Introduction
The treatment of metastatic, locally unresectable
renal cell carcinoma (RCC) remains a challenge for urologists; one
reason is the poor response rate of the disease to many therapeutic
approaches, such as chemotherapy or radiotherapy. Although targeted
molecular approaches including vascular endothelial growth factor
monoclonal antibodies, tyrosine kinase inhibitors and mammalian
target of rapamycin inhibitors that have shown promising results,
the overall response rates remain low (1). Thus, drugs targeting new signal
transduction pathways are desired.
Ubenimex (Bestatin) is a low-molecular-weight
dipeptide molecule that enhances the function of immunocompetent
cells and has diverse effects on the production of cytokines. It is
also known as an inhibitor of aminopeptidase N (APN), which is
identical to the cell surface molecule CD13 (2). APN is involved in various cellular
processes, including cell cycle control, cell differentiation and
motility, angiogenesis, cellular attachment and invasion/metastasis
of various malignancies (3).
Previous studies have shown that ubenimex has antitumor activity.
It inhibits the invasion of human metastatic tumor cells and
induces apoptosis in lung cancer and leukemic cell lines (4–6). In
tumor-bearing mice, ubenimex inhibits metastasis or tumor growth
and prolongs survival (7,8). In clinical studies, the drug has shown
beneficial effects in the treatment of leukemia, non-small cell
lung cancer, gastric cancer and cervical cancer (9–12). A
potential therapeutic effect of ubenimex in RCC is suggested by the
5-year remission in a case of residual lymph node metastasis of
RCC, following postoperative single administration of ubenimex
(13). However, there is little
data concerning the mechanism of ubenimex in suppressing tumor
cells in RCC. The purpose of the present study was to determine the
effects of ubenimex on the proliferation, migration and invasion of
RCC cells and the possible mechanism.
Materials and methods
Cell culture
The 786-O and OS-RC-2 RCC cell lines were purchased
from the cell bank of the Chinese Academy of Sciences. Cells were
maintained in RPMI-1640 (HyClone Biotechnology, Carlsbad, CA, USA)
supplemented with penicillin, streptomycin and 10% FBS. The cells
were incubated at 37 °C in a humidified atmosphere with 5%
CO2.
Western blotting
To determine the expression of APN, proteins were
extracted from the cells or tissues by suspension in RIPA buffer.
Samples were centrifuged at 15,000 rpm at 4°C for 10 min and the
supernatants were recovered for analysis. The protein
concentrations were determined using the Bradford protein method
and the BCA protein assay kit (Sigma, St. Louis, MO, USA). Protein
(40 μg) was electrophoresed on a pre-cast Bis-Tris polyacrylamide
gel (8%) and then transferred to a PVDF membrane. The membranes
were blotted with rabbit anti-APN (1:1,000; Epitomics
Biotechnology, Burlingame, CA, USA), rabbit anti-LC3B (1:1,000;
Sigma) rabbit anti-Caspase3 (1:1,000, Abgent; San Diego, CA, USA)or
mouse anti-GAPDH (1:3,000 TA08; ZsBio), followed by horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:5,000; ZB2306
and ZB2301; both from ZsBio, Beijing, China). Immunoblots were
visualized by enhanced chemiluminescence (LAS4000).
Enzyme activity assay
The APN activity in the RCC cells was detected
spectrophotometrically using L-leucine-p-nitroanilide (Peptide
Institute, Inc., Osaka, Japan) as an APN substrate. Cells
(5×104) were incubated in a 96 well microtiter plate
with 0.1, 0.25 or 0.5 mg/ml ubenimex at 37°C for 24 h. After
culture, the medium was aspirated, the cells were washed with PBS
and then 200 μl of 1 mM alanine-p-nitroanilide was added per well.
Each well was then incubated at 37°C for 60 min. The APN enzyme
activity was estimated by measuring the absorbance at 405 nm using
a microplate reader (Labsystems, Multiskan Bichromatic, Helsinki,
Finland).
Growth curve analysis
Cells were trypsinized and 1.0×104 cells
were plated in individual wells of a 24-well plate containing
RPMI-1640 with 10% FBS. Cells were treated with 0.1, 0.25 or 0.5
mg/ml ubenimex. Every 24 h, the medium was removed, adherent cells
were trypsinized and the total number of adherent cells in each
well was quantified using a hematocytometer. The cell counts for 3
wells/time-point were averaged for each group and the data were
used to draw growth curves.
WST-8 cell proliferation assay
Cells in an exponential phase of growth were
harvested and seeded in 96-well plates at a density of 3,000
cells/well in RPMI-1640 supplemented with different concentrations
of ubenimex. After a 24 or 48 h culture, a 10 μl WST-8 solution
(WST-8 cell proliferation and cytotoxicity assay kit; Dojindo,
Kumamoto, Japan) was added into each well. Plates were then
incubated for an additional 1 h at 37°C and the absorbance was
determined using a microplate reader (EL340 Bio-Tek Instruments,
Hopkinton, MA, USA) at 450 nm.
Wound healing migration assays
The RCC cells were plated in 6-well culture plates
and grown to ~100% confluency before scratching with a sterile P200
pipette tip across the monolayer. The cell debris were removed by
being washed with PBS and the cells were cultured in RPMI-1640 and
2% FBS supplemented with different concentrations of ubenimex. The
area of the scratch was measured at 0, 12 and 18 h and
quantification was performed by measuring the area of cell
migration at different time points compared to the scratch area at
0 h. Each experiment was repeated 3 times.
Matrigel invasion assay
Invasion assays were performed using Transwell
chambers that were pre-coated with 40 μl of 1 mg/ml Matrigel matrix
(BD Bioscience, Bedford, MA, USA). Control untreated cells or cells
treated with ubenimex (0.25 mg/ml for 24 h) were trypsinized and
1.0×105 cells were plated in the upper wells in a
serum-free medium, while medium with 10% FBS was added to the lower
well as a stimuli. After 12 h of incubation, the cells on the
Matrigel side of the chambers were removed with a cotton swab. The
inserts were fixed in methanol and stained with H&E staining.
The number of the invading cells attached to the other side of the
inserts was counted under a light microscope using 8 random fields
at ×200 magnification. The experiment was performed in
triplicate.
LDH cytotoxicity assay
The levels of LDH release were assessed as a method
for determining the extent of cell death irrespectively of the type
of death. A 200 μl volume of cell suspension in complete medium
(5×103 cells/well) was dispensed in each well of a
96-well plate. Ubenimex (0.25 or 0.5 mg/ml), 3-MA (2 mM) and/or
rapamycin (0.1 μM) was added for 24 h. The 96-well plates were
centrifuged for 5 min at 400 × g and then 120 μl of the supernatant
from each well was transferred to a new plate. The plates were
incubated at room temperature for 30 min in the dark and then the
absorbance was spectrophotometrically measured at a wavelength of
560 nm.
Electron microscopy
The RCC cells were treated with 0.25 mg/ml ubenimex
for 12 h. They were then fixed with 3% glutaraldehyde and 2%
paraformaldehyde in a 0.1 M PBS buffer for 30 min, postfixed with
1% osmium tetroxide for 1.5 h, washed and stained in 3% aqueous
uranyl acetate for 1 h, then dehydrated in an ascending series of
ethanol and acetoned and embedded in Araldite. Ultrathin sections
were cut on a Reichert ultramicrotome, double stained with 0.3%
lead citrate and examined on a JEOL-1200EX electron microscope
(Jeol, Tokyo, Japan).
Human tissues
RCC tissues were obtained from 76 patients diagnosed
with RCC (median age, 63.8 years; range, 19–85) who were treated by
radical nephrectomy at Shandong Provincial Hospital (Jinan, China)
between July 2009 and January 2011. Formalin-fixed and
paraffin-embedded specimens were used in our analysis. Ten
specimens of non-neoplastic renal tissues were also obtained from
these patients. This study was approved by the Ethics Committee of
the Shandong Provincial Hospital.
Construction of tissue microarray blocks
and immunohistochemistry
Tissue microarrays were constructed using a manual
tissue arrayer (Beecher, Silver Spring, MD, USA). Three cylindrical
core biopsies (0.6-mm in diameter) were taken from different sites
of each tumor and precisely arrayed using a recipient paraffin
tissue microarray block. Ten specimens of the non-neoplastic renal
tissues were also resected from adjacent regions of the RCCs and
analyzed for comparison. To assess APN expression, 4-μm tissue
microarray sections were deparaffinized, rehydrated and
subsequently incubated with monoclonal rabbit antibodies targeting
human APN (1:500; Epitomics Biotechnology). Hematoxylin served as a
counter-stain. Incubation without the primary antibody was used as
a negative control. The expression of APN was evaluated by two
independent assessors at ×200 magnification and scored as follows:
−, negative; +, weak; ++, moderate and +++, strong.
Statistical analysis
Data were analyzed statistically by the Student’s
t-test, χ2 test or Fisher’s exact test and analysis was
performed using the Statistical Package for Social Science (SPSS
for Windows package release 10.0; SPSS Inc., Chicago, IL, USA).
P<0.05 was considered statistically significant.
Results
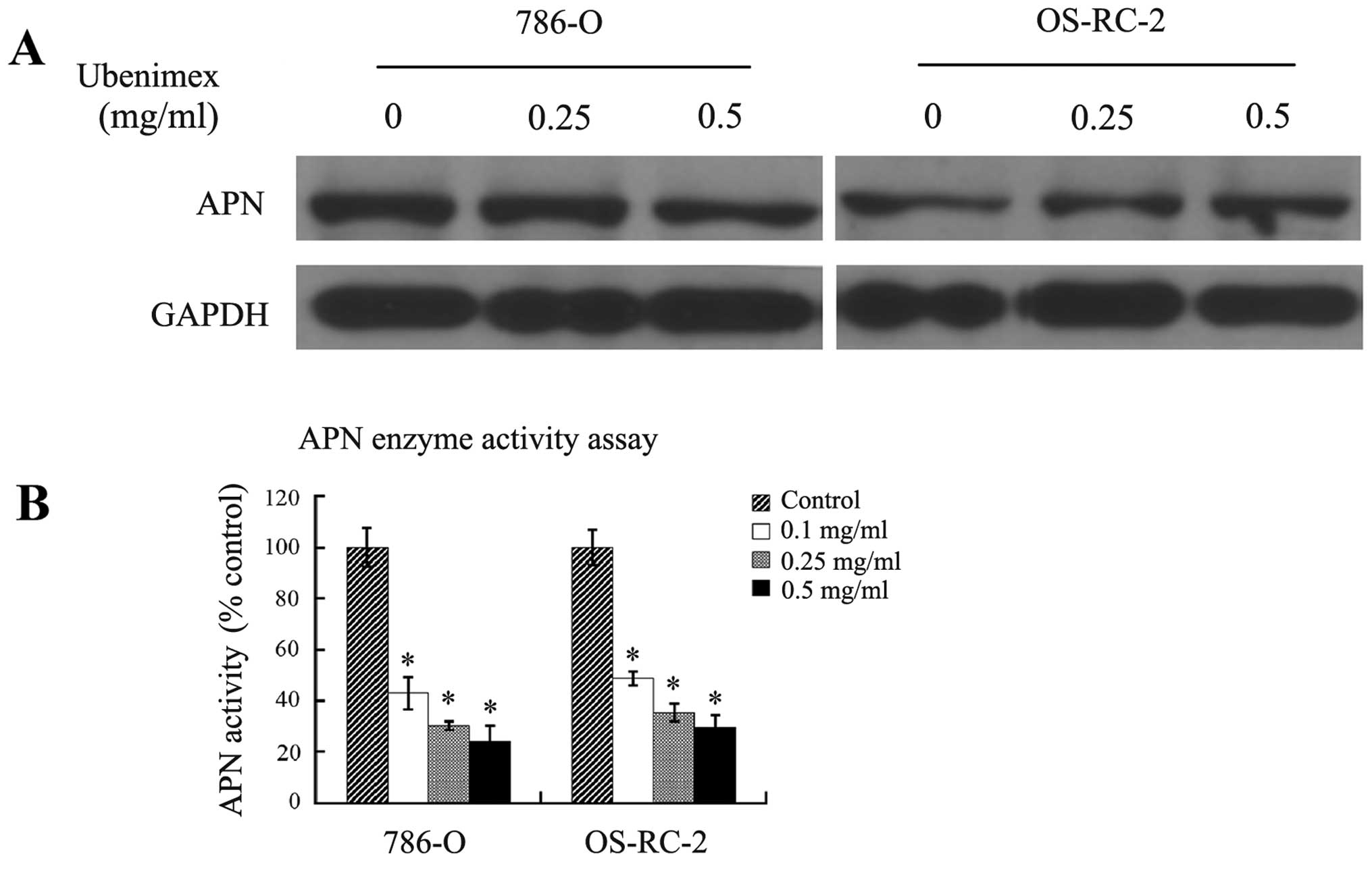
Ubenimex inhibits APN activity in RCC
cells without reducing expression
Ubenimex is known as an inhibitor of APN, which is
identical to the cell surface molecule CD13 (2). To confirm the expression of APN in two
RCC cell lines, 786-O and OS-RC-2, and to evaluate the effects of
ubenimex on APN expression and activity, we performed western
blotting and enzymatic activity assays. Both cell lines expressed
APN, although expression appeared to be consistently higher in the
786-O cells. Furthermore, the expression remained high after a 24-h
treatment with ubenimex (Fig. 1A).
Conversely, APN activity was reduced by ubenimex in a
dose-dependent manner (Fig. 1B).
These results verify that APN is expressed in the RCC cells and
suggest that ubenimex targets the activity, but not the expression
of APN.
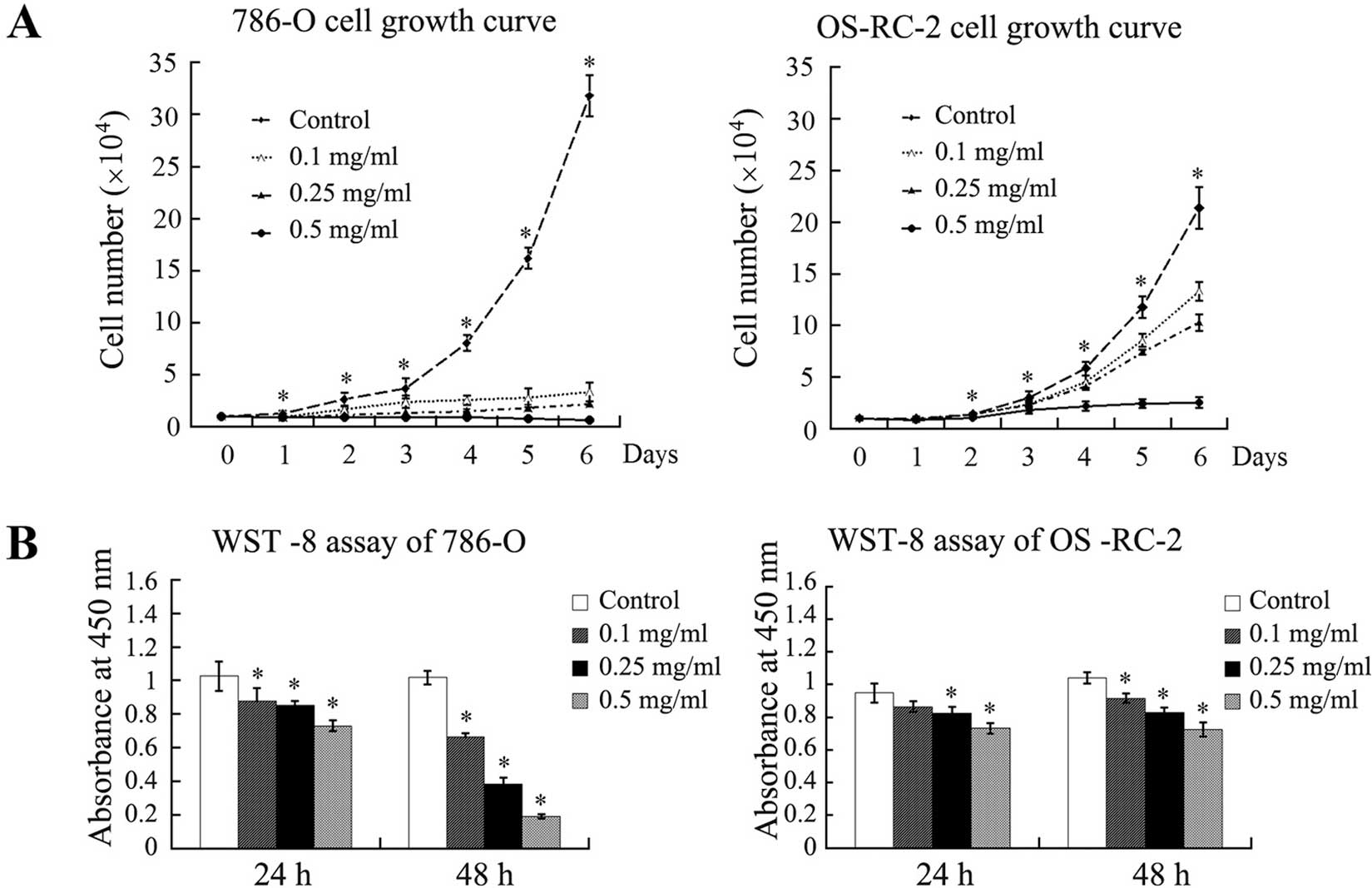
Ubenimex inhibits proliferation,
migration and invasion of the RCC cell lines
To examine the effects of ubenimex on the
proliferation of RCC cells, the 786-O and OS-RC cells were treated
with a range of concentrations of ubenimex and the cell growth was
assessed over a 6-day time course. The cell growth was
significantly decreased for both the cell lines in a
concentration-dependent manner, although the effect was more
obvious in the 786-O cells (Fig.
2A). These results were verified by the WST-8 assay after a 24-
and 48-h exposure to ubenimex (Fig.
2B).
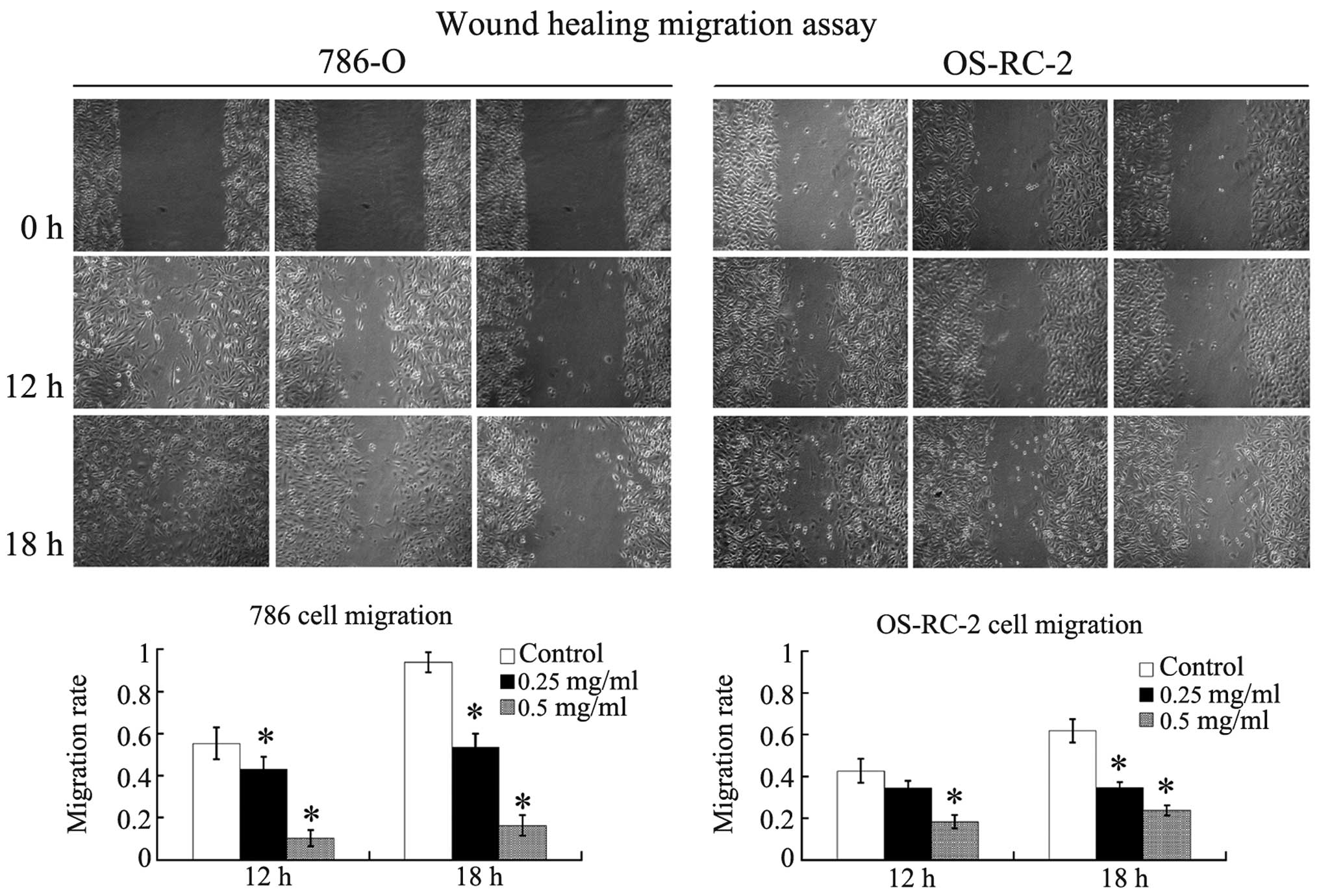
To determine whether ubenimex affects the migratory
ability of RCC cells, we performed scratch wound-healing migration
assays. The migratory abilities of the 786-O and OS-RC-2 cells were
significantly suppressed by ubenimex in a concentration-dependent
manner after a 12- or 18-h exposure (Fig. 3). We further examined the effect of
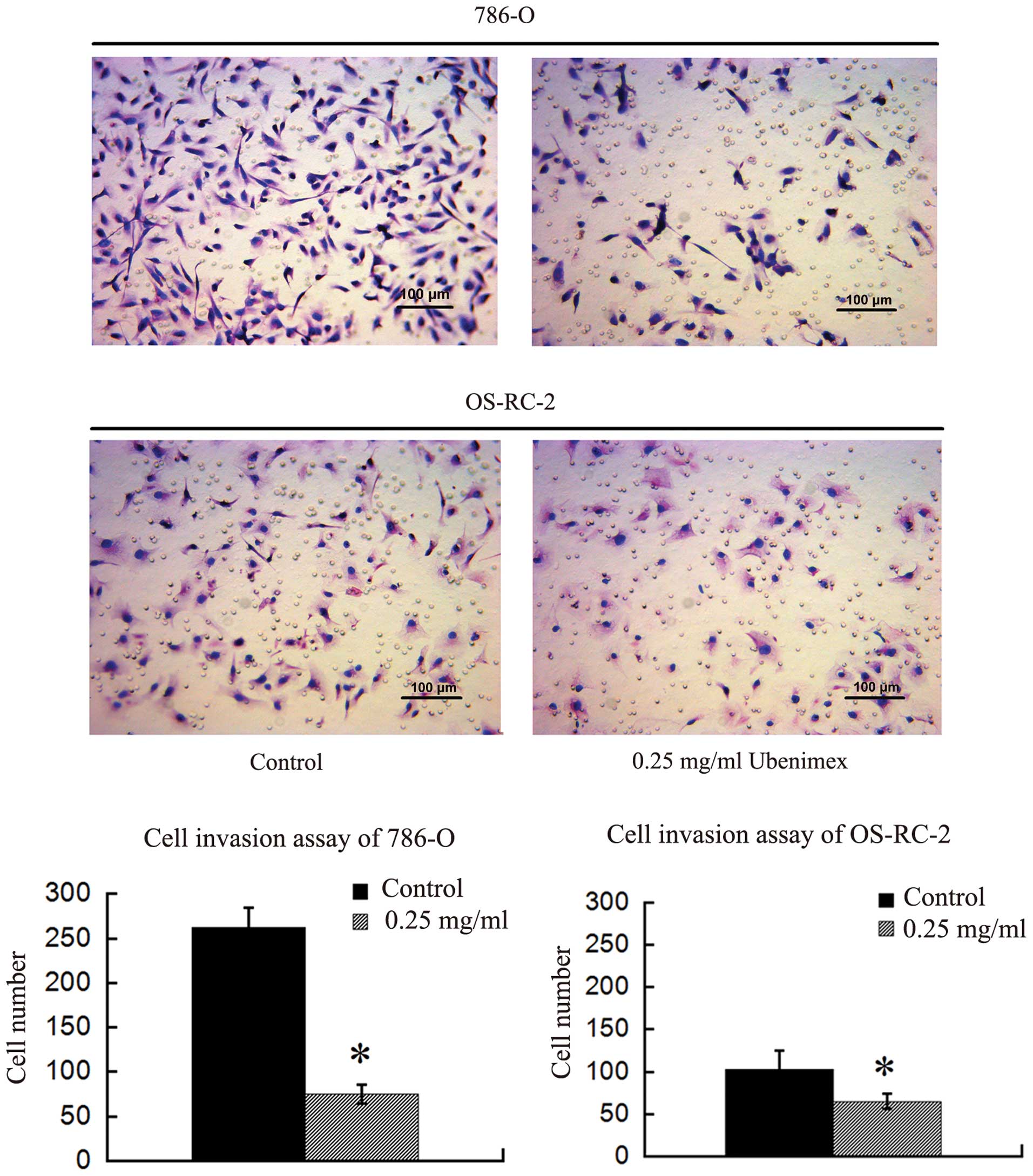
ubenimex on the invasion activity of RCC cells using Matrigel
invasion assays. Pretreatment of ubenimex markedly inhibited the
invasive abilities of the 786-O and OS-RC-2 cells, although once
again, the effects appeared more dramatic in the 786-O cells
(Fig. 4). Collectively, these
results suggest that ubenimex inhibits the tumorigenic properties
of RCC cells, including proliferation, migration and invasion of
RCC cells, although the extent of the effect may vary according to
the RCC cell line.
Ubenimex induces autophagic death of RCC
cells
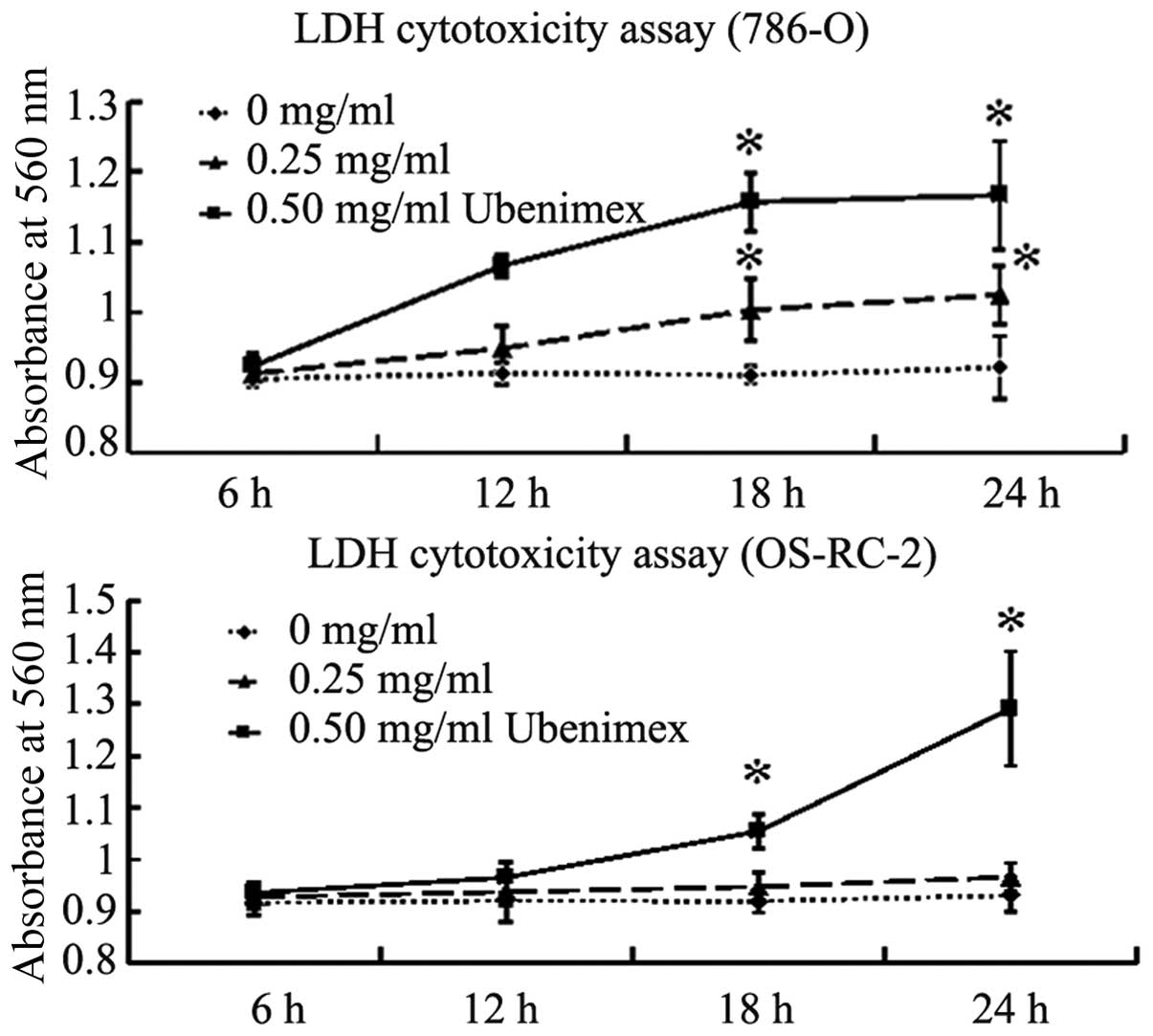
To determine whether ubenimex induces the cell death
of RCC cells, we performed LDH cytotoxicity assays following
ubenimex treatment. The LDH assay determines the extent of cell
death irrespectively of the type of cell death. The mortality of
both the RCC cells was significantly increased after treatment with
0.5 mg/ml ubenimex for 18 or 24 h; the mortality of the 786-O cells
was also significantly increased after treatment with 0.25 mg/ml
ubenimex for 18 or 24 h (Fig.
5).
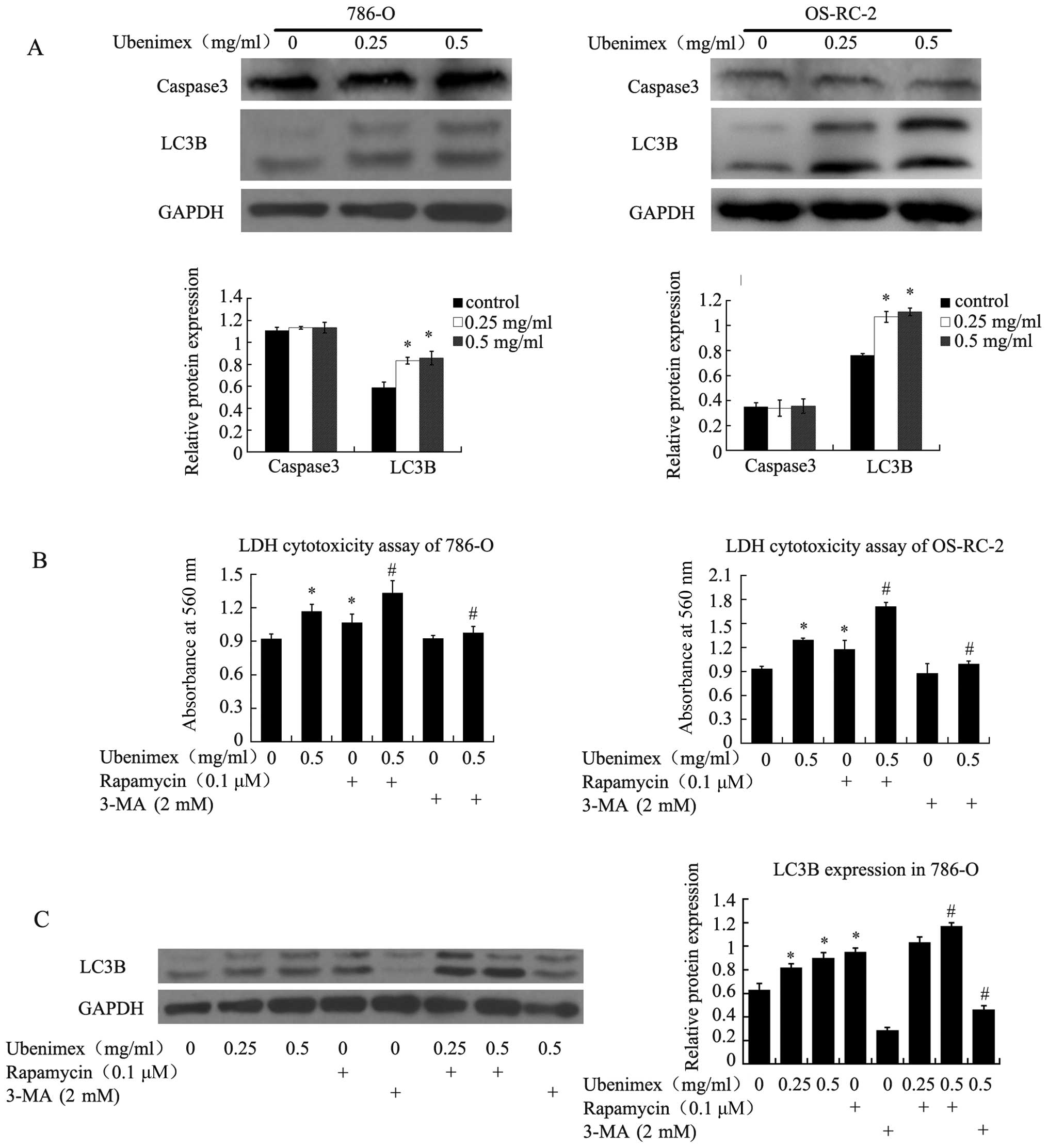
To investigate the mechanism of this effect, we
assessed the levels of an apoptosis marker (Caspase3) and an
autophagy marker (LC-3B). LC-3B expression increased after ubenimex
treatment, while the expression of Caspase3 remained unchanged
(Fig. 6A) These results indicated
that autophagic cell death, rather than apoptotic cell death may be
the predominate mode of ubenimex-induced RCC cell cytotoxicity. To
verify this hypothesis, an LDH cytotoxicity assay was performed
after pretreating the RCC cells with rapamycin (an inducer of
autophagy) or 3-methyladenine (an inhibitor of autophagy).
Rapamycin enhanced the levels of ubenimex-induced cell death while
3-methyladenine reversed the effect in both cell lines (Fig. 6B). Western blotting confirmed the
relationship between ubenimex and autophagy in 786-O cells
(Fig. 6C). These results indicate
that ubenimex promotes significant levels of cell death in RCC
cells and that the death occurs via an autophagic mechanism.
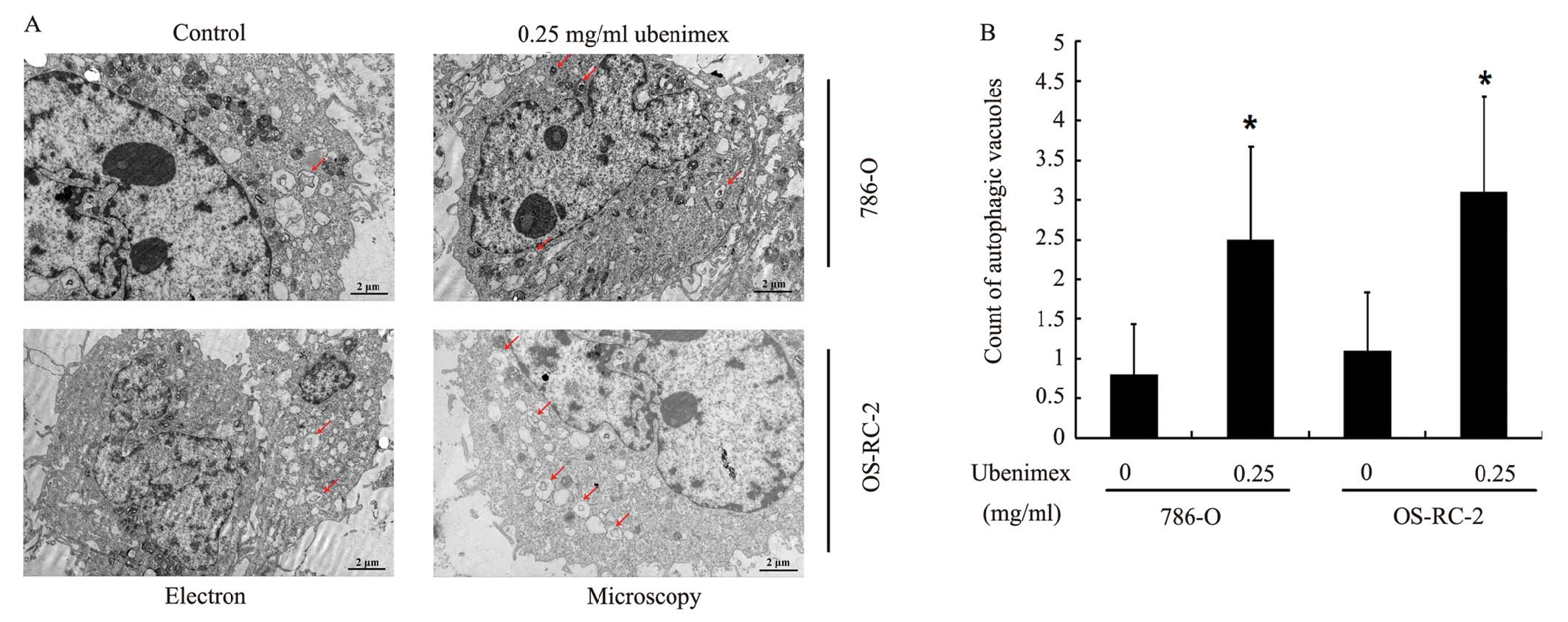
In order to confirm this theory, electron microscopy
was used to visualize the cell morphology after both cell lines
were treated with ubenimex compared to the control cells. Ubenimex
treatment increased the presence of autophagosomes filled with
debris in both the cell lines; only a few vacuoles were observed in
the control cells (Fig. 7A).
Quantification of the autophagosomes indicated higher autophagy
levels after ubenimex treatment (Fig.
7B).
APN is expressed in patient RCC tissues
at reduced levels
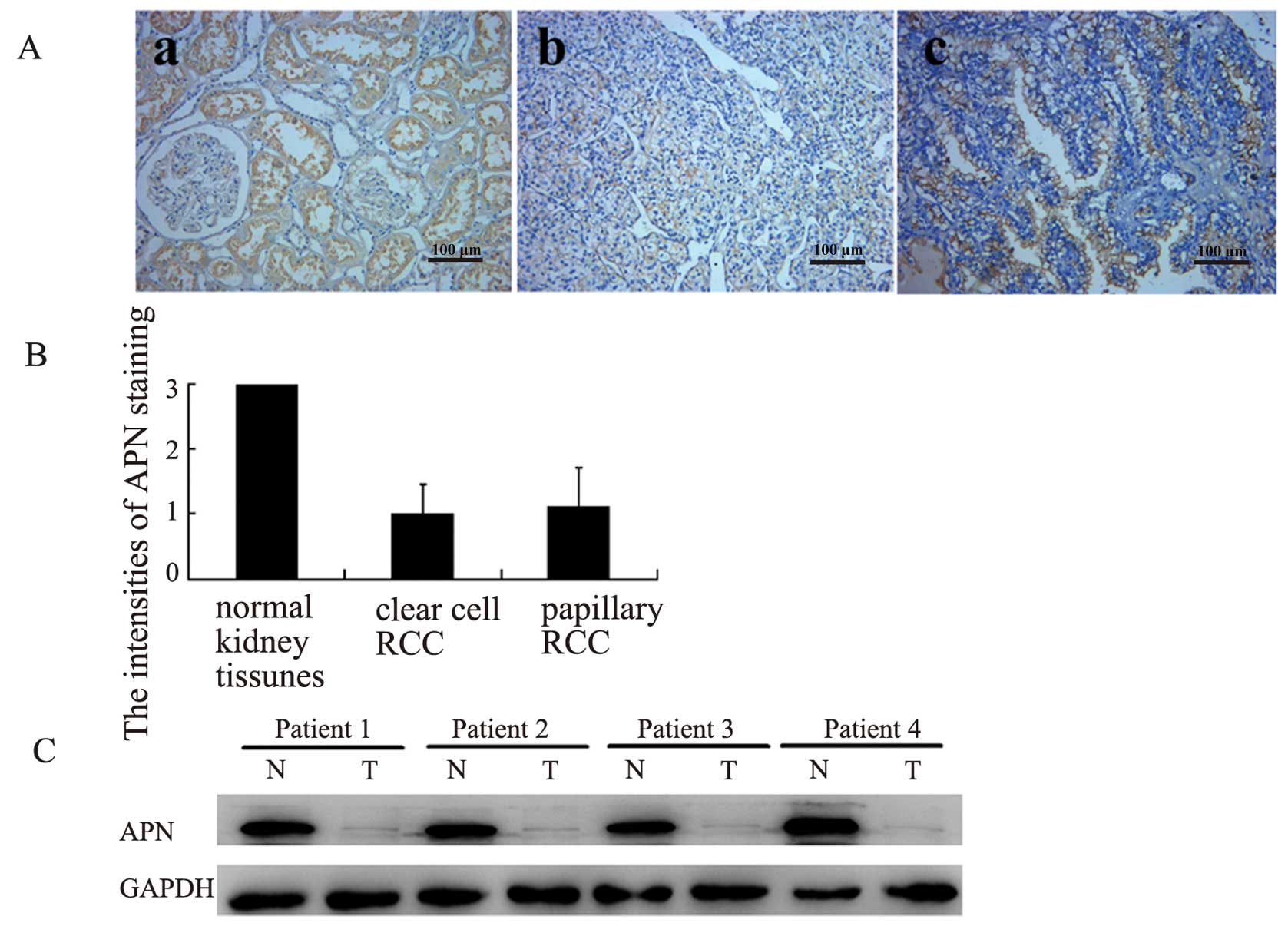
Immunohistochemical analysis showed strong
expression of APN in the epithelial cells of the renal proximal
tubules of the normal kidney tissue. Most of the RCC tumor tissues
(59/76, 77.6%) also showed positive staining, although the staining
was comparably weak (Fig. 8A and
B). Reduced expression of APN in the RCC tissue was verified by
western blotting of 4 representative normal adjacent kidney
tissues/RCC tissue pairs (Fig. 8C).
The intensity of APN was not significantly different between the
clear cells and the papillary RCC and was not associated with tumor
size grade or stage (Table I).
These results suggest that APN was expressed in the RCC, but the
levels of expression was reduced as compared to the normal tissues.
The differential expression of APN may be suggestive of a function
of this protein in RCC. Furthermore, the expression of APN in some
RCC tumors provides a rationale for considering ubenimex for the
treatment of those tumors that express APN, although the extent of
expression may limit the efficacy.
 | Table IImmunohistochemistry of APN in RCC
tissue microarray blocks. |
Table I
Immunohistochemistry of APN in RCC
tissue microarray blocks.
| | Intensity of APN
expression | |
|---|
| |
| |
|---|
| No. of patients | − | + | ++ | +++ | P-value |
|---|
| Histological
type |
| Clear cell | 67 | 15 | 37 | 15 | − | |
| Papillary | 9 | 2 | 4 | 3 | − | 0.576 |
| Tumor grade |
| 1 | 10 | 1 | 8 | 1 | − | |
| 2 | 54 | 14 | 27 | 13 | − | |
| 3,4 | 12 | 2 | 6 | 4 | − | 0.264 |
| Tumor stage |
| 1 | 40 | 9 | 19 | 10 | − | |
| 2 | 18 | 5 | 11 | 2 | − | |
| 3,4 | 18 | 3 | 11 | 4 | − | 0.565 |
| Tumor diameter
(cm) |
| ≤4 | 21 | 4 | 11 | 6 | − | |
| >4 and ≤7 | 28 | 9 | 14 | 5 | − | |
| >7 |
Discussion
Previous studies indicate that APN plays an
important role in the control of the growth and differentiation of
cancer cells (3). Inhibition of APN
expression or activity reduces the proliferation of various types
of cells (2). Here, we gained
insight into the effect of ubenimex treatment and the role of APN
activity in the growth and development of RCC cells.
Our data demonstrated that treatment of the 786-O
and OS-RC-2 cells with ubenimex had no effect on the APN expression
level, but decreased the APN enzyme activity. We also demonstrated
that ubenimex treatment significantly inhibited the RCC cell
growth, proliferation, migration and invasion, which may explain
its antitumorigenic properties. The effect of ubenimex was more
pronounced in the 786-O cells, which express higher amounts of APN.
These results suggest that the inhibitory effects of ubenimex may
be associated with its ability to block APN enzyme activity.
The role of APN in the cell motility and metastasis
of cancer cells is suggested to involve the degradation of
neuropeptides, cytokines and immunomodulatory peptides, as well as
angiotensins (6). Inhibition of APN
suppresses the progressive potential in many malignant solid tumor
cells (7,14–16).
The present study demonstrated that ubenimex inhibited RCC cell
migration and invasion, which is consistent with the role of APN in
these processes. The invasion activity of the RCC cell line SKRC-1,
which expresses moderate amounts of the APN, may also be suppressed
by ubenimex (17). Thus, these
results suggest that the APN may play a role in the metastasis of
RCC.
We also showed that ubenimex induced a
concentration-dependent cytotoxicity of the RCC cells. Several
studies have demonstrated a function of ubenimex in inducing cancer
cell apoptosis through activating Caspase3 (11,18,19);
however, in the present study, ubenimex had no significant effect
on the Caspase3 expression. Instead, LC-3B, which is a key protein
marker of autophagy-dependent cell death (20), was upregulated after ubenimex
treatment; the cytotoxic effect of ubenimex was attenuated after
blocking autophagy with 3-MA, indicating that ubenimex induces the
autophagic cell death of RCC cells; furthermore, the autophagy
occured after ubenimex treatment in both RCC lines, as evidenced by
electron microscopy. To our knowledge, this is the first
demonstration that ubenimex induces the autophagy in RCC cells.
Autophagy plays a significant role in tumorigenesis and it is the
basis of alternative trials evaluating the effectiveness of other
drug regimes (20–23). In many cases, the expression of
autophagy-related genes in cancer cells can inhibit cell
proliferation (24). Reduced levels
of autophagy liberate cancer cells from suppression and may further
accelerate their proliferation rate (25,26).
Therefore, our data are consistent with the possibility that growth
inhibition by ubenimex may partly be caused by autophagy.
To assess the feasibility of using ubenimex to treat
primary human RCC, we also assessed the APN levels in the RCC
tissues from patients undergoing nephrectomy. Immunohistochemistry
and western blotting showed that APN was expressed in most RCC
tumors, but at lower levels than in the surrounding tissues.
Although we did not observe any correlations between APN expression
and the clinicopathological parameters, downregulation of APN in
the tumor tissues compared with normal kidney tissues may suggest a
potential role for APN in RCC development. Studies concerning
several other cancer types indicate that APN functions in a highly
cell type- and context-specific manner: high expression is an
adverse prognostic factor for non-small cell lung cancer,
pancreatic and colon cancer (27–29),
but low APN expression is associated with aggressive disease in
meningioma, gastric cancer and prostate cancer (17,30,31).
Further studies are needed to determine the physiological effects
of APN down-regulation in RCC and the potential efficacy of the
ubenimex in treating RCC.
In conclusion, the present study demonstrated that
ubenimex inhibits proliferation, migration and invasion of RCC
cells. Ubenimex may induce autophagy, which may partly explain its
effect on growth arrest and cell death. Further studies on APN
function and the effect of ubenimex treatment in RCC will provide
insight into the mode of action of ubenimex in RCC.
Acknowledgements
This study was supported by the Science and
Technology Development Plan Project of Shandong Province, P.R.
China.
References
|
1
|
Su D, Stamatakis L, Singer EA and
Srinivasan R: Renal cell carcinoma: molecular biology and targeted
therapy. Curr Opin Oncol. 26:321–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hitzerd SM, Verbrugge SE, Ossenkoppele G,
Jansen G and Peters GJ: Positioning of aminopeptidase inhibitors in
next generation cancer therapy. Amino Acids. 46:793–808. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wickström M, Larsson R, Nygren P and
Gullbo J: Aminopeptidase N (CD13) as a target for cancer
chemotherapy. Cancer Sci. 102:501–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishii K, Usui S, Sugimura Y, Yoshida S,
Hioki T, Tatematsu M, Yamamoto H and Hirano K: Aminopeptidase N
regulated by zinc in human prostate participates in tumor cell
invasion. Int J Cancer. 92:49–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fontijn D, Duyndam MC, van Berkel MP,
Yuana Y, Shapiro LH, Pinedo HM, Broxterman HJ and Boven E:
CD13/Aminopeptidase N overexpression by basic fibroblast growth
factor mediates enhanced invasiveness of 1F6 human melanoma cells.
Br J Cancer. 94:1627–1636. 2006.PubMed/NCBI
|
|
6
|
Mina-Osorio P: The moonlighting enzyme
CD13: old and new functions to target. Trends Mol Med. 14:361–371.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terauchi M, Kajiyama H, Shibata K, Ino K,
Nawa A, Mizutani S and Kikkawa F: Inhibition of APN/CD13 leads to
suppressed progressive potential in ovarian carcinoma cells. BMC
Cancer. 7:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin-Padura I, Marighetti P, Agliano A,
Colombo F, Larzabal L, Redrado M, Bleau AM, Prior C, Bertolini F
and Calvo A: Residual dormant cancer stem-cell foci are responsible
for tumor relapse after antiangiogenic metronomic therapy in
hepatocellular carcinoma xenografts. Lab Invest. 92:952–966. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ichinose Y, Genka K, Koike T, Kato H,
Watanabe Y, Mori T, Iioka S, Sakuma A and Ohta M: NK421 Lung Cancer
Surgery Group: Randomized double-blind placebo-controlled trial of
bestatin in patients with resected stage I squamous-cell lung
carcinoma. J Natl Cancer Inst. 95:605–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wakita A, Ohtake S, Takada S, Yagasaki F,
Komatsu H, Miyazaki Y, Kubo K, Kimura Y, Takeshita A, Adachi Y,
Kiyoi H, Yamaguchi T, Yoshida M, Ohnishi K, Miyawaki S, Naoe T,
Ueda R and Ohno R: Randomized comparison of fixed-schedule versus
response-oriented individualized induction therapy and use of
ubenimex during and after consolidation therapy for elderly
patients with acute myeloid leukemia: the JALSG GML200 Study. Int J
Hematol. 96:84–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsukamoto H, Shibata K, Kajiyama H,
Terauchi M, Nawa A and Kikkawa F: Aminopeptidase N (APN)/CD13
inhibitor, Ubenimex, enhances radiation sensitivity in human
cervical cancer. BMC Cancer. 8:742008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu JW, Li CG, Huang XE, Li Y and Huo JG:
Ubenimex capsule improves general performance and chemotherapy
related toxicity in advanced gastric cancer cases. Asian Pac J
Cancer Prev. 12:985–987. 2011.PubMed/NCBI
|
|
13
|
Sugano O, Muto A, Kato M and Ono K: A case
of renal cell carcinoma with lymph node metastasis keeping
remission for five years by adjuvant immunotherapy with ubenimex.
Gan To Kagaku Ryoho. 30:1519–1522. 2003.(In Japanese). PubMed/NCBI
|
|
14
|
Saitoh Y, Koizumi K, Minami T, Sekine K,
Sakurai H and Saiki I: A derivative of aminopeptidase inhibitor
(BE15) has a dual inhibitory effect of invasion and motility on
tumor and endothelial cells. Biol Pharm Bull. 29:709–712. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mawrin C, Wolke C, Haase D, Krüger S,
Firsching R, Keilhoff G, Paulus W, Gutmann DH, Lal A and Lendeckel
U: Reduced activity of CD13/aminopeptidase N (APN) in aggressive
meningiomas is associated with increased levels of SPARC. Brain
Pathol. 20:200–210. 2010. View Article : Google Scholar
|
|
16
|
Inagaki Y, Tang W, Zhang L, Du G, Xu W and
Kokudo N: Novel aminopeptidase N (APN/CD13) inhibitor 24F can
suppress invasion of hepatocellular carcinoma cells as well as
angiogenesis. Biosci Trends. 4:56–60. 2010.PubMed/NCBI
|
|
17
|
Ishii K, Usui S, Sugimura Y, Yamamoto H,
Yoshikawa K and Hirano K: Inhibition of aminopeptidase N (AP-N) and
urokinase-type plasminogen activator (uPA) by zinc suppresses the
invasion activity in human urological cancer cells. Biol Pharm
Bull. 24:226–230. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sekine K, Fujii H and Abe F: Induction of
apoptosis by bestatin (ubenimex) in human leukemic cell lines.
Leukemia. 13:729–734. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sekine K, Fujii H, Abe F and Nishikawa K:
Augmentation of death ligand-induced apoptosis by aminopeptidase
inhibitors in human solid tumor cell lines. Int J Cancer.
94:485–491. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eum KH and Lee M: Crosstalk between
autophagy and apoptosis in the regulation of paclitaxel-induced
cell death in v-Ha-ras-transformed fibroblasts. Mol Cell Biochem.
348:61–68. 2011. View Article : Google Scholar
|
|
21
|
Natsumeda M, Aoki H, Miyahara H, Yajima N,
Uzuka T, Toyoshima Y, Kakita A, Takahashi H and Fujii Y: Induction
of autophagy in temozolomide treated malignant gliomas.
Neuropathology. 31:486–493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Evangelisti C, Ricci F, Tazzari P,
Tabellini G, Battistelli M, Falcieri E, Chiarini F, Bortul R,
Melchionda F, Pagliaro P, Pession A, McCubrey JA and Martelli AM:
Targeted inhibition of mTORC1 and mTORC2 by active-site mTOR
inhibitors has cytotoxic effects in T-cell acute lymphoblastic
leukemia. Leukemia. 25:781–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crowley LC, Elzinga BM, O’Sullivan GC and
McKenna SL: Autophagy induction by Bcr-Abl-expressing cells
facilitates their recovery from a targeted or nontargeted
treatment. Am J Hematol. 86:38–47. 2011. View Article : Google Scholar
|
|
24
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mathew R, Kongara S, Beaudoin B, Karp CM,
Bray K, Degenhardt K, Chen G, Jin S and White E: Autophagy
suppresses tumor progression by limiting chromosomal instability.
Genes Dev. 21:1367–1381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karantza-Wadsworth V, Patel S, Kravchuk O,
Chen G, Mathew R, Jin S and White E: Autophagy mitigates metabolic
stress and genome damage in mammary tumorigenesis. Genes Dev.
21:1621–1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hashida H, Takabayashi A, Kanai M, Adachi
M, Kondo K, Kohno N, Yamaoka Y and Miyake M: Aminopeptidase N is
involved in cell motility and angiogenesis: its clinical
significance in human colon cancer. Gastroenterology. 122:376–386.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ikeda N, Nakajima Y, Tokuhara T, Hattori
N, Sho M, Kanehiro H and Miyake M: Clinical significance of
aminopeptidase N/CD13 expression in human pancreatic carcinoma.
Clin Cancer Res. 9:1503–1508. 2003.PubMed/NCBI
|
|
29
|
Tokuhara T, Hattori N, Ishida H, Hirai T,
Higashiyama M, Kodama K and Miyake M: Clinical significance of
aminopeptidase N in non-small cell lung cancer. Clin Cancer Res.
12:3971–3978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawamura J, Shimada Y, Kitaichi H, Komoto
I, Hashimoto Y, Kaganoi J, Miyake M, Yamasaki S, Kondo K and
Imamura M: Clinicopathological significance of aminopeptidase
N/CD13 expression in human gastric carcinoma.
Hepatogastroenterology. 54:36–40. 2007.PubMed/NCBI
|
|
31
|
Sørensen KD, Abildgaard MO, Haldrup C,
Ulhøi BP, Kristensen H, Strand S, Parker C, Høyer S, Borre M and
Ørntoft TF: Prognostic significance of aberrantly silenced ANPEP
expression in prostate cancer. Br J Cancer. 108:420–428. 2013.
View Article : Google Scholar : PubMed/NCBI
|