Introduction
Breast cancer in men is a rare disease, accounting
for ~1% of all breast cancer cases worldwide (1). An important predisposing genetic
factor for male breast cancer (MBC) includes germline mutations in
the BRCA genes, particularly in BRCA2, although other
genes are implicated (2). Partner
and localizer of BRCA2 (PALB2) is the third hereditary
breast cancer susceptibility gene; it is mutated in approximately
1–2% of cases, with higher rates in certain populations (3,4).
PALB2 is also the second most commonly
mutated gene noted in hereditary pancreatic cancer (5). Indeed, deleterious germline
PALB2 mutations have been reported in families with breast
and pancreatic cancer from the USA (6) and Europe (7,8).
Other studies have reported that various
PALB2 mutations also predispose to hereditary prostate
cancer (9,10).
The role of PALB2 in MBC predisposition still
remains to be clarified. Several studies have reported truncating
mutations of PALB2 in cases of MBC (11–14).
In contrast, no evidence of PALB2 pathogenic mutations in
MBC was found in other studies (15–17).
Thus, the aim of the present study was to determine
the prevalence of PALB2 mutations in Italian families with
at least one male breast cancer case.
Patients and methods
Patients
We selected 8 patients affected with MBC, or who had
at least one MBC case in the pedigree, from a group of 181 patients
affected with hereditary breast and/or ovarian cancer from
Campania, a region of southern Italy. Of the patients, 5 were males
with breast cancer and 3 were females with breast cancer who had a
first-degree relative with MBC. Patients were selected according to
the selection criteria for hereditary breast cancer based on the
Breast Cancer Linkage Consortium (18).
Ethics committee approval was obtained for the
study. Informed consent for molecular analysis was obtained from
all subjects, and the main clinical and histopathological data were
generated by genetic counseling. Peripheral blood samples were
collected from all patients.
All patients were previously screened for
BRCA1 and BRCA2 mutations.
Mutation analysis
Genomic DNA was isolated from peripheral blood
lymphocytes, using the Wizard Genomic DNA purification kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer’s
instructions.
PALB2 mutational analysis was conducted by
direct sequencing of 13 exons and adjacent intronic regions. One
set of primers was used to amplify each exon, except for exons 4
and 5, which were amplified in four and two PCR products,
respectively.
The primers used to amplify the coding exonintron
boundaries of PALB2, were previously reported (19).
All PCR products were sequenced on both strands
using the ABI Prism di-Deoxy Terminator Cycle Sequencing kit in the
ABI 9700 thermal cycler and an ABI Prism 3100 automatic sequencer
(both from Life Technologies, Carlsbad, CA, USA). The results were
analyzed using Mutation Surveyor® software, version 3.24
(Softgenetics, State College, PA, USA).
GenBank reference sequences used for naming the
novel mutation were NM_024675.3 and NT_010393.15. The sequence
variant was named and referred to in the text according to the
nomenclature used by the Human Genome Variation Society (HGVS;
http://www.hgvs.org), using the descriptions suggested
by den Dunnen and Antonarakis (20).
RNA analysis
The patient with the novel mutation was subjected to
a second peripheral blood sample, to confirm the presence of the
mutation also in mRNA.
Total-RNA was isolated from peripheral blood
lymphocytes using TRIzol reagent and reverse transcribed with
SuperScript First-Strand Synthesis System (both from Life
Technologies) according to the manufacturer’s protocol.
From our set of primers used for the mutation
analysis, we selected a forward primer in exon 4 and a reverse
primer in exon 5 to amplify from total cDNA the region spanning the
mutation. The RT-PCR product was electrophoresed on agarose gel and
then sequenced.
Results
We observed 8 cases of MBC in a cohort of 181
hereditary breast and/or ovarian cancer probands. The prevalence of
MBC in our group of patients was 4.4%.
Mutation analysis of the PALB2 gene showed
the presence of a mutation in 1/8 (12.5%) breast cancer patients.
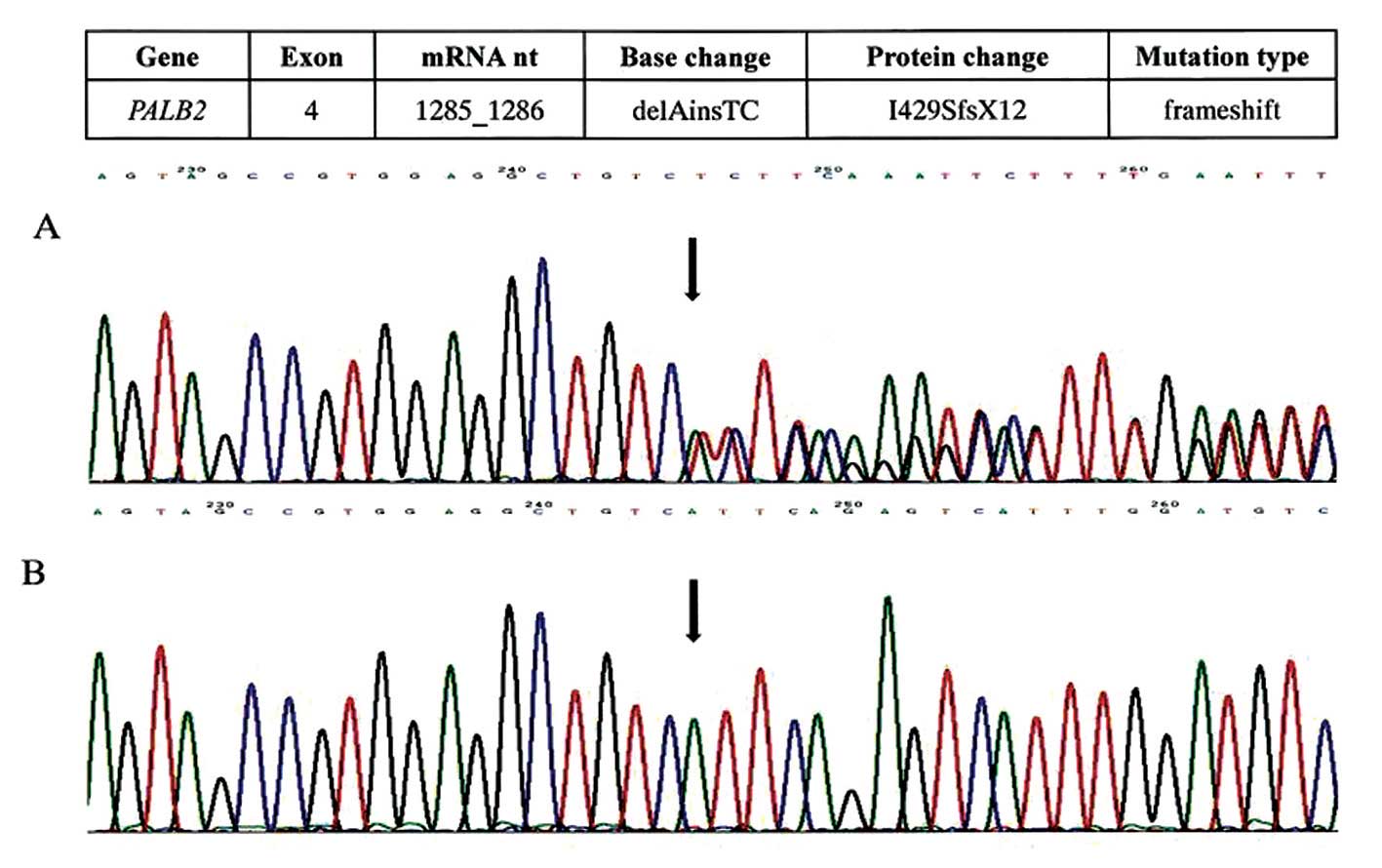
This mutation, not previously described, was named
c.1285_1286delAinsTC (p.I429SfsX12). It was localized within exon 4
and consisted of an A deletion and TC insertion at position
c.1285_1286, which shifted the reading frame at the 429 codon and
led to a premature termination 12 codon downstream, at codon 441
(Fig. 1).
The presence of the mutation was confirmed by
resequencing a second DNA sample from the patient. RNA analysis
showed the same mutation.
In Table I, we
document the clinical and histopathological characteristics of the
8 analyzed patients.
 | Table IMutation and receptor status of
patients affected with MBC or with BC and MBC cases in the
family. |
Table I
Mutation and receptor status of
patients affected with MBC or with BC and MBC cases in the
family.
| Patient no. | Gender | Age at diagnosis
(years) | Diagnosis | Receptor status | BRCA1/2
mutation | PALB2
mutation |
|---|
| 1 | M | 65 | MBC |
ER+/PR+/HER2− | - | - |
| 2 | M | 83 | MBC |
ER+/PR+/HER2− | - | - |
| 3 | M | 69 | MBC |
ER+/PR+/HER2− | UV in
BRCA2 | - |
| 4 | M | 40 | MBC |
ER+/PR+/HER2− | UV in
BRCA2 | - |
| 5 | M | 58 | MBC |
ER+/PR+/HER2− | UV in
BRCA2 | - |
| 6 | F | 29 | BC (father
MBC) |
ER−/PR−/HER2− | - |
c.1285_1286delAinsTC (p.I429SfsX12) |
| 7 | F | 29 | BC (grandfather
MBC) |
ER+/PR−/HER2+ | - | - |
| 8 | F | 38 | BC (grandfather
MBC) |
ER−/PR−/HER2− | 5382insC in
BRCA1 | - |
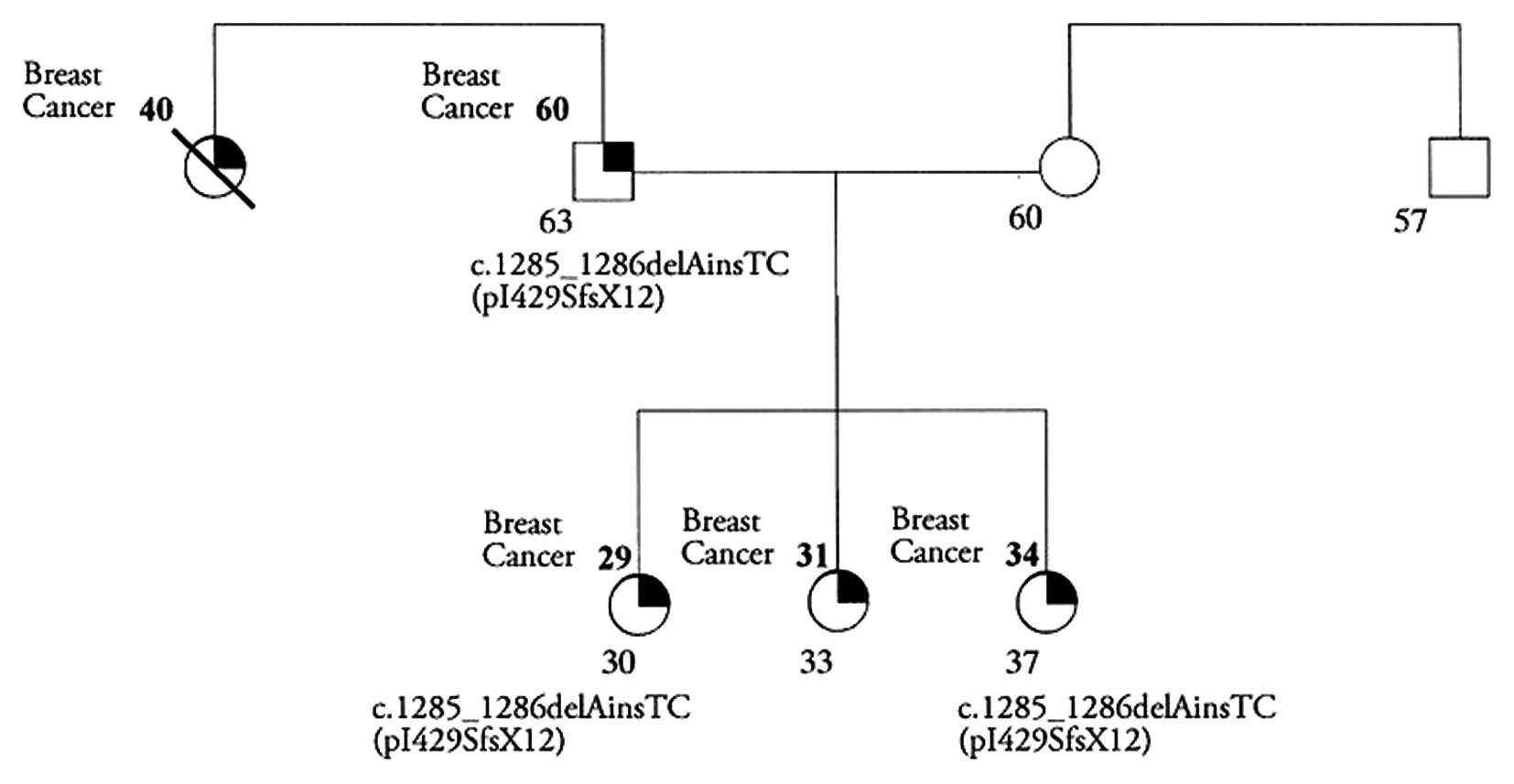
The c.1285_1286delAinsTC (p.I429SfsX12) mutation was
identified in a 30-year-old woman affected with hereditary breast
cancer, with one MBC case in the family. The pedigree is reported
in Fig. 2. This family showed
multiple affected members of the same disease. Breast cancer was
diagnosed in the patient at the age of 29, her father at the age of
60 and two sisters at the age of 31 and 34 years, respectively; in
addition a paternal aunt with breast cancer died at 40 years of
age.
Molecular testing for PALB2 was performed in
the father and in one of the two sisters that agreed with testing.
The analysis showed that both subjects were carriers of this
mutation.
Discussion
PALB2, the partner and localizer of BRCA2, binds
BRCA1 and BRCA2 to promote efficient DNA repair by homologous
recombination (21).
PALB2 is a moderate penetration breast cancer
susceptibility gene with a variable estimated risk of breast
cancer. The Finnish founder c.1952delT mutation increased the risk
of breast cancer 6-fold by the age of 70 years (22), and five PALB2 mutations
reported in the UK confer a 2.3-fold increased risk of breast
cancer (23).
We found a novel PALB2 truncating mutation in
an Italian family with an MBC case. Few studies have analyzed the
PALB2 status in MBC. Particularly, in Italy, only one study
has been conducted (16). In this
study, based on a series of 108 MBC cases, of which 97 cases were
BRCA1/2-negative, no PALB2 mutation was found
(16). In contrast, we found a
mutation in 1/8 (12.5%) patients. The high frequency of the
PALB2 mutation observed in our study was similar to that
described in a US study that reported two truncating mutations in
13 proband males (16%) (12),
whereas other authors showed a lower rate, ranging from 1 to 9%
(11,13,14).
In agreement with these studies, we confirmed the presence of the
PALB2 mutations in MBC cases, suggesting that PALB2
might play an important role in hereditary MBC.
Most of the truncating mutations previously reported
were mapped to exons 4 and 5 of the PALB2 gene, probably as
they are the largest two exons in PALB2 (24). Importantly, all of the mutations
discovered in a Chinese population occurred in exons 4 of
PALB2, suggesting a potential hotspot (25). The c.1285_1286delAinsTC
(p.I429SfsX12) mutation is localized in exon 4, whereas other
Italian studies reported the PALB2 mutations localized in
exon 4 and 5 (26,27), as well as in exon 2 and 13 (8), suggesting no mutational hot spot in
the PALB2 gene for the Italian population.
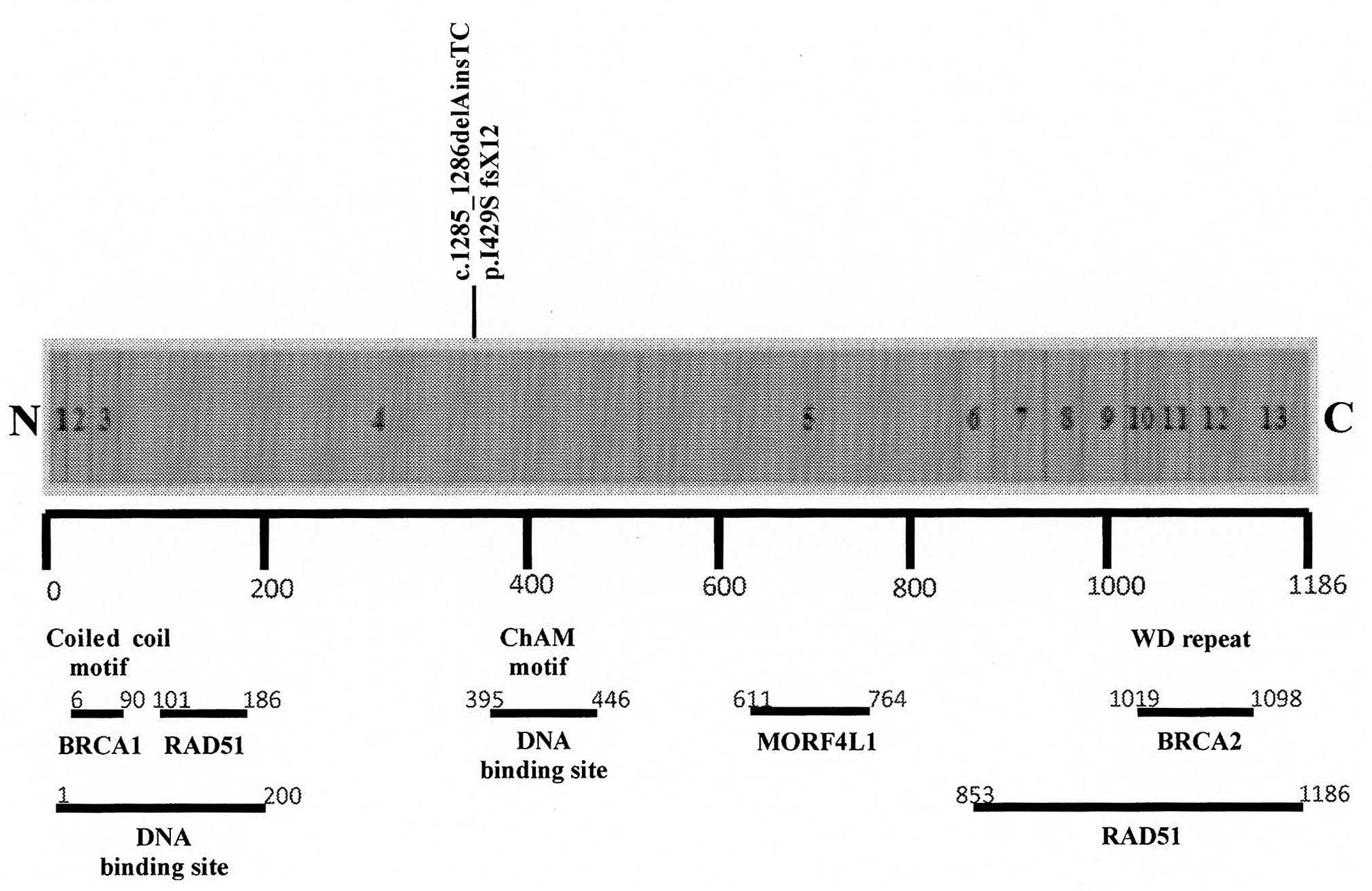
To map the BRCA1-interacting region in PALB2, 13
internal deletion mutants of PALB2 were generated with the P1
mutant, deleted for amino acids 6–90, that failed to associate with
BRCA1 (28). These results showed
that PALB2 protein has a coiled-coil motif at the N terminus,
required for interaction with BRCA1 (Fig. 3). Other biochemical studies showed
that PALB2 binds DNA via two separate regions in the N-terminus of
the protein, called PALB2 truncation 1 (P2T1) and PALB2 truncation
3 (P2T3), respectively (29,30).
Particularly, P2T3 contains an evolutionarily conserved PALB2
motif, named chromatin-association motif (ChAM), which is localized
at amino acid region 395–446. ChAM is important for the efficient
association of PALB2 to chromatin and for recruitment of the BRCA
complex to accumulate RAD51 at double-strand break sites (31). Furthermore, the amino acidic region
611–764 of PALB2 interacts with mortality factor 4 like protein 1
(MORF4L1) which is important for promoting the function of the BRCA
complex. PALB2 interacts with RAD51 by two regions (amino acids
101–184 and 853–1186) and has a C-terminal domain, containing four
WD repeats which mediate the interaction with BRCA2 (32).
The novel mutation c.1285_1286delAinsTC
(p.I429SfsX12) was found to be localized in exon 4 of PALB2,
in the region that encodes for the ChAM motif (Fig. 3). It introduces a premature stop at
codon 441 position; therefore, it may be expected that this
mutation reduces the capacity of the ChAM motif for mediating PALB2
chromatin association thus altering DNA damage repair processes. In
addition, this mutation induces the lack of interaction between
MORF4L1 and BRCA2.
The prevalence of MBC in our patients was 4.4% in
agreement with other studies that reported frequencies ranging from
4.1 to 4.6% (1). Out of 7 breast
cancer patients, negative for PALB2 mutations, one was a
carrier of the 5382insC mutation in BRCA1, as previously
reported in our study (33).
Instead, 3 MBC cases were carriers of unclassified variants in
BRCA2 (Table I). However,
the patients with the BRCA1 mutation or with unclassified
variants in BRCA2 were additionally tested for a
PALB2 mutation in order to not underestimate a possible
condition of double heterozygosity (DH) in the BRCA and
PALB2 genes. In a recent study, Pern et al described
DH for BRCA1 and PALB2 mutations in one German
patient with triple-negative breast cancer (34).
On the basis of our findings, PALB2 could be
added to the list of breast cancer susceptibility genes, not only
in families with recurring breast and pancreatic cancers but also
in families with MBC.
Acknowledgements
The authors thank Mrs. Anna Cuomo for technical
assistance in the mutation analysis.
References
|
1
|
Ottini L, Palli D, Rizzo S, Federico M,
Bazan V and Russo A: Male breast cancer. Crit Rev Oncol Hematol.
73:141–155. 2010. View Article : Google Scholar
|
|
2
|
Mohamad HB and Apffelstaedt JP: Counseling
for male BRCA mutation carriers: a review. Breast. 17:441–450.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erkko H, Dowty JG, Nikkilä J, Syrjäkoski
K, Mannermaa A, Pylkäs K, Southey MC, Holli K, Kallioniemi A,
Jukkola-Vuorinen A, Kataja V, Kosma VM, Xia B, Livingston DM,
Winqvist R and Hopper JL: Penetrance analysis of the PALB2
c.1592delT Founder mutation. Clin Cancer Res. 14:4667–4671. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foulkes WD, Ghadirian P, Akbari MR, Hamel
N, Giroux S, Sabbaghian N, Darnel A, Royer R, Poll A, Fafard E,
Robidoux A, Martin G, Bismar TA, Tischkowitz M, Rousseau F and
Narod SA: Identification of a novel truncating PALB2 mutation and
analysis of its contribution to early-onset breast cancer in
French-Canadian women. Breast Cancer Res. 9:R832007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones S, Hruban RH, Kamiyama M, et al:
Exomic sequencing identifies PALB2 as a pancreatic cancer
susceptibility gene. Science. 324:2172009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hofstatter EW, Domchek SM, Miron A, Garber
J, Wang M, Componeschi K, Boghossian L, Mitron PL, Nathanson KL and
Tung N: PALB2 mutations in familial breast and pancreatic cancer.
Fam Cancer. 10:225–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slater EP, Langer P, Niemczyk E, Strauch
K, Butler J, Habbe N, Neoptolemos JP, Greenhalf W and Bartsch DK:
PALB2 mutations in European familial pancreatic cancer families.
Clin Genet. 78:490–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peterlongo P, Catucci I, Pasquini G,
Verderio P, Peissel B, Barile M, Varesco L, Riboni M, Fortuzzi S,
Manoukian S and Radice P: PALB2 germline mutations in familial
breast cancer cases with personal and family history of pancreatic
cancer. Breast Cancer Res Treat. 126:825–828. 2011. View Article : Google Scholar
|
|
9
|
Tischkowitz M, Sabbaghian N, Ray AM, Lange
EM, Foulkes WD and Cooney KA: Analysis of the gene coding for the
BRCA2-interacting protein PALB2 in hereditary prostate cancer.
Prostate. 68:675–678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pakkanen S, Wahlfors T, Siltanen S,
Patrikainen M, Matikainen MP, Tammela TL and Schleutker J: PALB2
variants in hereditary and unselected Finnish prostate cancer
cases. J Negat Results Biomed. 8:122009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
García MJ, Fernández V, Osorio A, Barroso
A, Llort G, Lázaro C, Blanco I, Caldés T, de la Hoya M, Ramón Y,
Cajal T, Alonso C, Tejada MI, San Román C, Robles-Díaz L, Urioste M
and Benítez J: Analysis of FANCB and FANCN/PALB2 Fanconi anemia
genes in BRCA1/2-negative Spanish breast cancer families. Breast
Cancer Res Treat. 113:545–1551. 2009. View Article : Google Scholar
|
|
12
|
Casadei S, Norquist BM, Walsh T, Stray S,
Mandell JB, Lee MK, Stamatoyannopoulos JA and King MC: Contribution
of inherited mutations in the BRCA2-interacting protein PALB2 to
familial breast cancer. Cancer Res. 71:2222–2229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding YC, Steele L, Kuan CJ, Greilac S and
Neuhausen SL: Mutations in BRCA2 and PALB2 in male breast cancer
cases from the United States. Breast Cancer Res Treat. 6:771–778.
2011. View Article : Google Scholar
|
|
14
|
Adank MA, van Mil SE, Gille JJ, Waisfisz Q
and Meijers-Heijboer H: PALB2 analysis in BRCA2-like families.
Breast Cancer Res Treat. 127:357–362. 2011. View Article : Google Scholar
|
|
15
|
Sauty de Chalon A, Teo Z, Park DJ, Odefrey
FA, Hopper JL and Southey MC: Are PALB2 mutations associated with
increased risk of male breast cancer? Breast Cancer Res Treat.
121:253–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silvestri V, Rizzolo P, Zanna I, Falchetti
M, Masala G, Bianchi S, Papi L, Giannini G, Palli D and Ottini L:
PALB2 mutations in male breast cancer: a population-based study in
Central Italy. Breast Cancer Res Treat. 122:299–301. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blanco A, de la Hoya M, Balmaña J, Ramón y
Cajal T, Teulé A, Miramar MD, Esteban E, Infante M, Benítez J,
Torres A, Tejada MI, Brunet J, Graña B, Balbín M, Pérez-Segura P,
Osorio A, Velasco EA, Chirivella I, Calvo MT, Feliubadaló L, Lasa
A, Díez O, Carracedo A and Vega A: Detection of a large
rearrangement in PALB2 in Spanish breast cancer families with male
breast cancer. Breast Cancer Res Treat. 132:307–315. 2012.
View Article : Google Scholar
|
|
18
|
Breast Cancer Linkage Consortium.
Pathology of familial breast cancer: differences between breast
cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases.
Lancet. 349:1505–1510. 1997. View Article : Google Scholar
|
|
19
|
Tischkowitz M, Xia B, Sabbaghian N,
Reis-Filho JS, Hamel N, Li G, van Beers EH, Li L, Khalil T,
Quenneville LA, Omeroglu A, Poll A, Lepage P, Wong N, Nederlof PM,
Ashworth A, Tonin PN, Narod SA, Livingston DM and Foulkes WD:
Analysis of PALB2/FANCN-associated breast cancer families. Proc
Natl Acad Sci USA. 104:6788–6793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
den Dunnen JT and Antonarakis SE: Mutation
nomenclature. Curr Protoc Hum Genet. Chapter 7(Unit 7): 132003.
View Article : Google Scholar
|
|
21
|
Apostolou P and Fostira F: Hereditary
breast cancer: the era of new susceptibility genes. Biomed Res Int.
2013:7473182013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erkko H, Xia B, Nikkilä J, Schleutker J,
Syrjäkoski K, Mannermaa A, Kallioniemi A, Pylkäs K, Karppinen SM,
Rapakko K, Miron A, Sheng Q, Li G, Mattila H, Bell DW, Haber DA,
Grip M, Reiman M, Jukkola-Vuorinen A, Mustonen A, Kere J, Aaltonen
LA, Kosma VM, Kataja V, Soini Y, Drapkin RI, Livingston DM and
Winqvist R: A recurrent mutation in PALB2 in Finnish cancer
families. Nature. 446:316–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahman N, Seal S, Thompson D, Kelly P,
Renwick A, Elliott A, Reid S, Spanova K, Barfoot R, Chagtai T,
Jayatilake H, McGuffog L, Hanks S, Evans DG and Eccles D; Breast
Cancer Susceptibility Collaboration (UK). Easton DF and Stratton
MR: PALB2, which encodes a BRCA2-interacting protein, is a breast
cancer susceptibility gene. Nat Genet. 39:165–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng Y, Zhang J, Niu Q, Huo D and Olopade
OI: Novel germline PALB2 truncating mutations in African American
breast cancer patients. Cancer. 118:1362–1370. 2012. View Article : Google Scholar
|
|
25
|
Cao AY, Huang J, Hu Z, Li WF, Ma ZL, Tang
LL, Zhang B, Su FX, Zhou J, Di GH, Shen KW, Wu J, Lu JS, Luo JM,
Yuan WT, Shen ZZ, Huang W and Shao ZM: The prevalence of PALB2
germline mutations in BRCA1/BRCA2 negative Chinese women with early
onset breast cancer or affected relatives. Breast Cancer Res Treat.
114:457–462. 2009. View Article : Google Scholar
|
|
26
|
Balia C, Sensi E, Lombardi G, Roncella M,
Bevilacqua G and Caligo MA: PALB2: a novel inactivating mutation in
a Italian breast cancer family. Fam Cancer. 9:531–536. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Papi L, Putignano AL, Congregati C,
Piaceri I, Zanna I, Sera F, Morrone D, Genuardi M and Palli D: A
PALB2 germline mutation associated with hereditary breast cancer in
Italy. Fam Cancer. 9:181–185. 2010. View Article : Google Scholar
|
|
28
|
Zhang F, Ma J, Wu J, Ye L, Cai H, Xia B
and Yu X: PALB2 links BRCA1 and BRCA2 in the DNA-damage response.
Curr Biol. l19:524–529. 2009. View Article : Google Scholar
|
|
29
|
Buisson R, Dion-Côté AM, Coulombe Y,
Launay H, Cai H, Stasiak AZ, Stasiak A, Xia B and Masson JY:
Cooperation of breast cancer proteins PALB2 and piccolo BRCA2 in
stimulating homologous recombination. Nat Struct Mol Biol.
17:1247–1254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dray E, Etchin J, Wiese C, Saro D,
Williams GJ, Hammel M, Yu X, Galkin VE, Liu D, Tsai MS, Sy SM,
Schild D, Egelman E, Chen J and Sung P: Enhancement of RAD51
recombinase activity by the tumor suppressor PALB2. Nat Struct Mol
Biol. 17:1255–1259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bleuyard JY, Buisson R, Masson JY and
Esashi F: ChAM, a novel motif that mediates PALB2 intrinsic
chromatin binding and facilitates DNA repair. EMBO Rep. 13:135–141.
2012. View Article : Google Scholar
|
|
32
|
Southey MC, Southey MC, Teo ZL and Winship
I: PALB2 and breast cancer: ready for clinical translation! Appl
Clin Genet. 6:43–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vietri MT, Molinari AM, Laura De Paola M,
Cantile F, Fasano M and Cioffi M: Identification of a novel
in-frame deletion in BRCA2 and analysis of variants of BRCA1/2 in
Italian patients affected with hereditary breast and ovarian
cancer. Clin Chem Lab Med. 50:2171–2180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pern F, Bougdanova N, Schurmann P, Lin M,
Ay A, Langer F, Hillemanns P, Christiansen H, Park-Simon TW and
Dörk T: Mutation analysis of BRCA1, BRCA2, PALB2 and BRD7 in a
hospital-based series of German patients with triple negative
breast cancer. PLoS One. 7:e479932012. View Article : Google Scholar
|