Introduction
Hepatocellular carcinoma (HCC) is a typical
malignant tumor with an enriched blood supply. At the early stage
of HCC, transarterial chemoembolization (TACE) is a treatment
option, whereas anti-angiogenic drugs (such as sorafenib) are an
option at the advanced stage of HCC (1,2).
Angiogenesis is a complicated process involving pro-angiogenic
factors [e.g. vascular endothelial cell growth factor (VEGF), basic
fibroblast growth factor, cyclooxygenase-2, angiopoietin-1
(Ang1)/Ang2)] and antiangiogenic factors (e.g. tumstatin,
hexastatin and endostatin), which constitute the ‘angiogenic
switch’ (3,4). Tumor angiogenesis is closely
associated with tumor growth, metastasis and chemoresistance.
Recently, cytotoxic and anti-angiogenic drugs have been applied
clinically and were found to improve the prognosis of HCC patients
(5,6). However, chemoresistance has attracted
general attention and its definite mechanisms are being studied
(7). Hypoxia induced by
anti-angiogenic treatment activates hypoxia-inducible factor-1α
(HIF-1α) and nuclear factor-κB (NF-κB) pathways (8). Indeed, hypoxia caused by
anti-angiogenic drugs not only induces chemoresistance, but also
results in local dissemination of tumor cells and distant
metastasis (9). It has been
suggested that sustained anti-angiogenic treatment may improve the
poor prognosis of HCC patients to some extent. Nevertheless, it
cannot be denied that high levels of Ang2 in HCC patient serum is
an important marker of the poor treatment effect of sorafenib
(10). Ang2 may be a potential
factor in the modulation of angiogenesis, metastasis and
chemoresistance of tumors under hypoxic conditions.
Ang2 is a member of the angiopoietin family which
induces EC destabilization, disassociation from the endothelial
cell medium (ECM), migration and sprouting (11). Ang2 is mainly stored in
Weibel-Palade (WP) bodies of endothelial cells (ECs), which is
released into the ECM by hypoxic stimulation, a hallmark of the
intratumor environment. Ang2 and VEGF are considered as the main
pro-angiogenic factors and Ang2 has been demonstrated to improve
the pro-angiogenic activity of VEGF (12,13).
Thus, Ang2 may be a key target molecule in future antitumor
treatments.
Tumstatin is an endogenic anti-angiogenic factor
derived from the noncollagenous 1 domain fragment of the type IV
collagen α3 chain (α3(IV)NC1), which binds to integrin αVβ3 and
α3β1 and further inhibits the activity of FAK/PI3K and NF-κB
pathways under hypoxia (14).
Downregulation of tumstatin is associated with enhanced cell
transformation as well as tumor invasion and angiogenesis (15). The active fragments in full-length
tumstatin are a current research focus. The T7 peptide, the 74–98
amino acid fragment of tumstatin, has an equivalent anti-angiogenic
activity to that of full-length tumstatin (16). On the other hand, the T7 peptide
exerts pro-apoptotic activity and inhibits the growth of tumors
(17). However the anti-angiogenic
mechanisms of the T7 peptide in tumor therapy are unclear.
Tumstatin treatment of a diabetic nephropathy mouse model was found
to decrease the expression of Ang-2, resulting in inhibition of
glomerular hyper-filtration (18).
Autophagy is a process in which subcellular
membranes undergo dynamic morphological changes that lead to
degradation of cellular proteins and cytoplasmic organelles
mediating the cell survival and metastatic ability of tumor cells
(19,20). Autophagy has been investigated as a
mechanism of cell protection against drug-induced apoptosis in
cancer cells by degradation of important proteins such as the
pro-apoptotic protein Bax (21).
Recently, it was reported that activation of autophagy induces
pro-angiogenic activity under different contexts (22). High mobility group box 1 (HMGB1) was
found to induce tube formation in vitro via induction of
autophagy under hypoxic conditions (23). Moreover, the autophagic pathway
crosstalks with the apoptotic pathway, which is considered to be a
possible mechanism that modulates angiogenesis (24). However, the effect of the T7 peptide
on autophagy and the role of autophagy in the T7 peptide-mediated
inhibition of angiogenesis still remain unclear.
Anti-angiogenic therapy is important for the
treatment of tumors, especially for tumors with an enriched blood
supply. The related endogenic anti-angiogenic factors and
pro-angiogenic factors have been examined in a previous study
(3). The mechanisms of the T7
peptide in regulation of angiogenesis and invasion of HCC are still
unknown. In the present study, we investigated the mechanisms of
the T7 peptide in the inhibition of angiogenesis and whether the T7
peptide modulates the expression of Ang2 and the associated
mechanisms. Moreover, we also examined the autophagy induced by the
T7 peptide in modulation of angiogenesis. Our data provide evidence
that Ang2, p-AKT, metalloproteinase-2 (MMP-2) and autophagy play
important roles in angiogenesis and invasion of HCC, which can be
directly or indirectly regulated by the T7 peptide.
Materials and methods
Cell lines and culture
Human umbilical vascular endothelial cells (HUVECs)
were purchased from the Typical Animal Reserve Center of China
(Shanghai, China) and cultured in ECM-2 medium (ScienCell Research
Laboratories, Carlsbad, CA, USA) supplemented with 5% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin and 1%
endothelial cell growth supplement (ECGS). The human HCC cell line
HepG2 was obtained from the American Type Culture Collection
(Rockville, MD, USA) and cultured in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% FBS, 100 U/ml penicillin and
100 μg/ml streptomycin. A hypoxia chamber (Billups-Rothenberg,
Inc.) was used to mimic hypoxic conditions (1% O2, 5%
CO2 and 94% N2). Acetic acid (30%) was used
to adjust the pH of each group.
Antibodies and reagents
An antibody against Ang2 was purchased from
Epitomics (Burlingame, CA, USA). Antibodies against CD31, Bcl-2,
Bax, Akt, phosphorylated (p)-Akt (Ser473), MMP-2, light chain 3
(LC3) and GAPDH were purchased from Cell Signaling Technology
(Danvers, MA, USA). An anti-VE-cadherin antibody was purchased from
Abcam (Cambridge, UK). MK2206 was from Santa Cruz Biotechnology
Inc. (Santa Cruz, CA, USA). Recombinant human Ang-2 (rhAng-2) was
from PeproTech (Rocky Hill, NJ, USA). Recombinant T7 peptide was
purchased from Bootech Bioscience and Technology (Shanghai, China)
and dissolved in 30% acetic acid. Matrigel was purchased from BD
Biosciences (San Jose, CA, USA).
Immunohistochemistry
HepG2 tumor samples from a xenogeneic nude mouse
model (following the approval of the Ethics Committee of Qianfoshan
Hospital) were fixed in 10% formalin, embedded in paraffin and then
processed for immunohistochemistry. Sections were deparaffinized in
graded xylene and rehydrated in graded ethanol, followed by 3
washes with phosphate-buffered saline (PBS) for 3 min each. After
heat-induced antigen retrieval in citrate buffer, endogenous
peroxidase was inhibited by treatment with 3% hydrogen peroxide at
room temperature for 10 min, followed by 3 washes with PBS for 3
min each. Primary anti-VE cadherin (1:50 dilution) and anti-CD31
(1:30 dilution) antibodies were applied overnight. After washing,
the sections were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (1:50 dilution; Beyotime,
Jiangsu, China) for 1 h at 37°C. Negative control sections were
incubated with PBS instead of the primary antibody.
Tube formation assay
HUVECs (5×103/well) were seeded in
96-well plates coated with Matrigel and allowed to adhere. The
cells were then incubated under normoxic or hypoxic conditions with
or without the T7 peptide and/or 3-MA (5 nM) for 24 h at 37°C with
5% CO2.
Cell viability assay
HUVECs (5×103/well) were seeded in two
96-well plates. After 12 h, the medium in the first plate was
replaced with medium containing various concentrations of the T7
peptide (0.25, 0.5, 1 and 2 μM) and then the cells were incubated
in the hypoxia chamber. HUVECs in the second plate were incubated
under hypoxic conditions with or without the T7 peptide (1 μM).
Control and treatment groups were adjusted to the same pH with 30%
acetic acid. At various time-points (plate 1: t=24 h; plate 2: t=12
and 24 h), the cells were applied to a Cell Counting Kit-8 (Dojindo
Molecular Technologies, Japan). The optical density (OD) at 450 nm
was measured with a Spectra Max 190 (Molecular Devices, Sunnyvale,
CA, USA).
Cell apoptosis assay
HUVECs (4×105/well) were seeded in a
6-well plate. After 12 h, the cells were incubated with fresh
medium under normoxic or hypoxic conditions with or without the T7
peptide and/or 3-MA (5 μM). After 24 h, the cells were trypsinized
and centrifuged at 1,000 × g for 10 min. Then, the cells were
washed twice with sterile PBS and resuspended with binding buffer.
Annexin V/FITC and propidium iodide (PI; NeoBioscience Ltd.) were
used to detect the apoptosis rate of cells according to the
manufacturer’s instructions. Finally, Annexin V- and PI-labeled
cells were analyzed by flow cytometry.
Western blot analysis
At 80% confluency, HUVECs were incubated under
normoxic or hypoxic conditions with or without the T7 peptide (1
μM) or MK2206 (5 μM) and/or 3-MA (5 nM), respectively. HepG2 cells
were incubated with the supernatants from the HUVECs divided into 4
groups: normoxic group, hypoxic groups with or without the T7
peptide plus rhAng2 (15 ng/ml) or not. After 24 h, the cells were
lysed in cold RIPA lysis buffer (Beyotime) with 1 nM
phenylmethylsufonyl fluoride, followed by centrifugation at 12,000
× g for 10 min at 4°C. The concentration of the protein samples was
measured with a BCA Protein Assay kit (Beyotime). Total proteins
(20–40 μg) were separated on 8–15% SDS-polyacrylamide gel
electrophoresis gels (Beyotime) and electrotransferred to
polyvinylidene fluoride membranes. The membranes were then
incubated with primary antibodies overnight at 4°C [1:3,000 for
Ang2; 1:1,000 for Bcl-2, Bax, Akt, p-Akt (Ser473), MMP-2 and LC3].
After 3 washes with PBS containing 0.1% Tween-20 for 15 min each,
the membranes were incubated with HRP-conjugated anti-rabbit IgG
(Beyotime) at 37°C for 1 h and then washed 3 times with PBS
containing 0.1% Tween-20. A TANON-4500SF chemiluminescence system
was used to detect the target proteins.
Migration assay
HUVECs were seeded in 24-well plates with ECM-2
medium. At confluency, a wound was induced by scratching the
monolayer with a 10-μl pipette tip. The cells were then washed 3
times with sterile PBS. HUVECs were incubated in serum-free ECM-2
medium under normoxic or hypoxic conditions with or without the T7
peptide (1 μM) plus rhAng2 (15 ng/ml) or not. Images were acquired
at 12 h post-scratching.
HUVECs (1×105 in 200 μl medium) were
added to the upper chamber of a Transwell plate, and 500 μl medium
as described for the scratch assay was added to the lower chamber.
After 24 h, the migrated cells were fixed with 95% methanol and
stained with 0.1% crystal violet for 30 min followed by 5 washes
with PBS.
Invasion assay of HepG2
HUVECs (2×105/well) were seeded in the
lower chamber of a Transwell plate, and HepG2 cells
(1×105/well) were added to the upper chamber coated with
Matrigel. After 12 h, the culture medium for the HUVECs was
replaced and the cells were incubated under normoxic or hypoxic
conditions with or without the T7 peptide (1 μM) plus rhAng-2 (15
ng/ml) or not. After 48 h, HepG2 cells in the upper chamber were
fixed with 95% methanol and stained with 0.1% crystal violet for 30
min followed by 5 washes with PBS.
Enzyme-linked immunosorbent assay
(ELISA)
HUVECs were seeded in 6-well plates and incubated
overnight. Then, the medium was changed and the HUVECs were
incubated under normoxic or hypoxic conditions with or without the
T7 peptide. After 24 h, the culture supernatants were collected.
Measurement of Ang2 levels in the culture supernatants was
performed using a commercially available ELISA kit (Gr Bio,
Shanghai, China) according to the manufacturer’s instructions. OD
values were obtained at 450 nm by the Spectra Max 190.
Acridine orange staining for cell
autophagy
HUVECs (5×103/well) were seeded in
24-well plates with ECM-2 medium. After 6 h, the cells were
incubated with new medium under normoxic or hypoxic conditions with
or without T7 peptide (1 μM) and/or 3-MA (5 nM) for 24 h. The cells
were then stained with an acridine orange solution (5 μM) for 10
min at 37°C and then washed 3 times with PBS. The cells were
observed and photographed under a fluorescence microscope.
Statistical analysis
The Student’s t-test was used for comparisons
between 2 groups or one-way analysis of variance for comparisons
between multiple groups. Statistical analysis was performed with
SPSS software (version 17.0, SPSS China, Shanghai, China).
P<0.05 was considered to indicate a statistically significant
result.
Results
T7 peptide inhibits angiogenesis in vivo
and in vitro
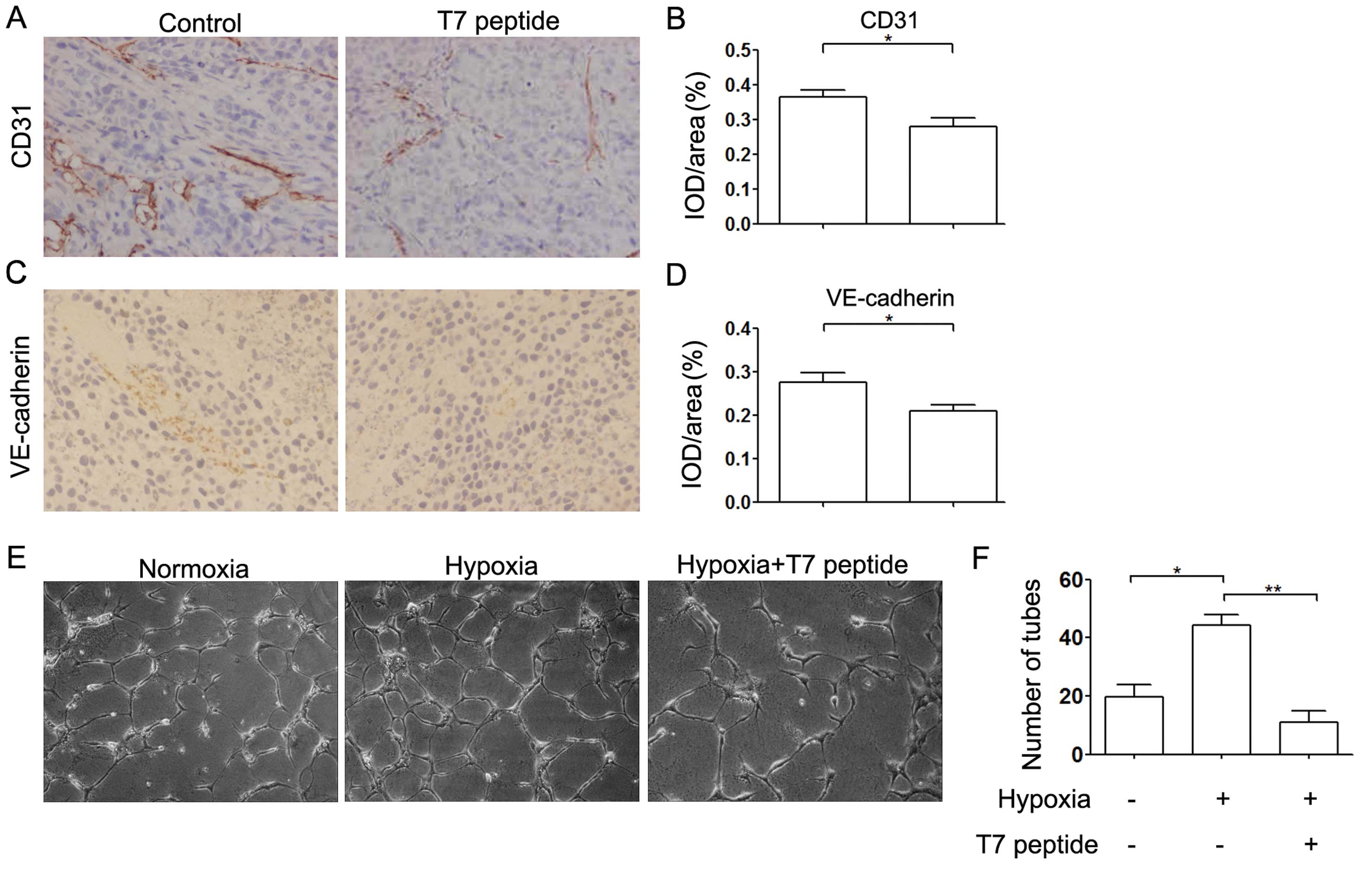
First, we examined the expression levels of CD31 and
VE-cadherin, which are hallmarks of angiogenesis, by
immunohistochemical analysis. The expression of both CD31 and
VE-cadherin was downregulated in the T7 peptide treatment group and
angiogenesis was decreased compared with that in the control group
(Fig. 1A–D). To further investigate
the effect of the T7 peptide on angiogenesis, we incubated HUVECs
in Matrigel-coated 96-well plates under various conditions for 24
h. The results indicated that capillary-like tube formation was
decreased significantly by the T7 peptide under hypoxic conditions
in vitro, which was consistent with the in vivo
results (Fig. 1E and F).
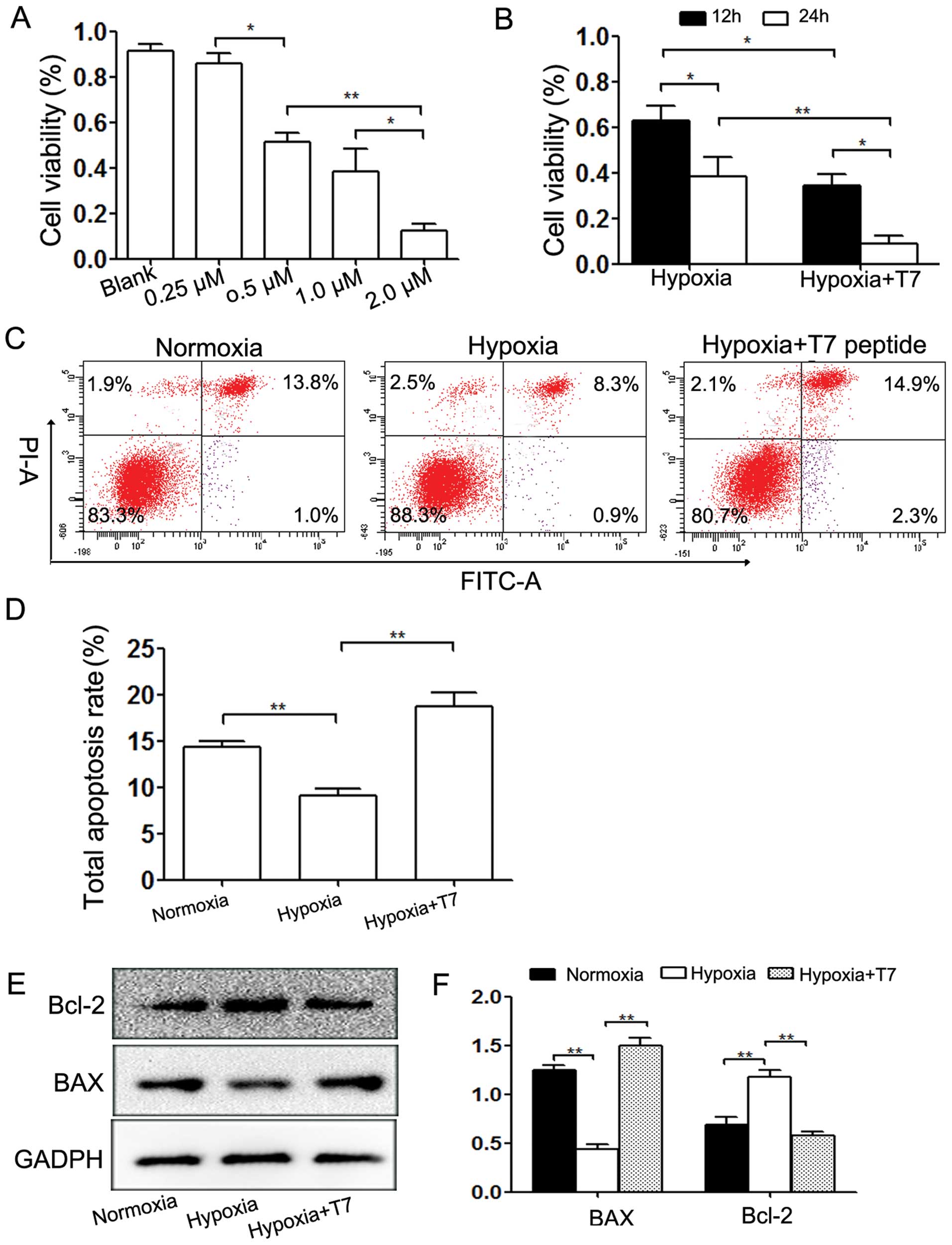
T7 peptide inhibits cell viability and
induces apoptosis under hypoxic conditions
Under hypoxic conditions, the T7 peptide contributed
to a significant decrease in cell viability in both dose- and
time-dependent manners (Fig. 2A and
B). We further detected the degree of apoptosis caused by the
T7 peptide under hypoxic conditions, which is one of the factors
affecting cell viability. The results revealed that the T7 peptide
enhanced the apoptosis rate significantly, whereas hypoxia
suppressed apoptosis (Fig. 2C and
D). Western blot analysis indicated that the T7 peptide reduced
the expression of the anti-apoptotic protein Bcl-2 and reversed the
decrease in the expression of pro-apoptotic protein Bax induced by
hypoxia (Fig. 2E and F).
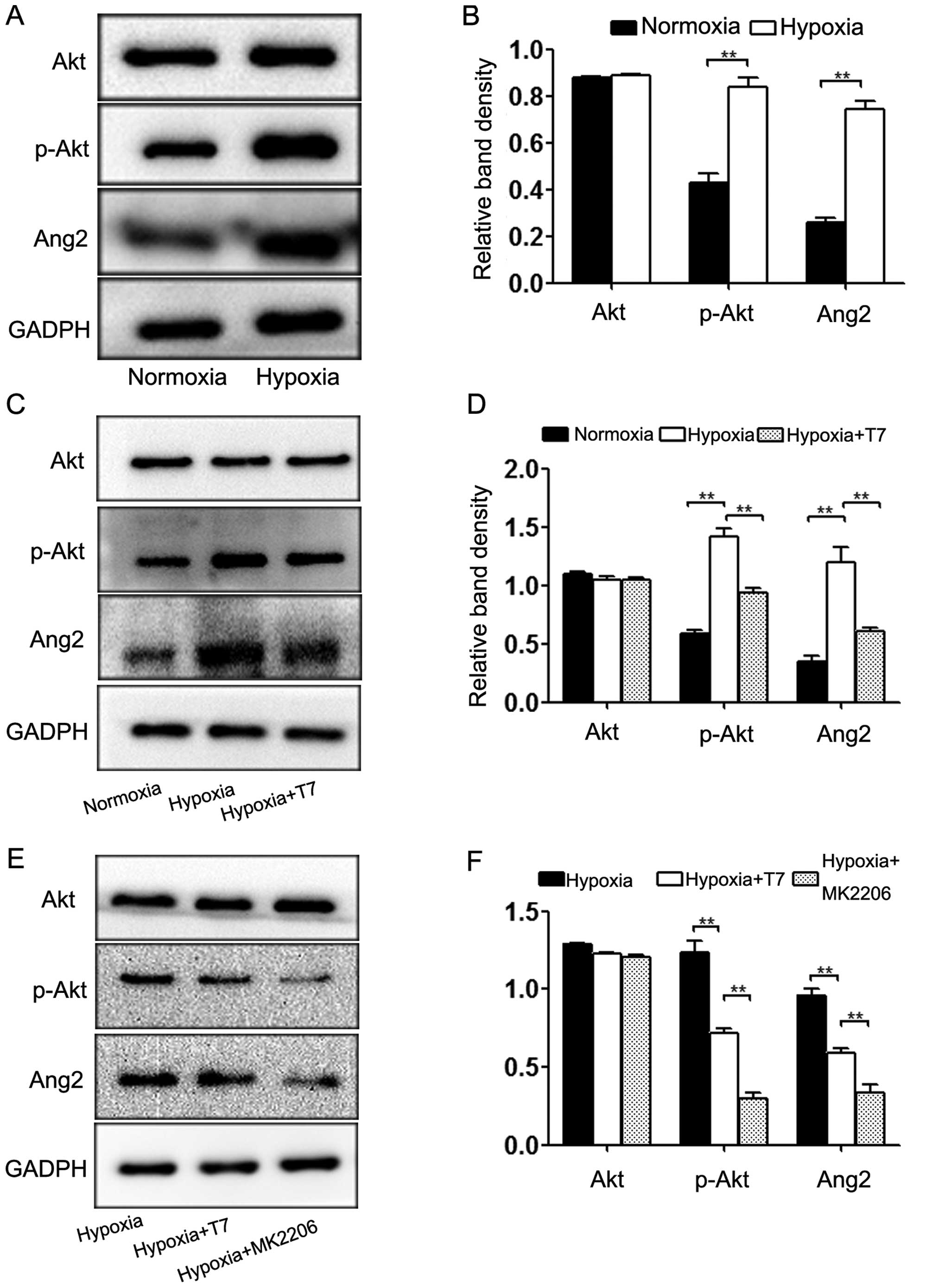
T7 peptide decreases Ang2 expression via
inhibition of AKT phosphorylation in ECs
Ang2 is crucial for disassociation of ECs from the
ECM, EC migration and sprouting and the induction of angiogenesis
in HCC (25). To explore the
mechanism of the T7 peptide in the modulation of Ang2 expression,
we first detected the expression levels of Akt, p-Akt and Ang2
under hypoxic conditions. Hypoxia significantly increased the
expression of Ang2, which was accompanied by Akt activation
(Fig. 3A and B). Based on these
results, we treated the cells with the T7 peptide under hypoxic
conditions. As a result, the Ang2 expression level was
downregulated significantly by the T7 peptide when the Akt pathway
was inhibited simultaneously, while the amount of total Akt was
unchanged (Fig. 3C and D). To
examine the relationship between Ang2 expression and the Akt
pathway, we incubated HUVECs under hypoxic conditions with the T7
peptide or MK2206 (positive control) for 24 h. Western blotting
showed that MK2206 significantly reduced the protein levels of Ang2
and p-Akt, which was consistent with the results of the T7 peptide
treatment (Fig. 3E and F). Based on
these data, blocking the phosphorylation of Akt contributed to the
downregulation of Ang2. Furthermore, the T7 peptide reduced the
expression of Ang2 via inhibition of Akt phosphorylation.
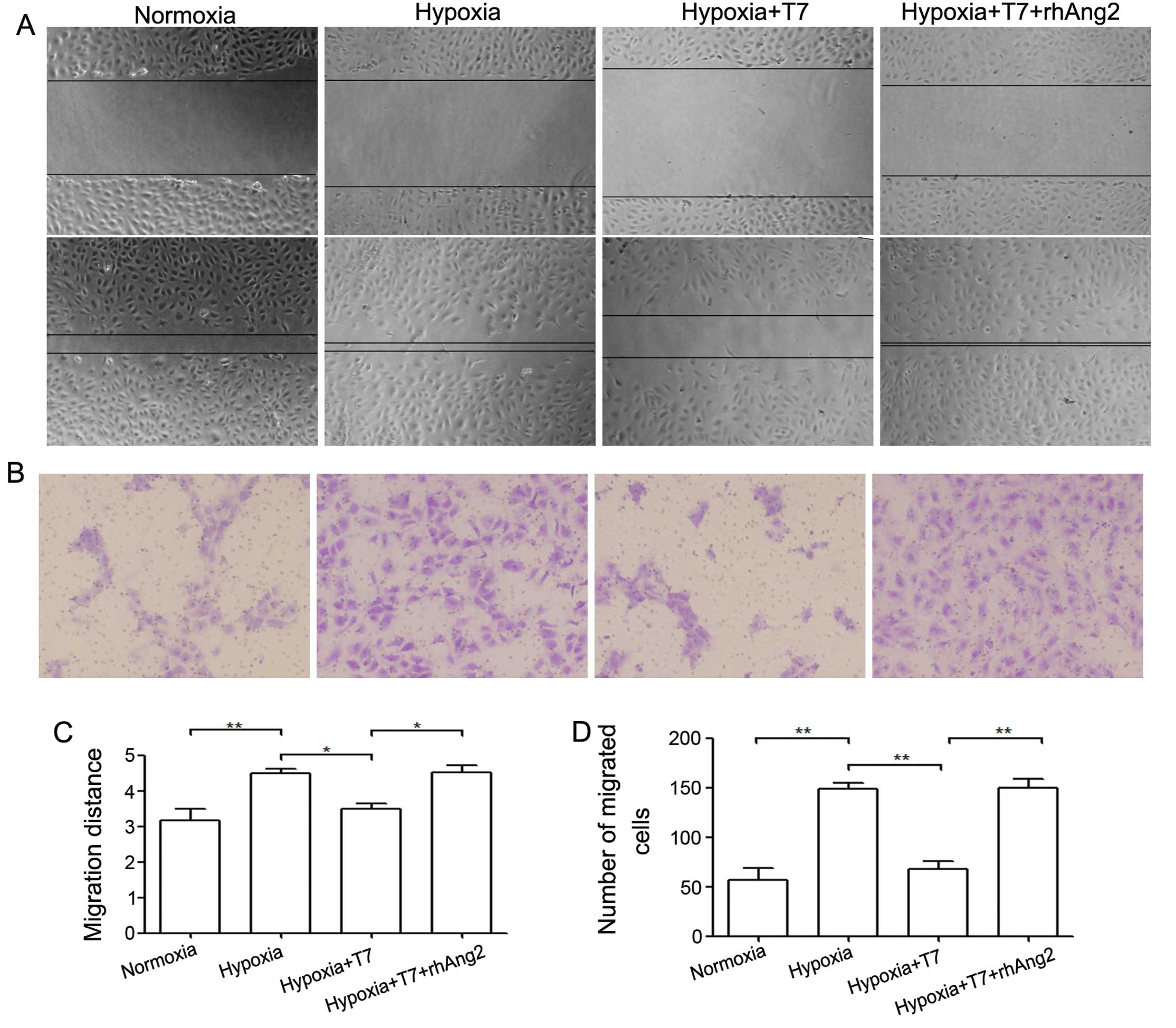
T7 peptide reduces EC migration via
downregulation of Ang2
The process of angiogenesis is initiated by
quiescent ECs that become activated by angiogenic signals.
Angiogenesis proceeds through vessel destabilization, EC migration,
proliferation and sprouting and coverage of pericytes and muscle
cells (26). Ang2 plays a key role
in modulation of EC disassociation and migration (27). To determine whether the T7 peptide
inhibits EC migration, we first analyzed HUVECs in a scratch assay.
Since we demonstrated that the T7 peptide reduced Ang2 expression
in a previous study, we treated the cells with the T7 peptide and
rhAng2. We found that the migration ability of HUVECs was enhanced
by hypoxia compared with that under normoxia, whereas the T7
peptide reduced cell migration ability significantly. Our data also
showed that rhAng2 significantly reversed the inhibition of the T7
peptide under hypoxic condition (Fig.
4A and C). To confirm these results, we applied HUVECs to a
migration assay and the results were consistent with those of the
scratch assay (Fig. 4B and D).
Based on these data, the T7 peptide inhibited EC migration by a
reduction in the protein level of Ang2, leading to inhibition of
angiogenesis.
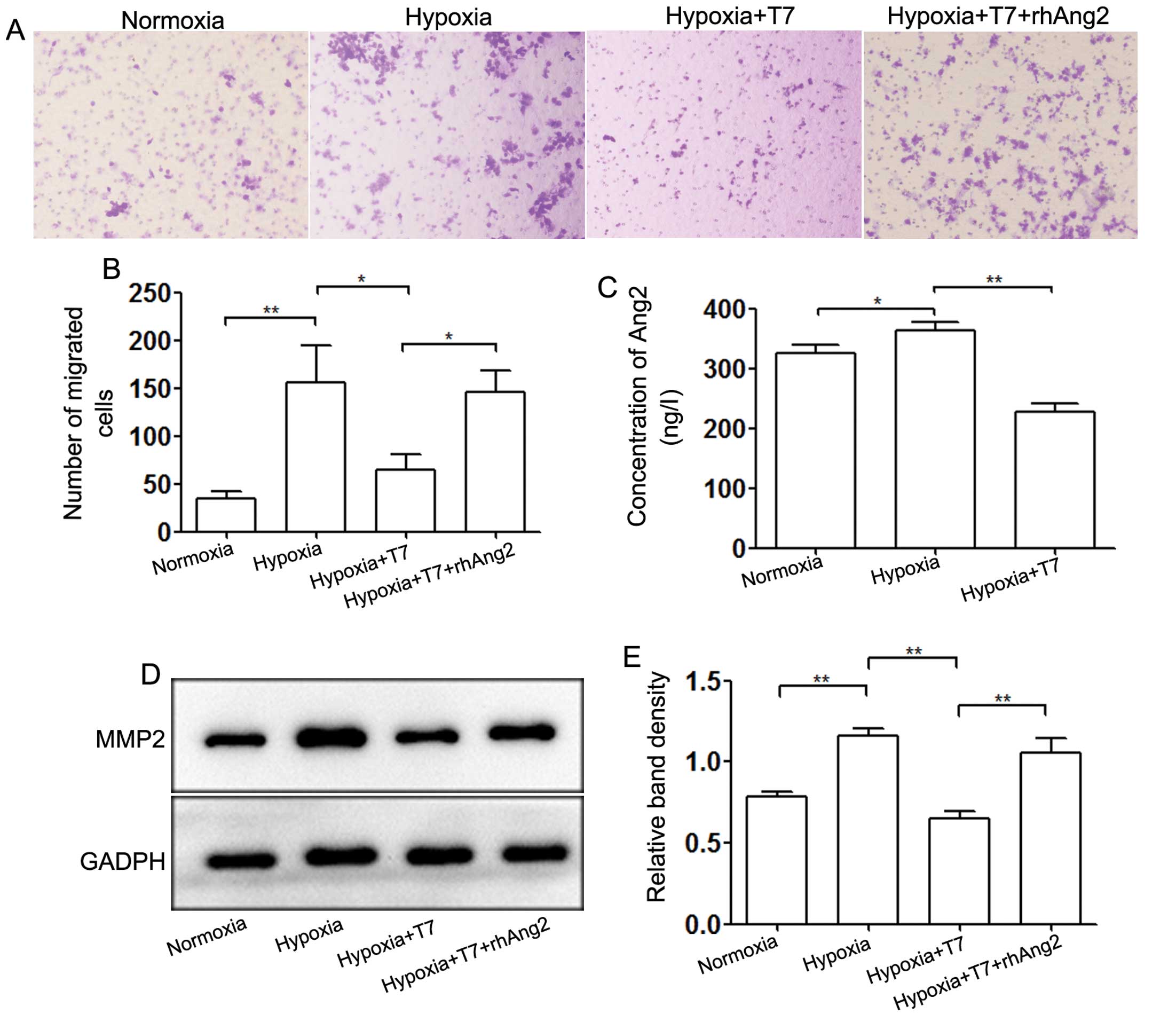
Ang2 mediates the invasion of HCC
cells
Ang2 plays an important role not only in the
angiogenesis of HCC, but also in the metastasis of HCC (24). We measured the concentration of Ang2
in culture supernatants of HUVECs by ELISA (Fig. 5C). Hypoxia induced Ang2 release from
WP bodies and the T7 peptide blocked this process significantly. To
explore the role of Ang2 in modulation of the invasive ability of
HepG2 cells, we incubated HepG2 cells for 24 h in the upper chamber
of a Transwell plate with HUVECs seeded in the lower chamber. Rapid
release of Ang2 induced by hypoxia enhanced the invasive ability of
HepG2 cells, whereas the T7 peptide abrogated HepG2 cell invasion
by downregulating the expression of Ang2 (Fig. 5A and B). Moreover, addition of
rhAng2 to the T7 peptide treatment group confirmed that Ang2 played
a key role in mediating tumor cell invasion regulated by the T7
peptide and hypoxia. We also detected the expression level of
MMP-2, which is related to tumor metastasis. The data showed that
downregulation of Ang2 induced by the T7 peptide decreased the
expression level of MMP-2 under hypoxic conditions, whereas rhAng2
partially neutralized the inhibition (Fig. 5D and E). These results indicated
that the T7 peptide inhibited the invasion of HepG2 cell by
reducing expression of MMP-2, which was mediated through Ang2.
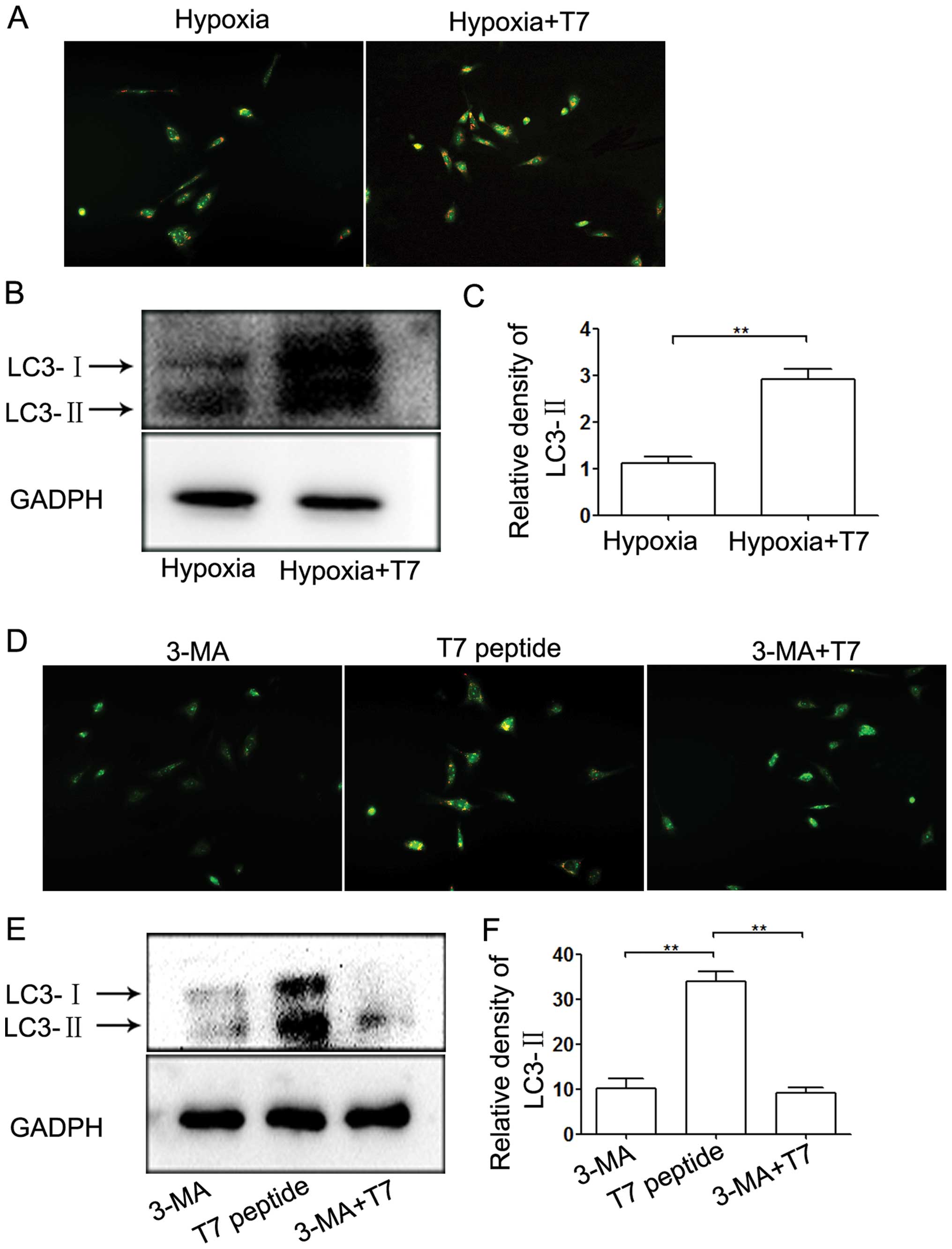
Inhibition of autophagy potentiates the
anti-angiogenic activity of the T7 peptide
The T7 peptide inhibited capillary-like tube
formation by inhibition of EC viability, migration mediated by Ang2
and induction of EC apoptosis. Previous studies have shown that
autophagy plays a dual role in regulating angiogenesis (22,28).
In our study, we incubated ECs under hypoxic conditions and found
that the T7 peptide induced autophagy compared with hypoxia
(Fig. 6A). LC3-II is a marker
protein of the level of autophagy (29). The results of our western blot
analysis of LC3-II were in accordance with the data from acridine
orange (AO) staining (Fig. 6B and
C). To further examine the role of autophagy in angiogenesis,
we incubated ECs under hypoxic conditions in a tube formation assay
followed by OA staining. The level of autophagy induced by the T7
peptide in HUVECs was significantly downregulated by 3-MA, a
specific inhibitor of autophagy (Fig.
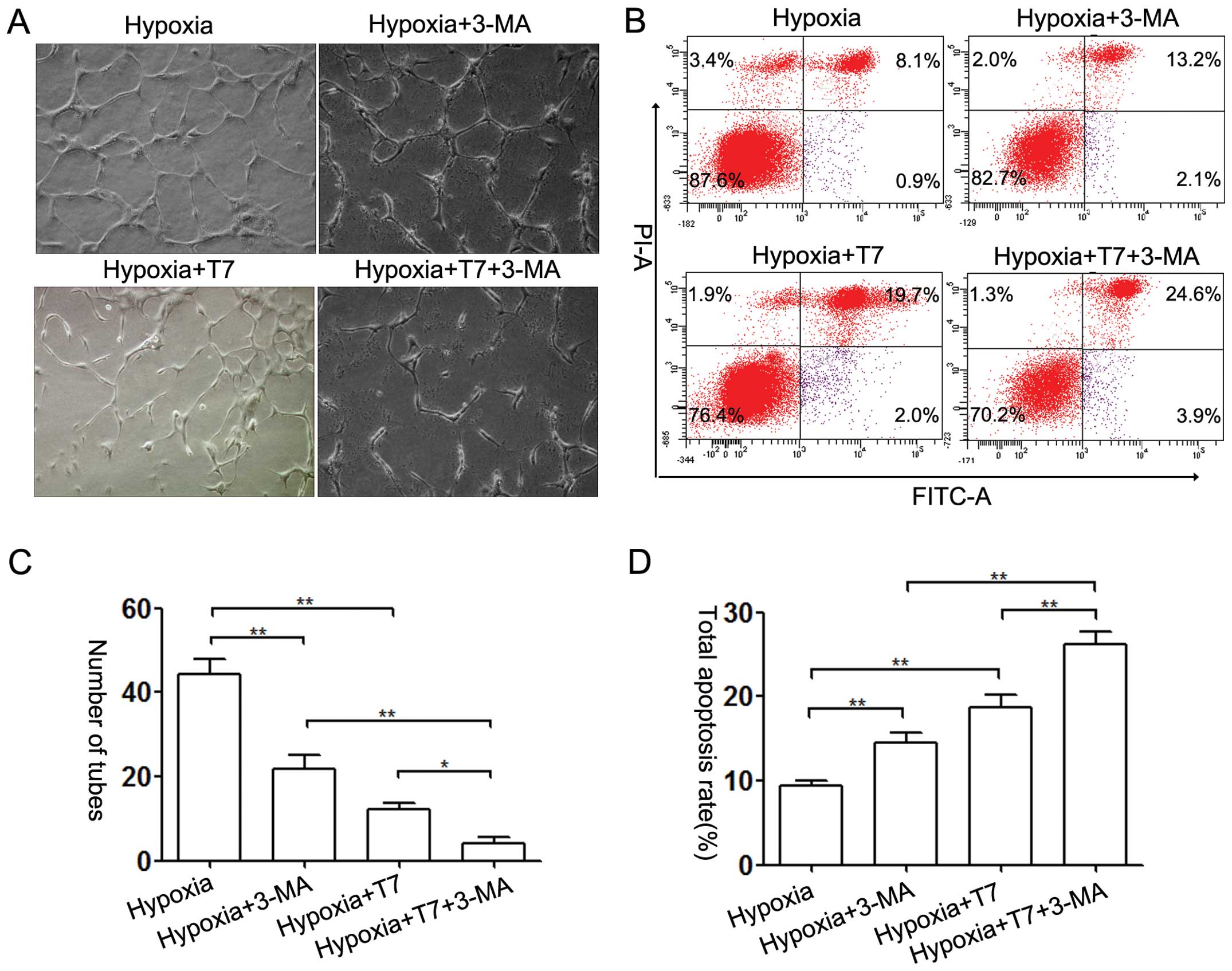
6D–F). Furthermore, 3-MA inhibited tube formation under hypoxic
conditions, and the anti-angiogenic ability of the T7 peptide was
enhanced by addition of 3-MA (Fig. 7A
and C). An apoptosis assay indicated that 3-MA increased the
pro-apoptotic activity of the T7 peptide (Fig. 7B and D). However, the effect of the
T7 peptide was more obvious than that of 3-MA (Fig. 7A and B). Based on these data, we
demonstrated that the T7 peptide reduced the tube formation in
vitro by induction of EC apoptosis and inhibition of autophagy.
3-MA synergistically enhanced the anti-angiogenic ability of the T7
peptide. Autophagy may be a protective mechanism against the
anti-angiogenic activity of the T7 peptide.
Discussion
Angiogenesis-targeted treatment is one of the most
appropriate therapeutic options for HCC patients, especially for
advanced patients (1).Tumor
angiogenesis mostly depends on the balance between pro-angiogenic
and anti-angiogenic factors (3,4).
Hypoxia, which is particularly present inside tumors, induces the
expression of pro-angiogenic factors such as VEGF and Ang2
(30). Anti-angiogenic treatment in
HCC patients is considered to be a factor that induces intratumor
hypoxia (8). The T7 peptide, an
active fragment of full-length tumstatin, exerts an anti-angiogenic
activity through its L, V and D amino acids (31). Compared with full-length tumstatin,
the T7 peptide owns lower molecular weight and better commercial
availability. Therefore, the T7 peptide may be a better prospect
for the anti-angiogenic therapy of HCC patients. Our data showed
that the T7 peptide inhibited angiogenesis in vivo. CD31 is
considered as a marker that indicates the degree of tumor
angiogenesis. VE-cadherin is a key component of endothelial
cell-to-cell adherens junctions and plays an important role in
vascular permeability and remodeling. VE-cadherin is required for
organization of a stable vascular system and controls vascular
permeability in adults (32).
Immunohistochemical staining of CD31 and VE-cadherin indicated that
treatment with the T7 peptide resulted in reduced vessel formation.
Moreover, the present study also indicated that the T7 peptide
exerts its anti-angiogenic ability through inhibiting EC viability
and migration and inducing apoptosis in vitro. The level of
Ang2 is rapidly elevated in tumor-associated endothelium (33), which promotes EC disassociation from
the ECM and migration. Blocking the Ang2/Tie2 axis inhibits tumor
angiogenesis, growth and dissemination in multiple tumor models,
including tumors that are prone to developing resistance against
anti-VEGF/VEGF receptor therapy (34). A previous study reported that AMG386
(an Fc fusion peptide that blocks the Ang-2/Tie2 axis) shows
antitumor activity in patients with clear cell metastatic renal
cell carcinoma (35). Ang2 blockade
also reduces tumor vessel sprouting, and ectopic high expression of
Ang2 inhibits the benefits of anti-VEGF receptor treatment
(36,37). Thus, a high Ang2 level is not only
responsible for the initiation of angiogenesis but also is
partially responsible for chemoresistance against sorafenib,
suggesting that Ang2, as a target of anti-angiogenic treatment,
should be investigated further. The present study demonstrated that
the T7 peptide significantly decreased the expression of Ang2 under
hypoxic conditions, which was significantly elevated by hypoxia.
Akt promotes tumorigenesis and drug resistance by disrupting
apoptosis (38). Akt
phosphorylation was induced by hypoxia, which indicates activation
of the Akt pathway, whereas the T7 peptide inhibited
phosphorylation of Akt. MK2206, an inhibitor of the Akt pathway,
also significantly downregulated the Ang2 expression level, which
was accompanied by inhibition of Akt phosphorylation. We conclude
that the T7 peptide reduced Ang2 expression by inhibiting
phosphorylation of Akt.
Angiogenesis involves EC dissociation, migration,
sprouting, vessel formation and coverage of pericytes. EC migration
is indispensable for angiogenesis (26). Our data showed that the T7 peptide
inhibited hypoxia-mediated cell migration and addition of rhAng2
mostly neutralized the effect of the T7 peptide. EC migration was
inhibited by the T7 peptide by downregulating Ang2 expression. It
has been reported that overexpression of Ang2 enhances tumor
metastasis and an Ang2-blocking antibody inhibits this process
(39). MMP-2 is responsible for
degradation of the ECM. High expression of MMP-2 is important for
invasion of tumor cells (25).
Consistent with previous studies, our study demonstrated that the
invasive ability of HepG2 cells was increased by hypoxia with high
expression of MMP-2, whereas the T7 peptide inhibited these
effects. The high expression of Ang2 under hypoxic conditions may
be related to the invasion of HepG2 cells and the mechanisms of the
T7 peptide in the inhibition of HepG2 cell invasion may involve
downregulation of Ang2. Moreover, the addition of rhAng2 partly
reversed the inhibitory activity of the T7 peptide, which verifies
our hypothesis. These results indicate that the anti-metastatic
ability of the T7 peptide was mediated by partially inhibiting
Ang2. However, the underlying mechanism of Ang2 in the enhancement
of tumor cell invasion still needs to be explored.
Autophagy is a self-catabolic cellular process in
which cytoplasmic proteins and organelles are sequestered and
delivered to lysosomes for degradation (40). In most cases, autophagy is
considered as a cell survival mechanism induced under certain
conditions of stress. A previous study reported that elevated
autophagy of ECs improves capillary-like tube formation in
vitro by potentiating the anti-apoptotic ability (22). Interestingly, it has also been
reported that autophagy increases angiogenesis by enhancing the
proliferation of ECs (28).
Therefore, autophagy has been suggested to play an adverse role in
angiogenesis under different contexts. Our present study indicated
that the T7 peptide induced autophagy of HUVECs and upregulated the
expression of LC3-II. 3-MA, a specific inhibitor of autophagy,
inhibited the angiogenesis. Compared with the T7 peptide, 3-MA had
a lesser effect on the tube formation and apoptosis of ECs.
Meanwhile, 3-MA enhanced the pro-apoptotic and anti-angiogenic
ability of the T7 peptide. The anti-angiogenic ability of the T7
peptide was partially antagonized by the autophagy induced by the
T7 peptide. There was an increase in EC apoptosis and reduction in
angiogenesis, which were accompanied by inhibition of autophagy.
Therefore, the combination of angiogenic and autophagic inhibitors
has a great prospect for future antitumor therapies.
In summary, the present study demonstrated that the
T7 peptide partially exerts its anti-angiogenic activity by
inhibiting tumor cell viability, migration and inducing the
apoptosis of ECs. Ang2 expression is upregulated by activation of
the Akt pathway and improves the invasive ability of HepG2 cells,
which is inhibited by the T7 peptide. Furthermore, inhibition of
autophagy enhances the antiangiogenic activity of the T7 peptide to
some extent, which provides evidence for the application of an
autophagic inhibitor in future antitumor therapy.
Acknowledgements
This research was supported from the National
Natural Scientific Foundation of China (http://www.nsfc.gov.cn) (30972890 and 81172331).
References
|
1
|
Psyrri A, Arkadopoulos N, Vassilakopoulou
M, Smyrniotis V and Dimitriadis G: Pathways and targets in
hepatocellular carcinoma. Expert Rev of Anticancer Ther.
12:1347–1357. 2012. View Article : Google Scholar
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Almog N, Ma L, Schwager C, et al:
Consensus microRNAs governing the switch of dormant tumors to the
fast-growing angiogenic phenotype. PLoS One. 7:e440012012.
View Article : Google Scholar
|
|
5
|
Welker MW and Trojan J: Anti-angiogenesis
in hepatocellular carcinoma treatment: current evidence and future
perspectives. World J Gastroenterol. 17:3075–3081. 2011.PubMed/NCBI
|
|
6
|
Edeline J, Boucher E, Rolland Y, et al:
Comparison of tumor response by Response Evaluation Criteria in
Solid Tumors (RECIST) and modified RECIST in patients treated with
sorafenib for hepatocellular carcinoma. Cancer. 118:147–156. 2012.
View Article : Google Scholar
|
|
7
|
Zhai B and Sun XY: Mechanisms of
resistance to sorafenib and the corresponding strategies in
hepatocellular carcinoma. World J Hepatol. 5:345–352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang Y, Zheng T, Song R, et al:
Hypoxia-mediated sorafenib resistance can be overcome by EF24
through Von Hippel-Lindau tumor suppressor-dependent HIF-1α
inhibition in hepatocellular carcinoma. Hepatology. 57:1847–1857.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paez-Ribes M, Allen E, Hudock J, et al:
Antiangiogenic therapy elicits malignant progression of tumors to
increased local invasion and distant metastasis. Cancer Cell.
15:220–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyahara K, Nouso K, Tomoda T, et al:
Predicting the treatment effect of sorafenib using serum
angiogenesis markers in patients with hepatocellular carcinoma. J
Gastroenterol Hepatol. 26:1604–1611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holash J, Maisonpierre PC, Compton D, et
al: Vessel cooption, regression and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Augustin HG, Koh GY, Thurston G and
Alitalo K: Control of vascular morphogenesis and homeostasis
through the angiopoietin-Tie system. Nat Rev Mol Cell Biol.
10:165–177. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saharinen P, Bry M and Alitalo K: How do
angiopoietins Tie in with vascular endothelial growth factors? Cur
Opin Hematol. 17:198–205. 2010.
|
|
14
|
Boosani CS, Mannam AP, Cosgrove D, et al:
Regulation of COX-2 mediated signaling by alpha3 type IV
noncollagenous domain in tumor angiogenesis. Blood. 110:1168–1177.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Xu CX, Hou GS, Chen YG, Xin JX and
Liu XX: Downregulation of tumstatin expression by overexpression of
ornithine decarboxylase. Oncol Rep. 30:2042–2048. 2013.PubMed/NCBI
|
|
16
|
Colorado PC, Torre A, Kamphaus G, et al:
Anti-angiogenic cues from vascular basement membrane collagen.
Cancer Res. 60:2520–2526. 2000.PubMed/NCBI
|
|
17
|
Maeshima Y, Sudhakar A, Lively JC, et al:
Tumstatin, an endothelial cell-specific inhibitor of protein
synthesis. Science. 295:140–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto Y, Maeshima Y, Kitayama H, et al:
Tumstatin peptide, an inhibitor of angiogenesis, prevents
glomerular hypertrophy in the early stage of diabetic nephropathy.
Diabetes. 53:1831–1840. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Glick D, Barth S and Macleod KF:
Autophagy: cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu H, Wang D, Zhang L, et al:
Upregulation of autophagy by hypoxia-inducible factor-1α promotes
EMT and metastatic ability of CD133+ pancreatic cancer
stem-like cells during intermittent hypoxia. Oncol Rep. 32:935–942.
2014.PubMed/NCBI
|
|
21
|
Dong X, Li R, Xiu P, et al: Meloxicam
executes its antitumor effects against hepatocellular carcinoma in
COX-2-dependent and -independent pathways. PLoS One. 9:e928642014.
View Article : Google Scholar
|
|
22
|
Roy A and Kolattukudy PE: Monocyte
chemotactic protein-induced protein (MCPIP) promotes inflammatory
angiogenesis via sequential induction of oxidative stress,
endoplasmic reticulum stress and autophagy. Cell Signal.
24:2123–2131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sachdev U, Cui X, Hong G, et al: High
mobility group box 1 promotes endothelial cell angiogenic behavior
in vitro and improves muscle perfusion in vivo in response to
ischemic injury. J Vasc Surg. 55:180–191. 2012. View Article : Google Scholar
|
|
24
|
Nguyen TM, Subramanian IV, Kelekar A and
Ramakrishnan S: Kringle 5 of human plasminogen, an angiogenesis
inhibitor, induces both autophagy and apoptotic death in
endothelial cells. Blood. 109:4793–4802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu B, Jarzynka MJ, Guo P, Imanishi Y,
Schlaepfer DD and Cheng SY: Angiopoietin 2 induces glioma cell
invasion by stimulating matrix metalloprotease 2 expression through
the alphavbeta1 integrin and focal adhesion kinase signaling
pathway. Cancer Res. 66:775–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fagiani E and Christofori G: Angiopoietins
in angiogenesis. Cancer Lett. 328:18–26. 2013. View Article : Google Scholar
|
|
27
|
Felcht M, Luck R, Schering A, et al:
Angiopoietin-2 differentially regulates angiogenesis through TIE2
and integrin signaling. J Clin Invest. 122:1991–2005. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim KW, Paul P, Qiao J and Chung DH:
Autophagy mediates paracrine regulation of vascular endothelial
cells. Lab Invest. 93:639–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimura S, Fujita N, Noda T and Yoshimori
T: Monitoring autophagy in mammalian cultured cells through the
dynamics of LC3. Methods Enzymol. 452:1–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eikesdal HP, Sugimoto H, Birrane G, et al:
Identification of amino acids essential for the antiangiogenic
activity of tumstatin and its use in combination antitumor
activity. Proc Natl Acad Sci USA. 105:15040–15045. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giannotta M, Trani M and Dejana E:
VE-cadherin and endothelial adherens junctions: active guardians of
vascular integrity. Dev Cell. 26:441–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thomas M and Augustin HG: The role of the
angiopoietins in vascular morphogenesis. Angiogenesis. 12:125–137.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mazzieri R, Pucci F, Moi D, et al:
Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis
by impairing angiogenesis and disabling rebounds of proangiogenic
myeloid cells. Cancer Cell. 19:512–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rini B, Szczylik C, Tannir NM, et al: AMG
386 in combination with sorafenib in patients with metastatic clear
cell carcinoma of the kidney: a randomized, double-blind,
placebo-controlled, phase 2 study. Cancer. 118:6152–6161. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hashizume H, Falcon BL, Kuroda T, et al:
Complementary actions of inhibitors of angiopoietin-2 and VEGF on
tumor angiogenesis and growth. Cancer Res. 70:2213–2223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chae SS, Kamoun WS, Farrar CT, et al:
Angiopoietin-2 interferes with anti-VEGFR2-induced vessel
normalization and survival benefit in mice bearing gliomas. Clin
Cancer Res. 16:3618–3627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wendel HG, De Stanchina E, Fridman JS, et
al: Survival signalling by Akt and eIF4E in oncogenesis and cancer
therapy. Nature. 428:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Holopainen T, Saharinen P, D’Amico G, et
al: Effects of angiopoietin-2-blocking antibody on endothelial
cell-cell junctions and lung metastasis. J Natl Cancer Inst.
104:461–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|