Introduction
Lung cancer is one of the most common malignancies
worldwide, and is the leading cause of cancer-related mortality
among both men and women (1). Lung
cancer can be divided into two main subtypes: small cell lung
cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC
accounts for ~85% of lung cancer cases. Most patients with NSCLC
are typically at an advanced stage when diagnosed (2). Therefore, uncovering the molecular
mechanisms of NSCLC and identifying new biomarkers may be crucial
for the diagnosis and treatment of NSCLC.
Sphingosine kinase (SphK) is a type of conserved
lipid kinase that can convert phosphorylation of sphingosine to
sphingosine-1-phosphate (S1P). S1P is an important bioactive lipid
mediator that regulates various aspects of cellular processes
during tumorigenesis (3,4). Thus, SphK may also play a crucial role
in the progression of cancer. There are two functional SphK
isoforms [sphingosine kinase 1 (SphK1; and SphK2)] that have been
identified and characterized in mammalian cells (5). As one member of the SphK family, SphK1
activity can be stimulated by a series of cytokines such as EGF and
VEGF (6), and acts as an oncogenic
enzyme in tumor cells (7).
Overexpression of SphK1 has been demonstrated in many cancer types
including breast, prostate, gastric and colon cancer, and is
closely related with cancer progression and the poor survival of
patients (8–11). Many reports have proved that SphK1
participates in the regulation of tumor cell antiapoptosis, growth
and survival (12,13). Studies also show that SphK1 promotes
breast cancer progression by stimulating angiogenesis and
lymphangiogenesis (14), and is
involved in the invasion and metastasis of esophageal carcinoma
(15). The expression of SphK1 is
significantly increased in NSCLC, and overexpression of SphK1
promotes tumor progression by enhancing resistance to apoptosis
(16). However, whether SphK1
participates in the invasion and metastasis processes of NSCLC has
not been previously reported.
In the present study, we examined the expression of
SphK1 in different NSCLC cell lines. We also investigated the role
of SphK1 in NSCLC cell invasion and migration, and attempted to
reveal the underlying mechanisms.
Materials and methods
Reagents and antibodies
LY294002 was obtained from Sigma (St. Louis, MO,
USA). Antibodies against SphK1, β-actin and E-cadherin were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Antibodies against Snail, phospho-AKT and AKT were purchased from
Cell Signaling Technology (Danvers, MA, USA).
Cell lines and culture conditions
All cell lines used in our experiments were
purchased from the cell bank of the Chinese Academy of Sciences
(Shanghai, China). The non-malignant lung epithelial cell line
BEAS-2B and the NSCLC cell lines H460, HCC827, H1299 and A549 were
all cultured in DMEM (Gibco, Grand Island, NY, USA) supplement with
10% fetal bovine serum (FBS) in a humidified atmosphere containing
5% CO2 at 37°C.
Real-time PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s
instructions. Total RNA was measured by NanoDrop 2000 (Thermo
Scientific, Waltham, MA, USA) and then 2 μg of total RNA was
reverse transcribed into cDNA using a cDNA synthesis kit (Promega
Corporation, Madison, WI, USA). Real-time PCR was performed with
the primers of SphK1 (sense, 5′-GGCTGCTGTCACCCATGAA-3′ and
antisense, 5′-TCACTCTCTAGGTCCACATCAG-3′); or β-actin (sense,
5′-TGAGCGCGGCTACAGCTT-3′ and antisense,
5′-TCCTTAATGTCACGCACGATTT-3′), respectively. The real-time PCR
thermal cycle conditions were as follows: 10 min at 95°C, 40 cycles
of 15 sec at 95°C and 1 min at 60°C. Finally, the relative gene
expression level of SphK1 was normalized to β-actin expression, and
then calculated using the 2−ΔΔCt method.
Western blotting
Cell lysate was extracted using RIPA buffer
containing protease inhibitors and phosphatase inhibitors (Applygen
Technologies Inc., Beijing, China). The concentration of total
protein was evaluated by the BCA method. Equal amounts of protein
were separated using SDS-PAGE gel and then electroblotted onto a
PVDF membrane. The PVDF membrane was blocked with 5% BSA diluted in
TBST [10 mM Tris (pH 7.4), 100 mM NaCl and 5% Tween-20], and then
the membrane was incubated separately with primary antibodies
against SphK1 (1:500), E-cadherin (1:500), Snail (1:1,000),
phospho-AKT (1:1,000), AKT (1:1,000) or β-actin (1:1,000) at 4°C
overnight. Next, the membrane was incubated with
peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology)
for 1 h at room temperature. The bands were visualized using an
enhanced chemiluminescence (ECL) kit (Applygen Technologies Inc.).
After exposure to X-ray film, the densitometry was quantified using
Quantity One software (Bio-Rad, Hercules, CA, USA).
Cell transfections
For overexpression of SphK1, a pcDNA3.1 vector
encoding full-length SphK1 was constructed and obtained from
GenePharma Co., Ltd. (Shanghai, Beijing). An empty pcDNA3.1 vector
was used as the negative control (NC). A549 cells were transfected
with the SphK1 vector or NC vector, respectively, using
Lipofectamine™ 2000 reagent (Life Technologies Co., Carlsbad, CA,
USA) according to the manufacturer’s instructions. Cells were used
48 h later for the following experiments.
For knockdown of SphK1, a siRNA targeting SphK1 was
used with the sequence as follows: SphK1 siRNA,
5′-GCAGGCAUAUGGAGUAUGA-3′. A scramble siRNA was used as the
negative control. A549 cells were transfected with the SphK1 siRNA
(siSphK1) or the control siRNA (siCtrl), respectively, using
Lipofectamine™ 2000 reagent. Forty-eight hours later, the cells
were used in the following experiments.
Invasion assay
Cell invasion ability was analyzed using a 24-well
Transwell chamber, which contained 8-μm pore size polyethylene
membranes (Costar, San Diego, CA, USA). In brief,
1.0×105 cells in 0.2 ml medium supplement with 1% FBS
were placed into the upper chamber, which was coated with 50 μl 1
μg/ml Matrigel before being used. The lower chamber was filled with
600 μl medium supplement with 30% FBS. Then the chambers were
incubated for 16 h at 37°C in a humidified atmosphere containing 5%
CO2. Cells that penetrated through the Matrigel-coated
membrane were stained with crystal violet after fixation in 4%
formaldehyde, and then photographed at ×200 magnification under a
light microscope. Cell number was counted from seven random fields,
and the mean number was calculated to indicate cell invasive
ability.
Migration assay
Cell migration ability was also evaluated in a
24-well Transwell chamber model. Briefly, the upper chamber was
seeded with 1×105 cells in 0.2 ml medium supplement with
1% FBS and the lower chamber was filled with 600 μl medium
supplement with 30% FBS as a chemoattractant. After incubation for
16 h at 37°C, cells on the upper chamber were removed with a cotton
swab, and cells that migrated through the membrane were stained
with crystal violet. Finally, the migrated cells were observed
under a light microscope at ×200 magnification, and the average
cell number was determined from seven random fields.
Statistical analysis
All experiments were performed at least three times.
The data were analyzed using the software package of SPSS 17.0, and
data are presented as means ± standard deviation. The significance
of the difference between two groups was assessed by the Student’s
t-test. Differences were considered to indicate a statistically
significant result at p<0.05.
Results
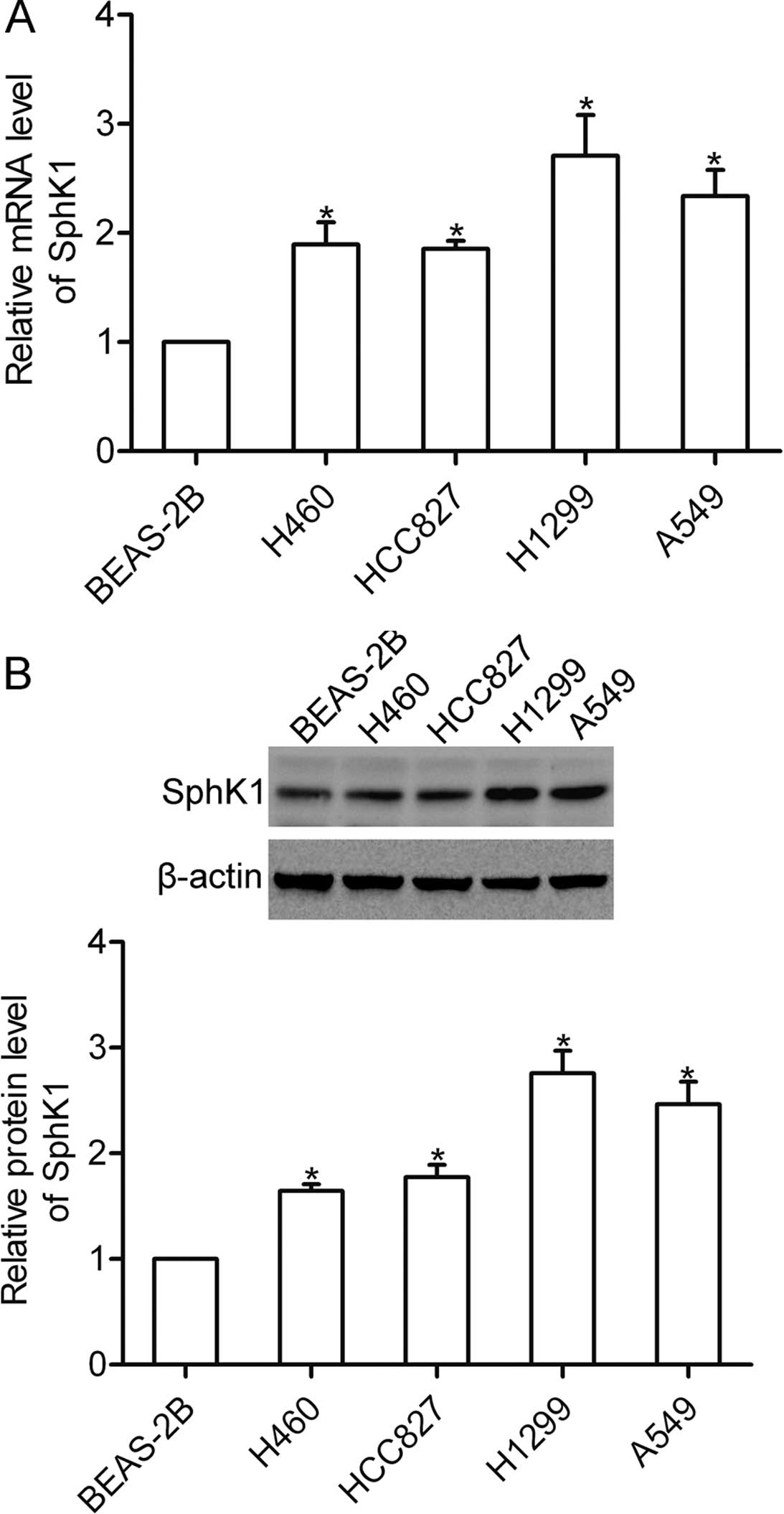
SphK1 is highly expressed in NSCLC
cells
Using real-time PCR, we firstly examined the mRNA
expression of SphK1 in the NSCLC cell lines H460, HCC827, H1299 and
A549 as well as in the non-malignant lung epithelial cell line
BEAS-2B. The results showed that the mRNA expression of SphK1 was
significantly expressed in the NSCLC cells (Fig. 1A). Furthermore, the protein level of
SphK1 was detected using western blotting, and the results showed
that SphK1 protein was highly expressed in the NSCLC cells
(Fig. 1B).
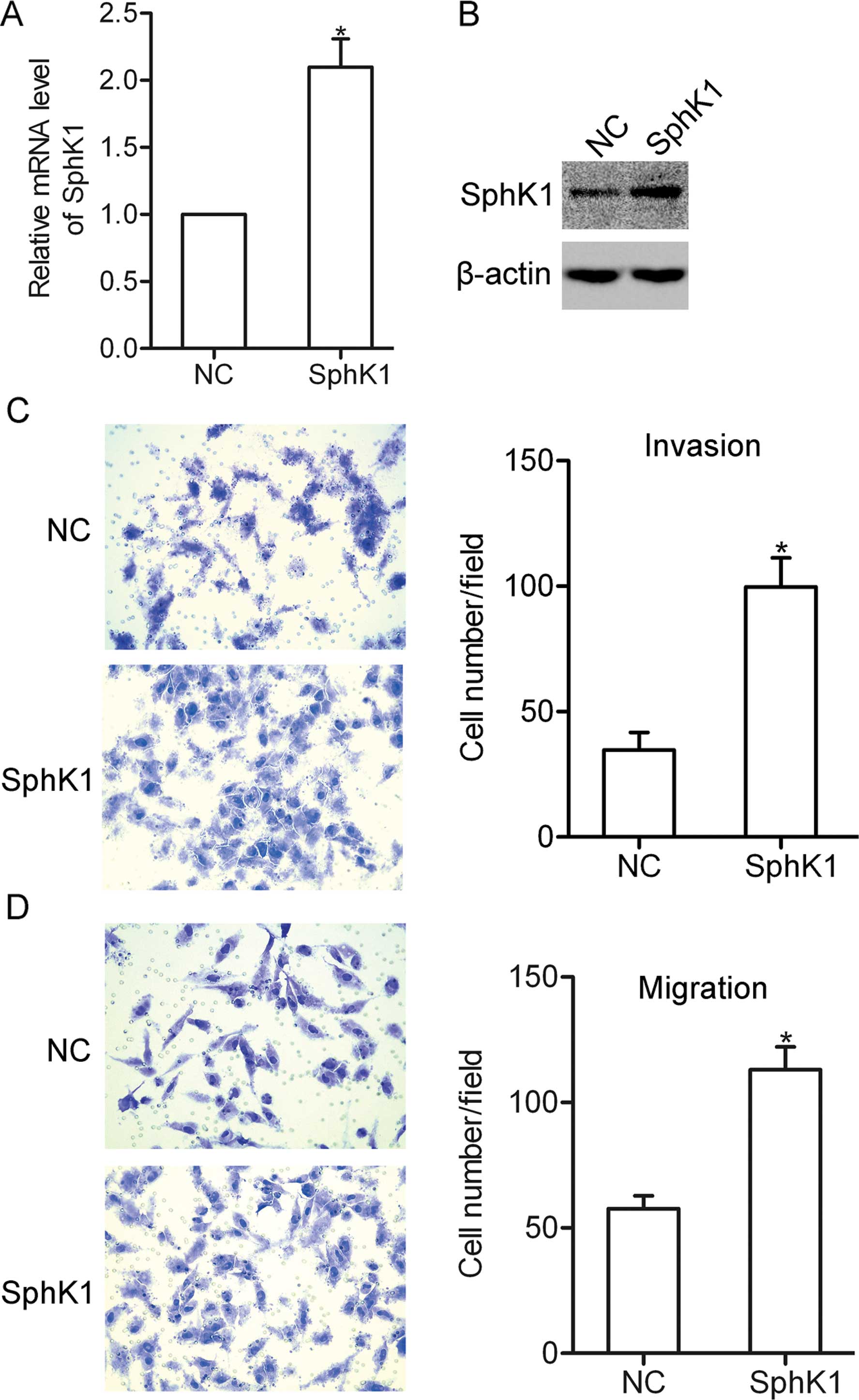
Overexpression of SphK1 promotes NSCLC
cell invasion and migration
To investigate the role of SphK1 in the biological
behavior of NSCLC cells, SphK1 was overexpressed in the A549 cells
by transfection with a pcDNA 3.1-SphK1 vector (Fig. 2A and B). Using invasion and
migration assays, we found that overexpression of SphK1 promoted
the invasion and migration of the A549 cells (Fig. 2C and D), suggesting that SphK1 is
involved in the invasion and migration of NSCLC cells.
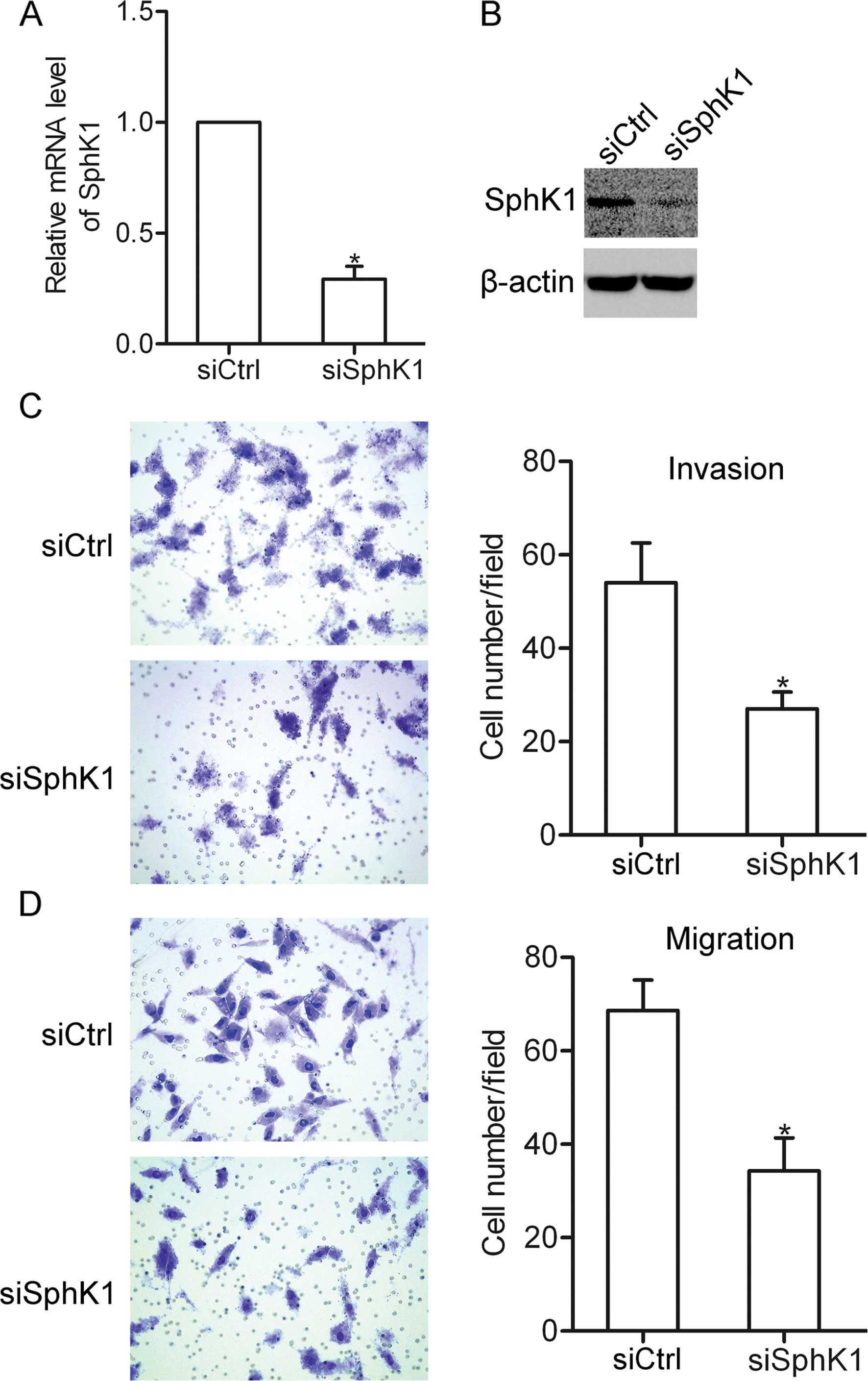
Knockdown of SphK1 inhibits NSCLC cell
invasion and migration
To further characterize the effect of SphK1 on NSCLC
cells, SphK1 siRNA was used to suppress the expression of SphK1 in
the A549 cells (Fig. 3A and B). The
results showed that transfection of SphK1 siRNA greatly suppressed
the invasion and migration of the A549 cells (Fig. 3C and D). These data further confirm
the involvement of SphK1 in NSCLC cell invasion and migration.
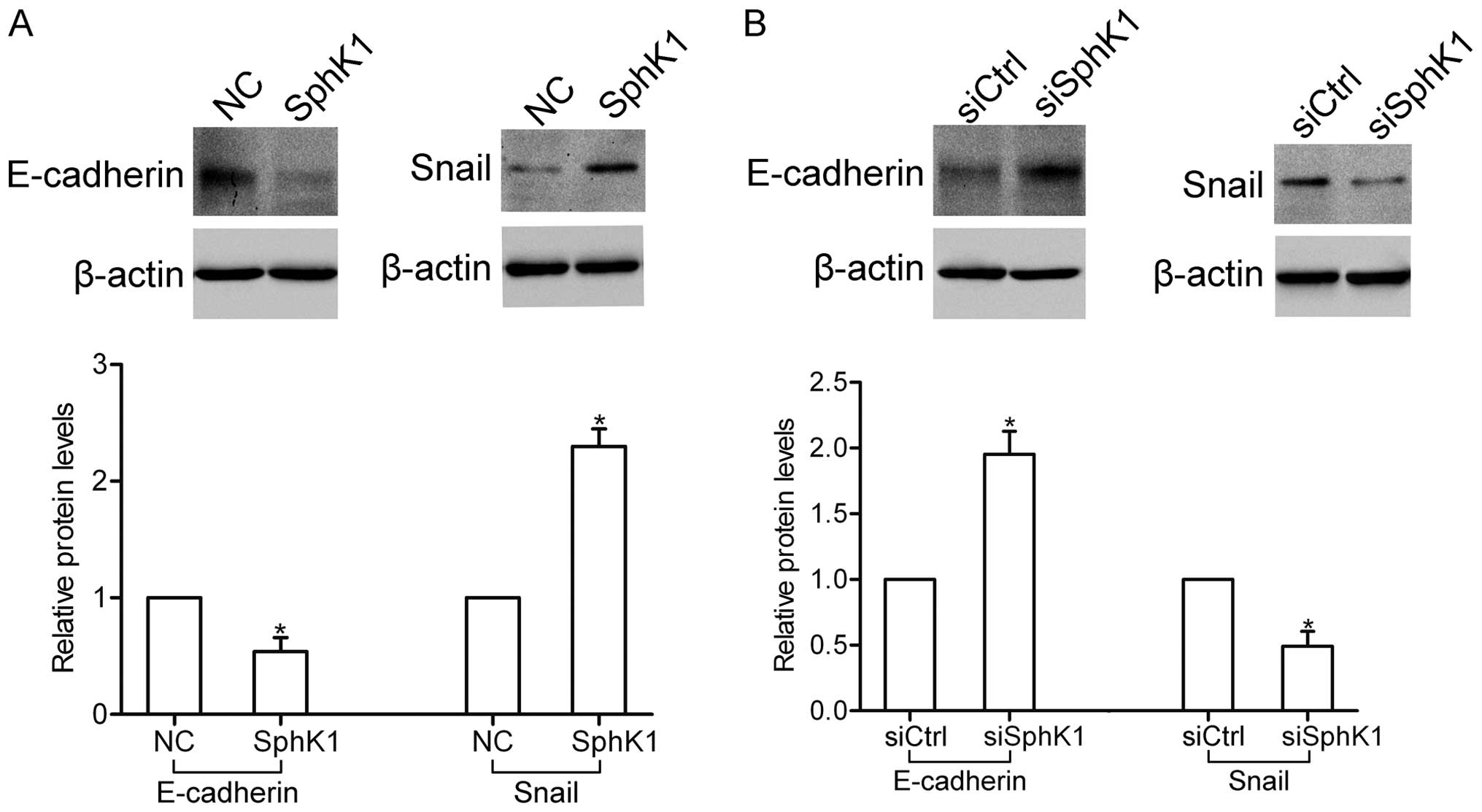
SphK1 participates in the regulation of
several EMT-related genes
The epithelial-mesenchymal transition (EMT) plays a
crucial role in the invasion and metastasis of NSCLC cells. Here,
western blot analysis showed that overexpression of SphK1 decreased
the expression of E-cadherin, yet increased the expression of Snail
(Fig. 4A). In contrast, knockdown
of SphK1 enhanced the expression of E-cadherin, yet inhibited the
expression of Snail (Fig. 4B).
These results suggest that SphK1 participates in the regulation of
E-cadherin and Snail expression.
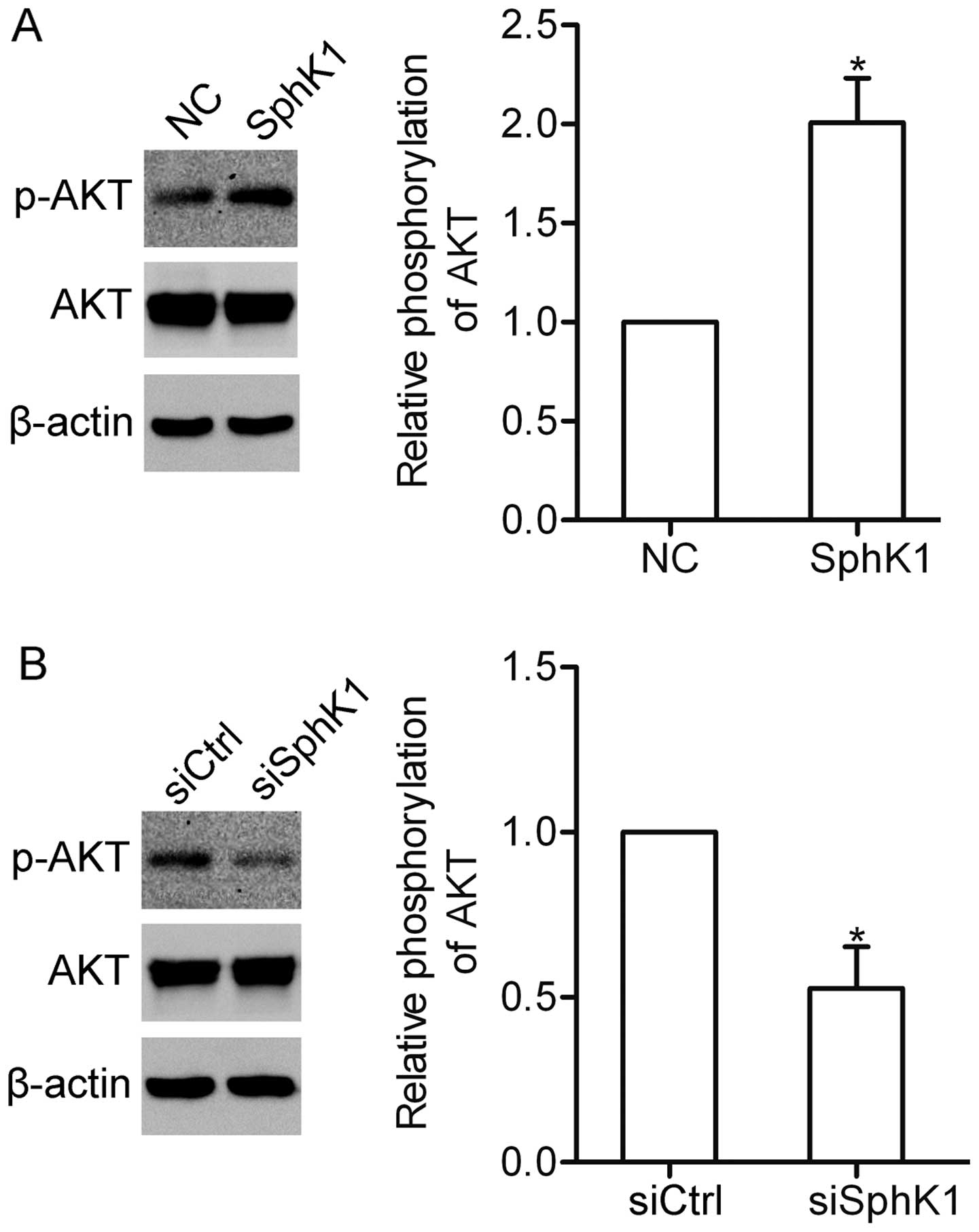
SphK1 contributes to the activation of
AKT in NSCLC cells
It has been demonstrated that SphK1 can activate the
PI3K/AKT pathway in glioma cells (17). We here wondered whether SphK1 could
affect the AKT pathway in NSCLC cells. Overexpression of SphK1
induced the activation of AKT in the A549 cells (Fig. 5A). In contrast, knockdown of SphK1
attenuated the activation of AKT (Fig.
5B). The data indicate that SphK1 participates in the
activation of the AKT pathway.
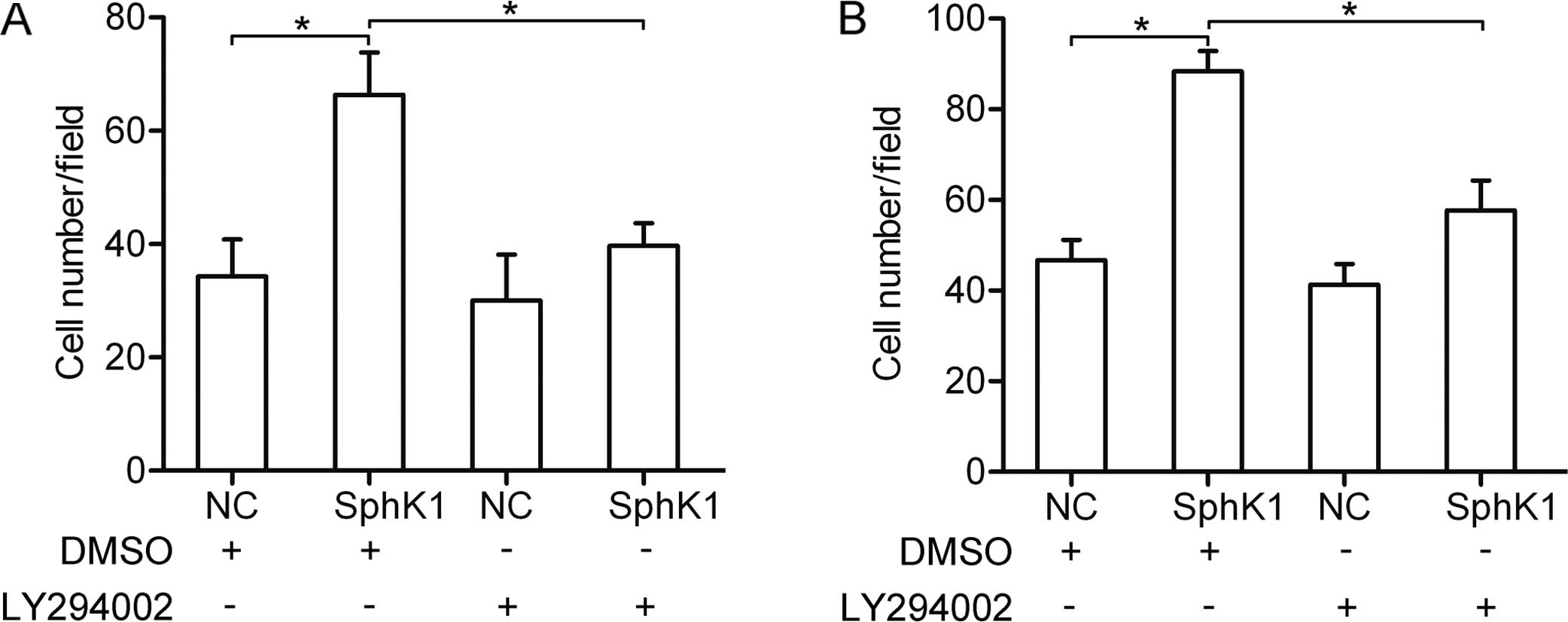
The AKT pathway is involved in
SphK1-enhanced invasion and migration
To determine whether the AKT pathway is associated
with the SphK1-enhanced invasion and migration, LY294002 (10 μM)
was used to block the AKT pathway in the A549 cells. Using invasion
and migration assays, we found that overexpression of SphK1
promoted the invasion and migration. However, the SphK1-enhanced
invasion and migration was greatly suppressed due to inhibition of
the AKT pathway (Fig. 6A and B).
These results suggest that the AKT pathway is required for
SphK1-enhanced invasion and migration.
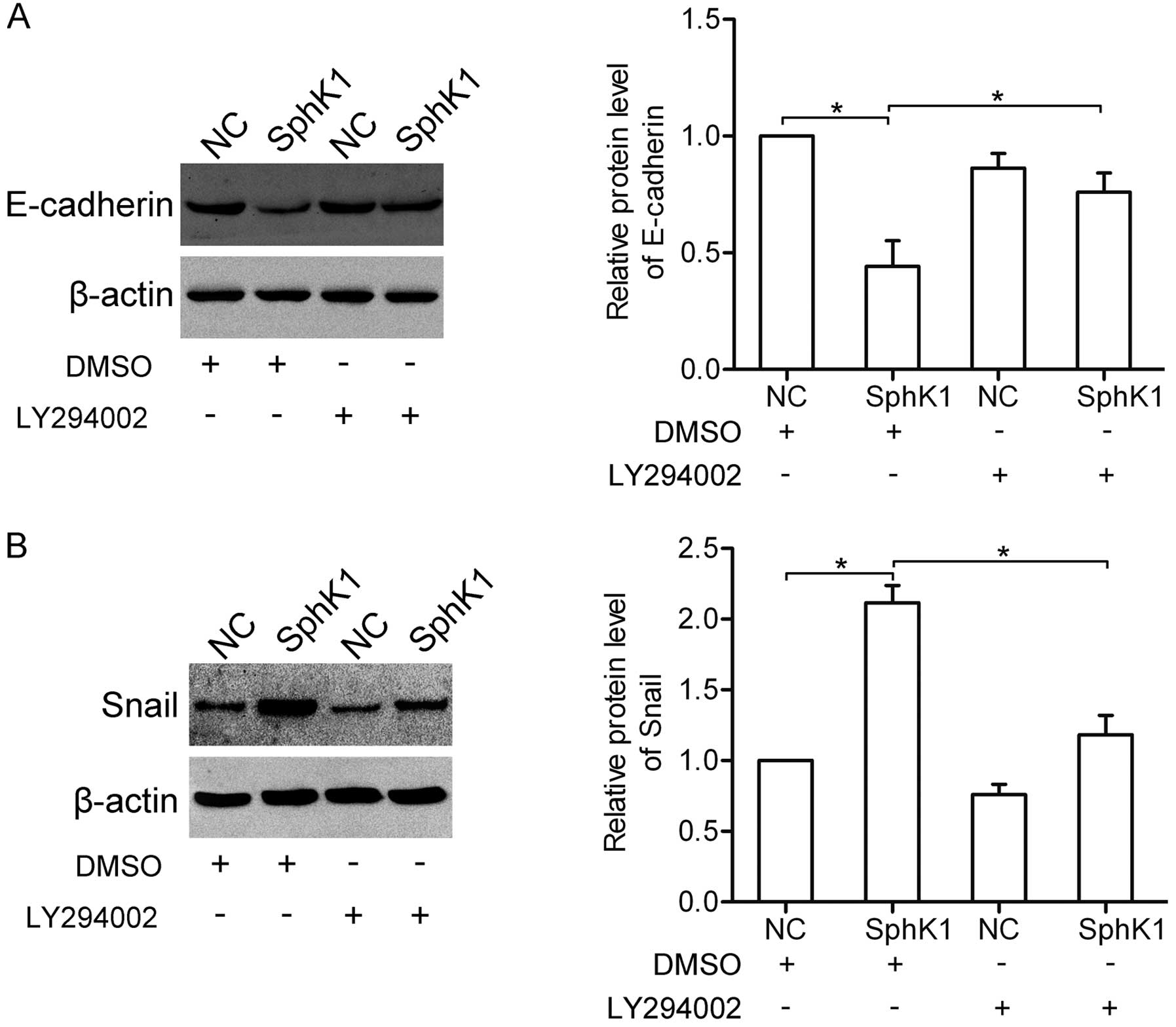
SphK1 regulates the expression of
EMT-related genes via the AKT pathway
We further examined the role of the AKT pathway in
the SphK1-mediated E-cadherin and Snail expression. Notably, the
results showed that overexpression of SphK1 decreased the
expression of E-cadherin, yet increased the expression of Snail in
the dimethyl sulfoxide (DMSO)-treated group. Nevertheless, after
inhibition of the AKT pathway by LY294002 (10 μM), the
SphK1-mediated expression changes in E-cadherin and Snail were
attenuated (Fig. 7A and B),
suggesting that SphK1 regulates the expressions of E-cadherin and
Snail via the AKT pathway.
Discussion
As one important member of the SphK family, SphK1
plays a crucial role in the regulation of intercellular and
intracellular signaling (18).
Overexpression of SphK1 has been observed in diverse tumors
(19–21). It is reported that the expression of
SphK1 is markedly increased in NSCLC tissues, and is correlated
with tumor progression and poor survival of patients with NSCLC
(16). In the present study, we
found that the expression of SphK1 was elevated in all detected
NSCLC cells as compared to normal lung epithelial BEAS-2B cells,
indicating a role of SphK1 in the progression of NSCLC cells.
SphK1 has been reported to act as a regulator in
diverse cellular processes in tumor. It is well known that SphK1
plays a positive role in regulating the proliferation of tumor
cells, including breast, prostate and thyroid cancer cells
(13,22,23).
Studies have also demonstrated that SphK1 contributes to the
apoptosis resistance in glioma and NSCLC cells (16,17),
and promotes tumor progression of colon cancer (24). In addition, SphK1 is required for
EGF-directed motility in breast cancer MCF-7 cells, and promotes
breast cancer progression by stimulating angiogenesis and
lymphangiogenesis (14,25). Further studies show that SphK1
accounts for the invasion and migration of colon cancer,
hepatocellular carcinoma and ovarian cancer cells (26–28),
suggesting the possible involvement of SphK1 in tumor metastasis.
In the present study, we investigated the role of SphK1 in NSCLC
cells. We found that overexpression of SphK1 greatly enhanced the
invasion and migration of NSCLC cells. In contrast, knockdown of
SphK1 significantly inhibited the invasion and migration. All of
our findings further confirm the notion that SphK1 is one of the
key modulators in NSCLC invasion and metastasis.
Accumulating evidence indicates that SphK1
participates in the activation of multiple signaling pathways in
tumor cells. It is reported that SphK1 contributes to the
regulation of CD44 protein expression through the ERK signaling
pathway (29), and enhances colon
cancer cell proliferation and invasiveness via activation of ERK1/2
and suppression of p38 MAPK pathways (26). In addition, SphK1 enhances
resistance to apoptosis through activation of the Akt/FOXO3a/Bim
pathway in glioma cells (17), and
blockage of SphK1 inhibits Akt signaling in human glioblastoma
cells and xenografts (30). In the
present study, our results showed that overexpression of SphK1
stimulated the activation of AKT, whereas knockdown of SphK1
attenuated the activation of AKT in A549 cells, suggesting that
SphK1 contributes to the activation of the AKT pathway in NSCLC
cells. The AKT pathway is frequently dysregulated in tumors, and
plays a pivotal role in tumor invasion and metastasis (31). Here, we found that inhibition of the
AKT pathway attenuated the invasion and migration induced by
overexpression of SphK1, suggesting that the AKT pathway is
required for SphK1-mediated invasion and migration.
The process of EMT is considered to be necessary for
the acquisition of increased motility and invasiveness of cancer
cells. E-cadherin, which is regulated by transcription factors such
as Snail and Slug, plays a crucial role in the maintenance of cell
polarity by mediating cell-cell adherence (32). However, whether SphK1 can affect the
expression of these EMT-related genes is still unclear. In the
present study, we found that SphK1 decreased the protein level of
E-cadherin, but increased the protein level of Snail. Furthermore,
blockage of the AKT pathway attenuated the SphK1-mediated
expression changes in above EMT-related genes. Thus, it is possible
that SphK1 can regulate the EMT process via the AKT pathway in
NSCLC cells.
In conclusion, our findings demonstrated that SphK1
increases the E-cadherin protein level but decreases Snail protein
level via the AKT pathway, which then participates in the invasion
and migration of NSCLC cells. Therefore, targeting SphK1 may
provide new strategies for the therapeutics of NSCLC.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
William WN Jr, Lin HY, Lee JJ, Lippman SM,
Roth JA and Kim ES: Revisiting stage IIIB and IV non-small cell
lung cancer: analysis of the Surveillance, Epidemiology, and End
Results data. Chest. 136:701–709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taha TA, Hannun YA and Obeid LM:
Sphingosine kinase: biochemical and cellular regulation and role in
disease. J Biochem Mol Biol. 39:113–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spiegel S and Milstien S:
Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol
Cell Biol. 4:397–407. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu H, Chakravarty D, Maceyka M, Milstien
S and Spiegel S: Sphingosine kinases: a novel family of lipid
kinases. Prog Nucleic Acid Res Mol Biol. 71:493–511. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shida D, Takabe K, Kapitonov D, Milstien S
and Spiegel S: Targeting SphK1 as a new strategy against cancer.
Curr Drug Targets. 9:662–673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cuvillier O, Ader I, Bouquerel P, et al:
Activation of sphingosine kinase-1 in cancer: implications for
therapeutic targeting. Curr Mol Pharmacol. 3:53–65. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li W, Yu CP, Xia JT, et al: Sphingosine
kinase 1 is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 15:1393–1399. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan SS, Khin LW, Wong L, et al:
Sphingosine kinase 1 promotes malignant progression in colon cancer
and independently predicts survival of patients with colon cancer
by competing risk approach in South Asian population. Clin Transl
Gastroenterol. 5:e512014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malavaud B, Pchejetski D, Mazerolles C, et
al: Sphingosine kinase-1 activity and expression in human prostate
cancer resection specimens. Eur J Cancer. 46:3417–3424. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruckhäberle E, Rody A, Engels K, et al:
Microarray analysis of altered sphingolipid metabolism reveals
prognostic significance of sphingosine kinase 1 in breast cancer.
Breast Cancer Res Treat. 112:41–52. 2008. View Article : Google Scholar
|
|
12
|
Yang YL, Ji C, Cheng L, et al: Sphingosine
kinase-1 inhibition sensitizes curcumin-induced growth inhibition
and apoptosis in ovarian cancer cells. Cancer Sci. 103:1538–1545.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dayon A, Brizuela L, Martin C, et al:
Sphingosine kinase-1 is central to androgen-regulated prostate
cancer growth and survival. PLoS One. 4:e80482009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagahashi M, Ramachandran S, Kim EY, et
al: Sphingosine-1-phosphate produced by sphingosine kinase 1
promotes breast cancer progression by stimulating angiogenesis and
lymphangiogenesis. Cancer Res. 72:726–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan J, Tao YF, Zhou Z, et al: A novel role
of sphingosine kinase-1 (SPHK1) in the invasion and metastasis of
esophageal carcinoma. J Transl Med. 9:1572011. View Article : Google Scholar
|
|
16
|
Song L, Xiong H, Li J, et al: Sphingosine
kinase-1 enhances resistance to apoptosis through activation of
PI3K/Akt/NF-κB pathway in human non-small cell lung cancer. Clin
Cancer Res. 17:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guan H, Song L, Cai J, et al: Sphingosine
kinase 1 regulates the Akt/FOXO3a/Bim pathway and contributes to
apoptosis resistance in glioma cells. PLoS One. 6:e199462011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pyne S, Lee SC, Long J and Pyne NJ: Role
of sphingosine kinases and lipid phosphate phosphatases in
regulating spatial sphingosine 1-phosphate signalling in health and
disease. Cell Signal. 21:14–21. 2009. View Article : Google Scholar
|
|
19
|
Meng XD, Zhou ZS, Qiu JH, Shen WH, Wu Q
and Xiao J: Increased SPHK1 expression is associated with poor
prognosis in bladder cancer. Tumour Biol. 35:2075–2080. 2014.
View Article : Google Scholar
|
|
20
|
Bayerl MG, Bruggeman RD, Conroy EJ, et al:
Sphingosine kinase 1 protein and mRNA are overexpressed in
non-Hodgkin lymphomas and are attractive targets for novel
pharmacological interventions. Leuk Lymphoma. 49:948–954. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuereder T, Hoeflmayer D, Jaeger-Lansky A,
et al: Sphingosine kinase 1 is a relevant molecular target in
gastric cancer. Anticancer Drugs. 22:245–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nava VE, Hobson JP, Murthy S, Milstien S
and Spiegel S: Sphingosine kinase type 1 promotes
estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp
Cell Res. 281:115–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan H, Liu L, Cai J, et al: Sphingosine
kinase 1 is overexpressed and promotes proliferation in human
thyroid cancer. Mol Endocrinol. 25:1858–1866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu SQ, Su YJ, Qin MB, Mao YB, Huang JA
and Tang GD: Sphingosine kinase 1 promotes tumor progression and
confers malignancy phenotypes of colon cancer by regulating the
focal adhesion kinase pathway and adhesion molecules. Int J Oncol.
42:617–626. 2013.
|
|
25
|
Sarkar S, Maceyka M, Hait NC, et al:
Sphingosine kinase 1 is required for migration, proliferation and
survival of MCF-7 human breast cancer cells. FEBS Lett.
579:5313–5317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu SQ, Huang JA, Qin MB, et al:
Sphingosine kinase 1 enhances colon cancer cell proliferation and
invasion by upregulating the production of MMP-2/9 and uPA via MAPK
pathways. Int J Colorectal Dis. 27:1569–1578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao M, Chen Z, Xu Y, et al: Sphingosine
kinase 1 promotes tumour cell migration and invasion via the
S1P/EDG1 axis in hepatocellular carcinoma. Liver Int. 32:331–338.
2012. View Article : Google Scholar
|
|
28
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6:842013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawahara S, Otsuji Y, Nakamura M, et al:
Sphingosine kinase 1 plays a role in the upregulation of CD44
expression through extracellular signal-regulated kinase signaling
in human colon cancer cells. Anticancer Drugs. 24:473–483. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kapitonov D, Allegood JC, Mitchell C, et
al: Targeting sphingosine kinase 1 inhibits Akt signaling, induces
apoptosis, and suppresses growth of human glioblastoma cells and
xenografts. Cancer Res. 69:6915–6923. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang H, Gao M, Shen Z, et al: Blocking
PI3K/Akt signaling attenuates metastasis of nasopharyngeal
carcinoma cells through induction of mesenchymal-epithelial
reverting transition. Oncol Rep. 32:559–566. 2014.PubMed/NCBI
|
|
32
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|