Sci. 114:4557–4565. 2001.
|
|
71
|
Yu JY, DeRuiter SL and Turner DL: RNA
interference by expression of short-interfering RNAs and hairpin
RNAs in mammalian cells. Proc Natl Acad Sci USA. 99:6047–6052.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang X, Zhang Y, Zhang L, et al: Silencing
alpha-fetoprotein expression induces growth arrest and apoptosis in
human hepatocellular cancer cell. Cancer Lett. 271:281–293. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chai H and Yang Y: Effects of MACC1 siRNA
on biological behaviors of HeLa. Arch Gynecol Obstet.
289:1271–1280. 2014. View Article : Google Scholar
|
|
74
|
Lui Z, Ma Q, Wang X and Zhang Y:
Inhibiting tumor growth of colorectal cancer by blocking the
expression of vascular endothelial growth factor receptor 3 using
interference vector-based RNA interference. Int J Mol Med.
25:59–64. 2010.
|