Introduction
Head and neck cancer is one of the most common
malignancies, accounting for ~274,000 new cancer cases and 145,000
cancer-associated mortalities annually worldwide (1). In the United States alone, an
estimated 42,440 new cases are expected to occur and 8,390 patients
are likely to succumb to head and neck cancer in 2014 (2). Tongue squamous cell carcinoma (TSCC)
is the most common malignant tumor in the head and neck region. The
greatest risk factors for TSCC are heavy alcohol consumption and
tobacco smoking (1), while a diet
lacking fresh fruits and vegetables also increases the risk of TSCC
(1). The long-term survival rate
for head and neck cancer is ~50%, and TSCC is prone to early
metastasis due to the rich blood supply, abundant lymphatic
circulation, and frequent contraction of the genioglossus (3). Thus, it is imperative to identify the
molecular mechanisms of TSCC development and progression in order
to develop novel-targeted therapies to improve the overall survival
of patients.
TSCC development, as with most other human cancers,
involves the alteration of oncogenic activation and tumor
suppressor gene inactivation by a variety of carcinogens or
cancer-promoting factors (4,5).
Interleukin-18 (IL-18), originally known as interferon-γ inducing
factor (IGIF), is a pleiotropic proinflammatory cytokine (6) and is produced by various cells,
including T and B cells as well as a range of antigen-presenting
cells, such as activated monocytes, dendritic cells, and
macrophages. IL-18 regulates innate and adaptive immune responses
(7,8). Evidence has indicated that IL-18
exerts anticancer effects by inhibiting tumor angiogenesis and
growth (9,10). Combination therapies of IL-18 with
granulocyte-macrophage colony-stimulating factor can facilitate
tumor antigen presentation and induce proliferation of
tumor-specific T cells (11).
Moreover, IL-18-containing adjuvant therapy promoted the induction
of antitumor immune responses through the effective accumulation
and interaction of mature dendritic cells and naive T cells within
lymph nodes, which is driven by the ccr7-ccL19/ccL21 chemokine
axis, thus inducing adaptive T cell immunity (12). However, the defined molecular
mechanism remains to be determined. Glycogen synthase kinase 3
(GSK-3) is a ubiquitously expressed serine/threonine kinase in most
epithelial cells (13), initially
identified for its role in regulating glycogen synthesis (14,15).
In mammals, the following isoforms exist: GSK-3, GSK-3α and GSK-3β.
Their overall homology is ~85%, with differences in their C and N
termini. Their functions are closely associated and play a similar
role in several signaling pathways (16). For example, GSK-3β plays a major
role in epithelial cell homeostasis (17), and the activity is regulated by a
site-specific phosphorylation of Tyr216/Ser9 residues (18). GSK-3β regulates a diverse range of
cell functions, from cytoskeleton maintenance (19) to gene transcription (20,21).
Altered GSK-3β expression has been associated with cell
proliferation, migration, and invasion (14,22,23).
However, GSK-3β may act either as a tumor promoter or tumor
suppressor, depending on the cell context (24–26).
In the present study, we assessed the effects of IL-18 expression
on the regulation of TSCC cell viability and apoptosis and then
explored the underlying molecular events, which may in turn provide
a molecular basis for applying IL-18 as a novel agent for the
clinical treatment of tongue cancer.
Materials and methods
Cell lines and culture
The CRL-1623 TSCC cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA) and maintained
at 37°C in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and
Ham’s F12 medium (both from Invitrogen Life Technologies, Carlsbad,
CA, USA) containing 1.2 g/l sodium bicarbonate (Sigma-Aldrich, St.
Louis, MO, USA), 2.5 mM L-glutamine (Invitrogen Life Technologies),
15 mM HEPES, and 0.5 mM sodium pyruvate supplemented with 400 ng/ml
hydrocortisone (all from Sigma-Aldrich) and 10% fetal bovine serum
(PAA Laboratories, Pasching, Austria) in a humidity incubator with
5% CO2 and 95% air. The culture medium was refreshed
every 2–3 days. For cell subculturing, the cells were digested with
0.25% trypsin and 0.03% EDTA solution (Invitrogen Life
Technologies).
Construction of an expression vector
carrying human IL-18 cDNA and gene transfection (27)
Human IL-18 cDNA was cloned and amplified from
leukocytes (from one healthy donor including the entire coding
sequence of IL-18; NM-001562.2). The polymerase chain reaction
(PCR) primers were: forward, 5′-GGG GTA CCA TGG CTG CTG AAC CAG TAG
AAG-3′ and reverse, 5′-CCG CTC GAG AGC TAG TCT TCG TTT TGA ACA
GTG-3′ with the restriction enzymes KpnI and XhoI
link. The cDNA was then subcloned into a linearized PMD-18T vector
[Takara Biotechnology (Dalian) Co., Ltd., Dalian, China], digested
and released with KpnI and XhoI (Fermentas,
Burlington, Ontario, Canada), and then cloned into pcDNA3.1 (+)
vector (Invitrogen Life Technologies). After amplification and DNA
sequence confirmation, this vector was designated as pcDNA3.1-IL-18
and used for the overexpression of IL-18 in TSCC cells.
pcDNA3.1 and pCDNA3.1-IL-18 plasmids were separately
transfected into CRL-1623 cells using Lipofectamine 2000
(Invitrogen Life Technologies) according to the manufacturer’s
instructions, and stable cell lines were selected with 650 μg/ml
G418 (Invitrogen Life Technologies). IL-18 transgene expression and
its function were confirmed by quantitative reverse
transcription-PCR (RT-qPCR), western blot analysis, and
immunofluorescence analyses.
Reverse transcription-PCR (RT-PCR) and
RT-qPCR
Total RNA was isolated from CRL1623-Vec or
CRL1623-IL-18 cells using TRIzol reagent (Takara Biotechnology Co.,
Ltd.) following the manufacturer’s instructions and then subjected
to RT-PCR for detection of IL-18 mRNA expression. The PCR primers
for IL-18 consisted of: 5′-GGG GTA CCA TGG CTG CTG AAC CAG TAG
AAG-3′ and 5′-CCG CTC GAG AGC TAG TCT TCG TTT TGA ACA GTG-3′.
RT-PCR was performed by using 1 μg of total RNA samples in the
Access RT-PCR System (Promega, Madison, WI, USA) under the
following conditions: first-strand DNA was synthesized at 48°C for
45 min and then denatured at 94°C for 5 min for the first cycle but
for 30 sec for the additional 30 cycles; annealing at 55°C for 45
sec and extension at 72°C for 2 min; and a final extension at 72°C
for 8 min. The PCR products were then subjected to electrophoresis
in a 1.2% agarose gel and stained with ethidium bromide.
For RT-qPCR, cDNA was synthesized by using 0.5 μg of
total RNA with a SuperScriptIII CellsDirect cDNA Synthesis kit
(Invitrogen Life Technologies). The levels of IL-18, cyclin D1,
cyclin A1, IFN-γ, caspase-3, -7, and -9, and cytochrome c
mRNA were amplified in triplicate using the SYBR-Green Real-time
PCR master mix (Toyobo, Osaka, Japan) on a
LightCycler®480 Real-Time PCR system (Roche, Basel,
Switzerland). The level of β-actin mRNA was used as an internal
control in all the experiments. The primer sequences are listed in
Table I. The qPCR program was set
to an initial denaturation at 94°C for 2 min; then 40 cycles of
denaturation at 94°C for 10 sec, annealing at 60°C for 15 sec, and
extension at 72°C for 30 sec; and a final extension at 72°C for 5
min. The relative levels of gene expression were quantified by
using the comparative CT method of -ΔΔCt
(28).
 | Table IPrimer sequences used in the qPCR
experiments. |
Table I
Primer sequences used in the qPCR
experiments.
| Gene (gene
product) | Primer
sequences | Tm (°C) |
|---|
| IL-18 | 5′-CTT CCA GAT CGC
TTC CTC TC-3′
5′-TCA AAT AGA GGC CGA TTT CC-3′ | 60 |
| CCND1
(cyclin D1) | 5′-GTG CTG CGA AGT
GGA AAC C-3′
5′-ATC CAG GTG GCG ACG ATC T-3′ | 60 |
| CCNA1
(cyclin A1) | 5′-ACC CCA AGA GTG
GAG TTG TG-3′
5′-GGA AGG CAT TTT CTG ATC CA-3′ | 60 |
| IFNG
(IFN-γ) | 5′-CTC TTG GCT GTT
ACT GCC AGG-3′
5′-CTC CAC ACT CTT TTG GAT GCT-3′ | 60 |
|
Caspase-3 |
5′-CAAACTTTTTCAGAGGGGATCG-3′
5′-GCATACTGTTTCAGCATGGCAC-3′ | 60 |
|
Caspase-7 |
5′-TGAGCCACGGAGAAGAGAAT-3′
5′-TTTGCTTACTCCACGGTTCC-3′ | 60 |
|
Caspase-9 | 5′-ATG GAC GAA GCG
GAT CGG-3′
5′-CCCTGG CCT TAT GAT GTT-3′ | 60 |
Protein extraction and western blot
analysis
Cells were lysed in RIPA lysis buffer (50 mM
Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium
deoxycholate, 1 mM EDTA, 0.1% sodium dodecyl sulfate, 1 mM sodium
vanadate, 1 mM NaF, 1 mM phenylmethanesulfonyl fluoride, 0.1 mg/ml
pepstatin, 0.1 mg/ml leupeptin, and 0.1 mg/ml aprotinin). The
protein concentration was determined by using a bicinchoninic acid
protein assay. Protein lysates (40 μg) were then resolved by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE),
transferred onto polyvinylidene difluoride membranes (PVDF;
Bio-Rad, Hercules, CA, USA), and blotted with different primary
antibodies [anti-IL-18; Abcam, Cambridge, UK; anti-GSK-3β,
anti-phosphorylated GSK-3β (p-GSK-3β), anti-caspase-3, anti-cleaved
caspase-3, anti-caspase-7, anti-cleaved caspase-7; all from Cell
Signaling Technology, Boston, MA, USA] overnight at 4°C. The
membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies and visualized with an
ECL reagent (GE Healthcare, London, UK).
Immunofluorescence
The cells were seeded onto glass coverslips in
12-well plates and cultured overnight. The following day, the cells
were washed with phosphate-buffered saline (PBS), fixed in 4%
paraformaldehyde for 10 min at room temperature, and then
permeabilized with 0.2% Triton X-100. The cells were then blocked
with 2% bovine serum albumin in PBS for 30 min and incubated with
the primary antibodies for 1 h, followed by incubation with
FITC/TRITC-conjugated secondary antibodies for 1 h (ZSGB-BIO,
Beijing, China) or directly stained for F-actin by TRITC-phalloidin
(Sigma-Aldrich). Cell nuclei were counterstained with
4′,6-diamidino-2-phenylindole (Sigma-Aldrich). The coverslips were
observed under a fluorescence or confocal microscope.
Flow cytometric Annexin V/propidium
iodide (PI) apoptotic assay
The cells were trypsinized, washed once in ice-cold
PBS, and incubated with Annexin V-fluorescein/PI (Boehringer
Mannheim, Mannheim, Germany) in a calcium-containing HEPES buffer,
according to the manufacturer’s instructions. The cells were
immediately analyzed by fluorescence-activated cell sorting (FACS;
Becton-Dickinson, Franklin Lakes, NJ, USA). For cell cycle
analysis, the cells were fixed and stained by PI. The DNA content
of each cell population was then analyzed by FACS. DNA synthesis
was measured by bromodeoxyuridine (BrdU) incorporation. Briefly,
the cells were pulse-labeled in a medium containing BrdU
(Becton-Dickinson) for 2 h, then fixed in 70% ethanol, followed by
staining with fluorescein-conjugated anti-BrdU antibody
(Becton-Dickinson) and subsequent microscopic and FACS
analysis.
Giemsa staining
The cells were collected, placed onto glass slides,
and then fixed with 4% paraformaldehyde for 10 min at room
temperature. The slides were rinsed with sterile water and flooded
with freshly prepared Giemsa’s stain solution (BDH Chemicals Co.,
Poole, UK) for 5 min. After rinsing three times in sterile water,
the cells were examined for morphological changes under a
microscope (TMS; Nikon, Tokyo, Japan) at a magnification of
×200.
Caspase-3/7 activity assay
Caspase-3/7 activity was assessed using the
Apo-One® Homogeneous Caspase-3/7 assay kit (Promega),
according to the manufacturer’s instructions. Briefly, an equal
volume of reagents at room temperature was directly added to cell
culture plates that had been equilibrated to room temperature. The
plates were agitated at 500 rpm for 30 sec and measured for
fluorescent or luminescent output at various time points following
addition of the reagent (up to 18 h). Between readings, the plates
were stored at room temperature in the dark. Fluorescence for the
Apo-One® Homogeneous Caspase-3/7 assay was measured
using a BMG POLARstar fluorescence plate reader (BMG Labtech,
Ortenberg, Germany) with a 480/520 excitation/emission filter and a
gain setting of 25.
Cell viability MTT assay
To assess the altered cell viability, a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed. Briefly, the cells were seeded in 96-well
plates at 5×103 cells/well containing 180 μl of medium
and cultured for up to 96 h. At the end of each experiment, 20 μl
of MTT solution (5 mg/ml) was added into each well, and the cells
were incubated for 4 h at 37°C. The growth medium was replaced with
200 μl of dimethyl sulfoxide in each well, and the cells were
incubated for 10 min. The optical density value was measured by
using an MR-7000 microplate reader (Dynatech Laboratories Inc.,
Chantilly, VA, USA) at 570 nm. The median inhibition concentration
(IC50) values were calculated using the probity model, and the
inhibition rate of cell proliferation was calculated as: inhibition
rate (%) = 1 - A570 (test)/A570 (control) × 100%. Data were
calculated from three independent experiments, each performed in
triplicate.
Statistical analysis
Data were presented as mean ± standard deviation
(SD). The Student’s t-test (two-tailed) was performed to determine
the statistical significance of differences between groups.
P<0.05 was considered statistically significant. Statistical
analysis was carried out using SPSS17.0 software (SPSS, Chicago,
IL, USA).
Results
IL-18 overexpression reduced viability
and induced apoptosis of TSCC cells
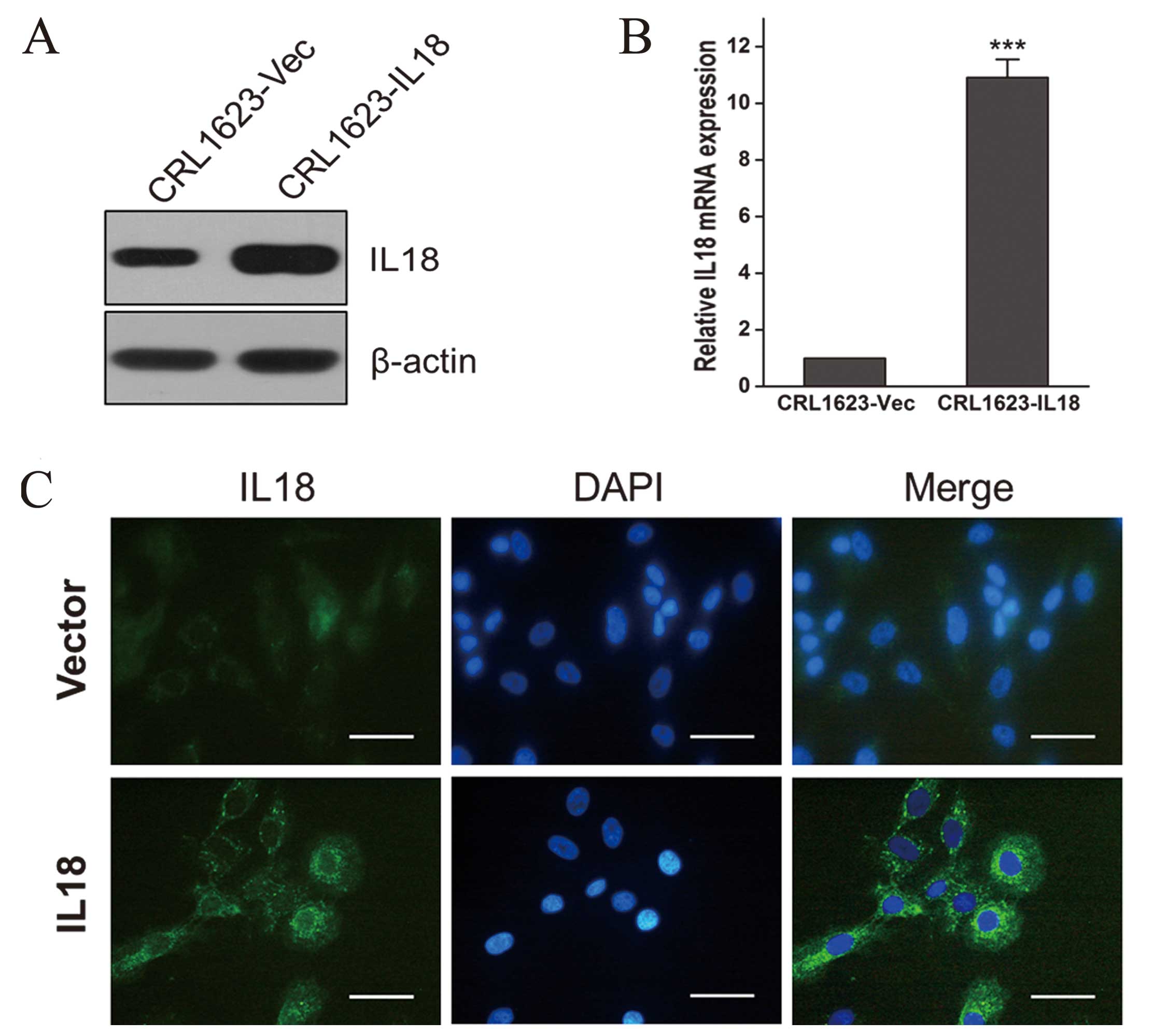
To overexpress IL-18 protein in TSCC cells, we
stably transfected pcDNA3.1 (+) -IL-18 (pIL-18) or control vector
[pcDNA3.1 (+)] in CRL1623 cells and performed immunofluorescence,
RT-qPCR, and western blot analysis experiments to confirm IL-18
expression. We found that CRL1623 cells overexpressed IL-18 mRNA
and protein (Fig. 1A and B) and
that IL-18 protein was mainly localized in the cytoplasm (Fig. 1C).
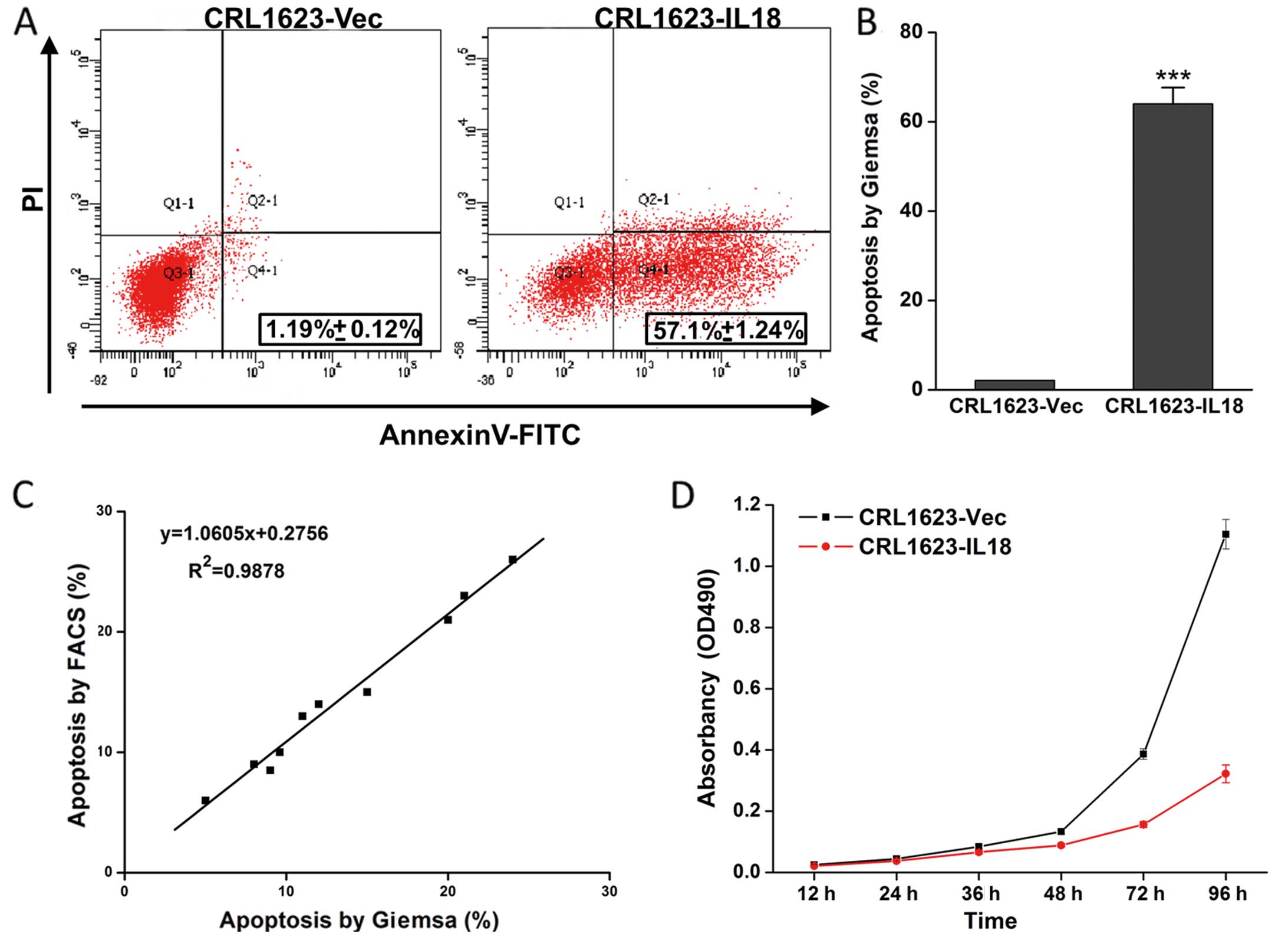
To assess the effect of IL-18 overexpression on TSCC
cells, we performed a cell viability assay and found that IL-18
expression reduced TSCC cell viability. Furthermore, the apoptosis
assay data showed that IL-18 induced TSCC cells to undergo
apoptosis (Fig. 2; P<0.05).
Overexpression of IL-18 protein modulated
the expression of apoptosis-associated genes
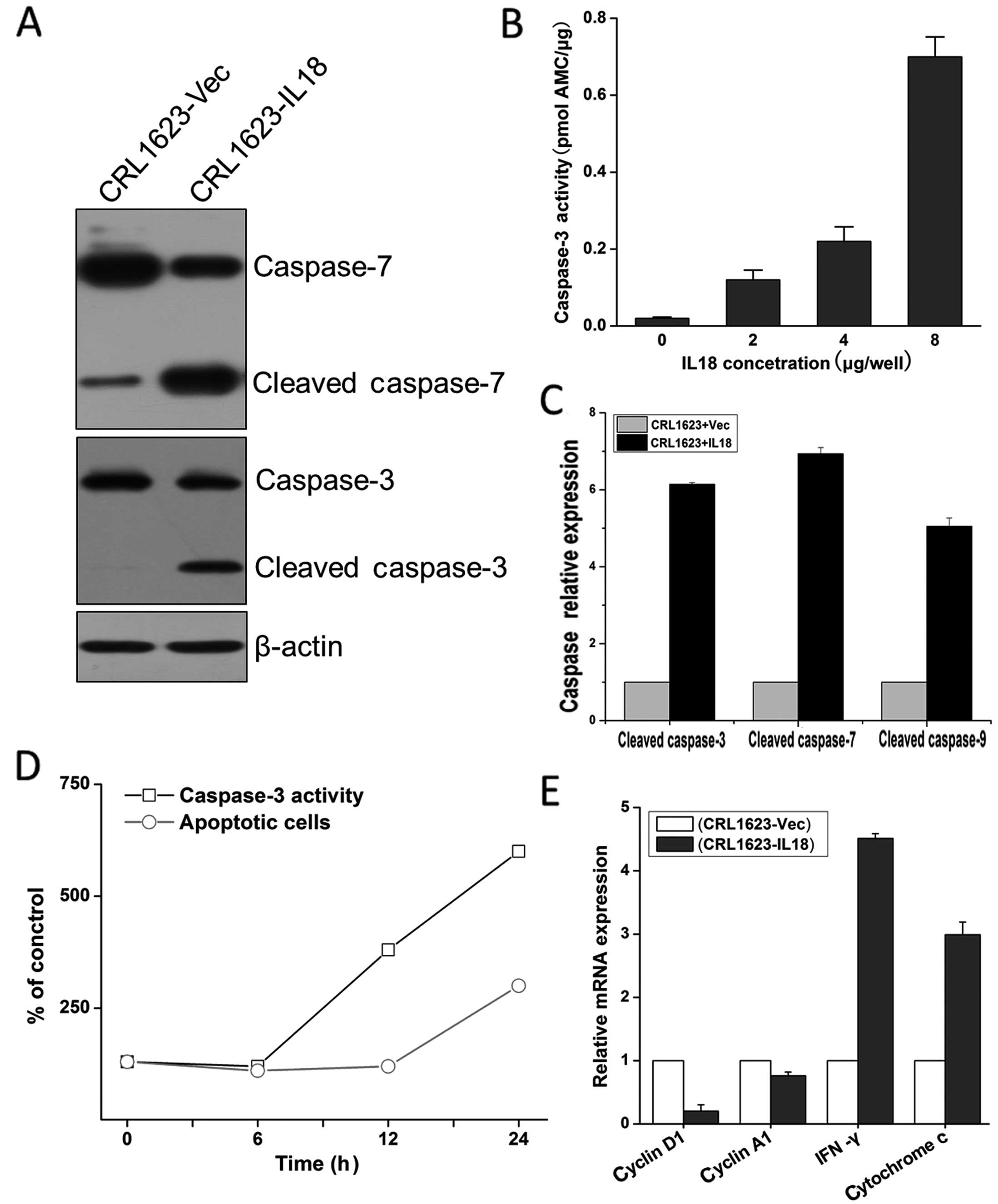
We assessed IL-18 protein modulation of
apoptosis-associated gene expression in TSCC cells. The data showed
that compared with cells transfected with the control vector, IL-18
expression activated caspase-3 and -7 (Fig. 3A and B) and subsequently induced
tumor cell apoptosis, as analyzed by flow cytometry (Fig. 3D). Further analysis showed that the
overexpression of IL-18 protein induced cleavage of caspase-3, -7,
and -9 and upregulated the expression of IFN-γ and cytochrome
c mRNA (Fig. 3C–E;
P<0.05), but reduced cyclin D1 (P<0.05) and A1 expression
(P>0.05) (Fig. 3E). These
results suggest that the overexpression of IL-18 induced caspase-
and cyclin-mediated cell apoptosis of TSCC cells.
GSK-3β activation mediated the effects of
IL-18 on TSCC cells
To further investigate the potential mechanisms of
IL-18 overexpression on the regulation of TSCC cell viability and
apoptosis, we detected the expression levels of GSK-3β and p-GSK-3β
proteins in CRL1623 cells. We found that the expression of p-GSK-3β
protein was upregulated and phosphorylated in CRL1623 cells
transfected with pIL-18 (Fig. 4A).
To further verify the inhibitory effect of activated GSK-3β on
tongue cancer, we used the selective GSK-3β inhibitor kenpaullone
(KP) to treat IL-18-transfected CRL1623 cells and found that KP
reduced GSK-3β phosphorylation and its activation, in turn
inhibiting the activity of caspase-3 and -7 (Fig. 4B). Moreover, cell viability (MTT)
and caspase activity assays, Giemsa staining, and RT-qPCR analysis
were performed to detect the effect of KP on CRL1623 cells. The
results confirmed that GSK-3β mediated the effects of IL-18 on TSCC
cells (Fig. 4C–E). We also observed
that the relative mRNA expression levels of cleaved caspase-3, -7,
and -9 were decreased following treatment with KP (Fig. 4F).
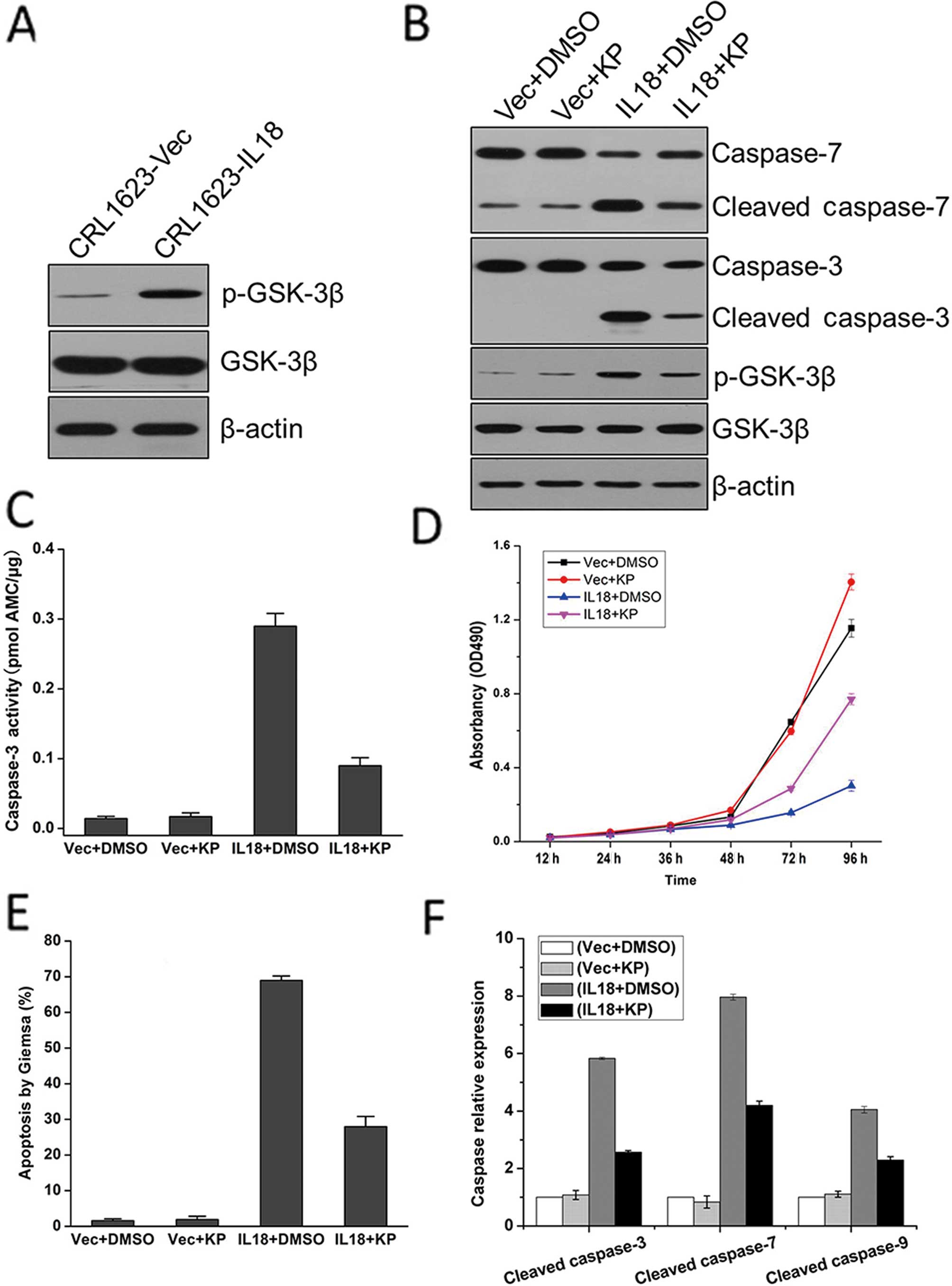 | Figure 4Expression of IL-18 protein inhibited
TSCC cell proliferation through GSK-3β phosphorylation. (A) Stable
IL-18-expressed CRL1623 cells or empty vector-control-transfected
cells were grown and subjected to immunoblot analysis of p-GSK-3β
and total GSK-3β protein levels. (B) Stable IL-18-expressed CRL1623
cells or empty vector-control-transfected cells were grown and
treated with 15 μM KP for 24 h and then subjected to immunoblot
analysis of p-GSK-3β, GSK-3β, caspase-3, and -7 and cleaved
caspase-3, and -7. (C) Stable IL-18-expressed CRL1623 cells or
empty vector-control-transfected cells were grown and treated with
15 μM KP for 24 h and then subjected to caspase-3 activity assay.
(D) Stable IL-18-expressed CRL1623 cells or empty
vector-control-transfected cells were grown and treated with 15 μM
KP for 24 h and then subjected to MTT assay. (E) Stable
IL-18-expressed CRL1623 cells or empty vector-control-transfected
cells were grown and treated with 15 μM KP for 24 h and then
subjected to Giemsa staining for detection of tumor cell apoptosis.
(F) Stable IL-18-expressed CRL1623 cells or empty
vector-control-transfected cells were grown and subjected to
RT-qPCR analysis of caspase-3, -7, and -9 mRNA. IL, interleukin;
RT-qPCR, quantitative reverse transcription-PCR; GSK, glycogen
synthase kinase; KP, kenpaullone. |
Discussion
Inactivation of the apoptotic pathway is one of the
features of tumor cells, and it may also be one of the important
mechanisms of the antitumor effect of IL-18. In the present study,
we assessed the effects of IL-18 expression by regulating the
viability and apoptosis of TSCC cells in vitro and then
explored the underlying molecular events. We found that IL-18
overexpression reduced viability and induced apoptosis of TSCC
cells. Moreover, we found that the overexpression of IL-18 protein
induced apoptosis-associated gene expression and its activation,
but inhibited cyclin D1 and A1 expression in TSCC cells. The
effects of IL-18 on TSCC cells were mediated by GSK-3β expression
and phosphorylation, whereas the selective GSK-3β inhibitor KP
antagonized the effects of IL-18 protein on TSCC cells. These
results, for the first time, provide evidence that IL-18
overexpression may be useful as a novel therapeutic approach for
tongue cancer treatment.
Previous studies have shown that IL-18 exhibits
significant antitumor activities by inducing IFN-γ expression in T
cells and natural killer cells (29), by ectopic expression of
hMSH2-induced oxidative stress (30), or via the modulation of cell cycle
progression leading to S-phase arrest (31). Data of those studies on IL-18
antitumor activity are consistent with our results, indicating that
IL-18 expression enhances the anticancer effects of TSCC. Moreover,
the combination of IL-12 (32),
IL-23 (33), or CpG (34) with IL-18 resulted in prominent tumor
growth inhibition. Thus, combination therapies of IL-18 with other
agents may improve their effects on the antitumor activity
(9,11).
Furthermore, to explore the potential mechanisms of
IL-18 antitumor activity in TSCC cells, we assessed the expression
of apoptosis- and cell cycle-associated genes as well as IFN-γ in
IL-18-overexpressed CRL1623 cells. We demonstrated that caspase-3,
-7, and -9 were activated in stable IL-18-transfected CRL1623
cells. The expression of IL-18 protein also upregulated IFN-γ
expression and reduced cyclin D1 and A1 expression. These data
indicate that IL-18 initiated the classical intrinsic apoptotic
pathway (also known as the mitochondrial apoptotic pathway)
(35). In normal human
keratinocytes, blockage of the IL-18 signaling pathway induced
mitochondrial damage or stress on the endoplasmic reticulum,
leading to the activation of caspase-3 and induction of apoptosis.
At the gene level, IL-18 was able to suppress activity of the
PI3K/Akt pathway (36). However, in
human cardiac endothelial cells, IL-18 expression has been
demonstrated to be accompanied by a decrease in anti-apoptotic
factors, such as Bcl-2 and Bcl-x, but an upregulation of Fas, FasL,
and caspase-3, -8, and -9 as well as cytochrome c (37). Moreover, chondrocyte apoptosis was
induced (38). Notably, Bcl-2
family proteins are known to determine the outcome of an intrinsic
apoptotic process (39). Caspase-3
has been identified as a key mediator of apoptosis by cleaving the
protein substrate poly (ADP-ribose) polymerase (PARP). The
inactivated PARP after cleavage can cause DNA fragmentation and
cell dysfunction, thus activated caspase-3 promotes cell death
(40). Furthermore, our results
also showed that IL-18 was a potent inducer of IFN-γ production.
IFN-γ is crucial for innate and acquired immunity against
intracellular pathogens as well as tumor control (41). Therefore, IL-18-induced IFN-γ
expression may be an important mechanism of IL-18 antitumor
activity in TSCC cells.
In addition, the cell cycle is a critical regulator
of cell proliferation and survival (42). Cyclin D1 is a multifunctional
oncoprotein and functions to regulate cell cycle progression
(43–45). Through activation of the
transcriptional factor E2F-1 by binding to cyclin-dependent kinase
4/6 (44), cyclin D1 promotes
transcription of the key cell cycle regulators, such as cyclin E
and A, to regulate the G1- to S-phase transition of the cell cycle.
Altered cyclin D1 expression contributes to the progression of
different tumors (46) and
participates in the invasion of head and neck squamous cell
carcinoma (4). A previous study has
shown that different growth factors or their receptors or
transcription factors (e.g., AP-1, NF-κB, and β-catenin) can
upregulate the expression of cyclin D1/E proteins and cyclin D1
protein stability/nuclear accumulation in oral squamous cell
carcinoma (25). In the present
study, IL-18 expression was able to reduce the levels of cyclin D1
mRNA in TSCC cells. However, how these cell cycle- and
apoptosis-associated genes are regulated by IL-18 remains to be
determined. In the present study, we found that IL-18
overexpression was able to induce GSK-3β expression and activation,
whereas the selective GSK-3β inhibitor KP antagonized the effects
of IL-18 expression in TSCC cells. Accumulating evidence suggests
that GSK3β is important in cell survival and resistance to
apoptosis (47,48). Activated GSK3β protein can block the
cAMP response element-binding-dependent expression of the
anti-apoptotic protein Bcl-2 (49).
By contrast, inactivation of the GSK3β protein (GSK-3β
phosphorylation at Ser9) inhibited MPTP opening and inactivated the
cytochrome c-caspase-3/9 apoptotic pathway, leading to
resistance to apoptosis (50).
Modulation of GSK3β expression can markedly increase p53-dependent
activation of Bax, leading to cytochrome c release and
initiation of the intrinsic apoptotic pathway (51). Again, GSK-3β can also regulate
cyclin D1 expression (52).
Phosphorylation of cyclin D1 protein on Thr286 by GSK-3β protein
facilitated cyclin D1 binding to CRM1, a nuclear protein that
mediates protein nuclear export, for exclusion of cyclin D1 protein
from the nucleus and proteasomal degradation (53). GSK-3β protein also suppresses cyclin
D1 transcription through inactivation of β-catenin, the
transcription factor of cyclin D1 (54). Findings of a recent study have
demonstrated that perinatal exposure to BDE-99 produces a decrease
in cyclin D1 protein levels in rat pup livers by altering the
Akt/GSK3β pathway, and the decrease may be due to disruption of the
non-genomic actions of thyroid hormone (TH) by BDE-99 and its
metabolites (55).
In summary, IL-18 activated GSK3β by site-specific
phosphorylation of Tyr216 residues to target intrinsic pathways and
cyclin D1 expression, thus inhibiting TSCC cell growth by promoting
apoptosis. GSK3β is central to numerous signaling pathways. Thus,
future studies are to focus on how IL-18 expression can activate
GSK3β protein to possess IL-18 antitumor activity in TSCC
cells.
Acknowledgements
We would like to thank Dr. Daxin Pang of Animal
Science and Veterinary Medicine, Jilin University (Changchun,
China) for technical support and helpful discussions. This study
was supported in part by grants from the Bethune Medical Research
Support Program of Jilin University, Health and Family Planning
Commission research projects of Jilin Province, Jilin Province
Department of Education Science and Technology Research Plan, and
Jilin Province Science and Technology Development Plan. We thank
Medjaden Bioscience Limited for assisting in the preparation of
this manuscript.
References
|
1
|
World Health Organization and
International Agency for Research on Cancer. World Cancer Report
2008. International Agency for Research on Cancer; Lyon: pp. 1–511.
2008
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bello IO, Soini Y and Salo T: Prognostic
evaluation of oral tongue cancer: means, markers and perspectives
(II). Oral Oncol. 46:636–643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giudice FS, Dal Vechio AM, Abrahao AC,
Sperandio FF and dos Pinto-Junior DS: Different expression patterns
of pAkt, NF-κB and cyclin D1 proteins during the invasion process
of head and neck squamous cell carcinoma: an in vitro approach. J
Oral Pathol Med. 40:405–411. 2011. View Article : Google Scholar
|
|
5
|
Squarize CH, Castilho RM, Abrahao AC,
Molinolo A, Lingen MW and Gutkind JS: PTEN deficiency contributes
to the development and progression of head and neck cancer.
Neoplasia. 15:461–471. 2013.PubMed/NCBI
|
|
6
|
Okamura H, Tsutsui H, Kashiwamura S,
Yoshimoto T and Nakanishi K: Interleukin-18: a novel cytokine that
augments both innate and acquired immunity. Adv Immunol.
70:281–312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Srivastava S, Salim N and Robertson MJ:
Interleukin-18: biology and role in the immunotherapy of cancer.
Curr Med Chem. 17:3353–3357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tschoeke SK, Oberholzer A and Moldawer LL:
Interleukin-18: a novel prognostic cytokine in bacteria-induced
sepsis. Crit Care Med. 34:1225–1233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HR, Yoon SY, Song SB, et al:
Interleukin-18-mediated interferon-gamma secretion is regulated by
thymosin beta 4 in human NK cells. Immunobiology. 216:1155–1162.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tse BW, Russell PJ, Lochner M, Forster I
and Power CA: IL-18 inhibits growth of murine orthotopic prostate
carcinomas via both adaptive and innate immune mechanisms. PloS
One. 6:e242412011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian H, Shi G, Yang G, et al: Cellular
immunotherapy using irradiated lung cancer cell vaccine
co-expressing GM-CSF and IL-18 can induce significant antitumor
effects. BMC Cancer. 14:482014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong JL, Muthuswamy R, Bartlett DL and
Kalinski P: IL-18-based combinatorial adjuvants promote the
intranodal production of CCL19 by NK cells and dendritic cells of
cancer patients. Oncoimmunology. 2:e262452013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Korur S, Huber RM, Sivasankaran B, et al:
GSK3beta regulates differentiation and growth arrest in
glioblastoma. PloS One. 4:e74432009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Atkins RJ, Dimou J, Paradiso L, et al:
Regulation of glycogen synthase kinase-3 beta (GSK-3β) by the Akt
pathway in gliomas. J Clin Neurosci. 19:1558–1563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grimes CA and Jope RS: The multifaceted
roles of glycogen synthase kinase 3beta in cellular signaling. Prog
Neurobiol. 65:391–426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi-Yanaga F: Activator or
inhibitor? GSK-3 as a new drug target. Biochem Pharmacol.
86:191–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim M, Datta A, Brakeman P, Yu W and
Mostov KE: Polarity proteins PAR6 and aPKC regulate cell death
through GSK-3beta in 3D epithelial morphogenesis. J Cell Sci.
120:2309–2317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doble BW and Woodgett JR: GSK-3: tricks of
the trade for a multi-tasking kinase. J Cell Sci. 116:1175–1186.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshimura T, Kawano Y, Arimura N, Kawabata
S, Kikuchi A and Kaibuchi K: GSK-3beta regulates phosphorylation of
CRMP-2 and neuronal polarity. Cell. 120:137–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu C, Li Y, Semenov M, et al: Control of
beta-catenin phosphorylation/degradation by a dual-kinase
mechanism. Cell. 108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu G and He X: Threonine 41 in
beta-catenin serves as a key phosphorylation relay residue in
beta-catenin degradation. Biochemistry. 45:5319–5323. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu Z, Sun D and Young W: Lithium promotes
neural precursor cell proliferation: evidence for the involvement
of the non-canonical GSK-3β-NF-AT signaling. Cell Biosci. 1:182011.
View Article : Google Scholar
|
|
23
|
Zhang X, Chen T, Zhang J, et al: Notch1
promotes glioma cell migration and invasion by stimulating
β-catenin and NF-κB signaling via AKT activation. Cancer Sci.
103:181–190. 2012. View Article : Google Scholar
|
|
24
|
Lu W and Li Y: Salinomycin suppresses LRP6
expression and inhibits both Wnt/β-catenin and mTORC1 signaling in
breast and prostate cancer cells. J Cell Biochem. 155:1799–1807.
2014. View Article : Google Scholar
|
|
25
|
Mishra R: Glycogen synthase kinase 3 beta:
can it be a target for oral cancer. Mol Cancer. 9:1442010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wen W, Ding J, Sun W, et al: Cyclin
G1-mediated epithelial-mesenchymal transition via phosphoinositide
3-kinase/Akt signaling facilitates liver cancer progression.
Hepatology. 55:1787–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu W, Han B, Sun B, Gao Y, Huang Y and Hu
M: Overexpression of interleukin-18 induces growth inhibition,
apoptosis and gene expression changes in a human tongue squamous
cell carcinoma cell line. J Int Med Res. 40:537–544. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Zheng JN, Pei DS, Sun FH, et al: Potent
antitumor efficacy of interleukin-18 delivered by conditionally
replicative adenovirus vector in renal cell carcinoma-bearing nude
mice via inhibition of angiogenesis. Cancer Biol Ther. 8:599–606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mo C, Dai Y, Kang N, Cui L and He W:
Ectopic expression of human MutS homologue 2 on renal carcinoma
cells is induced by oxidative stress with interleukin-18 promotion
via p38 mitogen-activated protein kinase (MAPK) and c-Jun
N-terminal kinase (JNK) signaling pathways. J Biol Chem.
287:19242–19254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nilkaeo A and Bhuvanath S: Role of
interleukin-18 in modulation of oral carcinoma cell proliferation.
Mediators Inflamm. 2006:671202006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shiratori I, Suzuki Y, Oshiumi H, et al:
Recombinant interleukin-12 and interleukin-18 antitumor therapy in
a guinea-pig hepatoma cell implant model. Cancer Sci. 98:1936–1942.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Kobayashi Y, Sato A, Kobayashi E
and Murakami T: Synergistic anti-tumor effect by combinatorial
gene-gun therapy using IL-23 and IL-18 cDNA. J Dermatol Sci.
36:66–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chaudhry UI, Kingham TP, Plitas G, Katz
SC, Raab JR and DeMatteo RP: Combined stimulation with
interleukin-18 and CpG induces murine natural killer dendritic
cells to produce IFN-gamma and inhibit tumor growth. Cancer Res.
66:10497–10504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu TP, Shen H, Liu LX and Shu YQ:
Plumbagin from Plumbago Zeylanica L induces apoptosis in human
non-small cell lung cancer cell lines through NF-κB inactivation.
Asian Pac J Cancer Prev. 14:2325–2331. 2013. View Article : Google Scholar
|
|
36
|
Hosotani Y, Kashiwamura S, Kimura-Shimmyo
A, et al: Interleukin-18 prevents apoptosis via PI3K/Akt pathway in
normal human keratinocytes. J Dermatol. 35:514–524. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chandrasekar B, Vemula K, Surabhi RM, et
al: Activation of intrinsic and extrinsic proapoptotic signaling
pathways in interleukin-18-mediated human cardiac endothelial cell
death. J Biol Chem. 279:20221–20233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
John T, Kohl B, Mobasheri A, Ertel W and
Shakibaei M: Interleukin-18 induces apoptosis in human articular
chondrocytes. Histol Histopathol. 22:469–482. 2007.PubMed/NCBI
|
|
39
|
Jin H, Liu AD, Holmberg L, et al: The role
of sulfur dioxide in the regulation of mitochondrion-related
cardiomyocyte apoptosis in rats with isopropylarterenol-induced
myocardial injury. Int J Mol Sci. 14:10465–10482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schoenborn JR and Wilson CB: Regulation of
interferon-gamma during innate and adaptive immune responses. Adv
Immun. 96:41–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu G, Li Y, Yoshimoto K, et al:
2,3,7,8-Tetrachlorodibenzo-pdioxin stimulates proliferation of HAPI
microglia by affecting the Akt/GSK-3β/cyclin D1 signaling pathway.
Toxicol Lett. 224:362–370. 2014. View Article : Google Scholar
|
|
43
|
Bienvenu F, Jirawatnotai S, Elias JE, et
al: Transcriptional role of cyclin D1 in development revealed by a
genetic-proteomic screen. Nature. 463:374–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Motokura T, Bloom T, Kim HG, et al: A
novel cyclin encoded by a bcl1-linked candidate oncogene. Nature.
350:512–515. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Witzel II, Koh LF and Perkins ND:
Regulation of cyclin D1 gene expression. Biochem Soc Trans.
38:217–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Beurel E and Jope RS: The paradoxical pro-
and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic
apoptosis signaling pathways. Prog Neurobiol. 79:173–189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin
O and Woodgett JR: Requirement for glycogen synthase kinase-3beta
in cell survival and NF-kappaB activation. Nature. 406:86–90. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Belkhiri A, Dar AA, Zaika A, Kelley M and
El-Rifai W: t-Darpp promotes cancer cell survival by up-regulation
of Bcl2 through Akt-dependent mechanism. Cancer Res. 68:395–403.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Q, Fu H, Zhang H, et al: Hydrogen
sulfide preconditioning protects rat liver against
ischemia/reperfusion injury by activating Akt-GSK-3β signaling and
inhibiting mitochondrial permeability transition. PLoS One.
8:e744222013. View Article : Google Scholar
|
|
51
|
Tan J, Zhuang L, Leong HS, Iyer NG, Liu ET
and Yu Q: Pharmacologic modulation of glycogen synthase
kinase-3beta promotes p53-dependent apoptosis through a direct
Bax-mediated mitochondrial pathway in colorectal cancer cells.
Cancer Res. 65:9012–9020. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee Y, Dominy JE, Choi YJ, et al: Cyclin
D1-Cdk4 controls glucose metabolism independently of cell cycle
progression. Nature. 510:547–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Takahashi-Yanaga F and Sasaguri T:
GSK-3beta regulates cyclin D1 expression: a new target for
chemotherapy. Cell Signal. 20:581–589. 2008. View Article : Google Scholar
|
|
54
|
Ye X, Guo Y, Zhang Q, et al: βKlotho
suppresses tumor growth in hepatocellular carcinoma by regulating
Akt/GSK-3β/cyclin D1 signaling pathway. PLoS One. 8:e556152013.
View Article : Google Scholar
|
|
55
|
Blanco J, Mulero M, Domingo JL and Sanchez
DJ: Perinatal exposure to BDE-99 causes decreased protein levels of
cyclin D1 via GSK3β activation and increased ROS production in rat
pup livers. Toxicol Sci. 137:491–498. 2014. View Article : Google Scholar
|