Introduction
Phytochemicals have emerged as a promising
therapeutic option in regulating cancer, as phytochemicals
reportedly exhibit safety and enhance anticancer activity (1–3). The
strategies for achieving anticancer effects from natural compounds
have led to much interest. Thus attention has focused on the
identification of new compounds with anticancer activities from
natural compounds without evident toxicities, and the establishment
of their precise mechanisms.
Tetrahydroisoquinoline (THI) alkaloids are
derivatives of higenamine, a major component of
Ranunculaceae that has been used as a herb for many diseases
without reported toxicities. It has been reported that these
alkaloids have pharmacological actions on inflammation. CKD-712, a
newly synthesized THI and an enantiomer (S form) of YS 49 (a
derivative of higenamine) has been reported to have
anti-inflammatory activity by suppressing nuclear factor-κB (NF-κB)
in normal cells (4). NF-κB is a
well-known transcription factor that is involved in cancer
proliferation, invasion, and angiogenesis. In addition, NF-κB
activation is closely associated with drug resistance by enhancing
the transcription of anti-apoptotic proteins that interfere with
death signaling. In that context, the agents harboring an
inhibitory effect on NF-κB can have anticancer activities. This
agent was developed for the cardiovascular disease, and has
anti-inflammatory effects (5).
Evidence suggested that inflammation is closely associated with
carcinogenesis. In addition, previous findings suggested that THI
alkaloids exert anticancer effects (6,7).
However, the anticancer effects of CKD-712 have not been reported
yet. Therefore, we hypothesized CKD-712 possesses a certain
anticancer property. In the present study, the anticancer effects
of CKD-712 on A549 human lung cancer cells were examined.
Materials and methods
Cell culture and chemicals
A549 human lung adenocarcinoma cells from the ATCC
(Rockville, MD, USA) were cultured in RPMI-1640 medium (Invitrogen,
Carlsbad, CA, USA) supplemented with 10% FBS (Gibco-BRL, Grand
Island, NY, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin
in an incubator at 37°C in a humidified atmosphere of 95% air and
5% CO2. Molecular mass markers for proteins were
obtained from Pharmacia Biotech (Saclay, France). Antibodies
against c-Myc, Bcl-2, Bcl-xL, XIAP, cIAP-1, cIAP-2, cyclin A,
cyclin B, Cyclin-dependent kinase (CDK-1), VEGF, poly (ADP-ribose)
polymerase (PARP), procaspase-3 and -9, and NF-κB (p65) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Antibody against β-actin was from Sigma (Beverly, MA, USA).
An enhanced chemiluminescence (ECL) kit was purchased from Amersham
(Arlington Heights, IL, USA). Any other chemicals not specifically
mentioned were purchased from Sigma Chemical Co., (St. Louis, MO,
USA).
Cell proliferation assays
A549 cells were seeded in 24-well plates at a
density of 5×104 cells/ml, grown to 70% conflu-ence and
then treated with the indicated concentrations of CKD-712 for 24 h.
Control cells were supplemented with media containing 0.1% DMSO
(vehicle control). Cell viability was determined by the MTT
assay.
Flow cytometric analysis
Cells (2×105) were plated in each well of
six-well plates, and treated with the indicated concentration of
CKD-712 (5–60 μg/μl) for 24 h. The cells were washed twice with
cold PBS and centrifuged at 1,200 rpm for 5 min. The pellet was
fixed in 75% (v/v) ethanol for 1 h at 4°C. The cells were washed
once with PBS and resuspended in cold PI solution (50 μg/ml)
containing RNase A (0.1 mg/ml) in PBS (pH 7.4) for 30 min in the
dark. Flow cytometric analysis was performed using FACSCalibur
(Becton Dickinson, San Jose, CA, USA).
Wound-healing assay
The cells were grown to 100% confluent monolayer and
then scratched to form a 100 μm ‘wound’ using sterile pipette tips.
The cells were then cultured in the presence or absence of CKD-712
in serum-free media for 24 h. The images were recorded at 12 and 24
h after scratching using an Olympus photomicroscope.
Cell invasion assay
The 5×104 cells/ml cultured in serum-free
media overnight were loaded onto pre-coated Matrigel 24-well
invasion chambers (BD Biosciences, San Jose, CA, USA) with or
without CKD-712. Then, 0.5 ml of medium containing 20% FBS was
added to the wells of the plate and incubated for 24 h at 37°C in
5% CO2. The cells on the bottom of the Matrigel were
fixed with 10% formalin, stained with DAPI, and counted. Stained
nuclei were then observed under fluorescent microscope using a blue
filter (magnification, ×400).
Gelatin zymography
The gelatinolytic activities of the culture medium
were assayed by electrophoresis with 10% poly-acrylamide gels
containing 1 mg/ml gelatin. Polyacrylamide gels were run at 120 V,
washed in 2.5% Triton X-100 for 1 h, and then incubated for 16 h at
37°C in activation buffer (50 mM Tris-HCl, pH 7.5, 10 mM
CaCl2). After staining with Coomassie blue (10% glacial
acetic acid, 30% methanol and 1.5% Coomassie brilliant blue) for
2–3 h, the gel was washed with a solution of 10% glacial acetic
acid and 30% methanol for 1 h. White lysis zones revealed by
staining with Coomassie brilliant blue indicated gelatin
degradation by MMP-2 and -9.
Western blot analysis
The cells were gently lysed for 30 min with lysis
buffer (20 mM sucrose, 1 mM EDTA, 20 μM Tris-HCl, pH 7.2, 1 mM DTT,
10 mM KCl, 1.5 mM MgCl2, 5 μg/ml pepstatin A, 10 μg/ml
leupeptin, and 2 μg/ml aprotinin) to prepare the total protein
Supernatants were collected and protein concentrations were
determined using a Bio-Rad Protein Assay kit (Bio-Rad, Hercules,
CA, USA). For the NF-κB (p65) experiment, cytoplasmic and nuclear
proteins were extracted using nuclear and cytoplasmic extraction
reagents according to the manufacturer’s instructions. For the
western blot analysis, an equal amount of protein was subjected to
electrophoresis on sodium dodecyl sulfate (SDS)-polyacrylamide gels
and transferred to a nitrocel-lulose membrane (Schleicher &
Schuell, Keene, NH, USA) by electroblotting. Blots were probed with
the indicated antibodies. The membranes were then incubated with
diluted enzyme-linked secondary antibodies for 1 h at room
temperature. After washing, the membranes were developed by
enhanced chemiluminescence.
Statistical analysis
Each experiment was performed in triplicate. The
results were presented as the mean values ± SD. Significant
differences were determined using the Student’s t-test. Statistical
significance was defined as P<0.05.
Results
CKD-712 significantly suppresses cancer
cell proliferation, but does not induce either apoptosis or cancer
cell death in A549 lung cancer cells
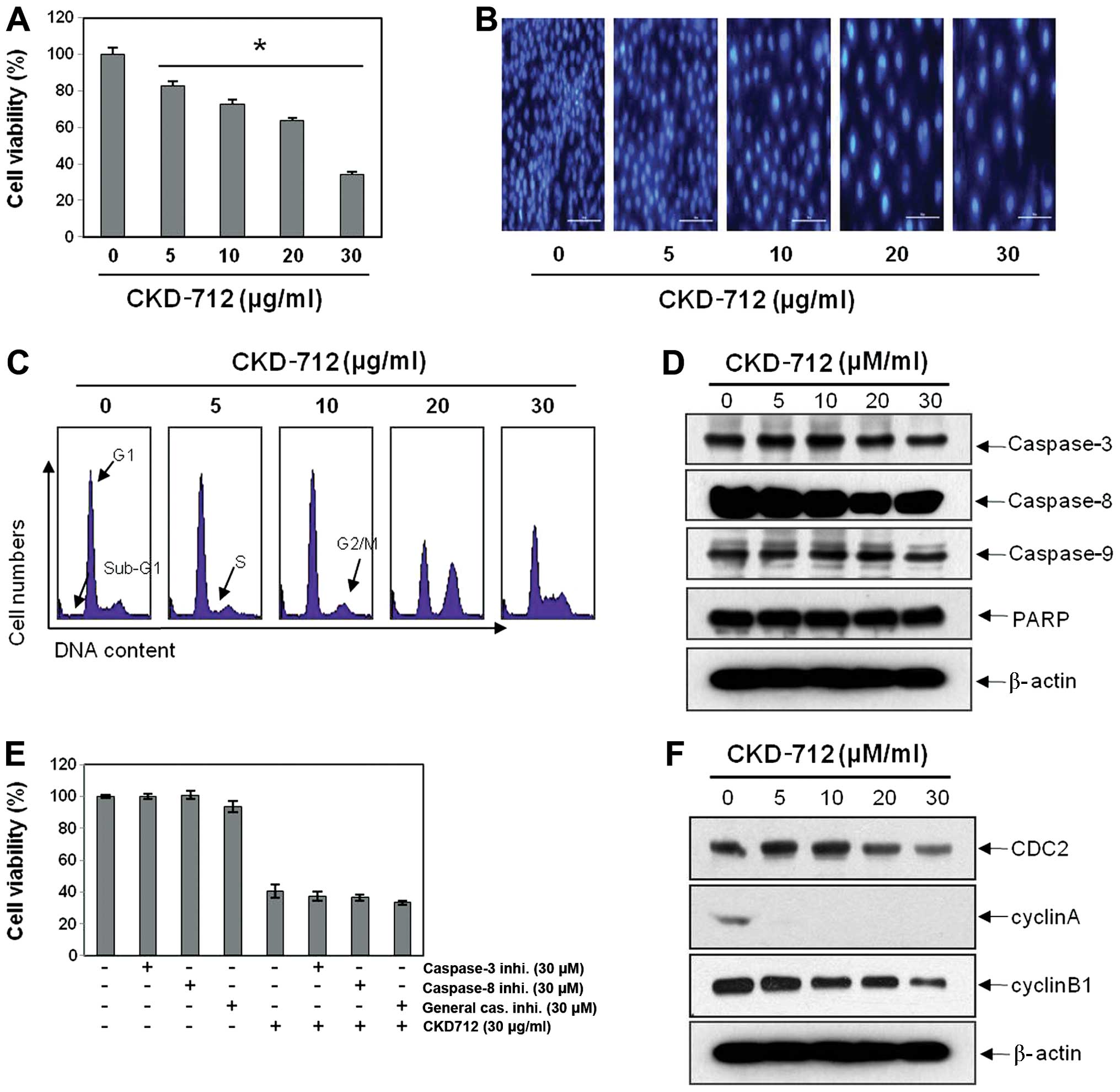
According to a previous report, we used an amount
<100 μg/ml where CKD-712 did not show cyctotoxicity in the
normal cells (5). We assessed the
anti-cancer effects at a concentrations of 5–60 μg/ml where the
growth of A549 cells was not significantly suppressed. The MTT
assay revealed that CKD-712 significantly suppressed cell
proliferation in a dose-dependent manner (Fig. 1A). To confirm whether CKD-712
induces apoptosis, we performed DAPI staining and measured cells
with sub-G1 DNA content. DAPI staining revealed that no
definite apoptotic cell death (Fig.
1B). However, marked cell cycle changes were noted at the
indicated concentrations of CKD-712 in 48 h (Fig. 1C). The increasing fraction of the
cells with sub-G1 DNA content as CKD-712 was negligible.
We assessed the anticancer effects of CKD-712 at the concentration
of 30 μg/ml. The MTT assay revealed that CKD-712 inhibited the cell
proliferation up to 60%, but did not induce caspase induction and
PARP cleavages (Fig. 1D). This
finding was confirmed by caspase inhibitors (Fig. 1E). These findings suggested that
CKD-712 suppresses A549 cell proliferation, but CKD-712 did
not induce apoptosis or any other type of cell death.
CKD-712 significantly suppresses cyclin A
and B expression as well as CDC2 expression in A549 lung cancer
cells
To investigate the molecular mechanisms for the S
and G2M arrest, we assessed the expression levels of cyclin A,
cyclin B, and CDK-1. Western blot analysis revealed that CKD-712
suppressed the expression of cyclin A, cyclin B, and CDK-1
(Fig. 1F), suggesting that
suppression of the expression of cyclin A, cyclin B, and CDK-1 is
one of the mechanisms for CKD-712-induced cell cycle arrest.
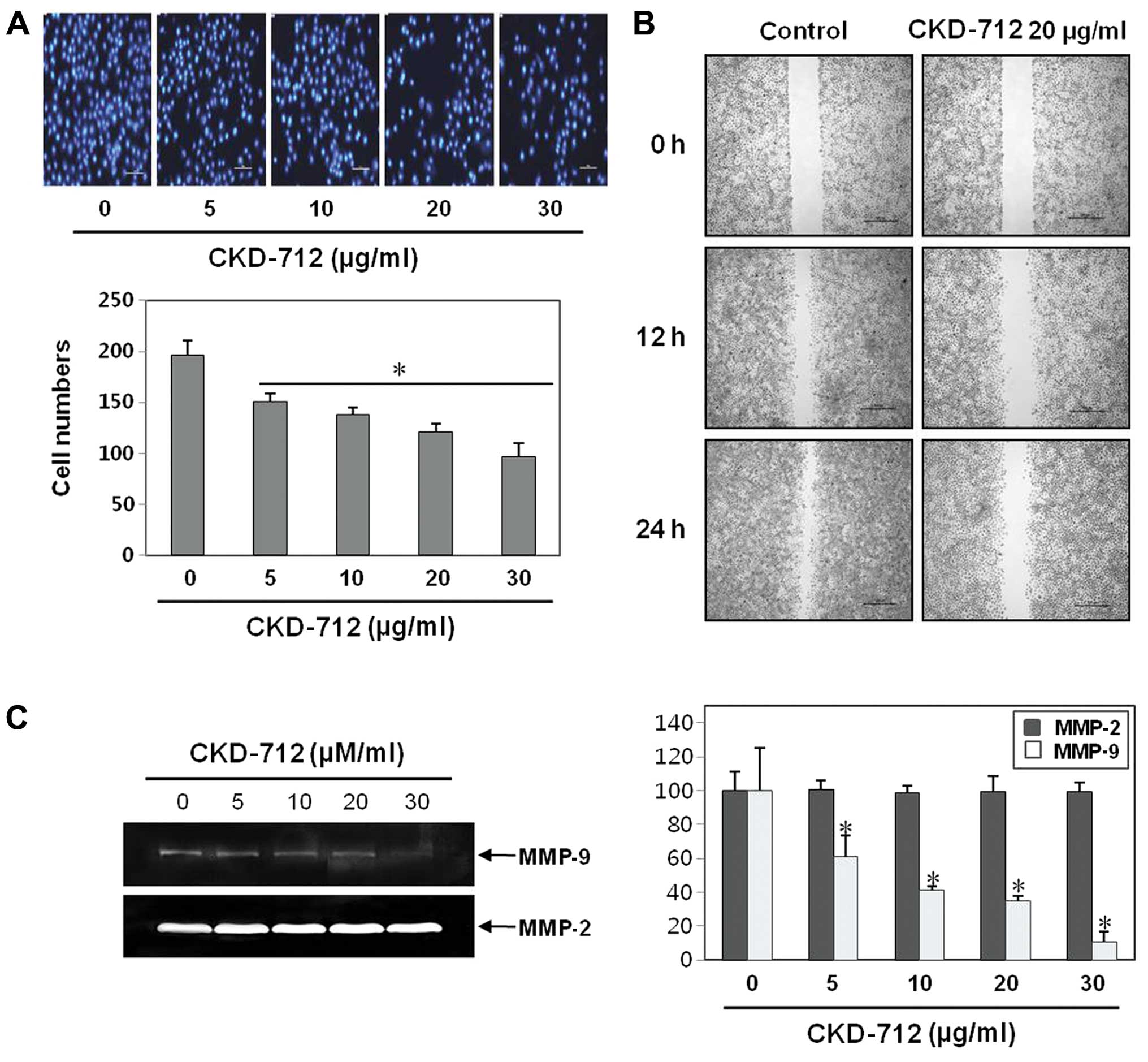
CKD-712 inhibits the invasion as well as
migration of A549 lung cancer cells
Cancer cell invasion is the key event in metastasis,
thus we performed the invasion test to determine the anticancer
effects of CKD-712 on A549 cells in vitro. CKD-712 inhibited
cell invasion in a dose-dependent manner as measured by Matrigel
invasion assays (Fig. 2A). In
addition, CKD-712 inhibited cancer cell migration in the
wound-healing assay at a concentration of 20 μg/ml (Fig. 2B). These findings suggest that
CKD-712 effectively suppressed A549 cell invasion and
migration.
CKD-712 inhibits the expression of MMP-9
in A549 lung cancer cells, but not MMP-2
To investigate the changes at the molecular level,
we assessed the expression levels of MMP-2 and -9, which are key
molecules for cancer invasion involved in by the proteolytic
digestion of the extracellular matrix (ECM) (8,9). The
activities of MMP-2 and -9 secreted in media using gelatin
zymography analyses were measured, which revealed that CKD-712
predominantly suppressed the expression of MMP-9 gene in a
dose-dependent manner, whereas no changes were identified in MMP-2
expression (Fig. 2C). These data
suggested that CKD-712 inhibits A549 cell invasion at least
in part by suppressing MMP-9.
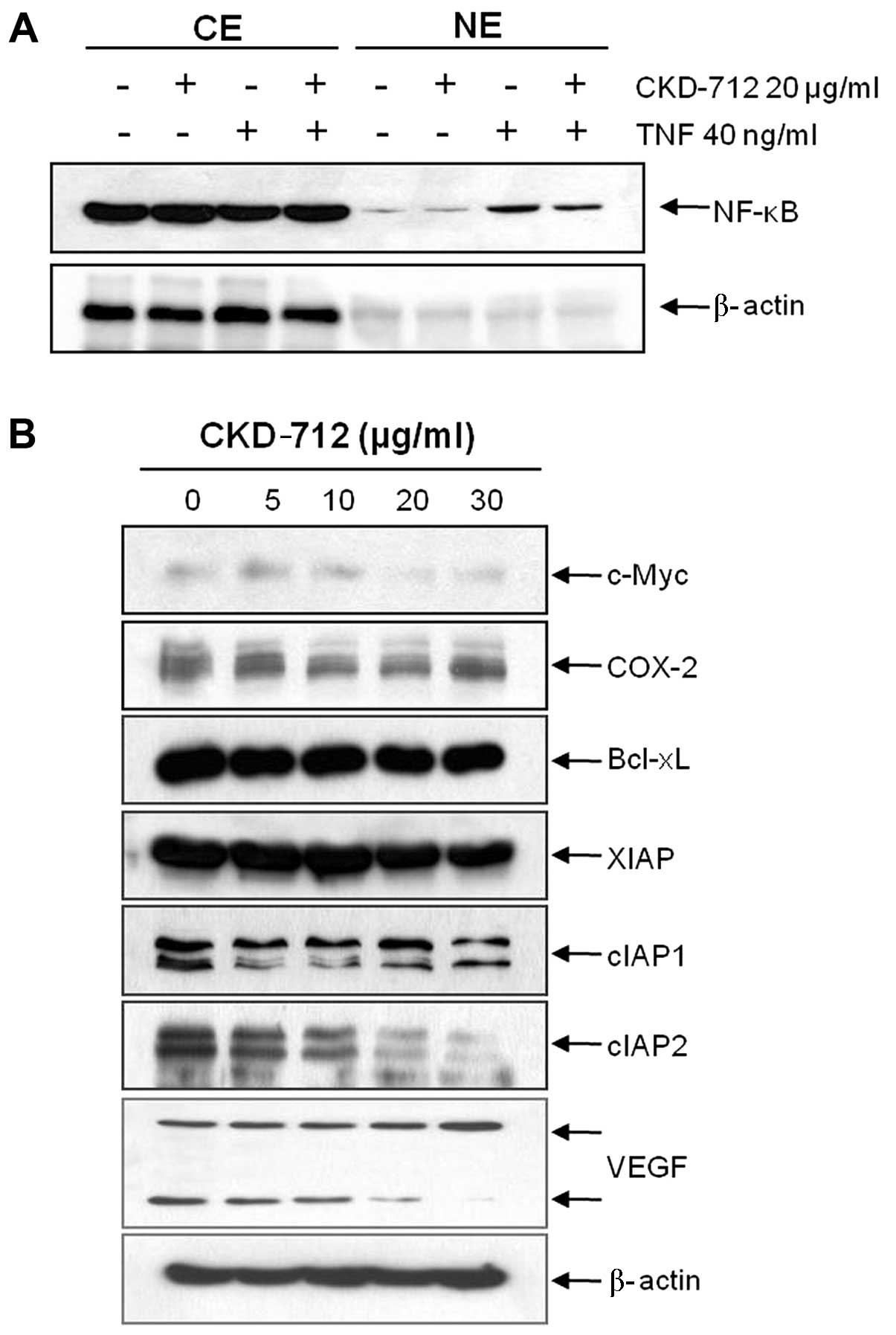
CKD-712 suppresses NF-κB activity, and
NF-κB-regulated proteins involved in cancer proliferation, and
angiogenesis in A549 lung cancer cells
The NF-κB pathway is involved in cancer cell
proliferation, invasion, and metastasis (10,11).
MMP-9 expression is preferentially regulated by NF-κB (12). We hypothesized that the stimulatory
effects of TNF on invasion and MMP-9 expression were driven by
NF-κB activation. In addition, we previously suggested that CKD-712
suppressed NF-κB activation in normal cells (5). In the present study, we investigated
again whether CKD-712 inhibits NF-κB activation using western blot
analysis, which revealed that TNF enhanced NF-κB translocation into
the nucleus and CKD-712 inhibited the TNF-induced NF-κB activation.
Additionally, the inhibitory effects on TNF-induced NF-κB
activation were not as marked as that shown in the cells treated
with CKD-712 alone (Fig. 3A). We
measured the levels of NF-κB-regulated proteins associated with
cancer proliferation, invasion, angiogenesis, and anti-apoptosis in
A549 cells. We found that CKD-712 suppressed NF-κB-regulated
proteins involved in cancer cell proliferation (c-Myc and COX-2),
anti-apoptosis (Bcl-xL, XIAP, cIAP1, cIAP2), and angiogenesis
(VEGF) (Fig. 3B). These gene
products are known to be regulated by NF-κB, thus we investigated
the effect of CKD-712 on these molecules. Western blot analysis
revealed that CKD-712 suppressed these proteins. The results
suggested that CKD-712 may suppress MMP-9 as well as other
NF-κB-regulated proteins that have been associated with cancer
metastasis.
Discussion
The aim of the present study was to investigate the
anti-cancer effects of CKD-712 on human lung cancer cells and, to
determine whether CKD-712 exerts anticancer effects at the doses
where the anti-inflammatory effects were shown (5). A previous report demonstrated that
CKD-712 has suppressive effects on the activated NF-κB in normal
cells (5). We found that CKD-712
suppressed cancer cell invasion and migration through suppression
of MMP-9 at the concentration where cytotoxicity was not evident in
normal cells. Invasion is the first step of cancer metastasis, for
which the process includes proteolytic digestion of the ECM, and
cell migration through the basement membranes to reach the
circulatory system. In particular, MMP-2 (gelatinase-A) and MMP-9
(gelatinase-B) are important in the proteolytic digestion of the
basement membrane. We clearly demonstrated that CKD-712 had
inhibitory activities by suppressing the expression of MMP-9 in
A549 cells. Gelatin zymography showed that CKD-712 predominantly
suppressed MMP-9 expression compared to MMP-2. This finding can be
supported by previous studies showing that MMP-9 appeared to be
much more influenced by NF-κB activation than was the case for
MMP-2 (13,14), and MMP-9 promoter contains NF-κB
binding sites (12). In addition,
we have demonstrated that CKD-712 inhibited VEGF, which is
important in cancer neovascularization and angiogenesis. Natural
compounds (15) or dietary agents
(16) carrying anti-NF-κB activity
have also shown similar effects on cancer cells although the
dominant target was different.
The present result have shown that CKD-712 inhibited
NF-κB activity and NF-κB-regulated proteins involved in cancer
survival and proliferation. The primary role of TNF consists in the
regulation of immune cells, and TNF was able to induce interleukin
(IL) production, apoptotic cell death, and inflammation to inhibit
tumorigenesis. TNF induces cell death through the extrinsic pathway
in certain cancer cells (13).
However, most cancer cells are resistant to TNF-induced cell death
by activation of NF-κB followed by the enhanced transcription of
anti-apoptotic proteins that interfere with cell death signaling
(14). Moreover, paradoxically the
patients with advanced and metastatic cancers have higher serum
levels of TNF than those with early stage cancer (12) that are associated with cancer
progression (12,15). Therefore, NF-κB can a good
therapeutic target for the treatment of advanced cancer. We
hypothesized that if NF-κB is suppressed by less toxic drugs, TNF
induces apoptosis of cancer cells. This may prove to be an
alternative approach for the treatment of patients with metastasis
or advanced patients without showing serious side effects. Thus, we
investigated the anticancer effects of CKD-712 with a special focus
on NF-κB and the NF-κB-regulated gene which is involved in cancer
progression and metastasis.
The study has certain limitations. First is that we
did not clearly examine the mechanisms by which CKD-712 manifests
its other anticancer effects such as cell cycle arrest. Most of the
agents with anti-NF-κB activity were associated with cyclin D1
suppression (17,18). Therefore, cell cycle arrest by
CKD-712 was considered a distinct anticancer activity. Another
limitation is that we did not fully elucidate the reason for
CKD-712 not exerting prominent anticancer effects in terms of
apoptosis. Further study is required to elucidate the underlying
mechanisms.
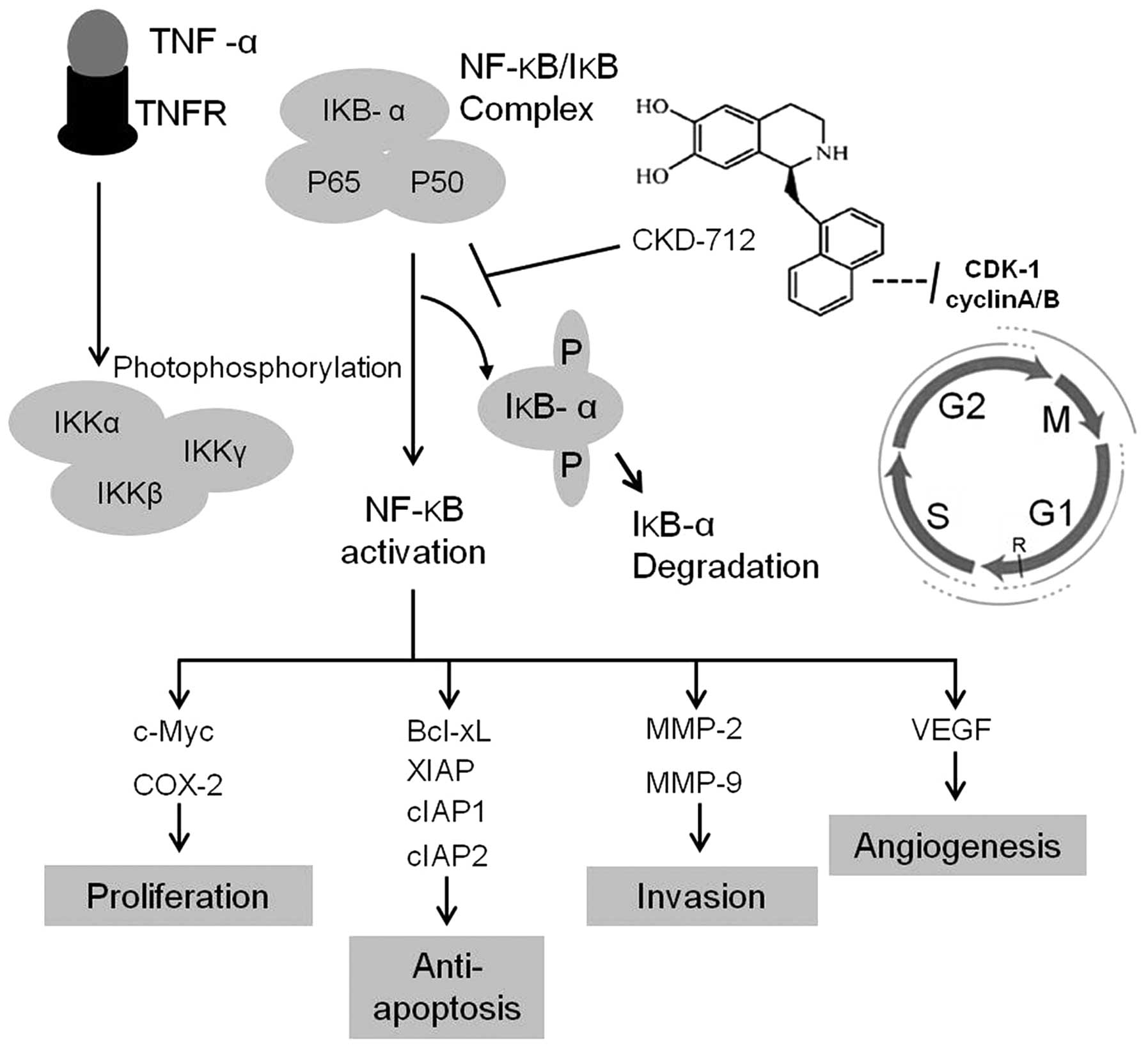
In conclusion, this study suggests that CKD-712
exerts anticancer effects at least in part by inhibiting NF-κB
activation and downstream proteins (Fig. 4). CKD-712-induced cell cycle arrest
is another mechanism for these anticancer effects. This study
therefore provides evidence that CKD-712 may exert anticancer
effects on human lung cancer.
Acknowledgements
This study was supported by a grant of the National
R&D Program for Cancer Control (0820050), Ministry for Health,
Welfare and Family Affairs.
References
|
1
|
Liu BL, Zhang X, Zhang W and Zhen HN: New
enlightenment of French Paradox: resveratrol’s potential for cancer
chemopre-vention and anti-cancer therapy. Cancer Biol Ther.
6:1833–1836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: from ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kundu JK and Chun KS: The promise of dried
fruits in cancer chemoprevention. Asian Pac J Cancer Prev.
15:3343–3352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin YC, Lee YS, Kim YM, Seo HG, Lee JH,
Kim HJ, et al:
(S)-1-(alpha-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline
(CKD712) reduces rat myocardial apoptosis against ischemia and
reperfusion injury by activation of phosphatidylinositol
3-kinase/Akt signaling and anti-inflammatory action in vivo. J
Pharmacol Exp Ther. 330:440–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsoyi K, Kim HJ, Shin JS, Kim DH, Cho HJ,
Lee SS, et al: HO-1 and JAK-2/STAT-1 signals are involved in
preferential inhibition of iNOS over COX-2 gene expression by newly
synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells
activated with lipopolysacchride. Cell Signal. 20:1839–1847. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabunoki H, Saito N, Suwanborirux K,
Charupant K and Satoh J: Molecular network profiling of U373MG
human glioblastoma cells following induction of apoptosis by novel
marine-derived anti-cancer 1,2,3,4-tetrahydroisoquinoline
alkaloids. Cancer Cell Int. 12:142012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yap TA, Cortes-Funes H, Shaw H, Rodriguez
R, Olmos D, Lal R, et al: First-in-man phase I trial of two
schedules of the novel synthetic tetrahydroisoquinoline alkaloid
PM00104 (Zalypsis) in patients with advanced solid tumours. Br J
Cancer. 106:1379–1385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davies B, Waxman J, Wasan H, Abel P,
Williams G, Krausz T, et al: Levels of matrix metalloproteases in
bladder cancer correlate with tumor grade and invasion. Cancer Res.
53:5365–5369. 1993.PubMed/NCBI
|
|
9
|
Bogenrieder T and Herlyn M: Axis of evil:
molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aggarwal BB: Nuclear factor-kappaB: the
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gilmore TD: Introduction to NF-kappaB:
players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor-inducing kinase plays a crucial role in
osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear
factor kappaB-mediated promatrix metalloproteinase-9 activation. J
Biol Chem. 279:38921–38935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng CY, Hsieh HL, Hsiao LD and Yang CM:
PI3-K/Akt/JNK/NF-κB is essential for MMP-9 expression and outgrowth
in human limbal epithelial cells on intact amniotic membrane. Stem
Cell Res. 9:9–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hozumi A, Nishimura Y, Nishiuma T, Kotani
Y and Yokoyama M: Induction of MMP-9 in normal human bronchial
epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am J
Physiol Lung Cell Mol Physiol. 281:L1444–L1452. 2001.PubMed/NCBI
|
|
15
|
Yun JW, Lee WS, Kim MJ, Lu JN, Kang MH,
Kim HG, et al: Characterization of a profile of the anthocyanins
isolated from Vitis coignetiae Pulliat and their anti-invasive
activity on HT-29 human colon cancer cells. Food Chem Toxicol.
48:903–909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji AJ, Liu SL, Ju WZ and Huang XE:
Anti-proliferation effects and molecular mechanisms of action of
tetramethypyrazine on human SGC-7901 gastric carcinoma cells. Asian
Pac J Cancer Prev. 15:3581–3586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Z and Andersson R: NF-kappaB
activation and inhibition: a review. Shock. 18:99–106. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aggarwal BB and Sung B: NF-κB in cancer: a
matter of life and death. Cancer Discov. 1:469–471. 2011.
View Article : Google Scholar
|