Introduction
Glioblastoma multiforme (GBM) is the most common and
malignant primary tumor that develops in the central nervous
system. Despite advances in surgery and adjuvant therapy, the
overall 5-year survival rate of GBM remains less than 5% and is
even worse for elderly patients due to the highly invasive ability
of these cancer cells (1,2).
Hypoxia-inducible factor 1α (HIF-1α) and
hypoxia-responsive genes play important roles in glioma migration
and invasion (3–5). Hypoxia is important in the biology and
aggression of human glial brain tumors (6,7).
Although HIF-1α is maintained at a low level in normoxic cells due
to the degradation mediated by the proteasome (8), it plays a critical role in the
cellular response to tumor hypoxia, which may be a major obstacle
to the killing of cancer cells by radiotherapy and chemotherapy
(6). It has been reported that
HIF-1α is upregulated in a variety of human cancers, including
prostate (9), breast (10) and glioma (3). Inhibition of HIF-1α expression with
antisense oligonucleotides was found to decrease the survival of
glioblastoma cells and induce p53-independent apoptosis (11). Moreover, silencing of HIF-1α by RNA
interference reduced human glioma migration and invasion (12,13).
Although the function of HIF-1α has been widely studied under
hypoxic stress, the mechanism regulating the activation of HIF-1α
is still unclear, and should be further understood for designing
more effective therapeutic strategies.
The protein seven in absentia (SINA) was firstly
discovered in R7 cells, and functions in the cell fate in the
Drosophila eye (14). Two
homologs of the SINA gene have been identified in humans, Siah1 and
Siah2 (15). Siah1 and Siah2 have
been shown to dominate the stability of several substrates,
including nuclear corepressor, β-catenin, TRAF2, α-ketoglutarate
dehydrogenase and prolyl hydroxylase 3 (PHD3), thereby affecting
diverse cellular processes, such as signaling, survival and
mitochondrial biogenesis (16–20).
In addition to the physiological function, Siah1 is found to be
involved in several types of cancer. It has been reported that
Siah1 plays a role as a tumor suppressor in hepatocellular
carcinomas and breast cancer (21,22).
However, in nasopharyngeal carcinoma, Siah1 has been reported as an
oncogene and is significantly correlated with advanced tumor status
and stage (23). To date, the role
of Siah1 in human glioma has not been elucidated.
In the present study, we investigated the role and
intracellular signaling pathway of Siah1 in the aggressive behavior
of glioma under hypoxic stress. We found that Siah1 levels in
glioma tissues were significantly upregulated, suggesting a
potential role of this protein in the progression of gliomas. Then,
we observed that the knockdown of Siah1 inhibited the migration and
invasion of human glioma cells under hypoxia, while overexpression
of Siah1 promoted it. Furthermore, we demonstrated that the
Siah1-regulated migration and invasion of human glioma cells under
hypoxia were mediated by decreasing the stability of PHD3, thereby
stabilizing the HIF-1α.
Materials and methods
Tissue samples
Six specimens of non-tumorous brain tissues
(internal decompression in cerebral trauma) and 16 specimens of
glioma tissues were obtained from the Affiliated Hospital of Xuzhou
Medical College (Xuzhou, China). Surgically removed tissues were
sampled for histological diagnosis, and the remaining tissues were
immediately frozen in liquid nitrogen and stored at −80°C. The
present study was approved by the Research Ethics Committee of
Xuzhou Medical College, and informed consent was provided by the
patients.
Cell culture
Cell lines (293T and U251) were purchased from the
Cell Bank of the Shanghai Institutes of the Chinese Academy of
Sciences and were cultured in Dulbecco’s modified Eagle’s medium
(DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Evergreen Biological Engineering Co., Hangzhou,
China) at 37°C in 5% CO2. For hypoxia, the cells were
cultured in a modular incubator chamber (150i; Thermo Fisher
Scientific, Waltham, MA, USA) at 37°C with 5% CO2, 1%
O2 and 94% N2.
Constructs and production of the
lentivirus
For overexpression of Siah1, the Siah1 cDNA was
inserted into the pWPXLd plasmid using BamHI and MluI
sites. For silencing of Siah1, two short hairpin RNA (shRNA)
duplexes were designed as follows: shSiah1A F,
TGATAGGAACACGCAAGCAATTCAAGAGATTGCTTGCGTGTTCCTATCTTTTTTC and
shSiah1A R,
TCGAGAAAAAAGATAGGAACACGCAAGCAATCTCTTGAATTGCTTGCGTGTTCCTATCA;
shSiah1B F, TCACACCTTTGAGCTTAATCTTCAAGAGAGATTAAGCTCAAAGGTGTGTTTTTTC
and shSiah1B R,
TCGAGAAAAAACACACCTTTGAGCTTAATCTCTCTTGAAGATTAAGCTCAAAGGTGTGA. The
shRNA oligomers and non-targeting oligomers (scramble) were
annealed and then subcloned into the pLL3.7 plasmid by the
HpaI and XhoI cloning site. Cell transfection was
performed with PolyJET (SignaGen, Gaithersburg, MD, USA) as
described in the manufacturer’s protocol. The viruses were
propagated in 293T cells by cotransfecting the corresponding
plasmids with the helper plasmids.
Establishment of the stable cell
lines
The formation of the stable cell lines was
previously described (24). For
stable overexpression of Siah1, the U251 cells were infected by GFP
or GFP-Siah1 viruses, respectively. Forty-eight hours after
infection, the cells were continuously cultured in medium
containing 2.5 μg/ml puromycin (Sigma, St. Louis, MO, USA). The
surviving cells were cultured into cell lines stably expressing GFP
or GFP-Siah1. For the silencing of Siah1, the scramble or shSiah1
virus-infected U251 cells were subjected to the sorting of
GFP-positive cells by flow cytometry. GFP-positive cells were
cultured to produce stable lines silenced for Siah1.
Western blotting
Equal amounts of protein lysates were subjected to
10% SDS-PAGE and then transferred to a PVDF membrane (Millipore,
Billerica, MA, USA) and probed with the primary antibodies (Siah1,
PHD3, HIF-1α, β-actin) at 4°C overnight and the secondary
antibodies at room temperature for 1 h. Bound antibodies were
detected by the ECL Plus Western Blotting Substrate (Thermo Fisher,
Waltham, MA, USA) and exposed to X-ray film. Band densities were
quantified by ImageJ software. The relative amount of the proteins
was determined by normalization of the densitometry.
Wound healing assay
The stable cell lines were cultured in a 6-well
plate under normal conditions for 24 h and starved by no serum for
12 h. Then, scratches were performed in the middle of the wells
with a pipette tip. Thereafter, the cells were cultured under a
hypoxic condition (5% CO2, 1% O2 and 94%
N2) for 12 and 24 h. The images were captured by an
inverted microscope (IX71; Olympus, Tokyo, Japan) at the designated
time points. The number of cells crossing the wound was normalized
according to the control.
Invasion assay
Cell invasion was assessed using Matrigel-precoated
Transwell inserts (8.0-μm pore size with polyethylene
tetraphthalate membrane; Invitrogen) according to the
manufacturer’s protocol. The pretreated cell suspension
(1×105) in serum-free culture media was added into the
inserts, and each insert was placed in the lower chamber filled
with culture media containing 10% FBS as a chemoattractant. The
invasion chambers were incubated under a hypoxic condition for 24
h. Then, the non-invasive cells were removed from the upper
chamber; filters were fixed with methanol for 15 min and stained
with a 0.1% crystal violet solution for 10 min. Five fields of
adherent cells in each well were randomly photographed under an
inverted microscope and counted.
Statistical analysis
Data are presented as the means ± SEM. Statistical
significance was determined using the Student’s t-test, and
P<0.05 was considered to indicate a statistically significant
result.
Results
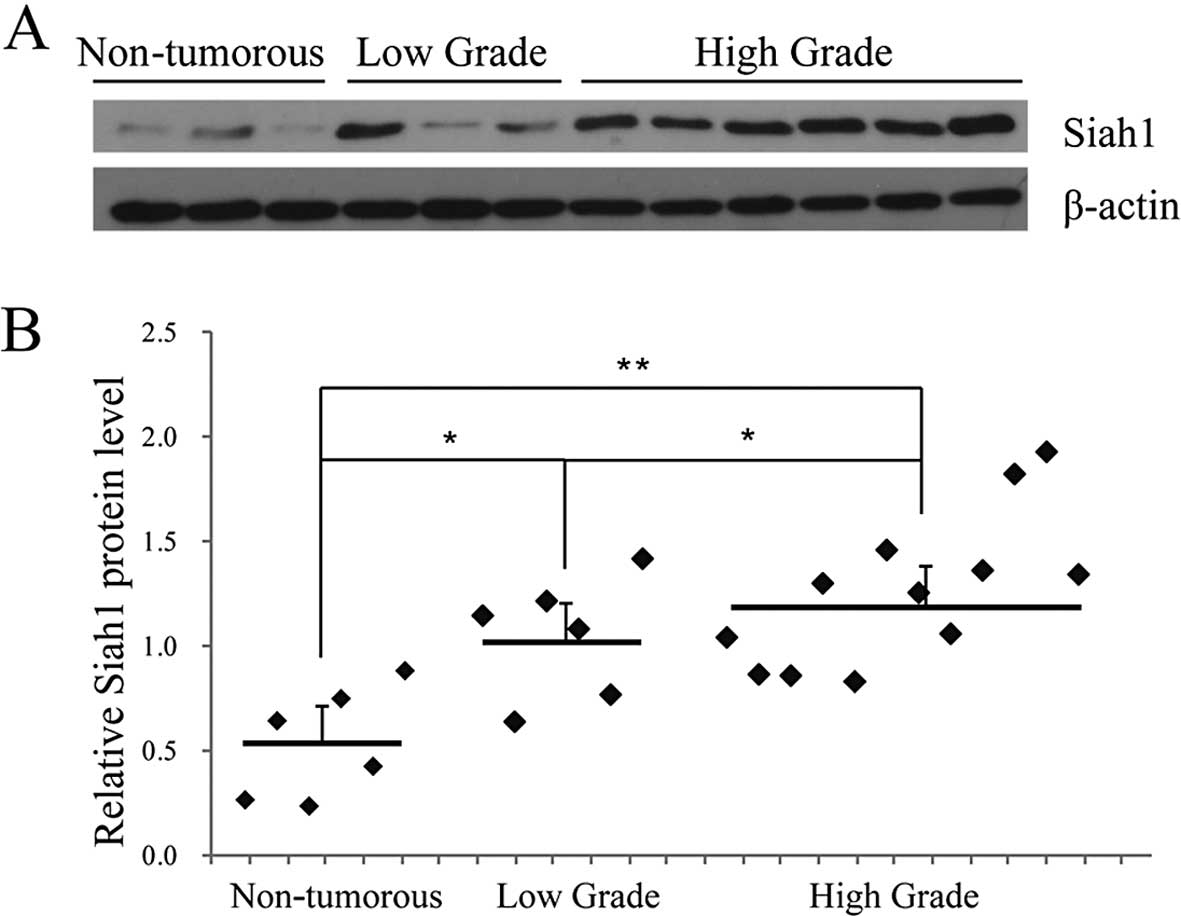
Siah1 is expressed increasingly in human
glioma tissues
In order to understand the possible role of Siah1 in
human glioma, we examined the Siah1 expression in 6 specimens of
non-tumorous brain tissues and 18 specimens of glioma tissues (6
low-grade and 12 high-grade) by western blotting. A representative
blot is shown in Fig. 1A. The
statistical results indicated that the protein level of Siah1 in
the glioma tissues was higher than that in the non-tumorous tissues
and was correlated with advanced tumor status and stage (Fig. 1A and B). These results suggest that
Siah1 expression is upregulated in human glioma, which provides us
with initial evidence that Siah1 plays a role in the development
and progression of human glioma.
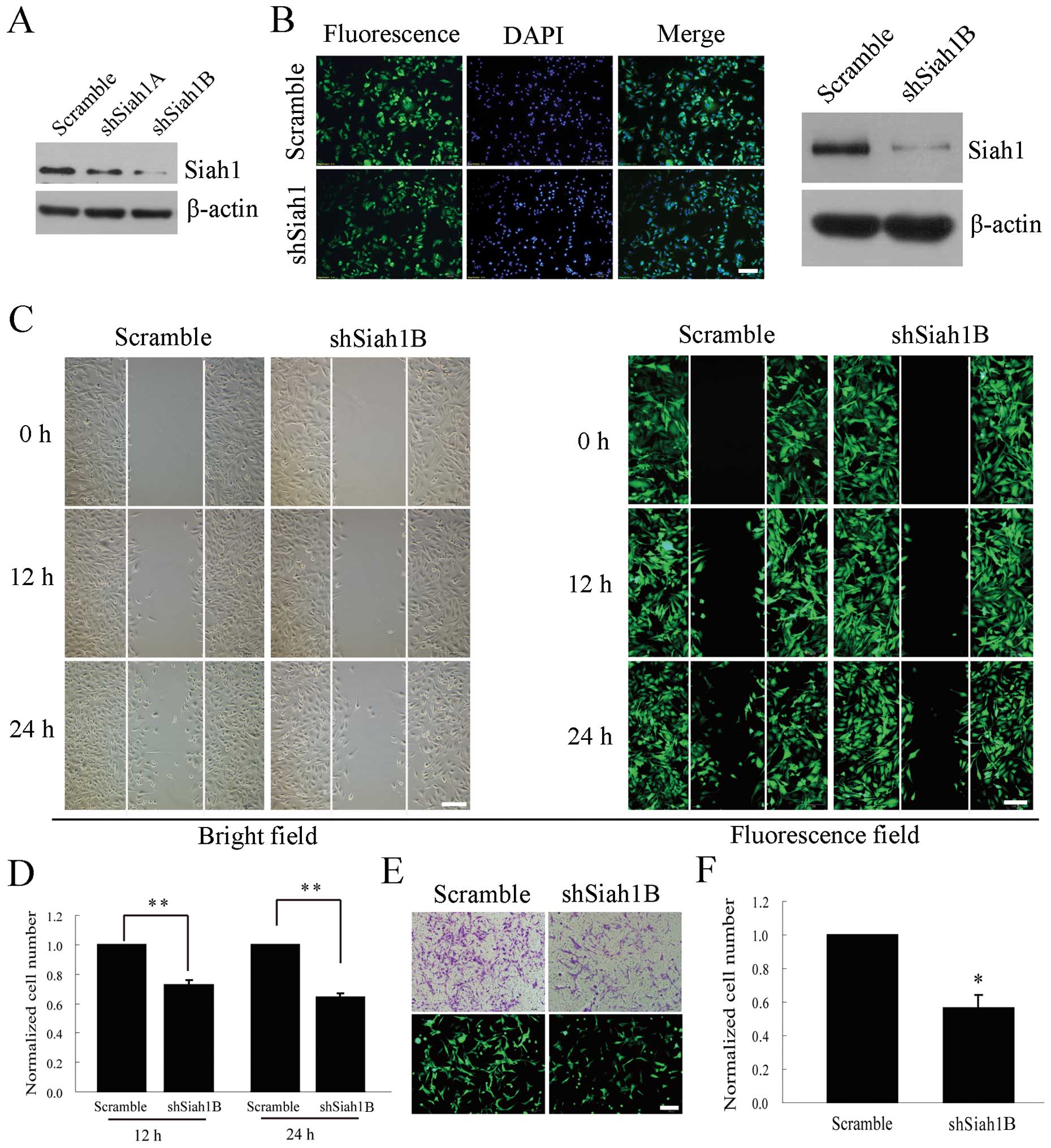
Downregulation of Siah1 inhibits glioma
cell migration and invasion under hypoxia
To investigate the possible role of Siah1 in the
development and progression of human glioma, we utilized loss-of-
and gain-of function approaches. Firstly, we downregulated Siah1
expression using its specific short hairpin RNA and observed the
effects on cell migration and invasion. For the silencing of Siah1,
two shRNA targets (shSiah1A and shSiah1B) were cloned into the
lentiviral vector pLL3.7 and screened for their efficacy in
suppressing Siah1 expression, and a negative control shRNA
(scramble) was used as a control. As shown in Fig. 2A, the silencing efficiency of
shSiah1B was ~80%. Thereafter, shSiah1B and the scramble were
packaged into the lentivirus in 293T cells and used to establish
the stable cell line with loss of Siah1 (Fig. 2B). Next, we investigated whether the
cell migration and invasion were affected by the silencing of Siah1
under hypoxia in the stable cell lines. The wound healing assay
showed that the number of migratory cells was reduced by 27 and 36%
at 12 and 24 h (Fig. 2C and D). The
Transwell assay showed that the invasive cells decreased by 43%,
compared with the corresponding controls (Fig. 2E and F). These results indicate that
Siah1 is involved in the migration and invasion of human glioma
cells and downregulation of Siah1 inhibits the glioma cell
migration and invasion under hypoxia.
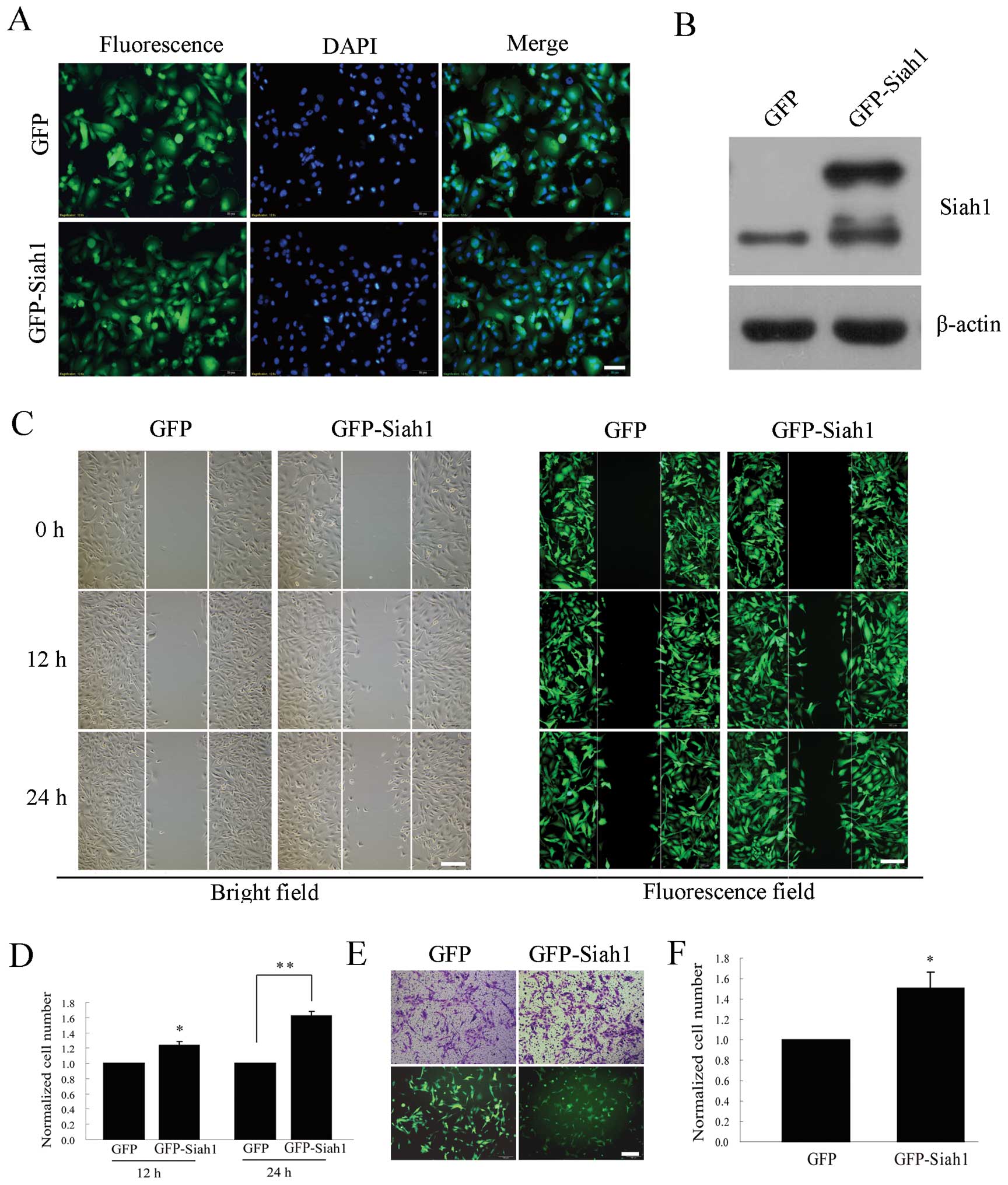
Overexpression of Siah1 promotes glioma
cell migration and invasion under hypoxia
To further investigate the role of Siah1, we tested
the effect of Siah1 on cell migration and invasion in U251 cells
transfected by a lentivirus overexpressing Siah1. The stable cell
lines overexpressing GFP or GFP-Siah1 were assessed by GFP imaging
and western blotting (Fig. 3A and
B). Then, we investigated whether the cell migration and
invasion were promoted upon Siah1 overexpression under hypoxia in
the stable lines expressing GFP or GFP-Siah1. The wound healing
assay showed that the number of migratory cells was increased by 25
and 62% at 12 and 24 h (Fig. 3C and
D) and the Transwell assay showed that the number of invasive
cells increased by 51%, when compared with the corresponding
controls (Fig. 2E and F). These
results further indicate that Siah1 is involved in the migration
and invasion of human glioma cells, and overexpression of Siah1
promotes glioma cell migration and invasion under hypoxia.
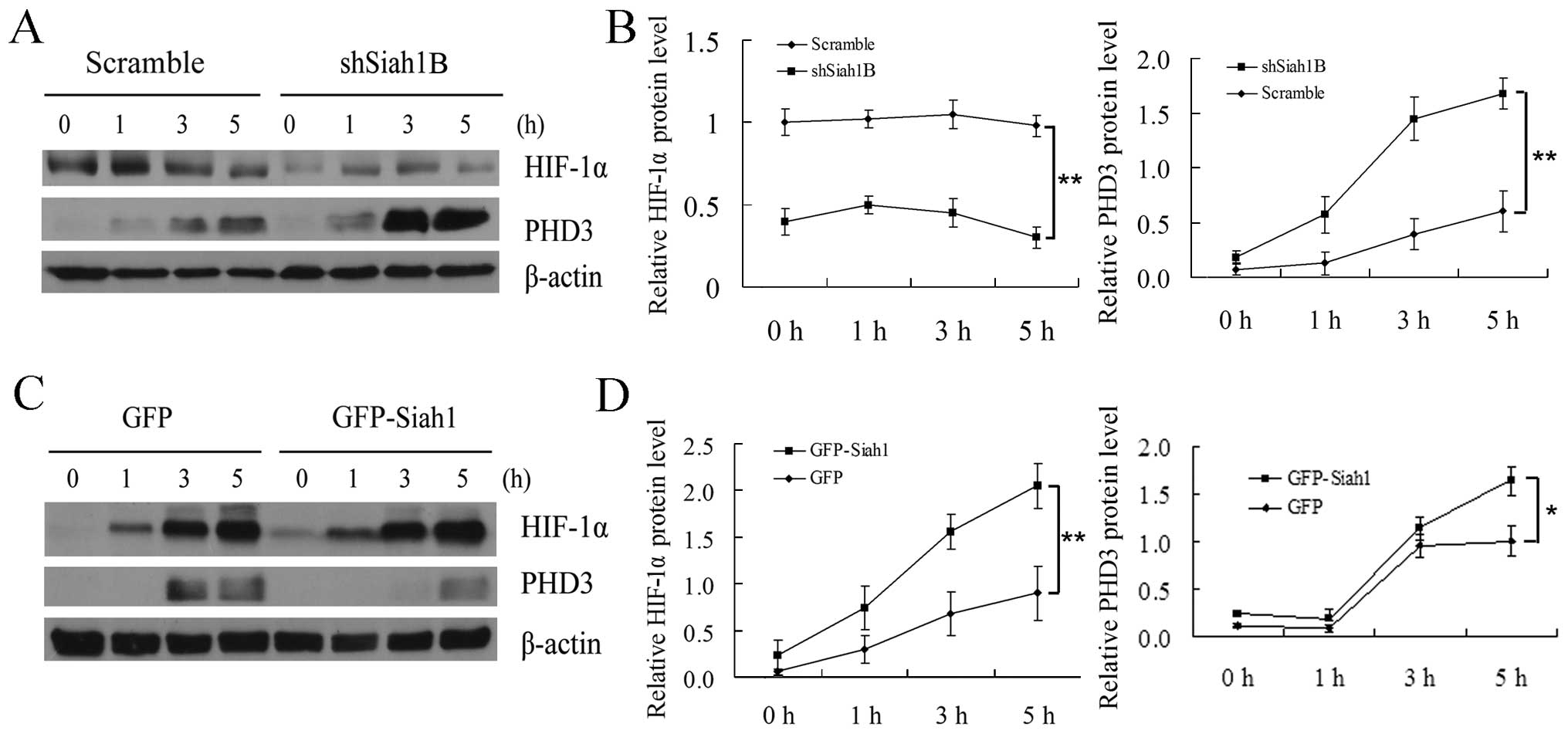
Siah1 induces HIF-1α by promoting the
degradation of PHD3 in human glioma cells under hypoxia
It has been reported that HIF-1α is activated under
hypoxia and plays a crucial role in glioma cell migration and
invasion (3); however, whether
Siah1 is involved in the regulation of HIF-1α in human glioma cells
is still unclear. For this reason, we detected the expression level
of PHD3 and HIF-1α in Siah1 loss-of- and gain-of-function studies
at several hypoxia time points by western blotting. We found that
loss of Siah1 led to a more rapid increase in oxygen sensor PHD3
thereby inhibiting the production of HIF-1α when compared with the
control (Fig. 4A and B). In
addition, we analyzed the role of Siah1 under the condition of
overexpression. As shown in Fig. 4C and
D, compared with the control, overexpression of Siah1 resulted
in a slower increase in PHD3 thereby promoting the rapid production
of HIF-1α. These results suggest that Siah1 promotes cell migration
and invasion by regulating the degradation of PHD3 thereby
improving the stability of HIF-1α.
Discussion
In the present study, we showed that Siah1
contributes to glioma cell migration and invasion by reducing the
stability of PHD3, thereby stabilizing HIF-1α. The following
results support our conclusion. Firstly, the western blotting
results showed that Siah1 was upregulated in the human glioma
tissues. Secondly, downregulation of Siah1 suppressed glioma cell
migration and invasion while Siah1 overexpression promoted it.
Thirdly, downregulation of Siah1 stabilized PHD3 and attenuated the
production of HIF-1α under hypoxia, while overexpression of Siah1
reduced the stability of PHD3 and accelerated the production of
HIF-1α.
Hypoxia is one of the important features of human
glioblastoma, which is associated with poor prognosis, increased
angiogenesis, tumor growth and resistance to radiotherapy and
chemotherapy (12). HIF-1α, a
pivotal hypoxia regulatory factor, has been shown to promote both
angiogenesis and invasion. Upregulation of HIF-1α is an important
physiological process of glioma adaptation to hypoxia (25). Our data showed that, when glioma
cells were in a hypoxic condition, downregulation of Siah1
inhibited the production of HIF-1α while overexpression of Siah1
promoted it. These data support that Siah1 plays an important role
in the regulation of HIF-1α under hypoxia and that inhibition of
Siah1 may be an attractive therapeutic strategy by which to target
the tumor microenvironment.
Although the role of HIF-1α in glioma cell
progression has been well studied (3,6,11–13),
the regulatory mechanism to date remains unclear. It has been
demonstrated that Rho small GTPases, Myc, mTOR and protein
prenylation transferase GGTI are involved in regulating HIF-1α
induction (26–31). Particularly, it has been reported
that ubiquitin ligase Siah2 contributes to the abundance of HIF-1α
by regulating PHD3 stability, thereby playing important roles in
the cellular response to hypoxia (19). As Siah1 possesses a high similarity
with Siah2, whether it plays an equivalent role under hypoxic
conditions is still unknown. Our results indicate that Siah1 is
involved in the regulation of HIF-1α in human glioma and plays a
similar role to that of Siah2.
In conclusion, the present study found that Siah1 is
upregulated in human glioma tissues, and plays important roles in
glioma cell migration and invasion in the cellular response to
hypoxia. To our knowledge, this is the first study to focus on the
role of Siah1 in glioma cells. The present study revealed the
function of Siah1 in glioma cells and indicates that Siah1 may be a
potential molecular target for the treatment of glioma based on the
interference of the Siah1-PHD3-HIF-1α signaling pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81201264 and 81272777), and the
China Postdoctoral Science Foundation.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 5:492–507. 2008. View Article : Google Scholar
|
|
2
|
Clarke J, Butowski N and Chang S: Recent
advances in therapy for glioblastoma. Arch Neurol. 67:279–283.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaur B, Khwaja FW, Severson EA, et al:
Hypoxia and the hypoxia-inducible-factor pathway in glioma growth
and angiogenesis. Neuro Oncol. 7:134–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vordermark D: Significance of hypoxia in
malignant glioma. Re: Evans et al Hypoxia is important in the
biology and aggression of human glial brain tumors. Clin Cancer
Res. 11:3966–3968. 2005. View Article : Google Scholar
|
|
5
|
Evans SM, Judy KD, Dunphy I, et al:
Hypoxia is important in the biology and aggression of human glial
brain tumors. Clin Cancer Res. 10:8177–8184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kessler J, Hahnel A, Wichmann H, et al:
HIF-1α inhibition by siRNA or chetomin in human malignant glioma
cells: effects on hypoxic radioresistance and monitoring via CA9
expression. BMC Cancer. 10:6052010. View Article : Google Scholar
|
|
7
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lipkowitz S and Weissman AM: RINGs of good
and evil: RING finger ubiquitin ligases at the crossroads of tumour
suppression and oncogenesis. Nat Rev Cancer. 11:629–643. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jans J, van Dijk JH, van Schelven S, et
al: Expression and localization of hypoxia proteins in prostate
cancer: prognostic implications after radical prostatectomy.
Urology. 75:786–792. 2010. View Article : Google Scholar
|
|
10
|
Wong CC, Zhang H, Gilkes DM, et al:
Inhibitors of hypoxia-inducible factor 1 block breast cancer
metastatic niche formation and lung metastasis. J Mol Med.
90:803–815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai S, Huang ML, Hsu CY and Chao KS:
Inhibition of hypoxia inducible factor 1α causes oxygen-independent
cytotoxicity and induces p53 independent apoptosis in glioblastoma
cells. Int J Radiat Oncol Biol Phys. 55:1027–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu H, Li Y, Shu M, et al:
Hypoxia-inducible factor-1α blocks differentiation of malignant
gliomas. FEBS J. 276:7291–7304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gillespie DL, Flynn JR, Ragel BT, et al:
Silencing of HIF-1α by RNA interference in human glioma cells in
vitro and in vivo. Methods Mol Biol. 487:283–301. 2009.
|
|
14
|
Carthew RW and Rubin GM: seven in
absentia, a gene required for specification of R7 cell fate in the
Drosophila eye. Cell. 63:561–577. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu G, Chung YL, Glover T, et al:
Characterization of human homologs of the Drosophila seven in
absentia (sina) gene. Genomics. 46:103–111. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Habelhah H, Frew IJ, Laine A, et al:
Stress-induced decrease in TRAF2 stability is mediated by Siah2.
EMBO J. 21:5756–5765. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Habelhah H, Laine A, Erdjument-Bromage H,
et al: Regulation of 2-oxoglutarate (α-ketoglutarate) dehydrogenase
stability by the RING finger ubiquitin ligase Siah. J Biol Chem.
279:53782–53788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuzawa SI and Reed JC: Siah-1, SIP, and
Ebi collaborate in a novel pathway for β-catenin degradation linked
to p53 responses. Mol Cell. 7:915–926. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakayama K, Frew IJ, Hagensen M, et al:
Siah2 regulates stability of prolyl-hydroxylases, controls HIF1α
abundance, and modulates physiological responses to hypoxia. Cell.
117:941–952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Guenther MG, Carthew RW and Lazar
MA: Proteasomal regulation of nuclear receptor corepressor-mediated
repression. Genes Dev. 12:1775–1780. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuo K, Satoh S, Okabe H, et al: SIAH1
inactivation correlates with tumor progression in hepatocellular
carcinomas. Genes Chromosomes Cancer. 36:283–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wen YY, Yang ZQ, Song M, et al: The
expression of SIAH1 is downregulated and associated with Bim and
apoptosis in human breast cancer tissues and cells. Mol Carcinog.
49:440–449. 2010.PubMed/NCBI
|
|
23
|
Kitagawa N, Kondo S, Wakisaka N, et al:
Expression of seven-in-absentia homologue 1 and hypoxia-inducible
factor 1 alpha: novel prognostic factors of nasopharyngeal
carcinoma. Cancer Lett. 331:52–57. 2013. View Article : Google Scholar
|
|
24
|
Shi H, Gao Y, Tang Y, et al: CacyBP/SIP
protein is important for the proliferation of human glioma cells.
IUBMB Life. 66:286–291. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Li YM, Tian RF, et al: The
expression and significance of HIF-1α and GLUT-3 in glioma. Brain
Res. 1304:149–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou J, Li K, Gu Y, et al: Transcriptional
up-regulation of RhoE by hypoxia-inducible factor (HIF)-1 promotes
epithelial to mesenchymal transition of gastric cancer cells during
hypoxia. Biochem Biophys Res Commun. 415:348–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue Y, Li NL, Yang JY, et al:
Phosphatidylinositol 3′-kinase signaling pathway is essential for
Rac1-induced hypoxia-inducible factor-1α and vascular endothelial
growth factor expression. Am J Physiol Heart Circ Physiol.
300:H2169–H2176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang P, Zhang X, Hao X, et al: Rac1
activates HIF-1 in retinal pigment epithelium cells under hypoxia.
Graefes Arch Clin Exp Ophthalmol. 247:633–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JW, Gao P, Liu YC, Semenza GL and Dang
CV: Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively
induce vascular endothelial growth factor and metabolic switches
hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol.
27:7381–7393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan G, Nanduri J, Khan S, et al:
Induction of HIF-1α expression by intermittent hypoxia: involvement
of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases,
and mTOR. J Cell Physiol. 217:674–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Liu Z, Shi Q, et al:
Geranylgeranyltransferase I regulates HIF-1α promoting glioblastoma
cell migration and invasion. J Neurooncol. 112:365–374. 2013.
View Article : Google Scholar : PubMed/NCBI
|