Introduction
Breast cancer cases (10–15%) are characterized by an
aggressive clinical with poor disease-free and overall survival and
show a high rate of metastasis when compared with patients with
other types of breast cancer (1–3).
Currently, there is no defined standard antimetastasis treatment
strategy for this disease other than traditional chemotherapy, to
which it is highly resistant.
Tumor metastasis is a multistep process involving
cell adhesion, cell migration and degradation of the extracellular
matrix. The metastatic potential of tumor cells is influenced by a
balance of the expression of proteases and their inhibitory
proteins. Matrix metalloproteinases (MMPs) are a family of 24
secreted and membrane-type proteases that mediate this process.
MMP-2 and MMP-9 are highly expressed in the breast and play an
important role in the invasive stages of cancer (4).
The motility and invasive potential of a number of
metastatic cancer cell lines has been inhibited by bioactive
substances such as flavonoids (5),
magnolol (6), tea catechins
(7) and apigenin (8). Inhibition of MMP expression was also
identified in a number of these studies.
The plant roots of Euphorbia fischeriana
Steud, known as ‘lang-du’ in traditional Chinese medicine, have
been used for the treatment of cancer for thousands of years
(9–11). In recent years, studies have focused
on Euphorbia fischeriana Steud, which has significant
activity of inhibiting the growth of many tumors cell lines
including leucocythemia K562, cervical carcinoma HeLa,
nasopharyngeal carcinoma CNE2 and breast cancer. The bioactive
substances in the roots of Euphorbia fischeriana Steud were
concentrated on diterpenoids (12–15).
The manner in which the bioactive constituent from Euphorbia
fischeriana Steud exactly facilitates understanding of its
involvement in cancer treatment remains to be determined. In the
present study, five monomers from the roots of Euphorbia
fischeriana Steud, and Ethyl gallate was identified as the
major constituent. The aim of the study was to systematically
analyze the potential activity of Ethy gallate on cancer cell lines
and its molecular targets of action. Highly invasive MDA-MB-231 and
low invasive MCF-7 human breast cell lines were employed, and
treated with Ethyl gallate. Cell proliferation and apoptosis were
analyzed, and the apoptotic-associated protein and expression of
Bcl-2/Bax was assayed.
Highly invasive MDA-MB-231 cells were found to be
highly sensitive to treatment. This observation led us to
hypothesize whether the activity of Ethyl gallate extends to
modulation of the metastatic potential as well. In the present
study, the effect of Ethyl gallate against human highly invasive
breast cancer, the abilities of cell adhesion, migration and
invasion of as well as mRNA levels of MMP-2 and MMP-9 and PI3K/Akt
and NF-κB signaling were evaluated. The results of the study
provide important information on the role of Euphorbia
fischeriana Steud as potential agents against the metastasis of
breast cancer.
Materials and methods
Preparation of Ethyl gallate
Ethyl gallate (molecular weight of 198.1727, purity
>99%), which is light brown powder, was extracted by our
laboratory and dissolved in phosphate-buffered saline (PBS) and
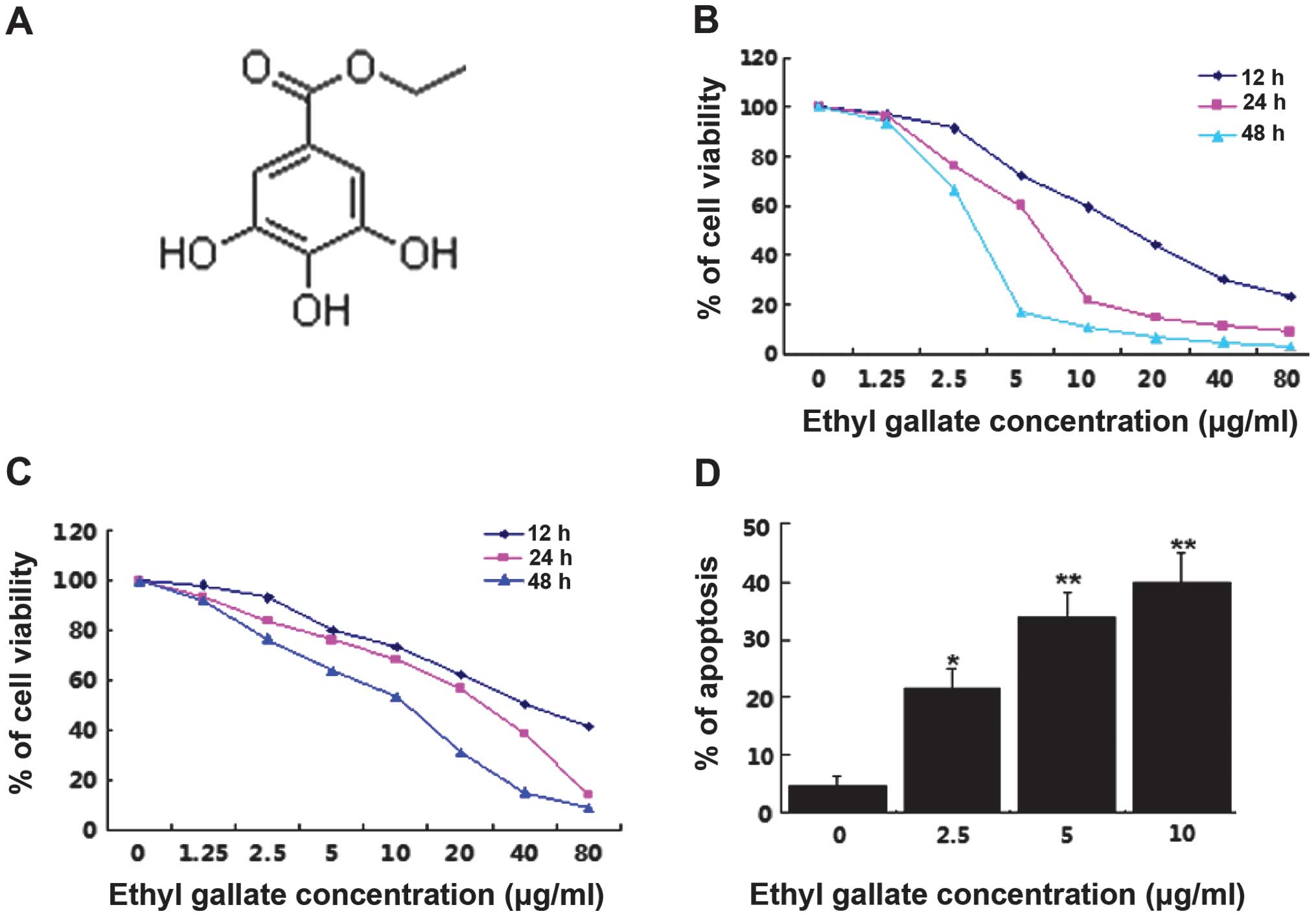
diluted in a serials concentration. The chemical structure is as
shown in Fig. 1A.
Cell lines and cell culture
Human breast cancer cell lines, estrogen
receptor-negative MDA-MB-231 cells and estrogen receptor-positive
MCF-7 cells were obtained from the Shanghai Cell Bank, Chinese
Academy of Sciences (Shanghai, China). MDA-MB-231 cells were
cultured in L15 medium (Gibco™ Invitrogen Corporation, Carlsbad,
CA, USA), while MCF-7 cells were cultured in DMEM medium with high
glucose. Both medium were supplemented with 10% heat-inactived
fetal bovine serum (FBS). The cells were cultured at 37°C in 5%
CO2 incubator and harvested by 0.25% trypsin.
Cell proliferation assay
The viability of breast cancer cells treated with
Ethyl gallate was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells (1.2×104)/well in 200 μl full-cultured
medium were seeded in 96-well plates and incubated at 37°C. After
24-h incubation, the medium was removed and replaced with
full-cultured medium containing the final concentration of Ethyl
gallate at 0, 0.625, 1.25, 2.5, 5, 10, 20, 40 and 80 μg/ml. All
groups were quadrupled. At 12, 24 and 48 h after treatment with
Ethyl gallate, MTT solution was added to each well (500 μg/ml final
concentrations) and incubated at 37°C for 4 h. The supernatant was
removed, and 150 μl of dimethyl sulfoxide (DMSO) was added. The
optical density value of the solution was read at 570 nm using a
microplate reader (Safire2; Tecan Group Ltd., Maennedorf,
Switzerland). The percentage of cell viability was determined by
comparing the cell density of the drug-treated cells with that of
untreated controls.
Analysis of apoptosis
MDA-MB-231 cells were treated with Ethyl gallate at
a concentration of 0, 2.5, 5.0 and 10.0 μg/ml for 24 h. Annexin V
assays were carried out using the Annexin V-FITC apoptosis
detection kit (Becton-Dickinson, San Jose, CA, USA). The treated
cells were collected, washed in cold PBS, and then resuspended
gently in 400 μl of binding buffer prior to the addition of Annexin
V-FITC and propidium iodide (PI). The cells were incubated for 15
min at room temperature, and 10,000 cells were analyzed for
apoptosis using flow cytometry (BD Biosciences, San Jose, CA, USA).
The percentage of apoptotic cells was quantified using CellQuest
software (Becton-Dickinson). The experiments were repeated in
triplicate separately.
Matrigel adhesion assay
The 96-well plates were precoated with 25 μg/well of
Matrigel (BD Biosciences). The MDA-MB-231 cells
(1×105/well) were incubated in 1 ml of serum-free medium
containing Ethyl gallate at concentrations of 0, 2.5, 5.0 and 10.0
μg/ml and labeled by derivatives of indocarbocyanine iodide (DiI; 3
μg/ml) for 2 h at 37°C, respectively. Then cells were washed twice
with PBS and seeded into the precoated wells and incubated for 30
min at 37°C in a 5% CO2 incubator. The cells were washed
twice with PBS gently to remove unadhered cells, and the number of
cells adhered to the Matrigel was counted in five randomly selected
microscopic fields per well and photographed by fluorescence
microscope. Independent experiments were performed at least three
times.
In vitro migration and invasion
assay
Tumor cell migration and invasion were examined
using Transwell chambers (Costar, Corning, Inc., Corning, NY, USA).
Briefly, Matrigel was coated the upper chamber for invasion (not
for migration). MDA-MB-231 cells (5×104 for invasion and
2×105 for migration) were incubated in 1 ml of
serum-free medium containing Ethyl gallate at concentrations of 0,
2.5, 5.0 and 10.0 μg/ml for 2 h, respectively, washed in PBS and
seeded in the upper chamber in 200 μl of serum-free medium, while
the lower chamber was filled with 600 μl of medium supplemented
with 10% of FBS. After 24-h incubation at 37°C, the cells on the
upper chambers were scraped with a cotton swab. Cells migrating or
invading to the lower surface were fixed with methanol for 30 min,
stained with 0.1% crystal violet for 20 min, washed with PBS,
photographed with fluorescence microscope and incorporated dye was
dissolved in 10% acetic acid. The optical densities of each well
were measured using a microplate reader at 570 nm. Experiments were
repeated three times.
Protein extraction and western blot
analysis
Cells were treated as described for the apoptosis
assay. Total cellular proteins were extracted. Equivalent amounts
of cellular protein were electrophoresed in 10% SDS-PAGE gel and
transfered to nitrocellullose membranes (Millipore, Billerica, MA,
USA) and blocked in 5% non-fat milk in TBST for 1 h at room
temperature. The cells were incubated with primary antibodies to
human Bcl-2 and Bax (both from Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), NF-κB p-65, Akt and p-Akt (Cell Signal
Technology, Inc., Danvers, MA, USA) and GAPDH in 5% non-fat milk at
4°C overnight. The membranes were washed with TBST and incubated
with horseradish peroxidase-conjugated secondary antibody in 5%
non-fat milk for 1 h at room temperature. Immune complexes were
detected by enhanced chemoluminescence techniques (Amersham Life
Science, Piscataway, NJ, USA). Band densities were quantified by
using BandScan software.
Semi-quantitative reverse
transcription-PCR
Total cellular RNA was extracted using TRIzol
reagent (Invitrogen) and quantified by spectrophotometry. RT-PCR
reaction was carried out using an RT-PCR kit (Takara Biotechnology,
Dalian, China) according to the manufacturer’s instructions. PCR
amplification was carried out with the following primers: MMP-2,
forward: 5′-GGATGATGCCTTTGCTCG-3′ and reverse:
5′-CAGTGGACATGGCGGTCT-3′; MMP-9, forward:
5′-TCCCTGGAGACCTGAGAACC-3′ and reverse:
5′-GGCAAGTCTTCCGAGTAGTTT-3′; β-actin, forward:
5′-ATCATGTTTGAGACCTTCAACACC-3′ and reverse:
5′-TAGCTCTTCTCCAGGGAGG-3′. The amplification products were
separated through a 1.5% agarose gel electrophoresis. The intensity
of each band was quantified using Scion Image software (Scion
Corporation, Frederick, MD, USA). Results for each detected band
intensity were normalized to β-actin band intensity values.
Statistical analysis
Values were expressed as means ± standard deviation
and analyzed by the SPSS 13.0 software to evaluate the statistical
difference. One-way analysis of variance (ANOVA) was used to
establish whether significant differences existed among multiple
groups. P <0.05 was considered to indicate a statistically
significant result.
Results
Ethyl gallate inhibits proliferation and
induces apoptosis of breast cancer cells in a dose- and
time-dependent manner
The effects of Ethyl gallate on the proliferation of
MDA-MB-231 [estrogen receptor-negative (ER−)] and MCF-7
[estrogen receptor-positive (ER+)] human breast cancer
cell lines were investigated using an MTT assay. The same amount of
cells was seeded and treated with a series of concentrations of
Ethyl gallate from 0 to 80 μg/ml. The results showed that Ethyl
gallate significantly inhibited cell proliferation in the
MDA-MB-231 and MCF-7 cells in a dose- and time-dependent manner
following treatment for 12, 24 and 48 h. The IC50 values
at 12, 24 and 48 h were 17.85, 6.55 and 2.95 μg/ml and 32.53, 16.88
and 9.40 μg/ml for MDA-MB-231 (Fig.
1B) MCF-7 (Fig. 1C) cells,
respectively. However, MDA-MB-231 cells (ER−) were more
sensitive to Ethyl gallate than MCF-7 cells (ER+).
To determine whether the decreased cell numbers
occurred due to the induction of cell death, apoptosis was
quantitatively analyzed by flow cytometry. Following treatment with
Ethyl gallate at concentrations of 2.5, 5.0 and 10.0 μg/ml for 24 h
in the MDA-MB-231 cells, the percentage of apoptosis was 21.50,
33.86 and 39.77%, respectively (Fig.
1D). The results suggested that apoptosis is an important
mechanism of Ethyl gallate in the induction of breast cancer cell
death.
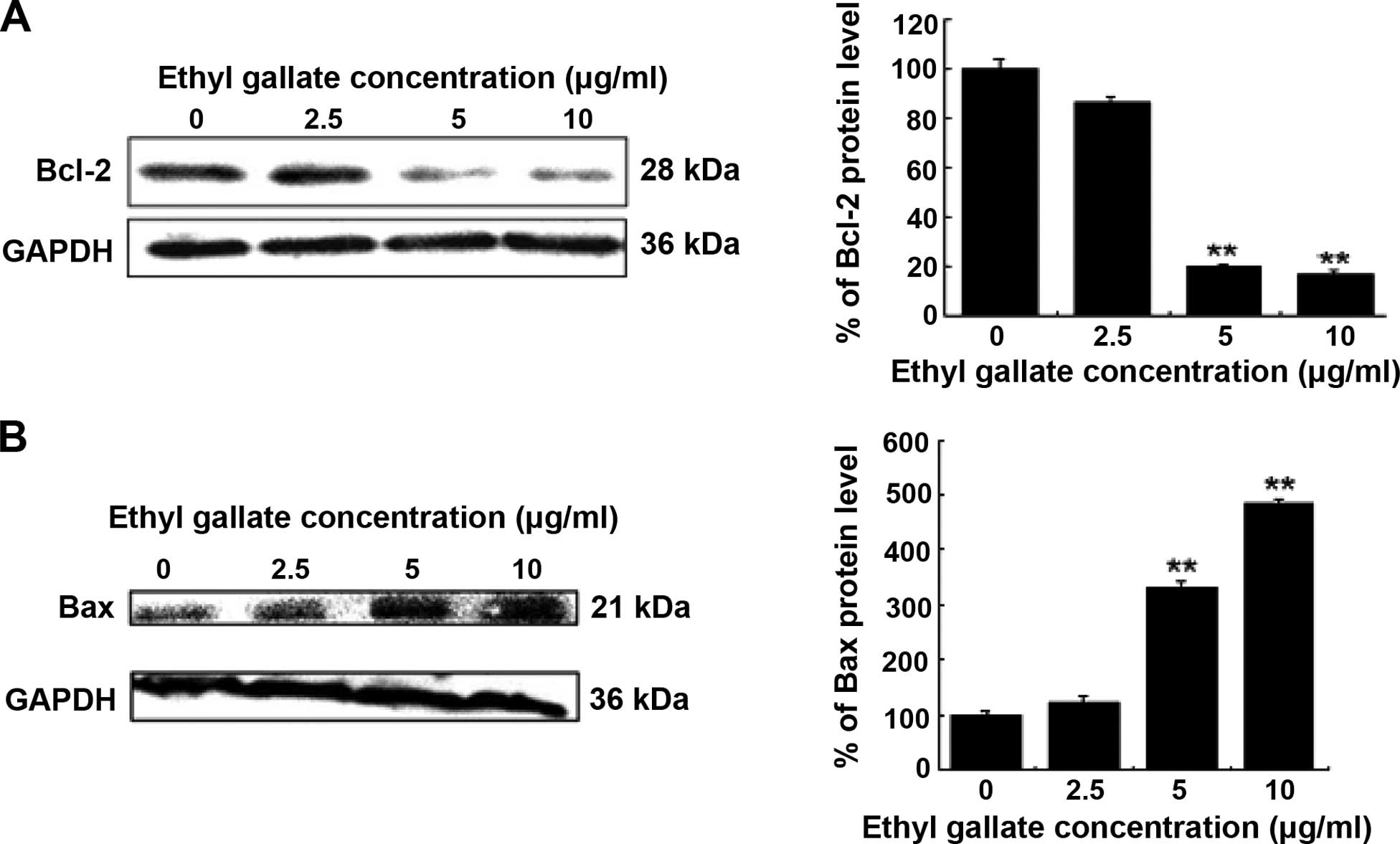
Effect of Ethyl gallate on Bcl-2 and Bax
proteins involved in apoptosis
Pro-apoptotic molecular mechanisms underlying Ethyl
gallate were examined. We investigated the expression of Bcl-2 and
Bax, which are pivotal regulators of cell growth and apoptosis.
Western blot analysis revealed that Ethyl gallate decreased Bcl-2
but increased Bax in a dose-dependent manner (Fig. 2). Thus, Ethyl gallate may induce
apoptosis by altering the Bax/Bcl-2 ratio favoring apoptosis.
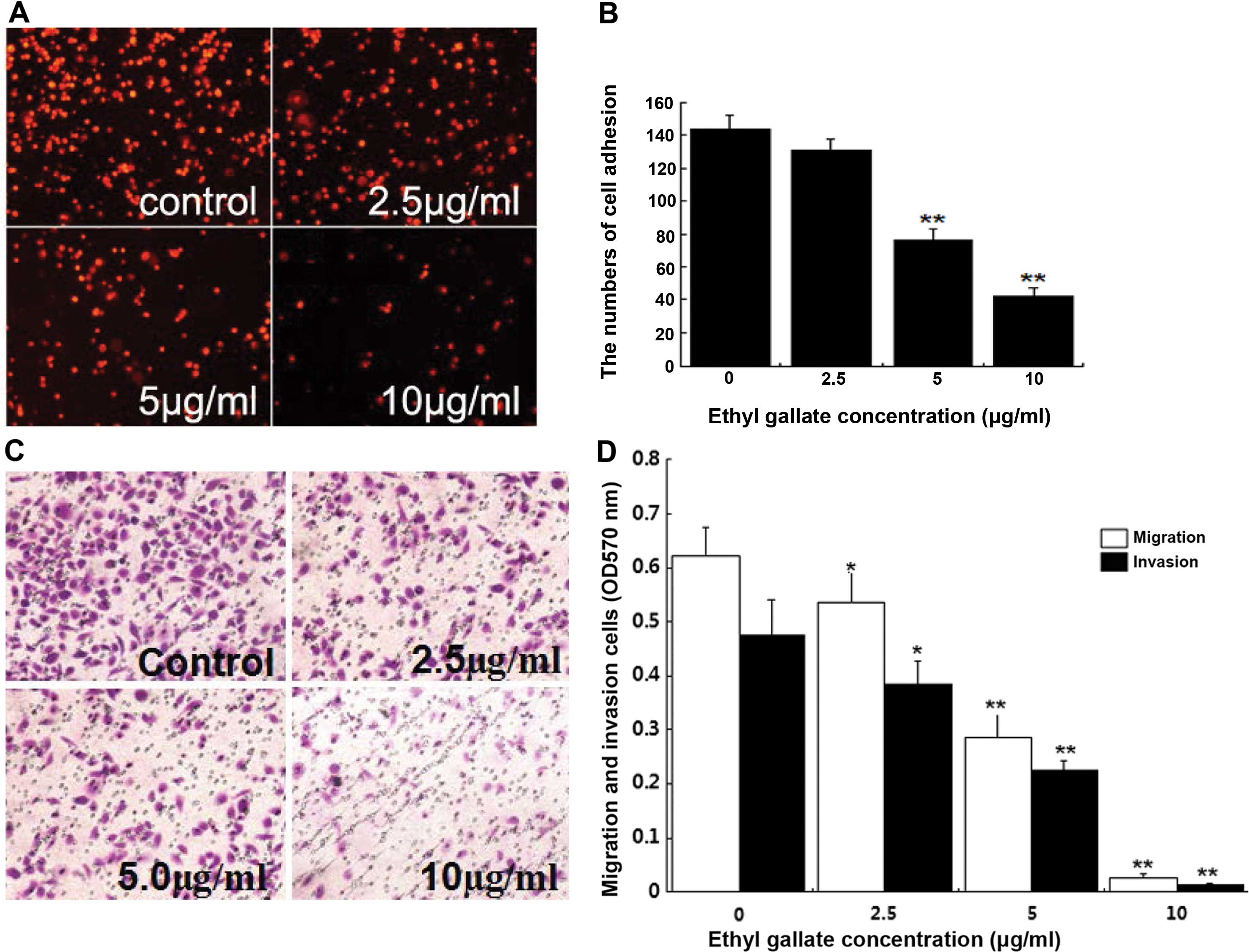
Ethyl gallate reduces cell adhesion,
migration and invasion
Considering that highly invasive breast cancer cells
were more sensitive to treatment with Ethyl gallate than poorly
invasive ones, we determined whether Ethyl gallate inhibited the
invasive behavior of breast cancer cells. Tumor invasion involve
cell adhesion to the extracellular matrix, migration and invasion
to Matrigel. To investigate the effect of Ethyl gallate on breast
cancer invasion potential, cell adhesion, migration and invasion
assays were examined in highly invasive MDA-MB-231 cells. As shown,
the number of cells that adhered to Matrigel was 143.78±8.53,
121.38±6.38, 76.34±6.32 and 42.0±5.18, respectively (Fig. 3A). Twenty-four hours after cell
migration and invasion, the optical density of MDA-MB-231 cells
were 0.623±0.053, 0.476±0.064, 0.536±0.059, 0.385±0.043,
0.284±0.048, 0.225±0.016, 0.027±0.006 and 0.014±0.002 respectively,
following cell treatment with Ethyl gallate at concentrations of
2.5, 5.0 and 10.0 μg/ml for 2 h (Fig.
3B). The results suggested that Ethyl gallate at short effect
time significantly suppressed the adhesion, migration and invasion
of MDA-MB-231 cells with the inhibition rate of 13.40, 13.89,
19.12, 46.90, 54.42, 52.74%, and 70.79, 95.67, 97.06% (Fig. 3C).
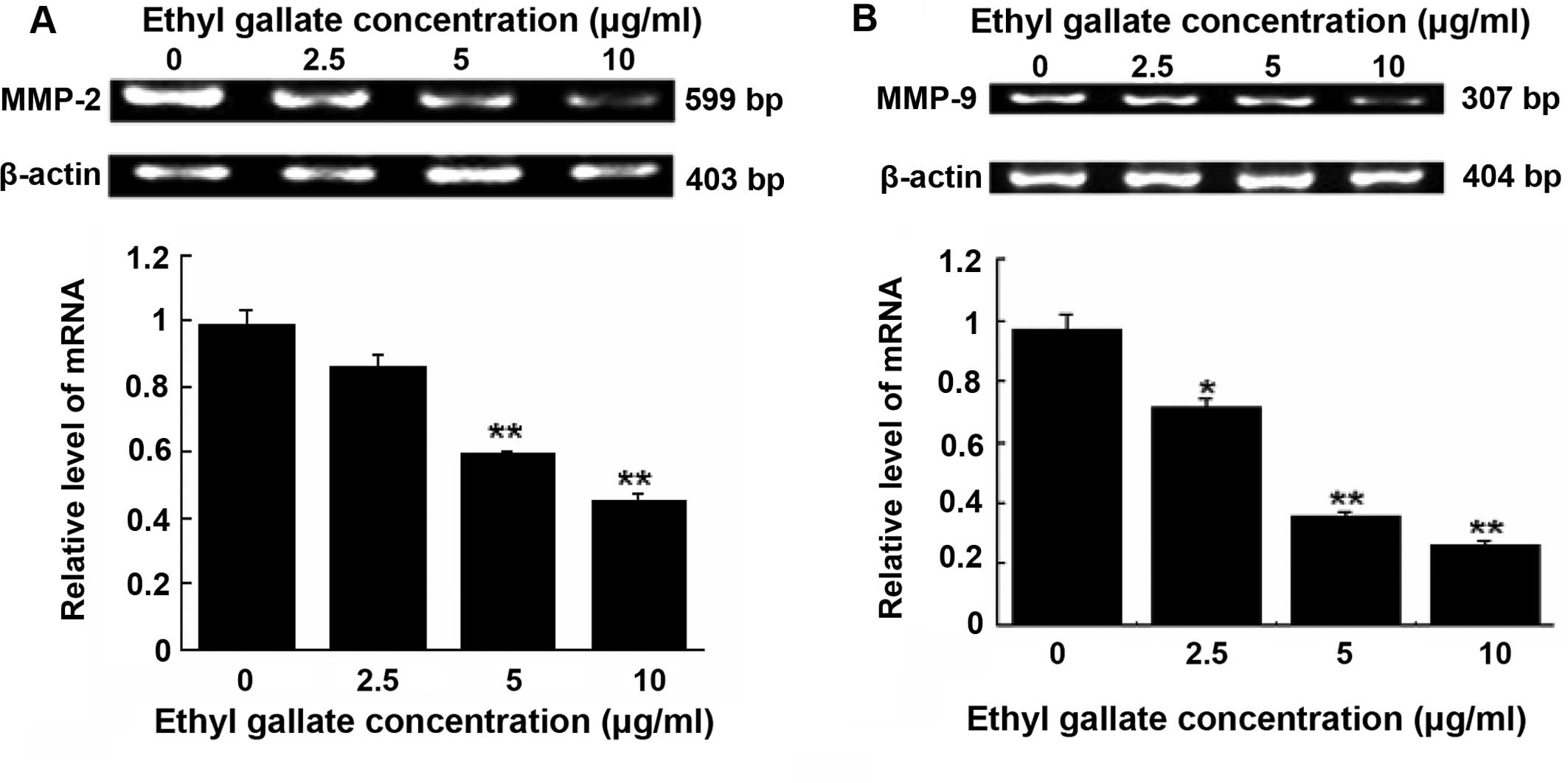
Inhibition of MMP-2 and MMP-9 expression
by Ethyl gallate
Degradation of the extracellular matrix relative
proteins, MMP-2 and MMP-9 is important in the invasive stages of
cancer. RT-PCR detection demonstrated that Ethyl gallate at 5.0 and
10.0 μg/ml significantly reduced the mRNA levels of MMP-2
(Fig. 4A) and MMP-9
(Fig. 4B) in MDA-MB-231 cells
following cell treatment for 24 h with Ethyl gallate.
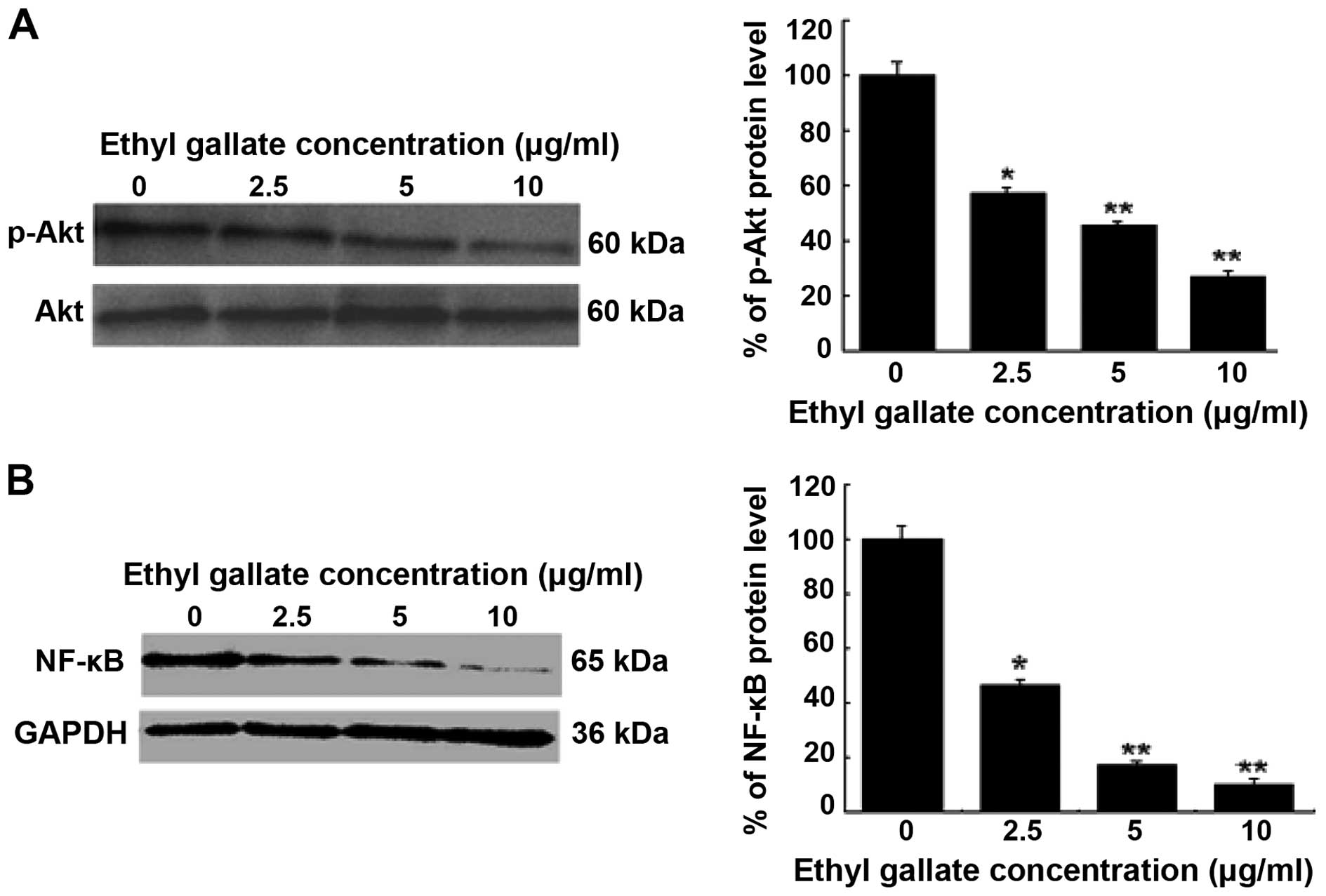
Inhibition of cell signaling pathway by
Ethyl gallate
The importance of the PI3K/Akt and NF-κB signaling
pathways to tumor cell growth and invasion was investigated. NF-κB
is a critical transcription factor involved in the regulation of
MMP-9 expression. To further understand the inhibitory mechanisms
of Ethyl gallate on MMP-9 transcriptional regulation, the
PI3K/Akt and NF-κB signaling pathways were investigated by western
blot analysis. The results revealed that Ethyl gallate
significantly reduced Akt phosphorylation at 5.0 and 10.0 μg/ml
(P<0.05 and 0.01; Fig. 5A), and
also decreased the activity of NF-κB at 5.0 μg/ml (P<0.05) and
10.0 μg/ml (P<0.01; Fig. 5B). By
contrast, the inhibition of PI3K/Akt leading to downregulation of
NF-κB activation may be one mechanism of Ethyl gallate against
MDA-MB-231 cell proliferation and invasion.
Discussion
Euphorbia fischeriana Steud is known to
inhibit tumor cell growth. The bioactive constituents from the
roots of Euphorbia fischeriana Steud were concentrated on
diterpenoids. However, in the present study, we found that Ethyl
gallate, which is identified as the major constituent in
Euphorbia fischeriana Steud extracts, potently inhibits
proliferation and induces apoptosis of breast cancer cells in dose-
and time-dependent manners in vitro. MDA-MB-231 cells
(ER−) were more sensitive to Ethyl gallate than MCF-7
cells (ER+) (Fig. 1).
This result shows that Ethyl gallate is an important bioactive
constituent in the chemotherapy of breast cancer through the
pathways of marked induction of cell apoptosis. Induction of
apoptosis was primarily due to altering Bax/Bcl-2 ratio (Fig. 2), providing evidence for
understanding the manner in which Euphorbia fischeriana
Steud functions in cancer treatment.
Metastasis is the main cause of mortality in cancer
patients (16). During the
complicated processes of metastasis, the adhesion to the
extracellular matrix (ECM), migration and invasion of cancer cells
are the most pivotal steps for motility to distant sites. Many
bioactive substances have been proven to inhibit an invasive
potential of metastatic cancer cell lines, including prostate, lung
and breast (17–19). In the present study, we have shown
that Ethyl gallate markedly inhibited cell adhesion in Matrigel
(Fig. 3) and the migratory
abilities of highly invasive MDA-MB-231 breast cells. The
anti-invasive capacity of Ethyl gallate may be important in
decreasing mortality and improving the survival time in ERbreast
cancer patients.
For tumor invasion, matrix metalloproteinases (MMPs)
are the best documented critical proteolytic enzymes associated
with degradation of ECM (20–22).
It is believed that this characteristic initiates the metastatic
process and enables tumor cells to invade and spread to various
secondary sites. To facilitate metastasis, tumor cells depend on
the activity of more than one MMP, enabling them to cross the
tissue barriers they encounter during the process of invasion.
Although many MMPs have been identified, MMP-2 (gelatinase A) and
MMP-9 (gelatinase B) are the enzymes most pivotal to degrading ECM
(23,24), and they are highly expressed in
highly metastatic tumors (25). MMP
activity is regulated by gene transcription, while its expression
level is directly associated with invasion and metastasis of tumor.
Thus, MMP-2 and MMP-9 are considered to be a target for breast
cancer therapy by suppressing breast cancer invasion (26). The present study demonstrated that
Ethyl gallate treatment significantly inhibited the activity of
MMP-2 and MMP-9 by downregulating mRNA expression
levels in MDA-MB-231 cells compared with the untreated control
(Fig. 4). Therefore, MMP-2 and
MMP-9 may be Ethyl gallate-responsive mediators whose degradation
of ECM may cause subsequent cancer migration and invasion.
In addition, the efficacy of Ethyl gallate can be
explained by interference with the PI3K/Akt and NF-κB signaling
pathways, which have been found to be important in growth, invasion
and metastasis of tumor cells (27). It has been shown that the NF-κB
signal a major pathway for modulating MMP-9 expression (28,29).
In the present study, Akt phosphorylation and NF-κB activation were
found to be inhibited by Ethyl gallate treatment, although there
was no effect on total Akt (Fig.
5). NF-κB is a nuclear transcription regulator for Bcl-2
transcription (30,31). The Bcl-2, Bax, p-Akt and NF-κB have
become the main targets of action by anticancer agents (32–34).
Ethyl gallate inhibits the PI3K/Akt and NF-κB/Bcl-2 signaling
pathways by suppressing the phosphorylation of Akt and NF-κB
expression in MDA-MB-231 cells. This inhibition may contribute to
downregulation of the Bcl-2/Bax ratio and mRNA expression levels of
MMP-2 and MMP-9 in human breast cancer cells. These
data provide a basic mechanism for Ethyl gallate chemotherapeutic
properties of human breast cancer cells.
Abnormal growth and metastasis are considered as
important biological properties of cancer cells. An agent that
efficiently inhibited these biological properties of cancer cells
is a potential candidate for the suppression of cancer progression.
Our data suggest that Ethyl gallate is an effective agent to target
breast cancer proliferation as well as migration and invasion.
These inhibitory effects are at least partially mediated by
interference with the Akt-NF-κB signaling pathway.
Future studies are required to analyze the precise
mechanism(s) of Ethyl gallate and to exploit its full potential for
breast cancer chemotherapy. In vivo studies are pivotal to
confirm these in vitro mechanisms and determine future
therapeutic applications of Ethyl gallate against breast
cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81374021), and the Youth Leading
Scholar Supporting Program in General Colleges and Universities of
Heilongjiang, China (no. 1253G067).
References
|
1
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van de Rijn M, Perou CM, Tibshirani R, et
al: Expression of cytokeratins 17 and 5 identifies a group of
breast carcinomas with poor clinical outcome. Am J Pathol.
161:1991–1996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foulkes WD, Brunet JS, Stefansson IM, et
al: The prognostic implication of the basal-like (cyclin
Ehigh/p27low/p53+/glomeruloid-microvascular-proliferation+)
phenotype of BRCA1-related breast cancer. Cancer Res. 64:830–835.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folgueras AR, Pendás AM, Sánchez LM and
López-Otín C: Matrix metalloproteinases in cancer: from new
functions to improved inhibition strategies. Int J Dev Biol.
48:411–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin YC, Tsai PH, Lin CY, Cheng CH, Lin TH,
Lee KP, Huang KY, Chen SH, Hwang JJ, Kandaswami CC and Lee MT:
Impact of flavonoids on matrix metalloproteinase secretion and
invadopodia formation in highly invasive A431-III cancer cells.
PLoS One. 8:e719032013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Cao W, Zhang B, Liu YQ, Wang ZY, Wu
YP, Yu XJ, Zhang XD, Ming PH, Zhou GB and Huang L: The natural
compound magnolol inhibits invasion and exhibits potential in human
breast cancer therapy. Sci Rep. 3:30982013.PubMed/NCBI
|
|
7
|
Ho YC, Yang SF, Peng CY, Chou MY and Chang
YC: Epigallocatechin-3-gallate inhibits the invasion of human oral
cancer cells and decreases the productions of matrix
metalloproteinases and urokinase-plasminogen activator. J Oral
Pathol Med. 36:588–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee WJ, Chen WK, Wang CJ, Lin WL and Tseng
TH: Apigenin inhibits HGF-promoted invasive growth and metastasis
involving blocking PI3K/Akt pathway and β4 integrin function in
MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol.
226:178–191. 2008. View Article : Google Scholar
|
|
9
|
Qin GW and Xu RS: Recent advances on
bioactive natural products from Chinese medicinal plants. Med Res
Rev. 18:375–382. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang YB, Huang R, Wang HB, Jin HZ, Lou LG
and Qin GW: Diterpenoids from the roots of Euphorbia fischeriana. J
Nat Prod. 69:967–970. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi HM, Williams ID, Sung HH, Zhu HX, Ip
NY and Min ZD: Cytotoxic diterpenoids from the roots of Euphorbia
ebracteolata. Planta Med. 71:349–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang JH, Zhang K, Niu HY, Shu LH, Yue DM,
Li D and He P: Jolkinolide B from Euphorbia fischeriana Steud
induces in human leukemic cells apoptosis via JAK2/STAT3 pathways.
Int J Clin Pharmacol Ther. 51:170–178. 2013. View Article : Google Scholar
|
|
13
|
Yan SS, Li Y, Wang Y, Shen SS, Gu Y, Wang
HB, Qin GW and Yu Q: 17- Acetoxyjolkinolide B irreversibly inhibits
IκB kinase and induces apoptosis of tumor cells. Mol Cancer Ther.
7:1523–1532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JH, Zhou YJ, Bai X and He P:
Jolkinolide B from Euphorbia fischeriana Steud induces apoptosis in
human leukemic U937 cells through PI3K/Akt and XIAP pathways. Mol
Cells. 32:451–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Ma X, Yan S, Shen S, Zhu H, Gu Y,
Wang H, Qin G and Yu Q: 17-Hydroxy-jolkinolide B inhibits signal
transducers and activators of transcription 3 signaling by
covalently cross-linking Janus kinases and induces apoptosis of
human cancer cells. Cancer Res. 69:7302–7310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hung SH, Shen KH, Wu CH, Liu CL and Shih
YW: α-Mangostin suppresses PC-3 human prostate carcinoma cell
metastasis by inhibiting matrix metalloproteinase-2/9 and
urokinase-plasminogen expression through the JNK signaling pathway.
J Agric Food Chem. 57:1291–1298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang
CL and Hsieh YS: Mulberry anthocyanins, cyanidin 3-rutinoside and
cyanidin 3-glucoside, exhibited an inhibitory effect on the
migration and invasion of a human lung cancer cell line. Cancer
Lett. 235:248–259. 2006. View Article : Google Scholar
|
|
19
|
Azios NG and Dharmawardhane SF:
Resveratrol and estradiol exert disparate effects on cell
migration, cell surface actin structures, and focal adhesion
assembly in MDA-MB-231 human breast cancer cells. Neoplasia.
7:128–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Das R, Philip S, Mahabeleshwar GH, Bulbule
A and Kundu GC: Osteopontin: it’s role in regulation of cell
motility and nuclear factor κB-mediated urokinase type plasminogen
activator expression. IUBMB Life. 57:441–447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu KW, Tsai ML, Chen JC, Hsu SC, Hsia TC,
Lin MW, Huang AC, Chang YH, Ip SW, Lu HF and Chung JG: Gypenosides
inhibited invasion and migration of human tongue cancer SCC4 cells
through down-regulation of NFκB and matrix metalloproteinase-9.
Anticancer Res. 28:1093–1099. 2008.PubMed/NCBI
|
|
23
|
Park SY, Kim JH, Lee YJ, Lee SJ and Kim Y:
Surfactin suppresses TPA-induced breast cancer cell invasion
through the inhibition of MMP-9 expression. Int J Oncol.
42:287–296. 2013.
|
|
24
|
Hojilla CV, Mohammed FF and Khokha R:
Matrix metalloproteinases and their tissue inhibitors direct cell
fate during cancer development. Br J Cancer. 89:1817–1821. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim Y, Kang H, Jang SW and Ko J: Celastrol
inhibits breast cancer cell invasion via suppression of
NF-κB-mediated matrix metalloproteinase-9 expression. Cell Physiol
Biochem. 28:175–184. 2011. View Article : Google Scholar
|
|
26
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor-inducing kinase plays a crucial role in
osteopontin-induced MAPK/IκBα kinase-dependent nuclear factor
κB-mediated promatrix metalloproteinase-9 activation. J Biol Chem.
279:38921–38935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi N, Ito T, Azuma S, Ito E, Honma
R, Yanagisawa Y, Nishikawa A, Kawamura M, Imai J, Watanabe S, Semba
K and Inoue J: Constitutive activation of nuclear factor-κB is
preferentially involved in the proliferation of basal-like subtype
breast cancer cell lines. Cancer Sci. 100:1668–1674. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang H, Lee M, Choi KC, Shin DM, Ko J and
Jang SW: N-(4-hydroxyphenyl)retinamide inhibits breast cancer cell
invasion through suppressing NF-KB activation and inhibiting matrix
metalloproteinase-9 expression. J Cell Biochem. 113:2845–2855.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thangapazham RL, Passi N and Maheshwari
RK: Green tea polyphenol and epigallocatechin gallate induce
apoptosis and inhibit invasion in human breast cancer cells. Cancer
Biol Ther. 6:1938–1943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Viatour P, Bentires-Alj M, Chariot A,
Deregowski V, de Leval L, Merville MP and Bours V: NF-κB2/p100
induces Bcl-2 expression. Leukemia. 17:1349–1356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marsden VS, O’Connor L, O’Reilly LA, Silke
J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ,
Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM and Strasser A:
Apoptosis initiated by Bcl-2-regulated caspase activation
independently of the cytochrome c/Apaf-1/caspase-9 apoptosome.
Nature. 419:634–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Emi M, Kim R, Tanabe K, Uchida Y and Toge
T: Targeted therapy against Bcl-2-related proteins in breast cancer
cells. Breast Cancer Res. 7:940–952. 2005. View Article : Google Scholar
|
|
33
|
Patel JB, Mehta J, Belosay A, Sabnis G,
Khandelwal A, Brodie AM, Soprano DR and Njar VC: Novel retinoic
acid metabolism blocking agents have potent inhibitory activities
on human breast cancer cells and tumour growth. Br J Cancer.
96:1204–1215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aggarwal BB: Nuclear factor-κB: the enemy
within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|