Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignant carcinoma worldwide and the third leading cause of
cancer mortality (1,2). Although its mortality decreased along
with great advances in surgical resection and chemotherapy, the
long-term prognosis remains unsatisfactory (3,4). Thus,
effective and innovative therapeutic procedures are required to
combat this disease. Evidence has shown that in addition to current
chemotherapy, gene therapy using small-interfering RNA (RNAi) is a
potent therapeutic approach in HCC therapy (5).
Telomerase is a ribonucleoprotein complex that is
involved in tumor growth and progression in part through
maintaining the ends of chromosomes (6). Human telomerase reverse transcriptase
(hTERT), an essential subunit of the telomerase complex, maintains
the length of telomeres by reverse transcription and the addition
of TTAGGG repeats onto the telomeric ends of the chromosomes
(7). Moreover, in various human
cancer cells, such as liver and ovarian cancer cells, a close
correlation between hTERT expression and telomerase activity has
been identified (8–10). Previous findings showed that the
hTERT appeared to be the major determinant of telomerase activity
(11). These observations indicated
that hTERT expression and telomerase activity serve as useful
diagnostic and/or prognostic markers in many types of human
malignancies. Accordingly, the potential of telomerase inhibition
by targeting hTERT as an effective therapy for cancer treatment has
been demonstrated (12,13). hTERT was previously found to be
expressed in most cancer cells but not in normal cells (14). However, previous results showed that
hTERT can be detected in both malignant and normal tissues
(15–17). Thus, to use this target by silencing
hTERT expression in the treatment of cancer requires identification
of a method with tumor cell-specificity and low toxicity, which can
effectively inhibit hTERT gene expression in tumor cells but cannot
affect hTERT activity in normal immortal cells.
Different approaches are currently under
investigation to develop gene therapy of cancer. However,
experience from clinical trials of cancer gene therapy indicates
that no single therapeutic strategy can effectively eradicate
cancer, whereas combined polygene therapy is a more reliable
approach to combating cancer. A well-established radionuclide-based
reporter gene system is the herpes simplex virus type-1-thymidine
kinase (HSV1-TK) enzyme, which can specifically infect a variety of
cancer cells but not normal mammalian cells (18,19).
This reporter gene can itself be the therapeutic gene, and
anticancer gene therapy using HSV1-TK can be coupled with imaging
of the accumulation of radio-labeled probes such as
9-[4-fluoro-3-(hydroxymethyl)butyl]guanine (FHBG) or
5-iodo-20-fluoro-20deoxy-1-b-D-arabinofuranosyluracil (FIAU)
(20,21), which can be imaged using PET. Thus,
co-expression of a reporter gene and a therapeutic gene may allow
for delivery of therapeutic gene to cancer cells efficiently and
safely and treat cancer effectively. Consequently, we constructed
triple-expressing vector in which the HSV1-TK gene and shTERT were
driven by the pLXSN and the U6 promoters and detected the antitumor
effects on cells in vitro as well as tumor inhibition in
vivo. In this study, the combination of hTERT shRNA/HSV1-TK
preferably targeted HCC cells and significantly suppressed the
tumor growth in liver tumor xenograft. Moreover, hTERT
shRNA/HSV1-TK showed virtually no toxicity in normal cells,
suggesting that the hTERT shRNA/HSV1-TK vector may be exploited as
a potential new therapeutic strategy for HCC.
Materials and methods
Cell lines and culture
Human HCC cell lines (BEL-7402, HCC36, HepG2, HA22T
and Hep3B), normal mammary epithelial (L-02, QSG-7701), and normal
lung fibroblasts (WI-38) were purchased from the American Type
Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum, 100 U/ml penicillin, 100 μg/ml streptomycin. The cells were
maintained at 37°C in a humidified atmosphere containing 5%
CO2/95% air.
Construction of recombinant retroviral
vectors and vector titration
The siRNA sequence targeting hTERT corresponded to
the coding region from 385 to 393: 5′-AAGCACTTCCTCT ACTCCTCA-3′.
The oligonucleotides with a sequence predicted to induce efficient
small-interfering RNA (RNAi) of hTERT (containing sense and
antisense sequences linked by the hairpin loop: CGAA) were
synthesized as follows: Forward: 5′-CGTCGACGCACTTCCTCTACTCCTCATT
CAAGAGATGAGGA-3′ and reverse: 5′-CAAGCTTCTCGA
GTCTAGAAAAAGCACTTCCTCTACTCCTCATCTC-3′. These oligonucleotides were
annealed in STE buffer (10 mM Tris pH 8.0, 50 mM NaCl, 1 mM EDTA)
at 94°C for 5 min and cooled gradually. The double-stranded product
was cloned downstream to the human U6 promoter of the pGEM/U6
vector (Genscripts). The recombinant retroviral vector pLXSN
(Clontech) was employed to develop the pLXSN-TK-U6-shTERT vector.
Full-length HSV1-TK cDNA (kindly provided by Dr Qiu Xinfang, FuDan
University, Shanghai, China) sequence coupled to U6 promoter was
removed from pRNAT-shTERT vector, and a NheI-SalI
restriction site was introduced into the pRNAT-shTERT vector by
PCR. HSV1-TK cDNA was amplified by PCR using the sense primer,
which contains a NheI restriction site, and the anti-sense
primer, which contains a SalI restriction site. Following
restriction digestion with NheI and SalI of
pRNAT-hTERT shRNA, the HSV1-TK sequence was ligated into the
pRNAT-shTERT vector. The resulting vector containing shTERT
sequence and HSV1-TK cDNA coupled to the U6, was designated as
pLXSN-TK-U6-shTERT vector, and confirmed by DNA sequencing. A
retroviral vector containing the shTERT sequence (pLXSN-U6-hTERT
shRNA) was also developed. The U6 promoter was inserted into the
vector as described above.
Plasmid vectors were transfected into the
amphotropic packaging cell line 293 by using the calcium phosphate
transfection system (Invitrogen Life Technologies, Carlsbad, CA,
USA) reagents, as previously described (22). Transfected cells were selected in a
medium containing G418 (Invitrogen) and single cell-derived clones
were isolated and expanded to cell lines. Viral titer, determined
by infection of NIH3T3 with virus-containing supernatants from
single cell-derived clones of 293 producer cells as described
previously (22), ranged from
104 to 106 cfu/ml. The supernatant from the
producer cell clones with higher viral titer was used to transduce
target cells.
MicroPET imaging of 18F-FHBG
in vivo
When flank tumors reached 200 mm3 in
size, 0.2 ml pLXSN-U6-shTERT (5×105 pfu/ml) or 0.2 ml
pLXSN-TK-U6-shTERT (5×105 pfu/ml) were injected directly
into the center of the tumor. Mice were anesthetized via inhalation
of isoflurane (1–1.5%) with an oxygen flow rate of 2 l/min. Depth
of anesthesia was monitored by respiratory rate and eye and footpad
reflex. A total of 150 MBq (0.2 ml) 18F-FHBG was
injected into the tail vein to acquire dynamic volumetric data for
210 min. Volumetric images were reconstructed with filtered back
projection after the data were corrected for uniformity, scatter,
attenuation, decay, and injected activity using the software AsiPro
4.1 provided by the manufacturer. Time-activity curves were
generated from the selected regions of interest including
pLXSN-U6-shTERT (on the left flank), pLXSN-TK-U6-shTERT tumor (on
the right flank).
Western blot analysis
The cells were lysed in mammalian protein extraction
reagent (Pierce Biotechnology, Rockford, IL, USA). Cell lysates
were collected and protein concentration of the cell lysates was
measured. Proteins (10–20 μg) were resolved by SDS-polyacrylamide
gel electrophoresis and transferred to PVDF membranes (Bio-Rad,
Hercules, CA, USA). The membranes were then incubated with primary
antibodies in 3% bovine serum albumin/Tris-buffered saline/Tween-20
at 4°C overnight, followed by incubation with secondary antibodies
at room temperature for 1 h. The protein signals were detected by
ECL method. Western blotting reagents were obtained from Pierce
Biotechnology. The antibodies to hTERT and β-actin were purchased
from Cell Signaling Technology (Danvers, MA, USA).
Cell viability assay
Cell viability was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, the cells were plated at a density of
5×103 cells/well in 96-well tissue culture plates and
subjected to different treatments. Following a 72-h incubation at
37°C in a humidified atmosphere containing 5% CO2/95%
air, the cells were incubated for another 4 h with MTT reagent. The
formazan product was dissolved in dimethyl sulfoxide and read at
570 nm on a Victor 3 Multi Label plate reader (PerkinElmer, Boston,
MA, USA).
In vivo combination therapy
When tumor masses reached average volume of ~200
mm3, pLXSN-U6-shTERT and pLXSN-TK-U6-shTERT were
initiated. Tumor xenograft mice were randomly divided into the
control, pLXSN-U6-shTERT and pLXSN-TK-U6-shTERT groups. The mice in
each group (n=5) received intratumor injections of either 0.2 ml
pLXSN-U6-shTERT (5×105 pfu/ml) or 0.2 ml
pLXSN-TK-U6-shTERT (5×105 pfu/ml) on continuous
injection for five days. The mice in the control group received
saline injections at the same time. Individual tumor size was
measured every 2 days by use of a caliper and tumor volumes were
determined by measuring the length (L) and width (W) of the tumors
and calculating using the following formula: V = LW2/2.
Animal maintenance and experimental procedures were approved by the
Nanfang Hospital Animal Ethics Committee.
In vivo apoptosis assays
At the end of the experiment (5 weeks after
intratumor injection), the mice were humanely euthanized and tumors
were surgically dissected. The tumor specimens were fixed in 4%
paraformaldehyde for TUNEL staining (Roche Applied Sciences,
Indianapolis, IN, USA) according to the manufacturer’s
instructions. Briefly, the slides were incubated with 50 ml of
TUNEL reaction mixture in a humidified atmosphere for 1 h at 37°C
in the dark. The percentage of TUNEL apoptotic cells was analyzed
by randomly selecting five independent fields for each sample.
Statistical analysis
The results are given as mean ± SD. Student’s t-test
was used to analyze the significance of differences. The
significance level was set at P<0.05.
Results
Construction and identification of
pLXSN-TK-U6-shTERT recombinant plasmid
Recombinant plasmids pGEM-T-shTERT, pGEM-T-U6,
pUC19-EGFP and pUC19-TK were constructed. BamHI/SalI
and SalI/HindIII double enzyme
digestionmethodswereselectedtoobtainpUC19-EGFP-U6and
pUC19-EGFP-U6-shTERT respectively, EcoRI/XhoI double
enzyme digested pUC19-EGFP-U6-shTERT and pLXSN plasmids to obtain
pLXSN-EGFP-U6-shTERT, BamHI/SalI double enzyme
digested pGEM-T-U6 and pUC19-TK plasmids to obtain pUC19-TK-U6, and
SalI/EcoRI double enzyme-digested recombinant
plasmids pLXSN-EGFP-U6-shTERT and pUC19-TK-U6 to obtain
pLXSN-TK-U6-shTERT (Fig. 1A).
Restriction enzyme identification of recombinant plasmids are shown
in Fig. 1B.
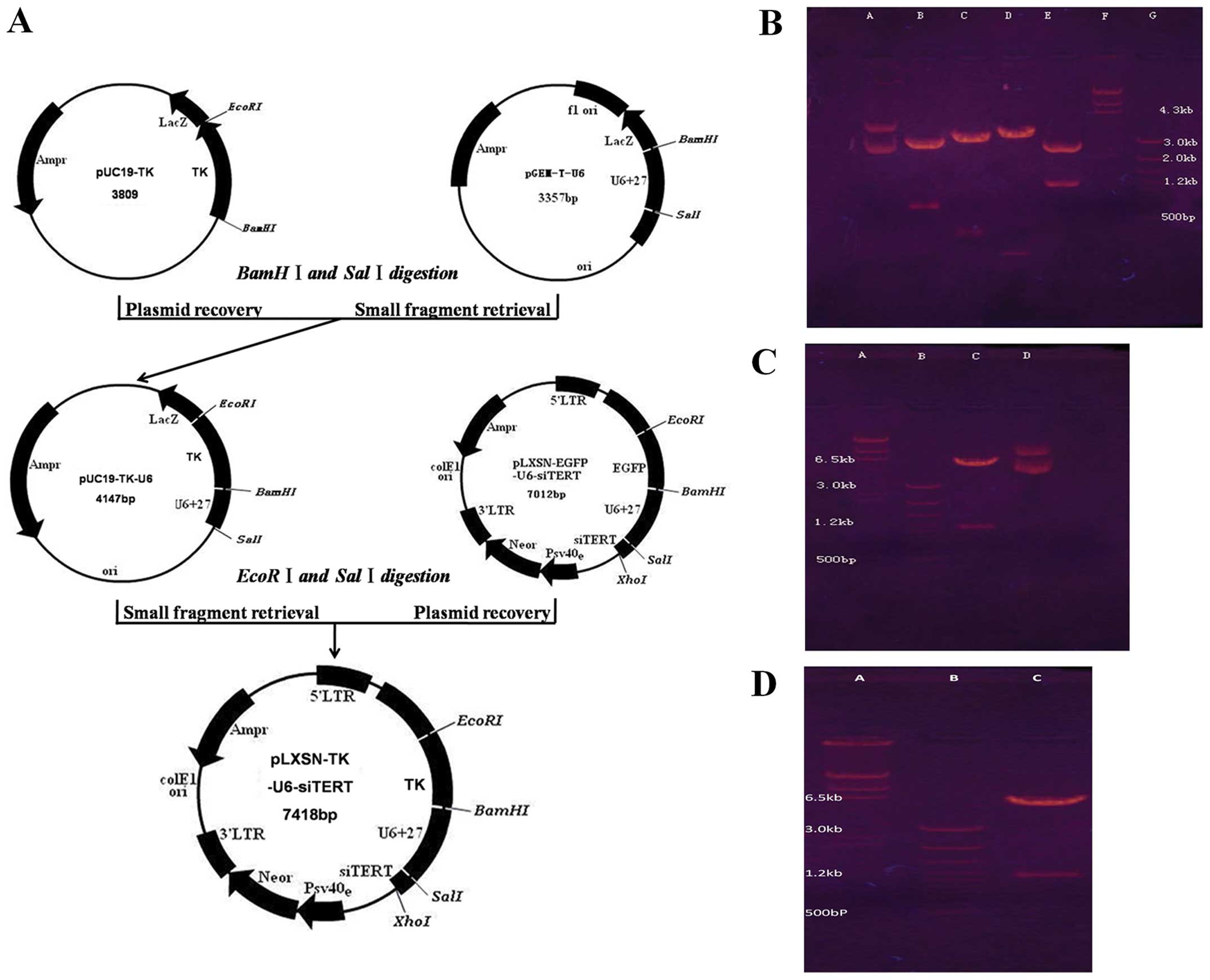 | Figure 1Successful construction and
identification of recombinant plasmids. (A) The schema of the
pLXSN-TK-U6-shTERT recombinant plasmid construction. (B)
Identification of pUC19-EGFP-U6-shTERT vector. Lane A, control
pUC19/EGFP-U6-shTERT; lane B, pUC19/EGFP-U6-shTERT/EcoRI,
BamHI; lane C, pUC19/EGFP-U6-shTERT/BamHI,
SalI; lane D, pUC19/EGFP-U6-shTERT/SalI, XhoI;
lane E, pUC19/EGFP-U6-shTERT/EcoRI, XhoI; lane F,
λDNA/HindIII markers; lane G, 100 bp DNA Ladder. (C)
Identification of pLXSN-EGFP-U6-shTERT vector. Lane A,
λDNA/HindIII markers; lane B, 100 bp DNA Ladder; lane C,
pLXSN/EGFP-U6-shTERT/EcoRI, XhoI; lane D, control
pLXSN/EGFP-U6-shTERT. (D) Identification of pLXSN/TK-U6-shTERT.
Lane A, λDNA/HindIII markers; lane B,
pLXSN/TK-U6-shTERT/EcoRI, BamHI. |
pLXSN-TK-U6-shTERT recombinant plasmid
preferentially inhibits HCC cell growth in vitro with extremely
limited toxicity in normal cells
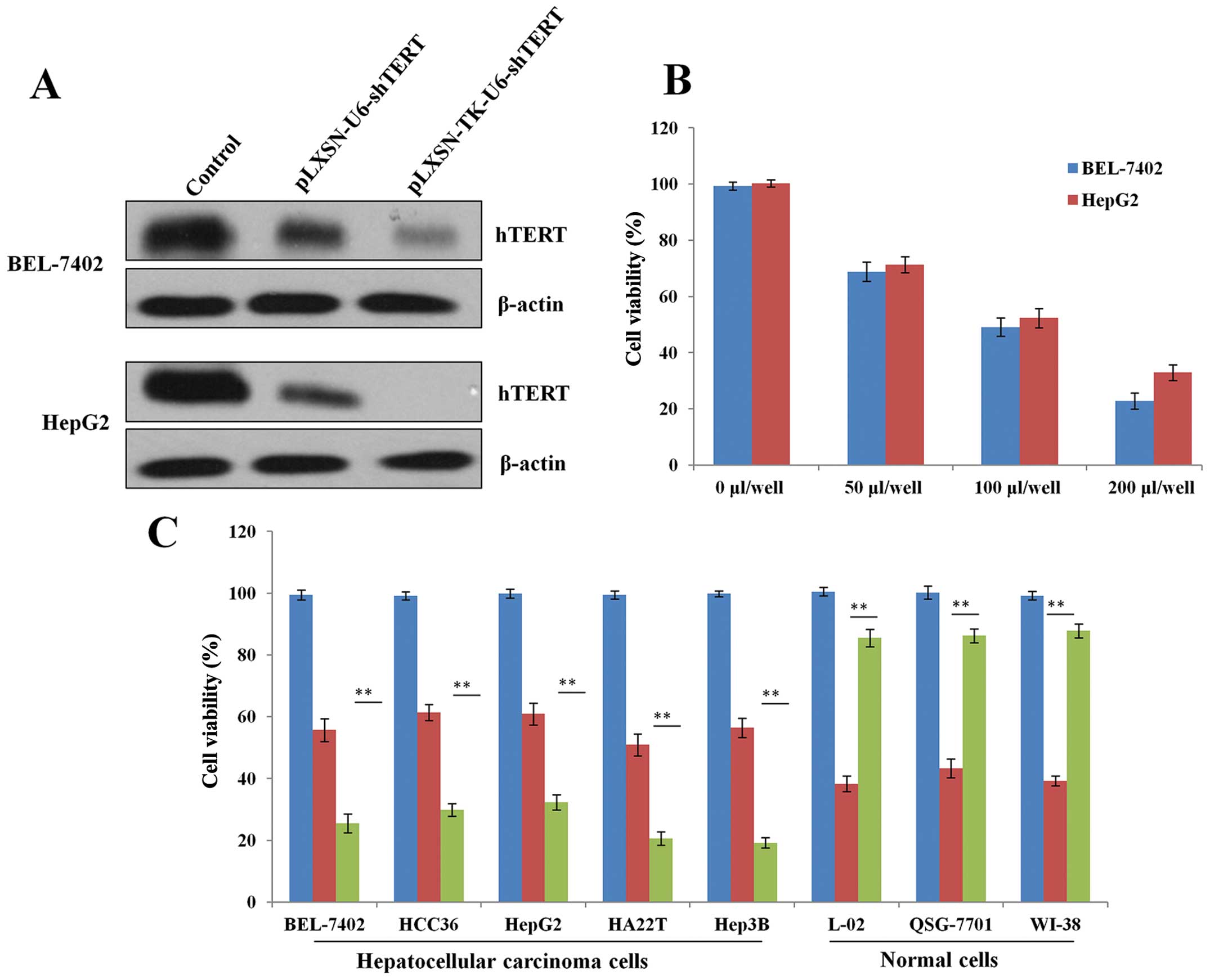
To determine whether the pLXSN-TK-U6-shTERT plasmid
can specifically target HCC cells, the expression of hTERT was
detected by western blotting 48 h after transient transfection in
BEL-7402 and HepG2 HCC cells (Fig.
2A). The results showed that pLXSN-TK-U6-shTERT inhibited cell
growth in vitro in a dose-dependent manner (Fig. 2B). Moreover, we examined the killing
effects of pLXSN-TK-U6-shTERT in a panel of HCC and normal cell
lines. The results showed that the pLXSN-TK-U6-shTERT inhibited
cell growth more effectively than pLXSN-U6-shTERT in vitro
(Fig. 2B). However, in normal
cells, the cell killing activity of pLXSN-U6-shTERT was more potent
than that of pLXSN-TK-U6-shTERT (Fig.
2C). Thus, the cytotoxic effect of pLXSN-TK-U6-shTERT is potent
in cancer cells but limited in normal cells, indicating that
pLXSN-TK-U6-shTERT is tumor-specific in vitro and is a
potential therapeutic agent that can be used for the treatment of
HCC.
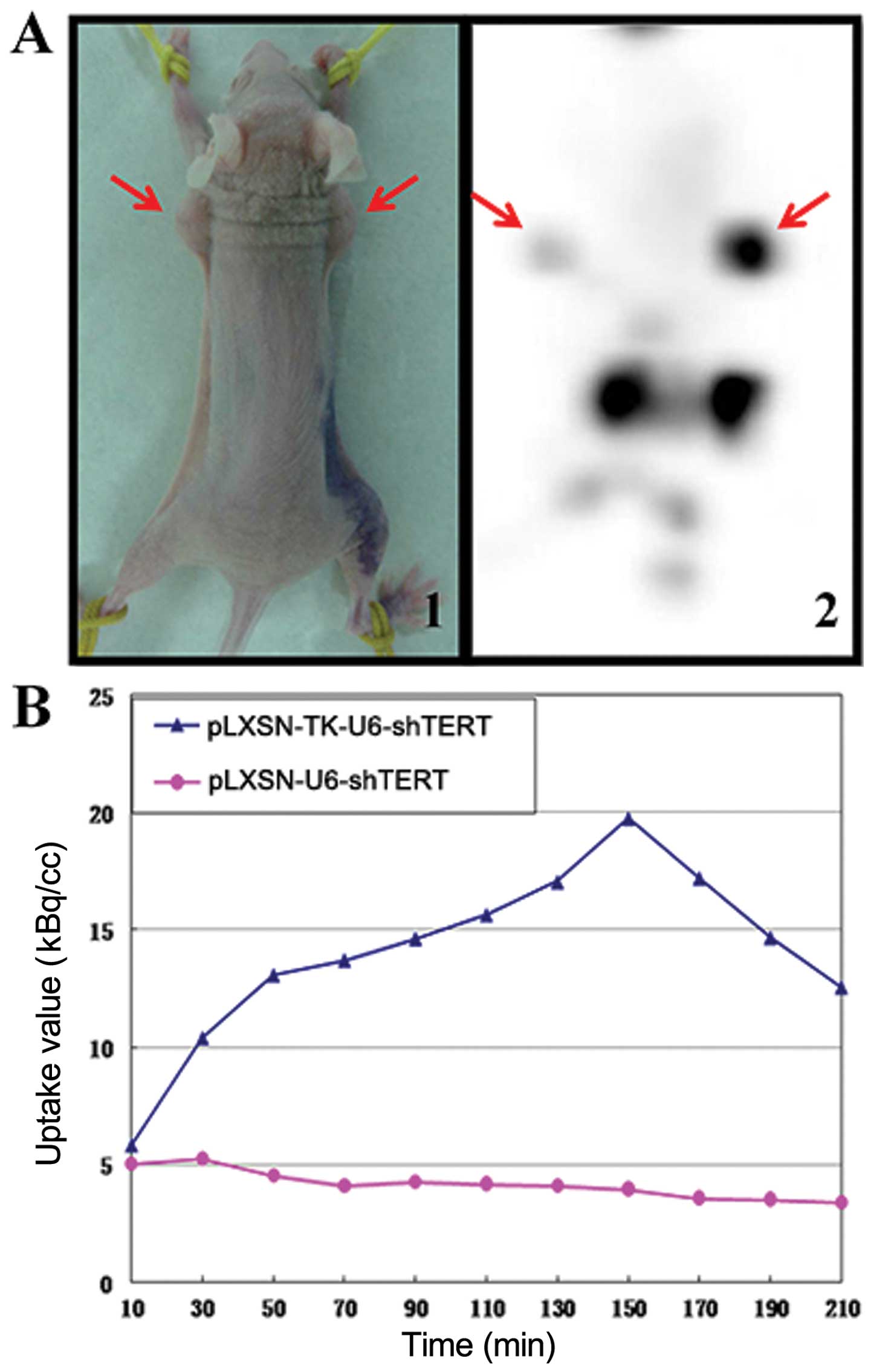
Detection of anatomic sites of
18F-FHBG sequestration with microPET
When tumor masses reached an average volume of ~200
mm3, the mice received intratumoral injections of 0.2 ml
pLXSN-U6-shTERT (5×105 pfu/ml, left flank) or 0.2 ml
pLXSN-TK-U6-shTERT (5×105 pfu/ml, right flank). Compared
with shTERT tumors (left flank), TK-shTERT tumors of mice showed
significant accumulation (right flank) over time as a result of
intracellular entrapment of HSV-TK-phosphorylated
18F-FHBG, 150 min after 18F-FHBG injection,
when the uptake value reached its greatest level (Fig. 3A and B). The liver and kidney
concentration of 18F-FHBG was significantly greater, and
no radioactive distribution was observed in the brain (Fig. 3B).
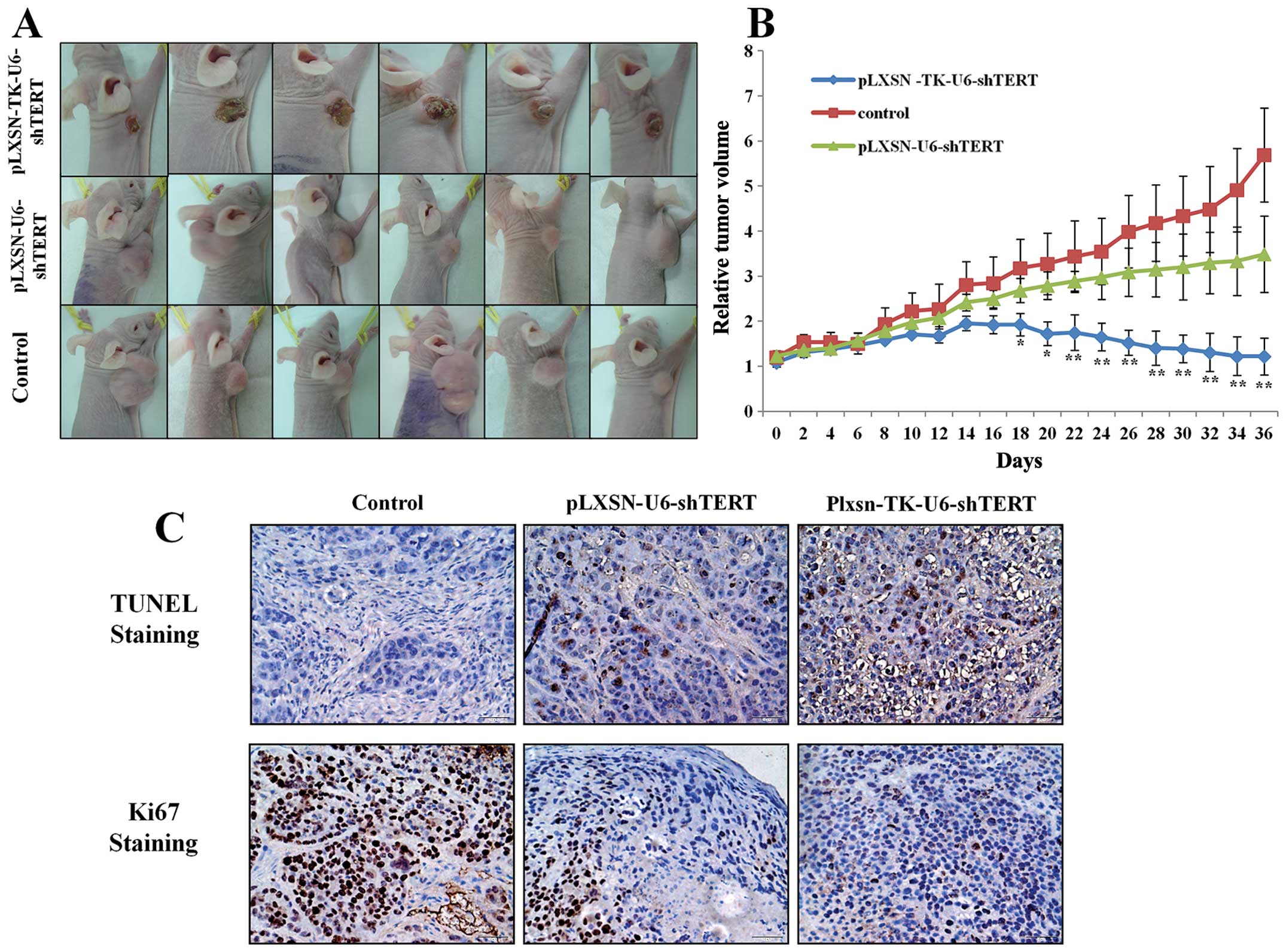
pLXSN-TK-U6-shTERT recombinant plasmid
exerts a significant antitumor effect in mouse xenograft model of
HCC
To investigate the therapeutic effects of
pLXSN-TK-U6-shTERT in vivo, we established a mouse xenograft
model with the BEL-7402 HCC cell line. When tumor masses reached an
average volume of ~400 mm3, the mice in each group
received intratumoral injections of pLXSN-U6-shTERT plasmid or
pLXSN-TK-U6-shTERT plasmid of continuous injection for 5 days. Five
weeks after the first treatment, the pLXSN-TK-U6-shTERT group
significantly inhibited tumor growth compared with the
pLXSN-U6-shTERT group (Fig. 4A and
B). These findings were further supported by the increase in
apoptosis and decrease in proliferation in the tumors of the
pLXSN-TK-U6-shTERT-treated groups. Notably, although the two
treatment groups showed a statistically significant trend of
increase in TUNEL-positive cells and decrease in Ki67-positive
cells compared with the control, pLXSN-TK-U6-shTERT showed
significantly higher activities than pLXSN-U6-hTERT shRNA,
suggesting that pLXSN-TK-U6-shTERT has higher targeting power over
pLXSN-U6-shTERT (Fig. 4C and D).
Collectively, these results showed that pLXSN-TK-U6-shTERT
consistently exerts strong antitumor effects on liver tumor in
vivo and induces apoptosis with high-tumor specificity.
Discussion
Cancer cells frequently overexpress hTERT, a
determinant of telomerase activity, resulting in enhanced
proliferation and tumor progression (23,24).
hTERT associates with human telomeres and may enhance genomic
stability and DNA repair in human cancer cells (25). Thus, hTERT is an attractive
therapeutic target in malignant tumor treatment. A variety of
gene-targeting approaches were found to interfere with hTERT
function (26–28). Among them, RNAi-mediated hTERT gene
silencing provides an efficient method to inhibit telomerase
activity for human cancer therapy (29–31).
RNAi is known to be very effective and selective in vitro,
however, issues, including incomplete suppression of target genes,
requirement of cytotoxic transfection reagents and/or enhancers,
positively impairing normal cellular functions and lack of
effective delivery system, hamper the development of this novel
therapy for cancer treatment. Thus, the development of low-toxic
and potent shRNA delivery systems is a crucial step for the success
of RNAi-based cancer therapy.
The most studied suicide gene is HSV1-TK.
HSV-TK-based suicide gene therapy has been used to target cancers
and its role has been assessed in several clinical trials. The
reporter gene can itself be the therapeutic gene or can be coupled
with the therapeutic gene (32).
Clinical trials using this approach have been conducted in patients
with gliomas and no serious adverse events were reported (33). The successful employment of several
candidate gene combinations for HSV1-TK suicide gene therapy have
been reported including IL-2 (34),
STAT3 (35), cytosine deaminase
(36), nitroreductase (37), and carboxylesterase (38). Non-invasive imaging should be
accompanied with gene therapy approaches for treatment response
monitoring as well as for assessment of distribution, extent and
duration of transgene expression. Rapid washout of activity from
the blood significantly decreased sensitivity and specificity of
tracer accumulation in HSV1-TK-expressing tumors.
18F-FHBG has emerged as the most reliable agent for
treatment response monitoring. In this study, we demonstrated the
applicability of non-invasive imaging using 18F-FHBG for
monitoring cancer gene therapy in an experimental animal model of
HSV1-TK-expressing tumor xenografts, which is also in concordance
with a previous report (39). In
the present study, we demonstrated the in vitro and in
vivo therapeutic efficacy of retroviral vector-mediated
combined HSV1-TK suicide and hTERT gene therapy for HCC. The use of
an LXSN-TK-based retroviral vector allows high transfection
efficiency of hTERT expression and selective targeting of cancer
cells, while sparing normal cells in vitro.
Although retrovirus vectors increasingly receiving
attention in the field of liver cancer gene therapy due to their
hepatic tropism and high titers, they have one major drawback of
in vivo delivery and transduction (40,41),
which may be relatively to obtain in liver cancer in which
percutaneous locoregional treatment is easy to perform. In the
current study, we also carried out in vivo transduction by
repeated intratumoral injection of the vector. When tumors were
transduced in vivo with TK-shTERT retroviral vector, we
observed a strong antitumor effect, with complete regression of
tumors composed of transduced cells compared with the shTERT group,
showing wide apoptotic areas and markedly decreased Ki67-positive
cells in the residual tumor (Fig.
4), thus confirming the efficacy of the vector and the
possibility to transduce a sufficient amount of tumor cells by
locoregional treatment, as also confirmed by the in vivo
experiment.
Taken together, our study has demonstrated enhanced
antitumor effects by the use of combination gene therapy using
TK-shTERT for tumor xenografts in mice as compared with shTERT
single therapeutic approach. Furthermore, therapeutic response
monitoring was possible by serial non-invasive in vivo
imaging using a reporter gene system. These results suggest that
shRNA targeting hTERT, coupled with the HSV-TK suicide gene, may be
therapeutically useful for HCC and potentially for other
malignancies.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China and the grant numbers are 30370426 and
81071174.
References
|
1
|
Roberts LR: Sorafenib in liver cancer -
just the beginning. N Engl J Med. 359:420–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM: Updated treatment approach to
hepatocellular carcinoma. J Gastroenterol. 40:225–235. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pang RW, Joh JW, Johnson PJ, Monden M,
Pawlik TM and Poon RT: Biology of hepatocellular carcinoma. Ann
Surg Oncol. 15:962–971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ and
Wu F: Solitary large hepatocellular carcinoma: a specific subtype
of hepatocellular carcinoma with good outcome after hepatic
resection. Ann Surg. 249:118–123. 2009. View Article : Google Scholar
|
|
5
|
Gao J, Chen H, Yu Y, et al: Inhibition of
hepatocellular carcinoma growth using immunoliposomes for
co-delivery of adriamycin and ribonucleotide reductase M2 siRNA.
Biomaterials. 34:10084–10098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng WDJ, Funk SS, Wang SL, et al: The RNA
component of human telomerase. Science. 269:1236–1241. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang PH, Zou L and Tu ZG: RNAi-hTERT
inhibition hepatocellular carcinoma cell proliferation via
decreasing telomerase activity. J Surg Res. 131:143–149. 2006.
View Article : Google Scholar
|
|
9
|
Sun PM, Wei LH, Luo MY, et al: The
telomerase activity and expression of hTERT gene can serve as
indicators in the anti-cancer treatment of human ovarian cancer.
Eur J Obstet Gynecol Reprod Biol. 130:249–257. 2007. View Article : Google Scholar
|
|
10
|
Toshikuni N, Nouso K, Higashi T, et al:
Expression of telomerase-associated protein 1 and telomerase
reverse transcriptase in hepatocellular carcinoma. Br J Cancer.
82:833–837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beattie TL, Zhou W, Robinson MO and
Harrington L: Reconstitution of human telomerase activity in vitro.
Curr Biol. 8:177–180. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herbert B, Pitts AE, Baker SI, Hamilton
SE, Wright WE, Shay JW and Corey DR: Inhibition of human telomerase
in immortal human cells leads to progressive telomere shortening
and cell death. Proc Natl Acad Sci USA. 96:14276–14281. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folini M and Zaffaroni N: Targeting
telomerase by antisense-based approaches: perspectives for new
anti-cancer therapies. Curr Pharm Des. 11:1105–1117. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meyerson M, Counter CM, Eaton EN, et al:
hEST2, the putative human telomerase catalytic subunit gene, is
up-regulated in tumor cells and during immortalization. Cell.
90:785–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pannone G, De Maria S, Zamparese R, et al:
Prognostic value of human telomerase reverse transcriptase gene
expression in oral carcinogenesis. Int J Oncol. 30:1349–1357.
2007.PubMed/NCBI
|
|
16
|
Li W, Li L, Liu Z, et al: Expression of
the full-length telomerase reverse transcriptase (hTERT) transcript
in both malignant and normal gastric tissues. Cancer Lett.
260:28–36. 2008. View Article : Google Scholar
|
|
17
|
Nagao K, Tomimatsu M, Endo H, Hisatomi H
and Hikiji K: Telomerase reverse transcriptase mRNA expression and
telomerase activity in hepatocellular carcinoma. J Gastroenterol.
34:83–87. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gil Z, Rein A, Brader P, Li S, Shah JP,
Fong Y and Wong RJ: Nerve-sparing therapy with oncolytic herpes
virus for cancers with neural invasion. Clin Cancer Res.
13:6479–6485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kelly K, Brader P, Rein A, Shah JP, Wong
RJ, Fong Y and Gil Z: Attenuated multimutated herpes simplex
virus-1 effectively treats prostate carcinomas with neural invasion
while preserving nerve function. FASEB J. 22:1839–1848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tjuvajev JG, Joshi A, Callegari J, et al:
A general approach to the non-invasive imaging of transgenes using
cis-linked herpes simplex virus thymidine kinase. Neoplasia.
1:315–320. 1999. View Article : Google Scholar
|
|
21
|
Gambhir SS, Barrio JR, Phelps ME, et al:
Imaging adenoviral-directed reporter gene expression in living
animals with positron emission tomography. Proc Natl Acad Sci USA.
96:2333–2338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barzon L, Bonaguro R, Castagliuolo I, et
al: Gene therapy of thyroid cancer via retrovirally-driven combined
expression of human interleukin-2 and herpes simplex virus
thymidine kinase. Eur J Endocrinol. 148:73–80. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bertorelle R, Briarava M, Rampazzo E, et
al: Telomerase is an independent prognostic marker of overall
survival in patients with colorectal cancer. Br J Cancer.
108:278–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Liu S, Wang H, et al: Genomic
amplification of the human telomerase gene (hTERC) associated with
human papillomavirus is related to the progression of uterine
cervical dysplasia to invasive cancer. Diagn Pathol. 7:1472012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma GG, Gupta A, Wang H, et al: hTERT
associates with human telomeres and enhances genomic stability and
DNA repair. Oncogene. 22:131–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kraemer K, Fuessel S, Kotzsch M, et al:
Chemosensitization of bladder cancer cell lines by human telomerase
reverse transcriptase antisense treatment. J Urol. 172:2023–2028.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hao ZM, Luo JY, Cheng J, et al: Intensive
inhibition of hTERT expression by a ribozyme induces rapid
apoptosis of cancer cells through a telomere length-independent
pathway. Cancer Biol Ther. 4:1098–1103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sachsinger J, Gonzalez-Suarez E, Samper E,
Heicappell R, Müller M and Blasco MA: Telomerase inhibition in
RenCa, a murine tumor cell line with short telomeres, by
overexpression of a dominant negative mTERT mutant, reveals
fundamental differences in telomerase regulation between human and
murine cells. Cancer Res. 61:5580–5586. 2001.PubMed/NCBI
|
|
29
|
Shay JW, Zou Y, Hiyama E and Wright WE:
Telomerase and cancer. Hum Mol Genet. 10:677–685. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Massard C, Zermati Y, Pauleau AL,
Larochette N, et al: hTERT: a novel endogenous inhibitor of the
mitochondrial cell death pathway. Oncogene. 25:4505–4514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kurvinen K, Syrjanen S and Johansson B:
Long-term suppression of telomerase expression in HeLa cell clones,
transfected with an expression vector carrying siRNA targeting
hTERT mRNA. Int J Oncol. 29:279–288. 2006.PubMed/NCBI
|
|
32
|
Gambhir SS, Herschman HR, Cherry SR, et
al: Imaging transgene expression with radionuclide imaging
technologies. Neoplasia. 2:118–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Northcott PA, Jones DT, Kool M, et al:
Medulloblastomics: the end of the beginning. Nat Rev Cancer.
12:818–834. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stefani AL, Barzon L, Castagliuolo I, et
al: Systemic efficacy of combined suicide/cytokine gene therapy in
a murine model of hepatocellular carcinoma. J Hepatol. 42:728–735.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahn YH, Yi H, Shin JY, et al: STAT3
silencing enhances the efficacy of the HSV. tk suicide gene in
gastrointestinal cancer therapy. Clin Exp Metastasis. 29:359–369.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Trinh QT, Austin EA, Murray DM, Knick VC
and Huber BE: Enzyme/prodrug gene therapy: comparison of cytosine
deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir
enzyme/prodrug systems in a human colorectal carcinoma cell line.
Cancer Res. 55:4808–4812. 1995.PubMed/NCBI
|
|
37
|
Bridgewater JA, Springer CJ, Knox RJ,
Minton NP, Michael NP and Collins MK: Expression of the bacterial
nitroreductase enzyme in mammalian cells renders them selectively
sensitive to killing by the prodrug CB1954. Eur J Cancer.
31:A2362–A2370. 1995. View Article : Google Scholar
|
|
38
|
Danks MK, Morton CL, Pawlik CA and Potter
PM: Overexpression of a rabbit liver carboxylesterase sensitizes
human tumor cells to CPT-11. Cancer Res. 58:20–22. 1998.PubMed/NCBI
|
|
39
|
Boerman OC, Oyen WJ and Corstens FH:
Progress in gene therapy: seeing is believing. J Nucl Med.
42:1235–1237. 2001.PubMed/NCBI
|
|
40
|
Huang TG, Savontaus MJ, Shinozaki K,
Sauter BV and Woo SL: Telomerase-dependent oncolytic adenovirus for
cancer treatment. Gene Ther. 10:1241–1247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishihara E, Nagayama Y, Mawatari F, et
al: Retrovirus-mediated herpes simplex virus thymidine kinase gene
transduction renders human thyroid carcinoma cell lines sensitive
to ganciclovir and radiation in vitro and in vivo. Endocrinology.
138:4577–4583. 1997. View Article : Google Scholar : PubMed/NCBI
|