Introduction
Colorectal cancer (CRC) is the third and second most
common cancer diagnosed in males and females, respectively, with
>1.2 million new cancer cases diagnosed and >0.6 million
mortalities annually (1). Although
patients diagnosed with localized disease have a 5-year survival
rate as high as 90.3%, this rate significantly decreases to 12.5%
in patients with distant metastasis (2). Mutation, phenotypic drift,
epithelial-mesenchymal transition (EMT), collective migration,
resistance to apoptosis and anoikis, vascularization, and
lymphangiogenesis are among the known mechanisms of metastasis
(3). Thus, mechanism-based targeted
therapies been on the increase and are thought to have high
efficacy that inhibits a target, while having relatively fewer
off-target effects and thus less non-specific toxicity. By
contrast, a targeted therapeutic agent that inhibits a key pathway
in a cancer cell may not completely eradicate the entire tumor,
allowing residual heterogenic cancer cells to survive, followed by
almost inevitable relapses (4).
Accumulating evidence suggests that microRNAs (miRNAs) are
important in carcinogenesis by affecting the expression of genes
that regulate cancer progression (5). When specific miRNAs involved in the
process of metastasis are identified, therapeutic strategies can be
developed to silence oncogenes or upregulate tumor-suppressor
genes.
microRNA-206, unlike other myomiR family members,
has been consistently identified based on Northern blot,
microarray, RNase protection assays, and RT-PCR to be highly
expressed in skeletal muscle (6).
Recently, the findings of a number of studies have shown that
miR-206 is frequently downregulated in many human malignancies,
including breast cancer (7),
rhabdomyosarcoma (8), renal
(9), lung (10), laryngeal (11), endometrioid (12), colorectal (13), gastric cancers (14) and melanoma (15), suggesting that miR-206 is associated
with a more malignant phenotype (10,13,16).
Furthermore, miR-206, in combination with miR-21, −135a, −335 and
let-7a, can be used as a prognostic panel for detecting the
presence of metastasis in human CRCs, with a specificity and
sensitivity of 87 and 76%, respectively (13). Oncogenes, including CCND1
(15), ESR1 (17), MET (18), and NOTCH3 (19), have been identified and confirmed to
be targets of miR-206, suggesting that miR-206 has a
tumor-suppressor role in CRC. Of these targets, NOTCH3,
identified as the third mammalian Notch receptor and expressed in
proliferating neuroepithelium (20), has been reported to be frequently
expressed in human CRC tissues and has been shown to play a role in
the modulation of CRC cell proliferation and tumorigenic potential
in xenograft models (21). Previous
studies have validated the inhibitory mechanism of miR-206 through
its seed match region binding to the NOTCH3 3′ untranslated
region, although in the context of HeLa and C2C12 cells (19,22).
However, whether the interplay between miR-206 and NOTCH3
occurring in CRC is important is unknown.
In the present study, the expression of miR-206 and
NOTCH3 in CRC tissues and cell lines was analyzed. Based on
an inverse association between the expression of this molecular
pair, miR-206 mimics were transiently transfected into the SW480
(and the metastatic strain) and SW620 colon cancer cell lines. The
results showed that upregulation of miR-206 inhibited cancer cell
proliferation and migration, blocked the cell cycle, and activated
apoptosis, even in SW620 cells, exhibiting a more aggressive
property and involving NOTCH3 signaling, EMT, and extracellular
matrix degradation. Therefore, our results confirmed miR-206 as a
tumor suppressor in CRC and suggested a potential therapeutic
target for clinical intervention.
Materials and methods
Patients and tissue samples
Approval from the Institutional Review Board of
Huai’an First People’s Hospital was obtained for the present study.
A total of 49 sequential patients who underwent surgery for the
first manifestation of CRC were included. Patients who underwent
preoperative radiation or chemotherapy were not included in this
study. Tumors were staged according to the AJCC Cancer Staging
Manual (6th edition). The primary tumors, but not the metastatic
lesions, were fixed in 10% formalin, embedded in paraffin and cut
(5–8 μm) for routine histopathology examination by the Department
of Pathology (Huai’an First People’s Hospital, China). The tissue
samples were snap-frozen and stored at −80°C for RNA and protein
extraction.
Immunohistochemistry
Tissue sections were deparaffinized and rehydrated,
and then incubated for heat-induced antigen retrieval in 0.01 M
citrate buffer (pH 6.0) at 100°C for 10 min. After cooling to room
temperature, 0.6% H2O2/80% methanol was
applied to the sections for 10 min at room temperature to eliminate
endogenous peroxidation. After washing with D-PBS, the sections
were blocked with 10% bovine serum albumin and 0.2% Triton X-100 in
D-PBS at room temperature for 30 min prior to incubation with
anti-NOTCH3 rabbit polyclonal antibody (AB61043a, Sangon, Shanghai,
China) diluted 100-fold at 4°C overnight. After washing with PBS-T,
the sections were applied with the Polink-1 HRP DAB detection
system (ZSGB-BIO, Beijing, China) for color development. After the
reaction was stopped by washing, the sections were counterstained
with hematoxylin, dehydrated with alcohol, mounted with neutral
balsam and imaged on a BX51 microscope with an E-620 digital camera
(Olympus, Tokyo, Japan).
In situ hybridization
After rehydration in water, the sections proceeded
to hybridization using an Enhanced Sensitive ISH detection kit
(Boster, Wuhan, China) according to the manufacturer’s
instructions. Briefly, the sections were treated with pepsin at
37°C for 10 min, washed with 0.5 M TBS and DEPC-treated water,
pre-hybridized at 40°C for 1 h, and hybridized with 100 nM
5′-digoxigenin (DIG)-labeled LNA™ probe against hsa-miR-206 at 50°C
for 8 h. After hybridization, the slides were washed with 2X, 0.5X
and 0.2X SSC, sequentially. The slides were then blocked for 30 min
at 37°C, and incubated with biotinylated anti-DIG antibody at 37°C
for 2 h. After 0.5 M TBS washing, alkaline phosphatase
(AP)-conjugated streptavidin was applied to the section for 1 h at
room temperature. Immediately after washing the slides, AP
substrate containing 0.2 mM levamisol was applied to the sections
protected from light at 30°C for 2 h. When the desired intensity of
chromogenic reaction was reached, the slides were washed with
water, mounted in aqueous medium and imaged on a BX51 microscope
with an E-620 digital camera (Olympus, Tokyo, Japan).
Cell lines, in vitro culture and
transfection
The SW480 and SW620 human colon cancer cell lines
were obtained from the Shanghai Institutes of Biological Sciences
(Shanghai, China). The cells were cultured in Leibovitz’s L-15
medium (Gibco, Grand Island, NY, USA) supplemented with 10%
HyClone® fetal bovine serum (Thermo Scientific, Beijing,
China). The cells were maintained at 37°C and 5% CO2 in
a humidified incubator to near confluence and were deprived of
serum for 16 h prior to use in the experiments. Experimental data
were obtained from cells passaged between 3 and 10.
For transient transfection, the cells were first
cultured to reach 50–75% confluence and transfected with 100 nM
miR-206 mimic or scrambled negative control (GenePharma, Shanghai,
China) with Lipofectamine 2000 (Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Tissue samples or cultured cells were homogenized in
TRIzol® reagent (Life Technologies). Total RNA
extraction was performed as described in the manufacturer’s
instructions with one modification, i.e., that isopropanol
precipitation was proceeded at −20°C overnight instead of at room
temperature for 10 min (23). RNA
concentration was measured by the NanoDrop 1000 UV/Vis
spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
Prior to reverse transcription, RNAs were treated
with DNase I (NEB, Beijing, China) according to the manufacturer’s
instructions. For amplification of ACTB, NOTCH3,
JAG1, HEY1, CDH2, MMP9 mRNA and RNU6-1,
cDNA was generated using the ReverTra Ace-α-® Reverse
Transcription kit (Toyobo, Japan) with random (dN)9
primer. According to the manufacturer’s instructions, the reaction
was performed on 250 ng of total RNA in 10 μl total volume as
follows: 30°C for 10 min, 42°C for 30 min, 99°C for 5 min and
stored at −20°C. Concerning miR-206, primers for reverse
transcription and qPCR were designed as previously described
(24). A pulsed gene-specific RT
reaction (46) in 10 μl total
volume containing 250 ng RNA and 4 nM stem-loop primer
[5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCACACAC-3′, (24)] was applied as follows: 16°C for 30
min, followed by 60 cycles at 20°C for 30 sec, 42°C for 30 sec and
50°C for 1 sec, then 99°C for 5 min and stored at −20°C.
DyNAmo™ ColorFlash SYBR-Green® qPCR kit
(Thermo Scientific, Espoo, Finland) was used with a modified
version relating to the volumes of reagents and cDNA in 10 μl total
volume: 5 μl of 2X master mix, 1 μl RT product, and 180 nM forward
and reverse primer pairs. The primers used in the qPCR were:
ACTB (001101.3), forward, 5′-CACGAAACTACCTTCAACTCC-3′ and
reverse, 5′-CATACTCCTGCTTGCTGATC-3′; NOTCH3 (008716.2)
forward, 5′-ACAGACTGGATGGACACAGAG-3′ and reverse,
5′-GATGTCAGCAGCAACCAGATG-3′; JAG1 (000214.2) forward,
5′-TGTCGGTCTTCCAGTCTCC-3′ and reverse, 5′-CACTGCAAATGTGCTCCGTAG-3′;
HEY1 (012258.3) forward, 5′-CATACGGCAGGAGGGAAAGG-3′ and
reverse, 5′-AACTCGAAGCGGGTCAGAGG-3′; CDH2 (001792.3)
forward, 5′-AGTCAACTGCAACCGTGTCT-3′ and reverse,
5′-AGCGTTCCTGTTCCACTCAT-3′; MMP-9 (004994.2) forward,
5′-TTCTACGGCCACTACTGTGC-3′andreverse,5′-AGAATCGCCAGTACTTCC
CATC-3′;RNU6-1(004394.1)forward,5′-GCAGCACATATACTAAAATTGGAACGA-3′
and reverse, 5′-AATATGGAACGCTTCACGAATTTGC-3′; and miR-206
(MIMAT0000239) forward, 5′-AGCTCGATTAAGGTGGAATGTAAGGAAGT-3′ and
reverse, 5′-CTCAACTGGTGTCGTGGAGTCGG-3′. Three-step qPCR for
ACTB, NOTCH3, JAG1, HEY1, CDH2
and MMP-9 cDNA was performed as follows: 95°C for 7 min; 40
cycles of denaturation at 95°C for 20 sec, annealing at 60°C for 20
sec, and extension at 72°C for 20 sec. Two-step qPCR for RNU6-1 and
miR-206 was performed as follows: 95°C for 7 min; 40 cycles of
denaturation at 95°C for 10 sec and extension (RNU6-1 at 68°C,
miR-206 at 59°C) for 30 sec.
RT-qPCR reactions, including RT-minus controls and
no-template controls, were run in triplicate on an CFX™ 96
real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The
quantification cycle (Cq) was defined as the fractional cycle
number at which fluorescence passes the fixed threshold determined
by CFX Manager™ software ver. 1.6 (Bio-Rad). The expression level
of mRNA and miRNA was calculated by the ΔCt method (25) using ACTB and RNU6-1,
respectively, as a reference.
Western blotting
Following removal of the RNA aqueous phase from
TRIzol® the remaining phenol-chloroform layer was used
for protein isolation according to the manufacturer’s instructions.
Proteins were dissolved in 0.5% SDS/3 M urea, quantified by the BCA
assay kit (CWBIO, China), and mixed with 5X SDS-PAGE sample buffer
(10% SDS/5% 2-mercaptoethanol). After the samples were denatured at
95°C for 5 min, 30 μg proteins per lane were separated by 10%
SDS-PAGE and transferred to Immobilon®-P membranes
(Millipore, Billerica, MA, USA). The membranes were blocked in
D-PBS/0.05% Tween-20 (PBST) containing 5% defatted milk for 1 h at
room temperature. The blocked membranes were then probed with
anti-ACTB rabbit polyclonal antibody (Proteintech, Chicago, IL,
USA), diluted at 2,000-fold; anti-NOTCH3 rabbit polyclonal antibody
(Sangon, China), diluted at 250-fold; or anti-Caspase-3 rabbit
monoclonal antibody (Cell Signaling Technology, Boston, MA, USA) at
4°C overnight. After washing with PBST, the membranes were then
incubated with HRP-conjugated goat anti-rabbit IgG antibody
(Boster, China), diluted at 4,000-fold for 1 h at room temperature.
After washing with PBST, the immunolabeling proteins were reacted
with chemiluminescent HRP substrate (Millipore) and visualized by
ChemiDoc™ XRS systems (Bio-Rad).
Cell proliferation and apoptosis
assay
For the proliferation assay, the transfected cells
were seeded in a flat-bottom 96-well plate at
1×104/well. After maintaining in serum-free medium for
16 h, the cells were recovered to the culture medium followed by
the addition of 20 μl/well of [3-(4,5-dimethylthiazol-2-yl) 2,5
diphenyl tetrazolium bromide)] (MTT) solution (5 mg/ml in PBS) for
4 h at 37°C. The reaction was terminated by adding 200 μl dimethyl
sulfoxide to each well, followed by gentle agitation for 20 min.
The absorbance of each well was then measured at 490 nm using an
Elx808 microplate reader (BioTek, Winooski, VT, USA).
At 36 h post-transfection, cells (5×105)
were resuspended and stained using an Annexin V-FITC Apoptosis
detection kit (BioBox, Nanjing, China), and then detected on a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). The
apoptotic cell level was calculated by dividing FITC-stained only
cells to gated cells.
Cell cycle analysis
The cells seeded in 6-well plates at
1×106cells/well were cultured and transfected as
mentioned above. Following serum starvation for 16 h, the cells
were digested with trypsin and neutralized with Leibovitz’s L-15
medium. Cell cycle analysis was performed using a CycleTest™ Plus
DNA reagent kit (BD Biosciences). Each sample was analyzed on a
FACSCalibur flow cytometer (BD Biosciences). The cell
subpopulations in G0/G1, S and
G2/M phases were calculated based on differences of DNA
content.
Wound-healing assay
The cells seeded at 5×105 cells/well were
cultured overnight in 6-well plates. The following day, 100 nM
miR-206 mimic or negative control was transfected using
Lipofectamine 2000. Untransfected cells were cultured in medium as
blanks. After 36 h, transfected cells were grown to confluence and
wounded by dragging a 0.2-ml pipette tip through the monolayer. The
cells were washed using pre-warmed PBS to remove cell debris and
allowed to migrate for 24 h. The rate of wound closure was
expressed as a percentage of the initial scraped gap.
Cell migration assay
Cells were seeded, cultured and transfected as
described above. At 36 h post-transfection, the cells collected and
resuspended in Leibovitz’s L-15 medium were placed in the upper
compartment at 1×105/100 μl. After 10 h of incubation,
the cells on the top of the membrane were removed by swiping with a
damp cotton swab. The membrane was fixed with 4% paraformaldehyde
for 30 min and stained with 0.1% crystal violet for 15 min. The
cells on the underside of the membrane were counted using a light
microscope. For each triplicate, the number of cells in 10 fields
(x200 magnification) was determined, and the counts were
averaged.
Statistical analysis
Data were presented as mean ± SD. A Shapiro-Wilk
normality test was used to evaluate the distribution of
quantitative observations. Skew distributional data expressed as
median (interquartile range) were analyzed using non-parametric
tests. The Wilcoxon matched-pairs signed-rank test was used to
compare between tumors and matched adjacent normal tissues. A
comparison between tissue samples with different stage was analyzed
with the Mann-Whitney test. The correlation between miR-206 and
NOTCH3 protein expression was analyzed using Spearman’s rank
correlation test. The in vitro cell experiment was repeated
at ≥3 times. Two-factor analysis of variance was performed using
two-way ANOVA. Data were analyzed using Stata/SE version 11.2
(StataCorp, TX, USA) and plotted in Prism® ver. 5
(GraphPad, College Station, TX, USA). P<0.05 was considered
statistically significant.
Results
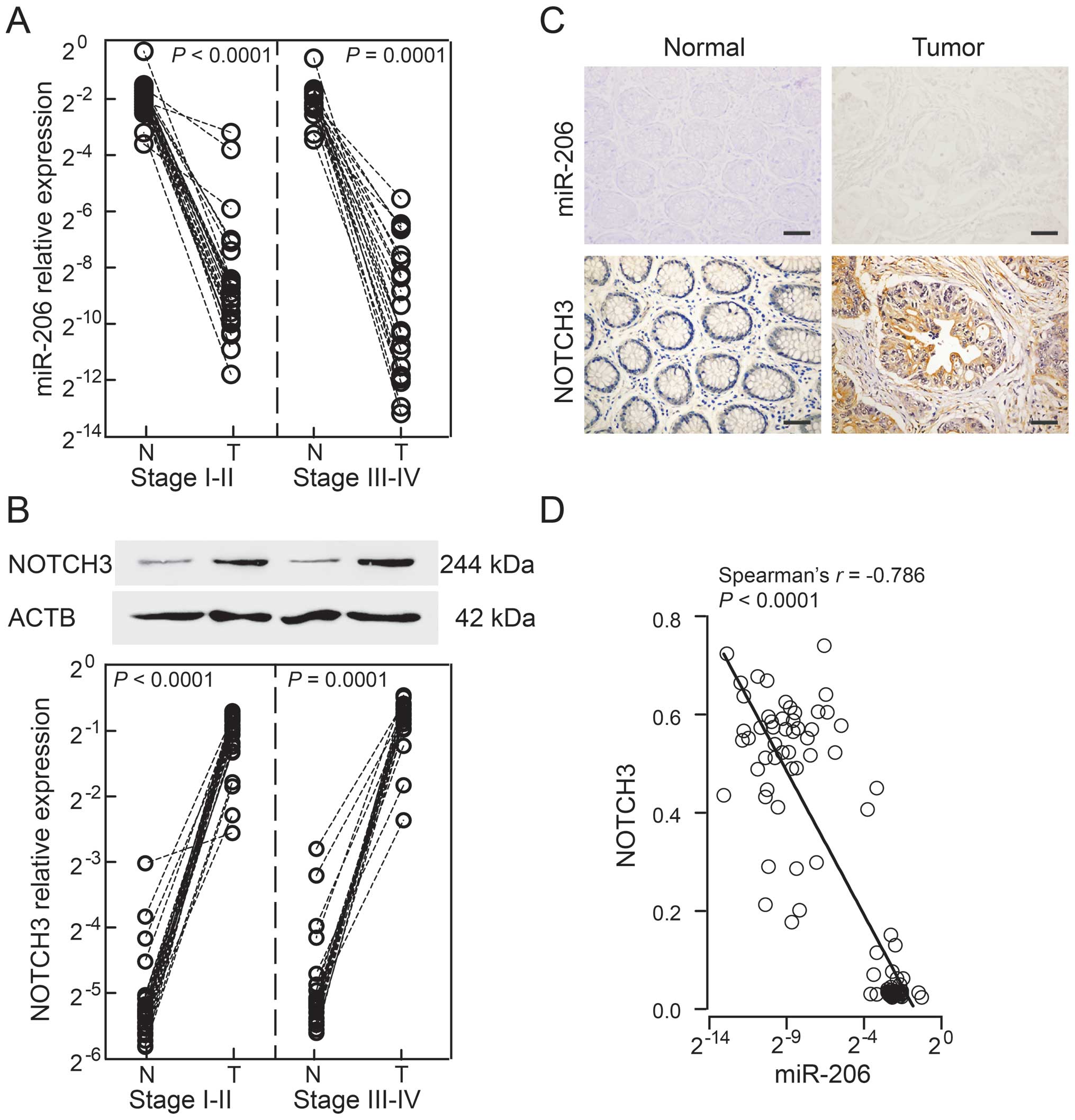
microRNA-206 expression is opposite to
NOTCH3 in primary CRCs
According to the occurrence of metastasis, patients
were enrolled in the following categories: stage I and II with
non-metastatic disease (n=28); and stage III and IV with metastatic
disease (n=21). Only primary tumors and matched adjacent normal
tissues, but not the metastatic lesions, were analyzed in the
present study. A significant difference was noted in the miR-206
expression of patients with stage I–II or stage III–IV disease
between tumors and normal tissues (stage I–II: P<0.0001; stage
III–IV: P=0.0001; Fig. 1A), while
there were no obvious differences between stage I–II and stage
III–IV patients in normal tissues or tumors (normal tissues,
P=0.4057; tumors, P=0.2577). By contrast, NOTCH3 protein was
expressed in opposition to miR-206, and was significantly
upregulated in tumors and minimally detected in normal tissues
(stage I–II, P<0.0001; stage III–IV, P=0.0001; Fig. 1B). However, patients with advanced
stages had a higher level of NOTCH3 in tumors and normal tissues
(normal tissues, P=0.0306; tumors, P=0.0068). Based on these data,
we further examined tissue distributions by ISH and
immunohistochemistry, respectively. microRNA-206 expression was
weak-to-moderate in normal mucosa, but was absent in tumor tissues.
By comparison, NOTCH3 was predominantly localized in the cytoplasm
and nucleus of tumor cells with strong staining, but was rarely
visible in normal mucosa (Fig. 1C).
Apparently, there was an inverse association between miR-206 levels
and NOTCH3 expression among the 49 CRC patients (Spearman’s rho
=−0.786, P<0.0001; Fig. 1D).
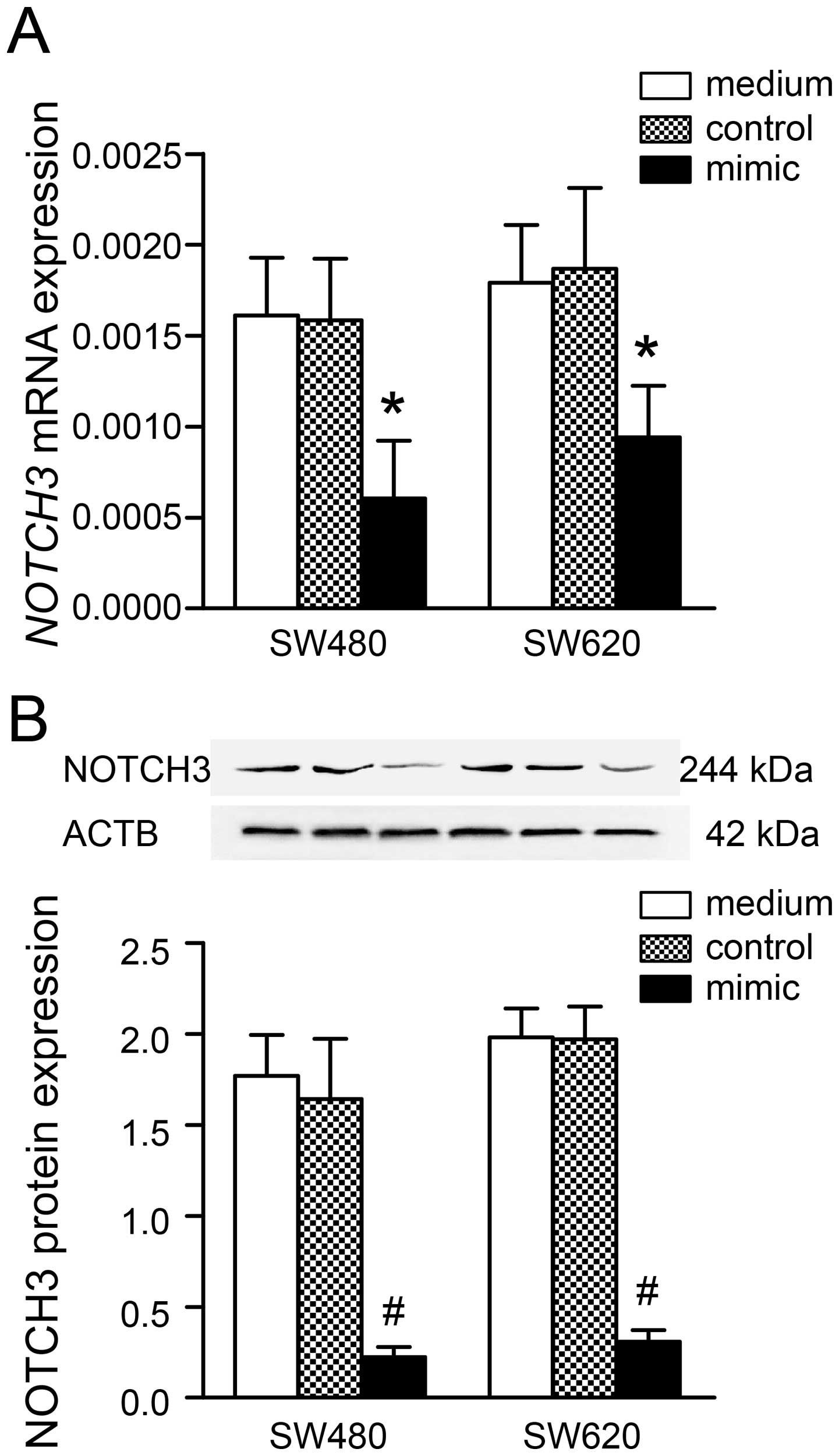
Upregulation of miR-206 alters NOTCH3
expression in colon cancer cells
A previous study had identified NOTCH3 as a
target of miR-206, resulting in apoptosis and migration in HeLa
cells (19). To investigate the
effect of miR-206 targeting NOTCH3 in CRC, we introduced a
colon cancer cell line (SW480) and a metastatic strain (SW620) for
subsequent experiments. Following transfection of cells with
miR-206 mimic (100 nM), NOTCH3 mRNA and protein levels were
significantly reduced in SW480 (mRNA, P<0.05; protein,
P<0.001) and SW620 (mRNA, P<0.05; protein, P<0.001), which
compared to the medium group (Fig.
2). As expected, treatment with scrambled negative control
under the same conditions had little effect on NOTCH3 mRNA
and protein expression in the two cell lines (all tests,
P>0.05). Repression of NOTCH3 expression by miR-206 was
more notable on protein translation than mRNA transcription. These
findings suggested an inverse association between miR-206 and
NOTCH3 expression in CRC.
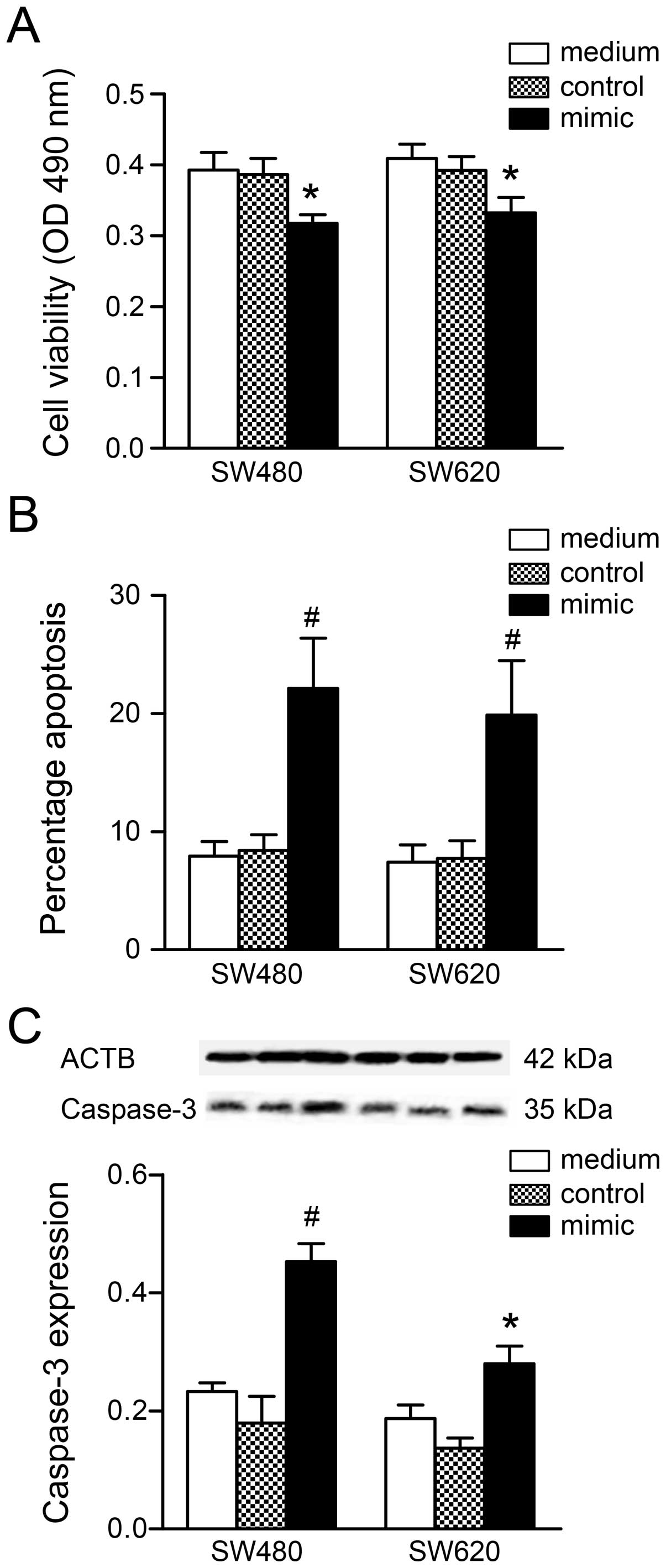
miR-206 inhibits cancer cell
proliferation and migration, and activates apoptosis
In view of the aforementioned data, recovery of
miR-206 levels attenuated NOTCH3 protein expression, making miR-206
a potential target for therapeutic intervention. Thus, the effects
of miR-206 on cell growth behavior require elucidation. Based on
the MTT assay, cell viability following transfection with a miR-206
mimic (100 nM) was significantly reduced when compared to the
medium group (SW480, P<0.01; SW620, P<0.01; Fig. 3A). Further examination of the cell
cycle (Table I) showed that the
percentage of transfected cells in the G0/G1
phase (SW480, P<0.01; SW620, P<0.01) was significantly
increased, accompanied by a reduction in the S (SW480, P<0.05;
SW620, P<0.05) and G2/M (SW480, P<0.05; SW620,
P<0.05) phases in the two cell lines compared to the medium
group. The cell cycle data obtained were consistent with the
proliferation results. In addition, miR-206 mimic transfection
increased the apoptotic cell number (SW480, P<0.001; SW620,
P<0.001; Fig. 3B) and
upregulated caspase-3 content (SW480, P<0.001; SW620, P<0.01;
Fig. 3C) in the two cell lines.
 | Table IEffect of miR-206 on the cell cycle
of colon cancer cells. |
Table I
Effect of miR-206 on the cell cycle
of colon cancer cells.
| SW480 | SW620 |
|---|
|
|
|
|---|
| Phase | Medium | Control | Mimic | Medium | Control | Mimic |
|---|
|
G0/G1 | 70.79±1.82 | 71.31±1.74 | 77.53±2.32b | 69.60±2.09 | 70.79±1.90 | 76.00±1.98b |
| S | 24.49±2.39 | 24.25±2.42 | 19.42±2.94a | 25.40±1.48 | 24.41±1.39 | 20.83±1.24a |
|
G2/M | 4.72±0.60 | 4.44±0.85 | 3.06±0.63a | 5.00±0.84 | 4.80±0.90 | 3.17±0.90a |
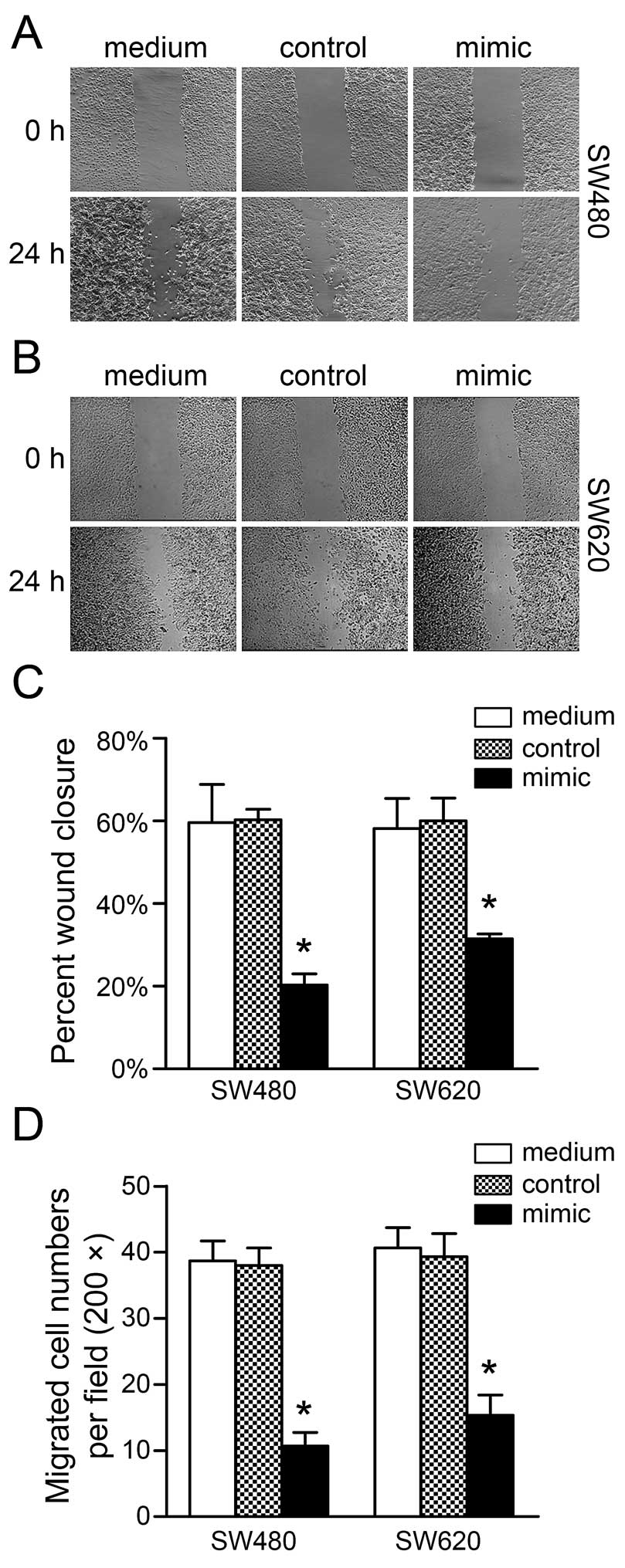
To determine whether miR-206 affects migration in
CRC, the wound-healing and Boyden chamber assays were used to
perform cell motility measurements. Cells transfected with miR-206
mimic presented a significant reduction in the percentage of wound
closure in SW480 and SW620 cells compared to the medium group
(SW480, P<0.001; SW620, P<0.001; Fig. 4A–C). Similar decreases in cell
migration in the two cell lines transfected with miR-206 mimic were
also observed using the Boyden chamber assay compared to the medium
group (SW480, P<0.001; SW620, P<0.001; Fig. 4D).
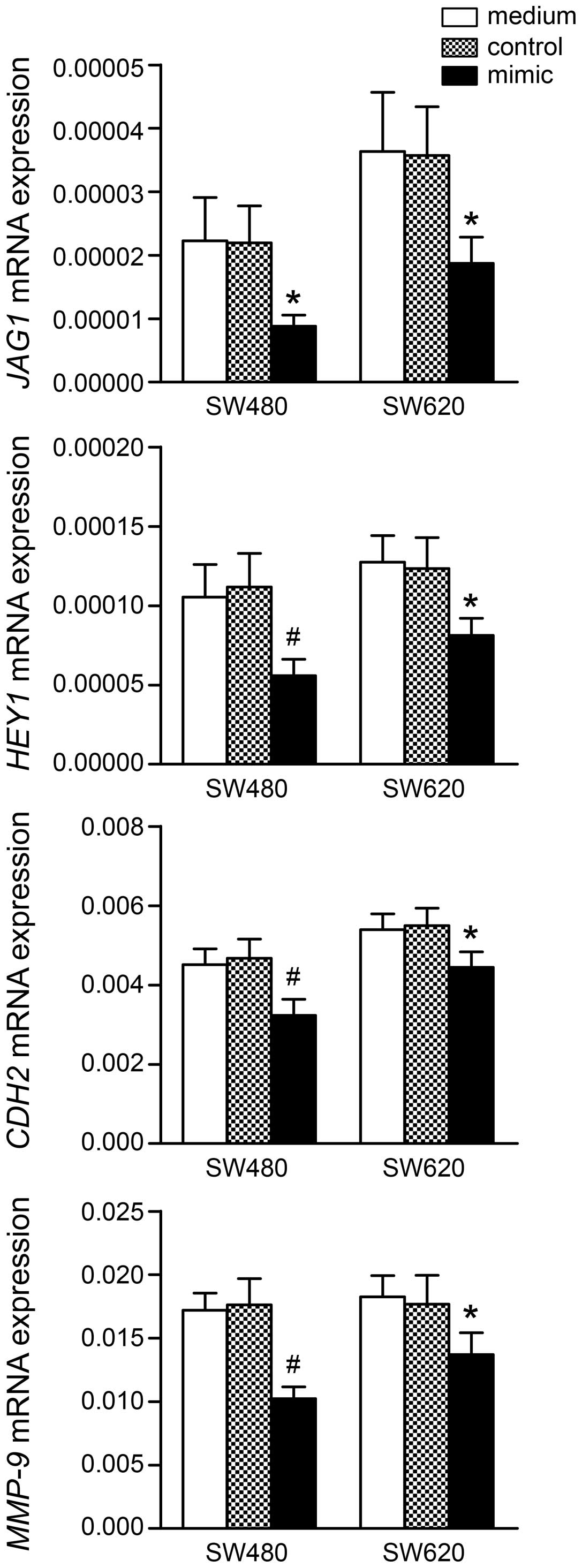
microRNA-206 affects NOTCH targets and
metastasis-associated genes
We determined whether NOTCH transcriptional targets
and metastasis-associated genes alter their expression following
miR-206 mimic transfection. As shown in Fig. 5, the mRNA levels of two NOTCH
transcriptional targets (JAG1 and HEY1) and two
metastasis-associated genes (CDH2 and MMP9) were
significantly downregulated 24 h post-transfection with miR-206
mimic in SW480 and SW620 cells. However, the basal expression of
these genes, with the exception of MMP-9, had a higher level in
SW620 cells, which may be relevant to its more aggressive property
than SW480 cells.
Discussion
Accumulating studies acknowledge that miR-206 is a
tumor suppressor that is downregulated in various tumors (7–13,15,26)
and is involved in cancer metastasis (13,27).
NOTCH3, a verified target of miR-206 (19), has also been reported to be
frequently expressed in human CRC samples, and has a capacity in
the modulation of CRC cell proliferation and tumorigenic potential
in xenograft models (21).
Therefore, we investigated the tumor suppressive and metastatic
effects of miR-206 and its target (NOTCH3) in CRC. First, we
evaluated the expression in two CRC cohorts grouped by the onset of
metastasis. An inverse association between the expression of this
molecular pair among the total CRC patients supported the negative
regulation of miR-206 targeting NOTCH3. miR-206 levels were
further reduced with CRC progression. However, the two CRC cohorts
did not differ in tumor tissue or normal mucosal miR-206
expression. This finding invalidates miR-206 as a prognostic marker
used independently for metastasis, as it was combined into a 5-miR
panel for predicting metastasis (13).
In CRC tissues, miR-206 was expressed at an
extremely low level and was scarcely probed by ISH. Even in normal
mucosa, miR-206 expression was minimal compared to tissue-specific
expression in skeletal muscle. It is plausible that miR-206 is not
identified in high-throughput profiling due to its low abundance in
intestinal tissue (28–30). This low-abundance miRNA may regulate
key components, the expression of which is markedly restricted
under normal circumstances. As one such component, NOTCH3 was shown
to have less expression in normal mucosa and overexpression in CRC
tissue in the present study, which coincided with findings of other
studies (21,31). In addition, only one mutation of the
NOTCH3 gene was detected in 48 CRC patients (32), suggesting the gene mutation may not
be important in tumorigenesis. A deficiency in miR-206 in CRC may
in turn make NOTCH3, one of its targets, lose control with
intensified upregulation. A high NOTCH3 expression has been shown
to be strongly correlated with metastasis in CRC (31), and may be an independent prognostic
factor for hepatocellular carcinoma as well (33). Compared to a decrease of the
overexpressed NOTCH3 by introducing an exogenic antagonist,
rescuing the reduced miR-206 in keeping with the physiologic
requirements may be more rational. Following transfection with
miR-206 mimics in colon cancer cells, the mRNA and protein levels
of NOTCH3 were all downregulated. The same phenomenon has
also been observed in HeLa (19)
and HepG2 (34) cells. This finding
supports the hypothesis that miRNAs are involved in gene regulation
via post-transcriptional silencing on its targets and interfering
with the transcription of target genes in an indirect manner.
Initially, studies on miR-206 mostly focused in muscle tissue,
which was due to the high tissue-specificity of miR-206 screened in
high-throughput analyses (6).
Occasionally, a significant expression of miR-206 was detected in
normal murine skin (24,35) and HPV8-mediated skin tumors
(36). Since the first evidence for
the post-transcriptional regulation of ESR1 by miR-206 in MCF-7
breast cancer cells, miR-206 has been denoted as a tumor suppressor
that is downregulated in many types of cancer, and a number of
target genes, including oncogenes and tumor-suppressor genes, have
been identified (37). Our results
confirmed the repressive effect of miR-206 on NOTCH3
expression at the mRNA and protein levels in CRC, which also
suggests that miR-206 coordinates molecular networks in cancer.
Overexpressed miR-206 has been shown to attenuate
colon cancer cell proliferation and migration in vitro. Such
inhibitory effects of miR-206 are evident in other cancer models in
which growth-associated targets, such as CCNC (15), CCND1 (15), CCND2 (14), CDC42 (38), CDK4 (15), MET (18), and VEGF (11), have been identified. The increase in
cell number in the G0/G1 phase caused by
miR-206 mimic transfection suggests that cyclin genes are involved
in the regulatory networks of miR-206, and probably account for the
downregulation of NOTCH3 at the mRNA level. Therefore,
underexpression of miR-206 in CRC may contribute to oncogenesis by
dysregulating the cell-cycle drivers.
In contrast to Notch1, Notch2 and Notch4, Notch3
does not appear to be essential for embryonic development as
Notch3-null mice are viable and fertile, but have impaired
maturation of vascular smooth muscle cells (39). NOTCH3 activation also protects
against apoptosis through CBF1/RBP-Jκ independently, but
cross-talks to the ERK/MAPK (40)
or PI3-kinase/AKT (41) pathway,
which emphasizes its indispensable role in maintaining cell
viability. Thus, the downregulation of NOTCH3 following miR-206
mimic transfection increases apoptosis and caspase-3 content.
Furthermore, MMP-2 and MMP-9, are crucial in tumor invasion and
metastasis because of specificity for type IV collagen (the
principal component of the basement membrane) (42), have been shown to be regulated by
NOTCH3 via the ERK1/2 pathway, and strongly correlate with
hepatocellular carcinoma metastasis (33). The invasion and migration of
MDA-MB-231 breast cancer cells in vitro were inhibited with
the downregulation of MMP-9 via miR-206 targeting CDC42
(38). Similarly, CDH2
(N-cadherin), mediating cell-cell interaction to prevent cell
apoptosis by activating the PI3-kinase/AKT pathway (43), was downregulated following miR-206
mimic transfection in the current study. CDH2 was upregulated in
dedifferentiated, invasive prostate carcinomas (44) and has been reported to participate
in TNF-α-induced EMT in CRC cells (45). The levels of expression of
JAG1, HEY1, and CDH2 mRNA were higher in SW620
than SW480 cells, which supports the metastatic property of SW620
compared to its primary strain (SW480). Nevertheless, the
upregulation of miR-206-induced tumor suppressive effects was
similar in the 2 cell lines, suggesting miR-206 plays a fundamental
role in the tumorigenesis of CRC involving multiple signaling
pathways.
Consistent with previous reports, our results
support the hypothesis that expressed miR-206 can decrease cell
proliferation and migration, block the cell cycle, and activate
apoptosis in CRC cells. The tumor suppressive capacity of miR-206
has a similar effect on CRC cells with different metastatic
potential and may be explained by its direct NOTCH3 signaling
inhibition and indirect cross-talk with other signaling pathways
involving CDH2 and MMP-9. These results emphasize the potential
therapeutic strategy for CRC by rescuing miR-206, which is
downregulated in CRC. Future studies may continue to evaluate the
effects of miR-206 on tumor growth in vivo, and to
understand how it affects the pathogenesis of CRC, which may reveal
feasible therapies for this disease.
Acknowledgements
The authors thank Dr Yuan Mu (Department of Clinical
Laboratory, Nanjing Children’s Hospital, Nanjing Medical
University) for the technical assistance and helpful
discussions.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, et al:
Cancer treatment and survivorship statistics, 2014. CA Cancer J
Clin. 64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eccles SA and Welch DR: Metastasis: recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar :
|
|
6
|
McCarthy JJ: MicroRNA-206: the skeletal
muscle-specific myomiR. Biochim Biophys Acta. 1779:682–691. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kondo N, Toyama T, Sugiura H, Fujii Y and
Yamashita H: miR-206 Expression is down-regulated in estrogen
receptor alpha-positive human breast cancer. Cancer Res.
68:5004–5008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taulli R, Bersani F, Foglizzo V, et al:
The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma
growth in xenotransplanted mice by promoting myogenic
differentiation. J Clin Invest. 119:2366–2378. 2009.PubMed/NCBI
|
|
9
|
Zhou L, Chen J, Li Z, et al: Integrated
profiling of microRNAs and mRNAs: microRNAs located on Xq27.3
associate with clear cell renal cell carcinoma. PLoS One.
5:e152242010. View Article : Google Scholar
|
|
10
|
Wang X, Ling C, Bai Y and Zhao J:
MicroRNA-206 is associated with invasion and metastasis of lung
cancer. Anat Rec. 294:88–92. 2011. View
Article : Google Scholar
|
|
11
|
Zhang T, Liu M, Wang C, Lin C, Sun Y and
Jin D: Down-regulation of MiR-206 promotes proliferation and
invasion of laryngeal cancer by regulating VEGF expression.
Anticancer Res. 31:3859–3863. 2011.PubMed/NCBI
|
|
12
|
Chen X, Yan Q, Li S, et al: Expression of
the tumor suppressor miR-206 is associated with cellular
proliferative inhibition and impairs invasion in ERα-positive
endometrioid adenocarcinoma. Cancer Lett. 314:41–53. 2012.
View Article : Google Scholar
|
|
13
|
Vickers MM, Bar J, Gorn-Hondermann I, et
al: Stage-dependent differential expression of microRNAs in
colorectal cancer: potential role as markers of metastatic disease.
Clin Exp Metastasis. 29:123–132. 2012. View Article : Google Scholar
|
|
14
|
Zhang L, Liu X, Jin H, et al: miR-206
inhibits gastric cancer proliferation in part by repressing
cyclinD2. Cancer Lett. 332:94–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Georgantas RW III, Streicher K, Luo X, et
al: MicroRNA-206 induces G1 arrest in melanoma by inhibition of
CDK4 and Cyclin D. Pigment Cell Melanoma Res. 27:275–286. 2014.
View Article : Google Scholar
|
|
16
|
Missiaglia E, Shepherd CJ, Patel S, et al:
MicroRNA-206 expression levels correlate with clinical behaviour of
rhabdomyosarcomas. Br J Cancer. 102:1769–1777. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adams BD, Furneaux H and White BA: The
micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen
receptor-alpha (ERalpha) and represses ERalpha messenger RNA and
protein expression in breast cancer cell lines. Mol Endocrinol.
21:1132–1147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan D, da Dong XE, Chen X, et al:
MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma
development. J Biol Chem. 284:29596–29604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song G, Zhang Y and Wang L: MicroRNA-206
targets notch3, activates apoptosis, and inhibits tumor cell
migration and focus formation. J Biol Chem. 284:31921–31927. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lardelli M, Dahlstrand J and Lendahl U:
The novel Notch homologue mouse Notch 3 lacks specific epidermal
growth factor-repeats and is expressed in proliferating
neuroepithelium. Mech Dev. 46:123–136. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Serafin V, Persano L, Moserle L, et al:
Notch3 signalling promotes tumour growth in colorectal cancer. J
Pathol. 224:448–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gagan J, Dey BK, Layer R, Yan Z and Dutta
A: Notch3 and Mef2c proteins are mutually antagonistic via Mkp1
protein and miR-1/206 microRNAs in differentiating myoblasts. J
Biol Chem. 287:40360–40370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang WX, Wilfred BR, Baldwin DA, et al:
Focus on RNA isolation: obtaining RNA for microRNA (miRNA)
expression profiling analyses of neural tissue. Biochim Biophys
Acta. 1779:749–757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mu Y, Zhou H, Li W, Hu L and Zhang Y:
Evaluation of RNA quality in fixed and unembedded mouse embryos by
different methods. Exp Mol Pathol. 95:206–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Guo R, Wu Q, Liu F and Wang Y: Description
of the CD133+ subpopulation of the human ovarian cancer cell line
OVCAR3. Oncol Rep. 25:141–146. 2011.
|
|
27
|
Tavazoie SF, Alarcon C, Oskarsson T, et
al: Endogenous human microRNAs that suppress breast cancer
metastasis. Nature. 451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bandres E, Cubedo E, Agirre X, et al:
Identification by Real-time PCR of 13 mature microRNAs
differentially expressed in colorectal cancer and non-tumoral
tissues. Mol Cancer. 5:292006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cummins JM, He Y, Leary RJ, et al: The
colorectal microRNAome. Proc Natl Acad Sci USA. 103:3687–3692.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Monzo M, Navarro A, Bandres E, et al:
Overlapping expression of microRNAs in human embryonic colon and
colorectal cancer. Cell Res. 18:823–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ozawa T, Kazama S, Akiyoshi T, et al:
Nuclear Notch3 expression is associated with tumor recurrence in
patients with stage II and III colorectal cancer. Ann Surg Oncol.
21:2650–2658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SH, Jeong EG and Yoo NJ: Mutational
analysis of NOTCH1, 2, 3 and 4 genes in common solid cancers and
acute leukemias. APMIS. 115:1357–1363. 2007. View Article : Google Scholar
|
|
33
|
Zhou L, Zhang N, Song W, et al: The
significance of Notch1 compared with Notch3 in high metastasis and
poor overall survival in hepatocellular carcinoma. PLoS One.
8:e573822013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu W, Xu C, Wan H, et al: MicroRNA-206
overexpression promotes apoptosis, induces cell cycle arrest and
inhibits the migration of human hepatocellular carcinoma HepG2
cells. Int J Mol Med. 34:420–428. 2014.PubMed/NCBI
|
|
35
|
Anderson C, Catoe H and Werner R: MIR-206
regulates connexin43 expression during skeletal muscle development.
Nucleic Acids Res. 34:5863–5871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hufbauer M, Lazic D, Reinartz M, Akgul B,
Pfister H and Weissenborn SJ: Skin tumor formation in human
papillomavirus 8 transgenic mice is associated with a deregulation
of oncogenic miRNAs and their tumor suppressive targets. J Dermatol
Sci. 64:7–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nohata N, Hanazawa T, Enokida H and Seki
N: microRNA-1/133a and microRNA-206/133b clusters: dysregulation
and functional roles in human cancers. Oncotarget. 3:9–21.
2012.PubMed/NCBI
|
|
38
|
Liu H, Cao YD, Ye WX and Sun YY: Effect of
microRNA-206 on cytoskeleton remodelling by downregulating Cdc42 in
MDA-MB-231 cells. Tumori. 96:751–755. 2010.
|
|
39
|
Domenga V, Fardoux P, Lacombe P, et al:
Notch3 is required for arterial identity and maturation of vascular
smooth muscle cells. Genes Dev. 18:2730–2735. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang W, Prince CZ, Mou Y and Pollman MJ:
Notch3 signaling in vascular smooth muscle cells induces c-FLIP
expression via ERK/MAPK activation. Resistance to Fas
ligand-induced apoptosis. J Biol Chem. 277:21723–21729. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang T, Holt CM, Xu C, et al: Notch3
activation modulates cell growth behaviour and cross-talk to
Wnt/TCF signalling pathway. Cell Signal. 19:2458–2467. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zeng ZS, Cohen AM and Guillem JG: Loss of
basement membrane type IV collagen is associated with increased
expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during
human colorectal tumorigenesis. Carcinogenesis. 20:749–755. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tran NL, Adams DG, Vaillancourt RR and
Heimark RL: Signal transduction from N-cadherin increases Bcl-2.
Regulation of the phosphatidylinositol 3-kinase/Akt pathway by
homophilic adhesion and actin cytoskeletal organization. J Biol
Chem. 277:32905–32914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tran NL, Nagle RB, Cress AE and Heimark
RL: N-Cadherin expression in human prostate carcinoma cell lines.
An epithelial-mesenchymal transformation mediating adhesion with
stromal cells. Am J Pathol. 155:787–798. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang H, Wang HS, Zhou BH, et al:
Epithelial-mesenchymal transition (EMT) induced by TNF-α requires
AKT/GSK-3β-mediated stabilization of snail in colorectal cancer.
PLoS One. 8:e566642013. View Article : Google Scholar
|
|
46
|
Tang F, Hajkova P, Barton SC, et al:
MicroRNA expression profiling of single whole embryonic stem cells.
Nucleic Acids Res. 34:e92006. View Article : Google Scholar : PubMed/NCBI
|