Introduction
Epidemiologic studies indicate that green tea
consumption decreases cancer risk (1–3).
Different tea preparations contain varying amounts of polyphenols
and epigallocatechin gallate (EGCG) is the most abundant,
best-studied and possibly most potent polyphenol against cancer
found in green tea (4–6). EGCG reportedly exerts cancer
preventive activity at a variety of organ sites, including oral
cavity, esophagus, stomach, colon, skin, lung, pancreas and mammary
gland (5,7–10).
Previous studies suggested that EGCG affects different signal
transduction pathways, including inhibition of various protein
kinases (11,12), suppression of the activation of
transcription factors such as AP-1 (13,14)
and NF-κB (15,16), blockade of growth factor
receptor-mediated pathways, and induction of cell-cycle arrest or
apoptosis (16,17). EGCG was also found to inhibit cell
transformation (18), and repress
angiogenesis in various cancer models (8,19,20).
However, the mechanisms explaining the cancer preventive activity
of EGCG remain to be elucidated.
Carcinoma of the oral tongue is the most common
cancer in the oral cavity (21).
The cause and incidence of tongue carcinoma are associated with
different factors, such as genetic (mutations in DNA oncogenes
and/or tumor-suppressor genes) and environmental (chemical,
physical, smoke and alcohol) (21–23).
Oral tongue carcinoma has a high propensity of lymph-node
metastasis, even in early local diseases. The incidence of occult
nodal metastasis is reportedly 30–40% (24–26).
Treatment of tongue carcinoma and conservation of important
functions such as language articulation, swallowing and respiration
remain a challenge. The overall 5-year disease-free survival rate
of tongue carcinoma has not changed substantially over the past
decades and has been estimated to be ~50% (26,27). A
major challenge in treating tongue carcinoma is to identify novel
therapeutic targets or develop new anticancer agents that can
complement current surgical treatment.
In general, even in the presence of oxygen, most
tumor cells are predisposed to consume glucose through the
glycolytic pathway as their major energy source to rapidly generate
ATP and biosynthetic intermediates to sustain unlimited growth
(28). This phenomenon, which is
defined as the Warburg effect, has been consistently observed in
most types of cancer, and is considered to be an emerging hallmark
of cancer (29). The canonical
oncogenic signaling pathways and transcription factors, including
PI3-K/Akt, c-Myc and hypoxia-inducible factor 1 (HIF1), are
involved in the regulation of numerous genes that are responsible
for the metabolic difference in the solid tumor, such as hexokinase
2 (HK2), lactate dehydrogenase A and cell transporters (30–32).
HK2, a rate-limiting enzyme of glycolysis, is upregulated in a
number of cancers. HK2 overexpression is associated with greater
glucose consumption and increased macromolecular biosynthesis. HK2
now has been recognized as a therapeutic target in various types of
cancer (33–35).
In the present study, we first reported that HK2 is
a key modulator of EGCG-induced glycolysis suppression in human
tongue carcinoma cells. We evaluated its underlying mechanism of
action and demonstrated that the Akt signaling pathway is involved.
The findings suggest that targeting metabolic enzymes may be a
novel preventive and therapeutic target in this type of tumor.
Materials and methods
Cell culture and transfection
The Tca8113 and TSCCa human tongue squamous cell
carcinoma cell lines were purchased from the China Center for Type
Culture Collection (Wuhan University) and cultured with Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% fetal bovine serum
(FBS) and 1% antibiotics. For transfection experiments, the
Lipofectamine™ 2000 transfection reagent (Invitrogen, Carlsbad, CA,
USA) was used according to the manufacturer’s instructions.
Reagents and antibodies
EGCG was obtained from Sigma (St. Louis, MO, USA),
and Myr-Akt1 was purchased from Addgene (Cambridge, MA, USA).
Wortmannin, PD98059, anti-p-EGFR (Tyr1068), anti-Akt (pan),
anti-Akt1, anti-p-Akt (S473), anti-ERK1/2, anti-p-ERK1/2
(Thr202/Tyr204), anti-HK2, anti-cleaved-PARP, anti-caspase3 and
anti-α-tubulin antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Anti-β-actin, anti-rabbit
IgG-HRP and anti-mouse IgG-HRP were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-VDAC was purchased
from Abcam (Cambridge, UK).
Measurement of glucose uptake and lactate
production
Cells (5×105) were seeded in 6-well
plates. After incubation for 4 h, the culture medium was discarded
and the cells were incubated in fresh medium for another 8 h.
Glucose and lactate levels were measured using the Automatic
biochemical analyzer (AU680; Beckman Coulter International, Brea,
CA, USA) at the Clinical Biochemical Laboratory of Xiangya Hospital
(Changsha, China). The relative glucose consumption and lactate
production rates were normalized by the protein concentration of
samples.
Western blotting
Cells were harvested by trypsinization and pelleted
by centrifugation. Cell pellets were lysed in Nonidet P-40 cell
lysis buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 0.5% Nonidet P-40
and protease inhibitor mixture). Protein concentrations were
determined using the Bradford assay (Bio-Rad, Hercules, CA, USA).
Proteins were separated by SDS-PAGE and electrically transferred to
a polyvinylidenedifluoride membrane (Millipore, Billerica, MA,
USA). After blocking in 5% non-fat dry milk in TBS, the membranes
were hybridized to specific primary antibodies overnight at 4°C,
washed three times with TBS Tween-20, and then incubated with
secondary antibodies conjugated with horseradish peroxidase for 1 h
at room temperature. The membranes were then washed three times in
TBS Tween-20 at room temperature. The protein bands were visualized
using ECL chemiluminescence reagents (Pierce Chemical Co.,
Rockford, IL, USA) according to the manufacturer’s
instructions.
Isolation of mitochondrial fractions
Following EGCG treatments, ~5×106 cells
from a 10-cm plate were harvested by trypsinization and centrifuged
at 800 rpm for 5 min at 4°C. The cell pellets were washed once with
ice-cold PBS and then resuspended in three volumes of isolation
buffer (20 mM HEPES, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1
mM sodium EDTA, 1 mM dithiothreitol, 10 mM phenylmethylsulfonyl
fluoride, 10 mM leupeptin and 10 mM aprotinin) in 250 mM sucrose.
After chilling on ice for 3 min, the cells were disrupted by 60
strokes of a glass homogenizer. The homogenate was centrifuged once
at 2,000 rpm at 4°C for 10 min to remove unbroken cells and nuclei.
The mitochondria-enriched fraction (supernatant) was then pelleted
by centrifugation at 13,000 rpm for 30 min. The pellets were lysed
in RIPA buffer [10 mM Tris-Cl (pH 8.0), 1 mM EDTA, 0.5 mM EGTA, 1%
Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl], and
analyzed by western blotting.
Soft agar colony formation assay
To assess the anchorage-independent growth, tongue
carcinoma cells were suspended (8×103 cells/ml) in 1 ml
of 0.3% agar with Eagle’s basal medium containing 10% FBS, 1%
antibiotics, and different concentrations of EGCG (0, 20, 40 and 80
μM/l) overlaid in 6-well plates containing a 0.6% agar base. The
cultures were maintained in a 37°C, 5% CO2 incubator for
1–2 weeks, and then colonies were counted under a microscope using
the Image-Pro Plus software program (Media Cybernetics, Silver
Spring, MD, USA).
Statistical analysis
Statistical analyses were performed using the SPSS
software (version 13.0). The experiments were performed in
triplicate. Quantitative data are presented as means ± standard
deviation. Significant differences between two groups were assessed
by a two-tailed Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
EGCG inhibits the anchorage-independent
growth of human tongue carcinoma cells
Anchorage-independent growth is one of the malignant
phenotypes of tumor cells and is considered to be one of the most
accurate and stringent in vitro assays for detecting the
malignant transformation of cells. Therefore, we first investigated
the effect of EGCG on the anchorage-independent growth of human
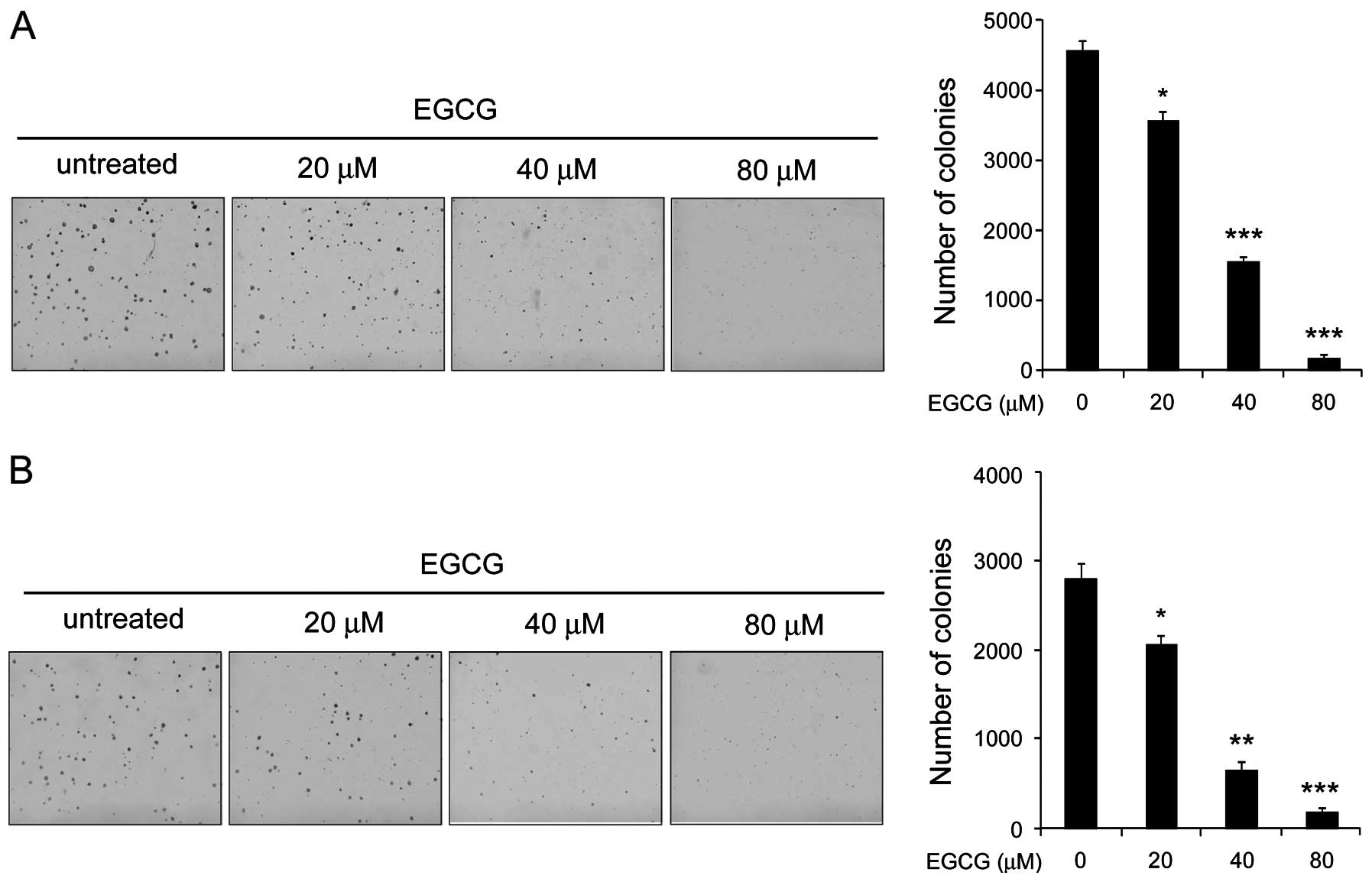
tongue carcinoma cells. Our results demonstrated that although EGCG
had little effect on colony formation at 20 μM, the inhibition rate
reached >60% after Tca8113 cells exposure to EGCG at the
concentration of 40 μM. More importantly, 80 μM of EGCG almost
blocked the colony growth in soft agar (Fig. 1A). We also found that EGCG had a
similar effect on the suppression of the anchorage-independent
growth of TSCCa cells in soft agar (Fig. 1B). These data indicated that EGCG
inhibits anchorage-independent growth of human tongue carcinoma
cells in a dose-dependent manner.
EGCG downregulates HK2 expression and
decreases human tongue carcinoma cell glycolysis
Elevated glycolysis is considered to be an emerging
hallmark of human cancer. HK2, a rate-limiting enzyme of
glycolysis, is upregulated in various cancers (31). Thus, in the present study, we
investigated whether EGCG-mediated antitumor activity is associated
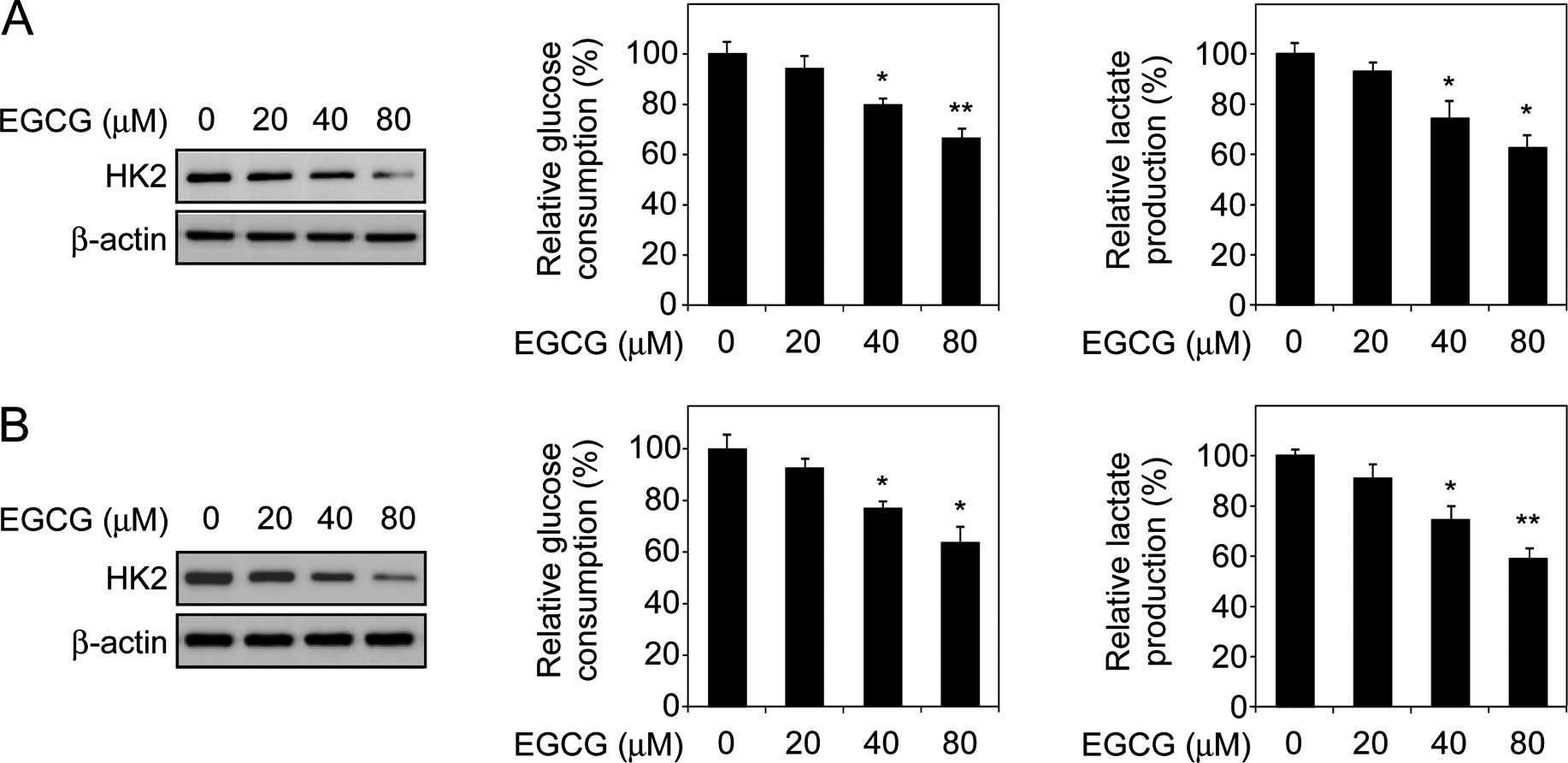
with tongue carcinoma cell glycometabolism. Immunoblot analysis
indicated that the HK2 protein level decreased in response to EGCG
treatment in Tca8113 cells (Fig.
2A, left) and a similar result was also observed in TSCCa cells
(Fig. 2B, left). To assess the
effect of EGCG on glycolysis, we examined the level of glucose
consumption and lactate production in the two human tongue
carcinoma cells. Consistent with the results of the immunoblot
analysis, EGCG dose-dependently inhibited the consumption of
glucose (Fig. 2A and B, middle) and
production of lactate (Fig. 2A and
B, right) in the Tca8113 and TSCCa cells. These results
suggested that EGCG-mediated tumor suppression is partially
dependent on glycolysis inhibition, and HK2 is involved in this
process.
EGCG decreases HK2 expression by
downregulating the EGFR-Akt signaling pathway
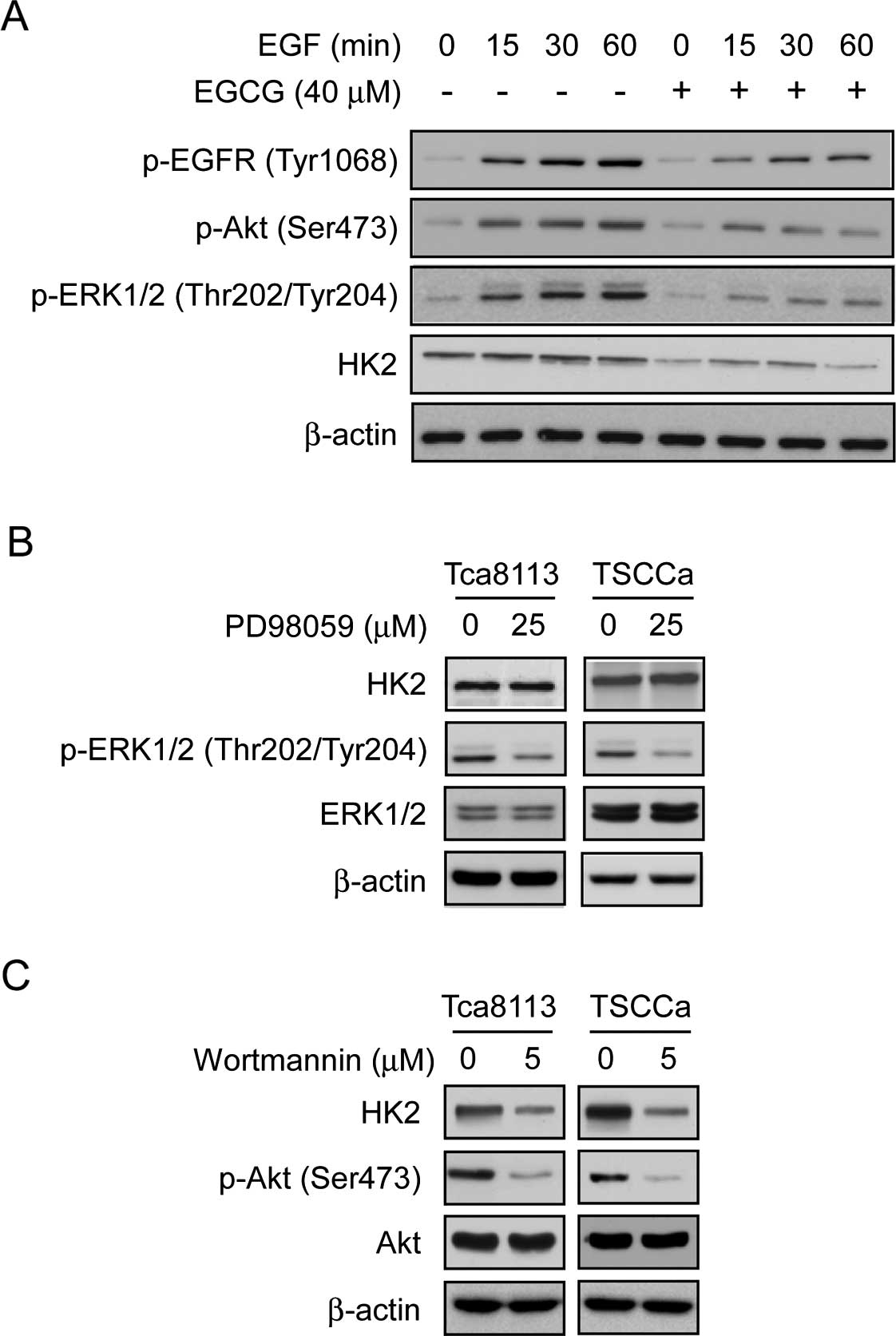
Findings of recent studies demonstrated that the Akt
signaling pathway is a hub in the regulation of cancer metabolism
(36,37). As an Akt upstream receptor tyrosine
kinase, the oncoprotein EGFR is always overexpressed in human
tongue carcinoma (38). The results
showed that EGCG strongly inhibited EGF-induced EGFR activation and
EGCG also significantly inhibited the phosphorylation of EGFR
downstream kinases Akt and ERK1/2. Furthermore, we found that the
protein levels of HK2 were inhibited following EGCG treatment
(Fig. 3A). To determine the
connection between ERKs/Akt and HK2 suppression in the presence of
EGCG, we used PD98059, a specific inhibitor of the ERKs pathway, to
treat Tca8113 and TSCCa cells and examined whether HK2 expression
would be affected by the inhibition of the ERKs signaling pathway
in these cells. Results indicated that treatment with PD98059
markedly decreased the phosphorylation level of ERKs, however,
there was no obvious effect on the expression of HK2 (Fig. 3B). By contrast, wortmannin, a
specific inhibitor of the Akt pathway, not only substantially
inhibited Akt activity, but also strongly suppressed HK2 expression
in these cells (Fig. 3C). These
results indicated that Akt, but not ERKs, was a key kinase of the
EGCG-mediated downregulation of HK2.
Constitutively activated Akt1 rescues
EGCG-mediated glycolysis inhibition
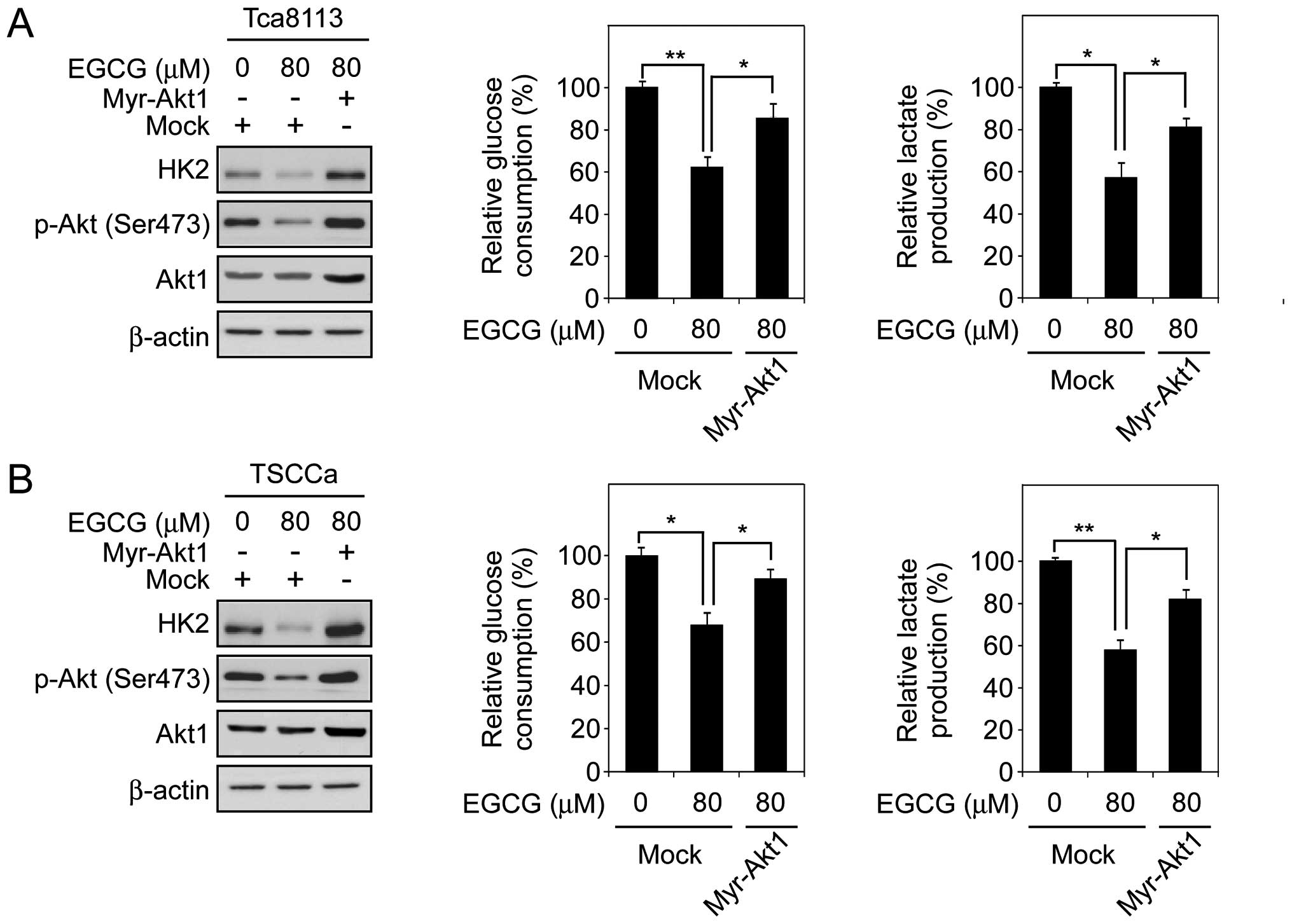
Based on the previous data, in order to confirm that
the regulation of glycolysis by EGCG in human tongue carcinoma
cells is dependent on Akt activity, we transfected constitutively
activated Akt1 (Myr-Akt1) into Tca883 and TSCCa cells. The results
indicated that 80 μM EGCG significantly decreased the expression of
HK2 in Tca8113 and TSCCa cells. However, Myr-Akt1 transfection
markedly upregulated HK2 expression in these EGCG-treated cells as
expected (Fig. 4A and B, left).
Moreover, Myr-Akt1 rescued over 70 and 60% of the deficient glucose
uptake (Fig. 4A and B, middle) and
lactate production (Fig. 4A and B,
right) in EGCG-treated Tca8113 and TSCCa cells, respectively. These
results suggested that EGCG regulates glycolysis in human tongue
carcinoma cells, and these biochemical processes are partly
mediated by Akt activation.
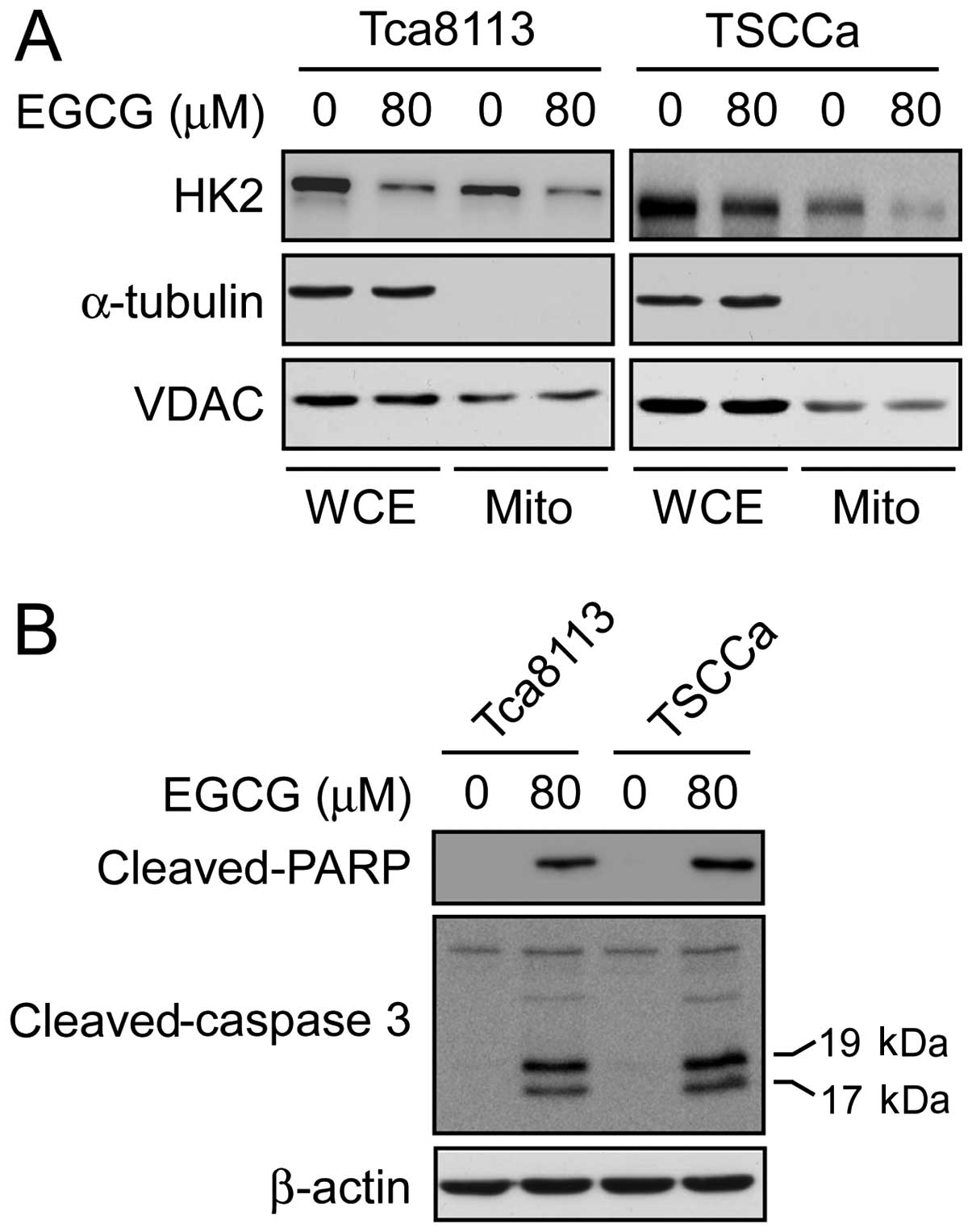
EGCG inhibits HK2 expression on
mitochondrial outer membrane and induces apoptosis
The HK2 protein is crucial in cell proliferation as
well as apoptosis resistance. In cancer cells, HK2 overexpression
is associated with hyperactivated glycolysis and upregulated cell
growth. More importantly, for HK2 to exert its anti-apoptotic
function, HK2 translocates to the mitochondrial outer membrane and
interacts with the voltage-dependent anion channel (VDAC) to block
the release of cytochrome c, eventually inhibiting the
apoptotic pathway (39–42). In order to assess the influence of
EGCG on the subcellular localization of HK2, mitochondrial
fractions from Tca8113 and TSCCa cells, with or without 80 μM EGCG
treatment, were extracted and analyzed by immunoblotting with
antibodies to detect HK2, α-tubulin and VDAC. The results indicated
that the translocation of HK2 in the mitochondrial outer membrane
fraction was decreased in the Tca8113 and TSCCa cells following
EGCG treatment (Fig. 5A).
Furthermore, we evaluated the apoptotic signaling pathway by
immunoblotting to examine the cleaved caspase-3 and cleaved
poly(ADP-ribose) polymerase (PARP) expression after EGCG treatment.
The results demonstrated that the levels of cleaved-caspase-3 and
cleaved-PARP in Tca8113 and TSCCa cells were markedly increased in
response to EGCG treatment (Fig.
5B). Based on these data, we suggested that the EGCG-mediated
downregulation of HK2 inhibits anti-apoptotic effects of human
tongue carcinoma cells.
Discussion
Oral cavity cancer is ranked as the sixth most
widespread cancer type worldwide. Carcinoma of the oral tongue, the
most common cancer in the oral cavity, exhibits invasion and/or
metastasis at a very early stage. Surgery, radiotherapy,
chemotherapy and combined modalities are the main treatment options
for the tongue carcinoma (21–24).
However, no molecular-targeted therapeutic drugs against tongue
carcinoma are currently licensed, and the prevention strategies to
decrease the incidence remain elusive. The limited therapeutic
options provide a strong stimulus for the development of novel
therapeutics. Preclinical studies have consolidated activity of
EGCG in cancer prevention and, it was shown that inhibition of
EGCG-induced proliferation and induction of apoptosis played an
important role in its antitumor activity (5). However, how the underlying mechanisms
of EGCG exert a tumor-suppressor effect through the metabolic
pathways remains to be determined. We have demonstrated that EGCG
had a profound antitumor activity in human tongue carcinoma cells
in vitro. EGCG treatment decreased the expression of HK2 and
inhibited its translocation into the mitochondrial outer membrane,
while EGCG exerted its function in an Akt activity
suppression-dependent manner.
In the present study, we first examined the
antitumor activity of EGCG in two tongue carcinoma cells. The
results indicated that EGCG markedly inhibited
anchorage-independent growth of Tca8113 and TSCCA cells in a
dose-dependent manner (Fig. 1A and
B). These data are consistent with those of a previous study
which reported that tea polyphenols have an inhibitory effect on
the growth of oral squamous carcinoma cells (43). For cancer cells to sustain their
rapid proliferation and gain a survival advantage, glycolysis has
been demonstrated as a hot spot for metabolic reprogramming in
tumor. This physiological process generates ATP for cell energy
supply and provides sufficient biosynthetic intermediates for
anabolic pathways (28). Although
metabolic control over the glycolytic rate can be applied at many
steps in the glycolytic pathway, most studies in cancer support the
hypothesis that control over glycolytic flux primarily resides at
the transport and phosphorylation steps (31). Findings of previous studies
indicated that EGCG is associated with glucose uptake inhibition by
regulating glucose transporter GLUTs, in different signaling
pathways (44–47). Those findings suggested that EGCG
may be involved in glycolysis regulation. Our results clearly
showed that EGCG dose-dependently inhibited glucose consumption and
lactate production in Tca8113 and TSCCa cells. Moreover, we found
that EGCG directly decreased the expression of HK2 in these cells.
These results suggest that EGCG regulates glycolysis in human
tongue carcinoma cells.
HK2, the first rate-limiting enzyme in glycolysis,
is considered to be required for tumor initiation and maintenance.
Although the underlying mechanisms of the deregulation of HK2 in
cancer cells have not yet been completely elucidated, the
PI3-K/Akt-related signaling pathway, as well as the transcription
factors, hypoxia inducible factors-1α (HIF-1α) and c-myc, have been
demonstrated to be involved in the regulation of HK2 and
HK2-mediated glycolysis (31). More
importantly, overexpression of the receptor tyrosine kinase EGFR,
accompanied by Akt hyperactivation is always observed in human
tongue carcinoma and associated with tumor cell malignant
properties (38,48). Thus, we hypothesized that the
EGFR-Akt signaling pathway may regulate glycolysis in human tongue
carcinoma. Our results show that EGCG substantially inhibited
EGF-induced EGFR and its downstream kinase Akt and ERK
phosphorylation as well as HK2 expression as expected. Subsequent
analysis verified that the downregulation of HK2 in Tca8113 and
TSCCa cells is mediated through the Akt signaling pathway, but is
not dependent on ERK activity. In order to confirm our
observations, a transient transfection of constitutively activated
Akt1 (Myr-Akt1) was conducted in EGCG-treated human tongue
carcinoma cells. Our results have demonstrated that Myr-Akt1
transfection promoted the expression of HK2 and rescued the glucose
consumption and lactate production in Tca8113 and TSCCa cells,
although in the presence of EGCG. These findings provide direct
evidence that downregulation of Akt signaling pathway is involved
in the suppression of EGCG-induced human tongue carcinoma
glycolysis.
Findings of laboratory and clinical investigations
have demonstrated that the high expression of HK2 is associated
with resistance to cancer cell apoptosis (39,40).
In this case, HK2 translocates to the mitochondrial outer membrane
and interacts with the VDAC to block the release of cytochrome
c, eventually inhibiting apoptosis (39–42).
Our results indicate that EGCG obviously inhibited HK2 expression
on the mitochondrial outer membrane and markedly promoted tongue
carcinoma cell apoptosis. These results reveal a previously unknown
mechanism of EGCG-induced tumor cell apoptosis, which may be
partially dependent on HK2 subcellular localization. In summary,
the present study suggests that glycolysis is involved in
EGCG-mediated antitumor activity, and identified HK2 as a new
potential target of EGCG. These results suggest that HK2 is a good
molecular target for the prevention and treatment of human tongue
carcinoma.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81371690), the International
Cooperation Program Funds of the China Hunan Provincial Science and
the Technology Department (project nos. 2012WK4005 and
2013FJ6009).
Abbreviations:
|
EGCG
|
epigallocatechin gallate
|
|
EGFR
|
EGF receptor
|
|
VDAC
|
voltage-dependent anion channel
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
HK2
|
hexokinase 2
|
References
|
1
|
Dreosti IE, Wargovich MJ and Yang CS:
Inhibition of carcinogenesis by tea: the evidence from experimental
studies. Crit Rev Food Sci Nutr. 37:761–770. 1997. View Article : Google Scholar
|
|
2
|
Katiyar S and Mukhtar H: Tea in
chemoprevention of cancer. Int J Oncol. 8:221–238. 1996.PubMed/NCBI
|
|
3
|
Yang CS, Maliakal P and Meng X: Inhibition
of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 42:25–54.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katiyar SK and Elmets CA: Green tea
polyphenolic antioxidants and skin photoprotection (Review). Int J
Oncol. 18:1307–1313. 2001.PubMed/NCBI
|
|
5
|
Yang CS: Inhibition of carcinogenesis by
tea. Nature. 389:134–135. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang CS and Wang H: Mechanistic issues
concerning cancer prevention by tea catechins. Mol Nutr Food Res.
55:819–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma YC, Li C, Gao F, et al:
Epigallocatechin gallate inhibits the growth of human lung cancer
by directly targeting the EGFR signaling pathway. Oncol Rep.
31:1343–1349. 2014.
|
|
8
|
Maruyama T, Murata S, Nakayama K, et al:
(−)-Epigallocatechin-3-gallate suppresses liver metastasis of human
colorectal cancer. Oncol Rep. 31:625–633. 2014.
|
|
9
|
Bode AM and Dong Z: Epigallocatechin
3-gallate and green tea catechins: united they work, divided they
fail. Cancer Prev Res. 2:514–517. 2009. View Article : Google Scholar
|
|
10
|
Shirakami Y, Shimizu M and Moriwaki H:
Cancer chemoprevention with green tea catechins: from bench to bed.
Curr Drug Targets. 13:1842–1857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh BN, Shankar S and Srivastava RK:
Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu M, Shirakami Y and Moriwaki H:
Targeting receptor tyrosine kinases for chemoprevention by green
tea catechin, EGCG. Int J Mol Sci. 9:1034–1049. 2008. View Article : Google Scholar
|
|
13
|
Khoi PN, Park JS, Kim JH, et al:
(−)-Epigallocatechin-3-gallate blocks nicotine-induced matrix
metalloproteinase-9 expression and invasiveness via suppression of
NF-κB and AP-1 in endothelial cells. Int J Oncol. 43:868–876.
2013.PubMed/NCBI
|
|
14
|
Dong Z, Ma W, Huang C and Yang CS:
Inhibition of tumor promoter-induced activator protein 1 activation
and cell transformation by tea polyphenols, (−)-epigallocatechin
gallate, and theaflavins. Cancer Res. 57:4414–4419. 1997.PubMed/NCBI
|
|
15
|
Rouzer CA and Marnett LJ: Green tea gets
molecular. Cancer Prev Res. 4:1343–1345. 2011. View Article : Google Scholar
|
|
16
|
Bode AM and Dong Z: Targeting signal
transduction pathways by chemopreventive agents. Mutat Res.
555:33–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jankun J, Selman SH, Swiercz R and
Skrzypczak-Jankun E: Why drinking green tea could prevent cancer.
Nature. 387:5611997. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Z, Tang F, Ermakova S, et al: Fyn is a
novel target of (−)-epigallocatechin gallate in the inhibition of
JB6 Cl41 cell transformation. Mol Carcinog. 47:172–183. 2008.
View Article : Google Scholar
|
|
19
|
Sakamoto Y, Terashita N, Muraguchi T,
Fukusato T and Kubota S: Effects of epigallocatechin-3-gallate
(EGCG) on A549 lung cancer tumor growth and angiogenesis. Biosci
Biotechnol Biochem. 77:1799–1803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katiyar S, Elmets CA and Katiyar SK: Green
tea and skin cancer: photoimmunology, angiogenesis and DNA repair.
J Nutr Biochem. 18:287–296. 2007. View Article : Google Scholar
|
|
21
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shah JP and Gil Z: Current concepts in
management of oral cancer - surgery. Oral Oncol. 45:394–401. 2009.
View Article : Google Scholar
|
|
23
|
Calabrese L, Tagliabue M, Maffini F,
Massaro MA and Santoro L: From wide excision to a compartmental
approach in tongue tumors: what is going on? Curr Opin Otolaryngol
Head Neck Surg. 21:112–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuen AP, Lam KY, Chan AC, et al:
Clinicopathological analysis of elective neck dissection for N0
neck of early oral tongue carcinoma. Am J Surg. 177:90–92. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rana M, Iqbal A, Warraich R, Ruecker M,
Eckardt AM and Gellrich NC: Modern surgical management of tongue
carcinoma - a clinical retrospective research over a 12 years
period. Head Neck Oncol. 3:432011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petersen PE: Global policy for improvement
of oral health in the 21st century - implications to oral health
research of World Health Assembly 2007, World Health Organization.
Community Dent Oral Epidemiol. 37:1–8. 2009. View Article : Google Scholar
|
|
27
|
Preis M, Hadar T, Soudry E, et al: Early
tongue carcinoma: analysis of failure. Head Neck. 34:418–421. 2012.
View Article : Google Scholar
|
|
28
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg’s contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tennant DA, Durán RV and Gottlieb E:
Targeting metabolic transformation for cancer therapy. Nat Rev
Cancer. 10:267–277. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar
|
|
32
|
Wallace DC: Mitochondria and cancer:
Warburg addressed. Cold Spring Harb Symp Quant Biol. 70:363–374.
2005. View Article : Google Scholar
|
|
33
|
Ros S and Schulze A: Glycolysis back in
the limelight: systemic targeting of HK2 blocks tumor growth.
Cancer Discov. 3:1105–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patra KC, Wang Q, Bhaskar PT, et al:
Hexokinase 2 is required for tumor initiation and maintenance and
its systemic deletion is therapeutic in mouse models of cancer.
Cancer Cell. 24:213–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wolf A, Agnihotri S, Micallef J, et al:
Hexokinase 2 is a key mediator of aerobic glycolysis and promotes
tumor growth in human glioblastoma multiforme. J Exp Med.
208:313–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yecies JL and Manning BD: Transcriptional
control of cellular metabolism by mTOR signaling. Cancer Res.
71:2815–2820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fruman DA and Rommel C: PI3K and cancer:
lessons, challenges and opportunities. Nat Rev Drug Discov.
13:140–156. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ribeiro FA, Noguti J, Oshima CT and
Ribeiro DA: Effective targeting of the epidermal growth factor
receptor (EGFR) for treating oral cancer: a promising approach.
Anticancer Res. 34:1547–1552. 2014.PubMed/NCBI
|
|
39
|
Cheung EC, Ludwig RL and Vousden KH:
Mitochondrial localization of TIGAR under hypoxia stimulates HK2
and lowers ROS and cell death. Proc Natl Acad Sci USA.
109:20491–20496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pastorino JG, Shulga N and Hoek JB:
Mitochondrial binding of hexokinase II inhibits Bax-induced
cytochrome c release and apoptosis. J Biol Chem. 277:7610–7618.
2002. View Article : Google Scholar
|
|
41
|
Majewski N, Nogueira V, Bhaskar P, et al:
Hexokinase-mitochondria interaction mediated by Akt is required to
inhibit apoptosis in the presence or absence of Bax and Bak. Mol
Cell. 16:819–830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Krasnov GS, Dmitriev AA, Lakunina VA,
Kirpiy AA and Kudryavtseva AV: Targeting VDAC-bound hexokinase II:
a promising approach for concomitant anti-cancer therapy. Expert
Opin Ther Targets. 17:1221–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Elattar TM and Virji AS: Effect of tea
polyphenols on growth of oral squamous carcinoma cells in vitro.
Anticancer Res. 20:3459–3465. 2000.PubMed/NCBI
|
|
44
|
Moreira L, Araújo I, Costa T, et al:
Quercetin and epigallocatechin gallate inhibit glucose uptake and
metabolism by breast cancer cells by an estrogen
receptor-independent mechanism. Exp Cell Res. 319:1784–1795. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ueda M, Nishiumi S, Nagayasu H, Fukuda I,
Yoshida K and Ashida H: Epigallocatechin gallate promotes GLUT4
translocation in skeletal muscle. Biochem Biophys Res Commun.
377:286–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ku HC, Tsuei YW, Kao CC, et al: Green tea
(−)-epigallocatechin gallate suppresses IGF-I and IGF-II
stimulation of 3T3-L1 adipocyte glucose uptake via the glucose
transporter 4, but not glucose transporter 1 pathway. Gen Comp
Endocrinol. 199:46–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Naftalin RJ, Afzal I, Cunningham P, et al:
Interactions of androgens, green tea catechins and the antiandrogen
flutamide with the external glucose-binding site of the human
erythrocyte glucose transporter GLUT1. Br J Pharmacol. 140:487–499.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ongkeko WM, Altuna X, Weisman RA and
Wang-Rodriguez J: Expression of protein tyrosine kinases in head
and neck squamous cell carcinomas. Am J Clin Pathol. 124:71–76.
2005. View Article : Google Scholar : PubMed/NCBI
|