Introduction
Lung cancer continues to be the leading cause of
cancer-related mortality worldwide. Non-small cell lung cancer
(NSCLC) accounts for ~85% of all the cases of lung cancer (1). Lung cancer is a highly metastatic
disease, and the mortality of this type of cancer is strongly
associated with its high tendency to metastasize to specific
organs. Most patients present locally advanced or metastatic
disease at the time of diagnosis. Chemotherapy remains the mainstay
of treatment for patients with metastatic NSCLC. The median
survival time for advanced NSCLC patients remains poor. Therefore,
it is necessary to develop new drugs to improve the survival rate
of lung cancer patients (2,3).
Nematode anticoagulant protein c2 (NAPc2) is an
85-residue polypeptide originally isolated from the hematophagous
hookworm, Ancylostoma caninum (3,4). NAPc2
is a potent inhibitor of factor X (FX) activation by the extrinsic
Xase complex composed of VIIa and tissue factor (5–7). It
targets a specific site in the primary stages of the coagulation
pathway; it binds to FX or activated factor X (FXa) prior to the
formation of an inhibitory complex with activated factor VII/tissue
factor (FVIIa/TF). This inhibits coagulation and decreases the
formation of fibrin. The high affinity interaction between NAPc2
and FX is decisive for its pharmacokinetics. NAPc2 is potentially
an attractive agent, for its elimination half-life was found to be
longer than 50 h following subcutaneous and intravenous
administration in healthy male volunteers (6). Recombinant rNAPc2 has been shown to be
very effective in reducing the incidence of deep venous thrombosis
without hemostatic compromise when administered prophylactically in
patients (8). Furthermore, rNAPc2
inhibits the growth of primary and metastatic tumors in mice
independently of its ability to initiate coagulation (9). rNAPc2 can inhibit colorectal
tumorigenesis, progression and metastasis in mice through a
TF-dependent mechanism, indicating that rNAPc2 is a potent
anticancer agent when used in combination with chemotherapy or
anti-angiogenic therapy in mouse models of colorectal cancer
(10).
The urokinase plasminogen activator (uPA) system has
been implicated in angiogenesis, growth factor activation,
mobilization, ECM remodeling, invasion and metastasis in tumors
(11,12). It is currently believed that the
expression and activation of uPA play an important role in
tumorigenicity, and high endogenous levels of uPA are associated
with advanced metastatic cancers (13,14).
Therefore, uPA is increasingly being recognized as a candidate
target for gene therapy in cancers (15–20).
The purpose of the present study was to examine the
effect of rNAPc2 treatment on the invasive ability of NSCLC cells.
In the present study, we showed that rNAPc2 inhibited cell invasion
and uPA protein production in NSCLC cells. Its inhibitory effect is
possibly dependent on the nuclear factor-κB (NF-κB) activation
pathway, indicating its potential use in cancer therapy.
Materials and methods
Materials
Isopropyl 1-thio-β-D-galactopyranoside (IPTG),
penicillin and streptomycin were purchased from Sigma-Aldrich (St.
Louis, MO, USA). SN50 was purchased from Biomol (Plymouth Meeting,
PA, USA). TRIzol, Dulbecco’s modified Eagle’s medium (DMEM) and
fetal bovine serum (FBS) were obtained from Life Technologies
(Carlsbad, CA, USA). Rabbit polyclonal NF-κB p65 antibody was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Secondary antibodies for western blotting and immunofluorescence
were obtained from Amersham Biosciences Corporation (Piscataway,
NJ, USA). Other reagents were obtained from Sigma-Aldrich unless
stated otherwise.
Generation of bioactive recombinant
Ancylostoma caninum anticoagulant peptide c2
For the expression of high levels of rNAPc2 in E.
coli, we designed and synthesized 14 oligonucleotides
corresponding to the protein sequence of NAPc2 using the
theoretically optimized codons in E. coli (21). These oligonucleotides cover the
full-length sense and antisense NAPc2 gene with a 20-bp overlap.
The NAPc2 cDNA was cloned into the pTWIN1, yielding pTWIN1-NAPc2.
The NAPc2 cDNA was fused with the genes for the intein and chitin
binding domain (CBD), which functions as an affinity tag for
expression in E. coli and purification of rNAPc2 by
pH-dependent cleavage. The plasmid was transformed into E.
coli ER2566 and was grown in LB media induced with 30 mM IPTG
for 3 h. The cell lysate was loaded onto a chitin column and the
target protein was collected after triggered splicing with 20 mM
Tris-HCl, pH 7.2. The target protein rNAPc2 was sequenced from the
N-terminus.
Cell culture
A549 and H1299 cells were purchased from the
American Type Culture Collection (ATCC) and were maintained in DMEM
supplemented with penicillin (50 U/ml), streptomycin (50 U/ml) and
10% FBS.
Cell invasion assay
A Transwell cell culture chamber (Merck Millipore,
Bedford, MA, USA) was coated with Matrigel, dried and reconstituted
at 37°C with culture medium. rNAPc2 (0, 3, 6 and 12 μg/ml) was
added into the upper chamber. We placed culture medium containing
20% FBS in the lower chamber (24-well plates). Then the cells at
1×105 cells/chamber were added to the upper chamber in
DMEM containing 10% FBS. After a 24-h incubation at 37°C, the
suspended media in the lower chamber were removed. The cells that
had invaded to the lower side of the filter were fixed in 4%
paraformaldehyde and stained with Giemsa solution. The number of
cells that passed through the pores into the lower chamber were
counted under a phase-contrast microscope. The invasion index is
expressed as the ratio of the percent invasion of test cells over
the percent invasion of the control cells.
Immunofluorescence
The cells were washed with phosphate-buffered saline
(PBS) twice and immediately fixed with 4% paraformaldehyde at 4°C
for 20 min. The cells were incubated with 3% BSA for 30 min and
then with the primary antibody against NF-κB p65 overnight at 4°C
followed by incubation with the fluorescence-conjugated secondary
antibodies for 1 h at room temperature.
Preparation of cytoplasmic and nuclear
extracts
Cytoplasmic and nuclear extracts were prepared
according to the instructions of the Nuclear Extract kit from
Active Motif (Carlsbad, CA, USA). Briefly, the cells were scraped,
washed with phosphate-buffered saline (pH 7.4), resuspended in
hypotonic buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl,
0.2 mM phenylmethylsulfonyl fluoride and 0.5 mM dithiothreitol) and
allowed to swell on ice for 10 min. The cells were homogenized in a
Dounce homogenizer. The nuclei were separated by spinning at 3,300
× g for 5 min at 4°C. The supernatant was used as the cytoplasmic
extract. The nuclear pellet was extracted in nuclear extraction
buffer [20 mM HEPES (pH 7.9), 0.4 M NaCl, 1.5 mM MgCl2,
0.2 mM EDTA, 25% glycerol, 0.5 mM phenylmethylsulfonyl fluoride and
0.5 mM dithiothreitol (DTT)] for 30 min on ice and centrifuged at
12,000 × g for 30 min. The supernatant was used as the nuclear
extract. The protein concentrations in the supernatants of both the
nuclear and cytoplasmic extracts were measured by the Bio-Rad
protein assay. The nuclear and cytoplasmic extracts (30 μg) were
resolved by SDS-PAGE, and the levels of phosphorylated IκBα were
detected by western blot analysis.
NF-κB transcription factor assay
NF-κB transcription factor assay was performed
according to the instructions of the TransAM NF-κB Family
Transcription Factor Assay kit from Active Motif.
Total RNA extraction and real-time PCR
analysis
Total cellular RNA was isolated using a single step
method with TRIzol (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Real-time PCR was performed in a final
volume of 20 μl containing 3 μl of cDNA sample, 20 pmol of each
primer and 2X IQ™ SYBR®-Green Supermix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The following
oligonucleotide primers were used: uPA-FP, 5′-CAG
GGCATCTCCTGTGCATG-3′; uPA-RP, 5′-AGCCCTGCCCTGAAGTCGTTA-3′; human
GAPDH-FP, 5′-ACATGTTCCAATATGATTCCA-3′; human GAPDH-RP, 5′-AGCCCTG
CCCTGAAGTCGTTA-3′. We used a denaturing step at 95°C for 3 min and
40 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec.
Each reaction was run in duplicate, and fluorescence data were
collected at the end of the extension step in every cycle.
uPA ELISA
Cells were treated with various concentrations (0,
3, 6 and 12 μg/ml) of rNAPc2 for 24 h. Levels of uPA in the
conditioned medium were measured using commercially available uPA
ELISA kits (American Diagnostica, Greenwich, CT, USA) according to
the manufacturer’s instructions.
Chromatin immunoprecipitation (ChIP)
An ChIP assay was performed according to the
instructions of the ChIP Assay kit from Active Motif. Sequences of
promoter-specific primers 5′-GAG GGG GCG GAA GGG GAG AA-3′ and
5′-TGT GGT CAG TTT TGT TTG GAT TTG-3′ were used to amplify the
region of the uPA promoter containing the NF-κB element.
Nude mouse xenograft model
All experimental procedures were conducted in
accordance with the Principles of Laboratory Animal Care (Law on
Animal Experiments in Denmark, publication 1306, November 23, 2007)
and were approved by the West China Second University Hospital
Animal Care and Use Committee. Female BALB/c nu/nu mice (4–5 weeks
of age) were purchased from the Institute of Experimental Animals,
Sichuan University (Chengdu, China). A549 or H1299 cells
(1×106) were suspended in 100 μl PBS and injected
subcutaneously in the right posterior flank region of the nude
mice. After 10 days, when the tumors reached 5–10 mm in diameter,
the mice were randomly divided into 3 groups (5 mice/group).
Animals received rNAPc2 as daily intravenous injections of 400
μg/kg or PBS, respectively. Tumor dimensions were measured twice
every 5 days by a linear caliper. Tumor volume (mm3) was
calculated using the formula: length × width2/2. All the
mice were sacrificed humanely on day 35 after implantation and the
extirpated tumors were weighed.
Statistical analysis
Continuous variables are expressed as mean ± SD.
Statistical differences were performed using t-test or Mann-Whitney
U test. The results are the mean ± SD of values obtained from at
least 3 separate experiments. All statistical analyses were
performed by the software SPSS for Windows (version 13.0).
Differences described as significant in the text correspond to
P<0.05.
Results
Expression, purification and
identification of rNAPc2
ER2566 E. coli cells transformed with the
pTWIN1-NAPc2 plasmid were grown in LB media at 37°C and induced
with 30 mM IPTG for expression of the rNAPc2-intein-CBD fusion
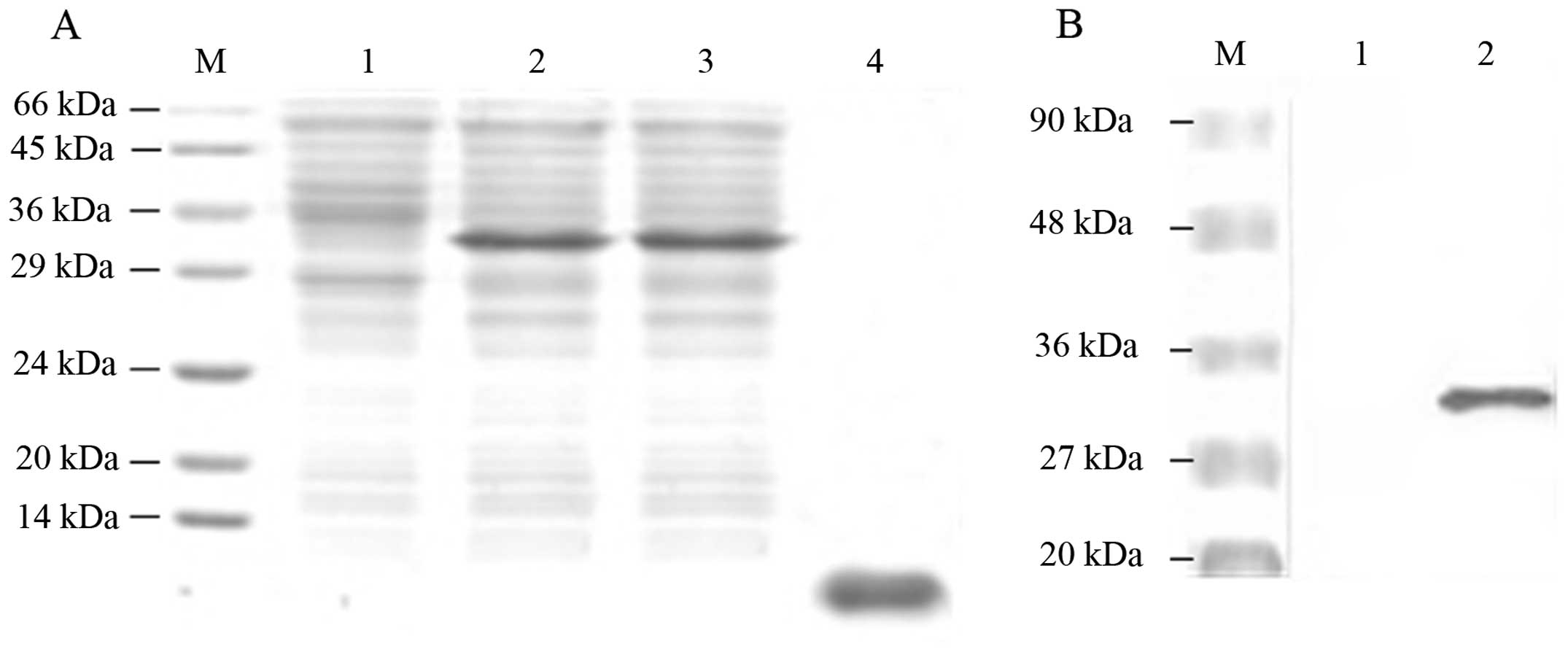
protein. Fig. 1A shows that a large
amount of rNAPc2 fusion proteins was detected in the supernatant
and the cell lysate. As shown in Fig.
1B, western blot analysis revealed that a product with a
molecular weight of 34 kDa was detected by the antibody against the
CBD. As the C-terminal cleavage of the intein was favored at a pH
7.2, following splicing of the fusion proteins at a pH 7.2, the
eluted rNAPc2 reached a concentration of 4 mg/ml with an expected
10-kDa molecular weight (Fig. 1A).
The rNAPc2 was identified by N-terminal sequencing (data not
shown).
rNAPc2 inhibits cell invasion and tumor
growth in vivo
To provide insight into the possible mechanisms
underlying the anticancer effect of rNAPc2, we examined whether
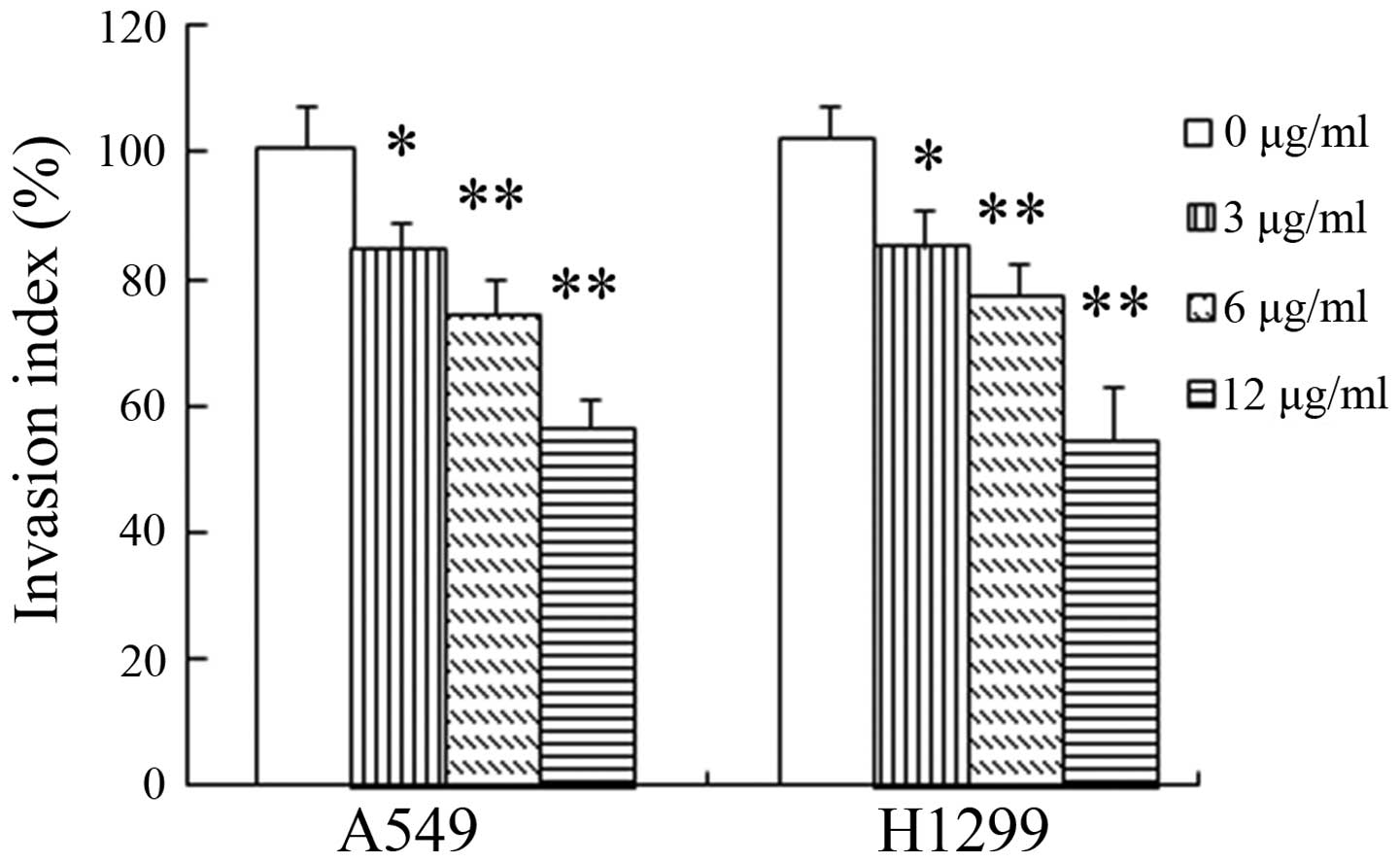
rNAPc2 impairs NSCLC cell invasion. In the in vitro invasion
assay, rNAPc2 obviously inhibited the invasive ability of the NSCLC
cells in a dose-dependent manner (Fig.
2). At doses of 3, 6 and 12 μg/ml, treatment with rNAPc2
significantly (P<0.05) reduced the cell invasion, respectively,
compared with the control group.
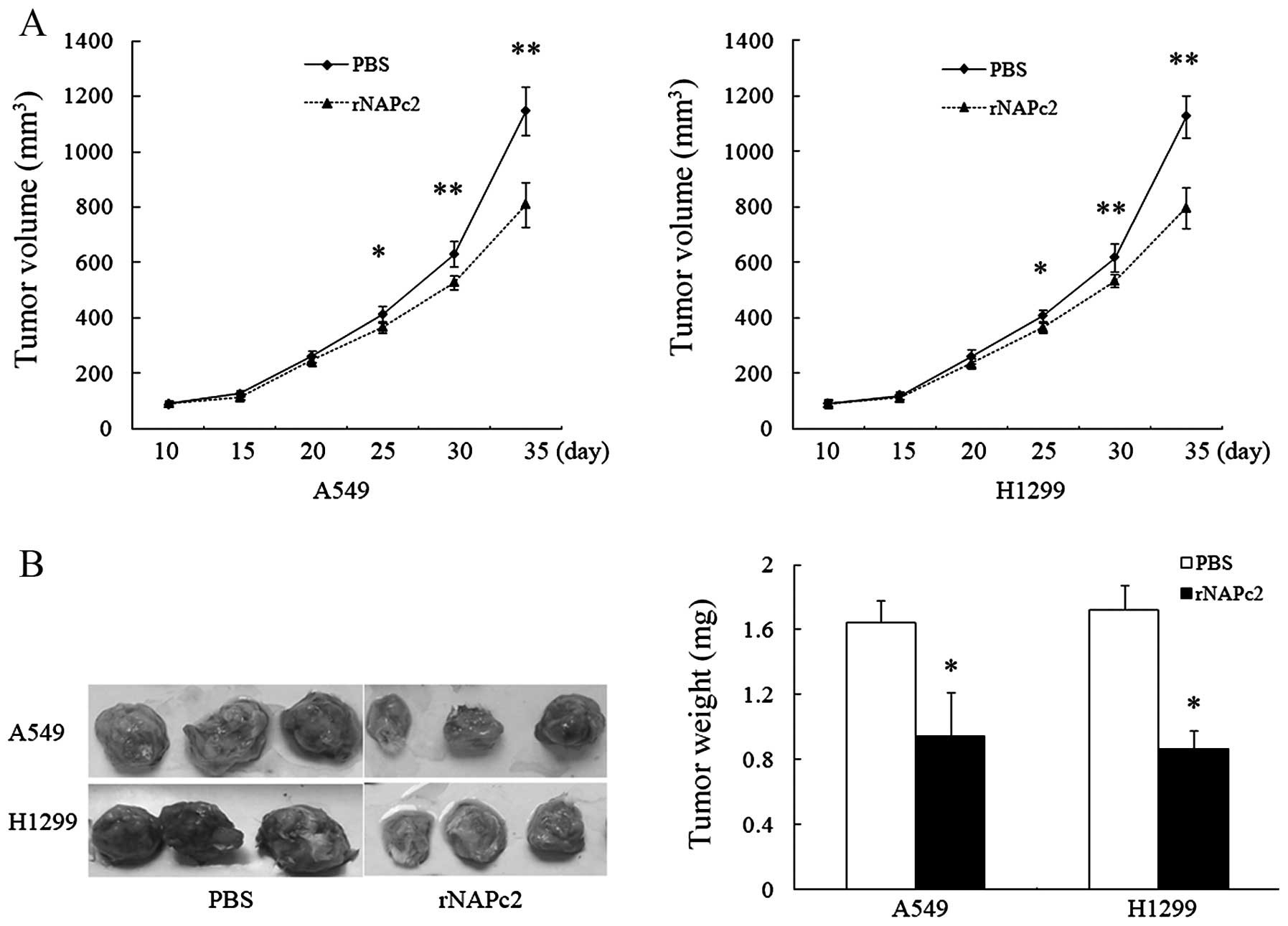
To further explore the effect of rNAPc2 on tumor
growth in vivo, we investigated the ability of rNAPc2 to
suppress tumor growth in vivo by daily intraperitoneal
injection of rNAPc2 at a dose of 400 μg/kg in A549 and H1299
xenograft models in nude mice. rNAPc2 at the dose of 400 μg/kg
showed strong inhibition of tumor growth (Fig. 3A). The tumor volume in the mice
injected with rNAPc2 was also significantly (P<0.05) smaller
than the tumor volume in the mice injected with PBS from day 35
after implantation. The average tumor weight in the mice treated
with rNAPc2 on day 35 after the implantation was significantly
lower than the tumor weight in the mice treated with PBS alone
(Fig. 3B). These results revealed
that rNAPc2 significantly inhibited tumor growth in a nude mouse
xenograft model.
rNAPc2 reduces cell invasion by
inhibiting uPA expression in the NSCLC cells
We hypothesized that rNAPc2 inhibition of NSCLC cell
invasion may be due to reduction in the uPA-uPAR system. To test
this hypothesis, we investigated uPA and uPAR mRNA expression
levels in A549 and H1299 cells following the rNAPc2 treatment. As
shown in Fig. 4A and B, rNAPc2
treatment significantly reduced the level of mRNA expression of uPA
in cells in a dose-dependent manner but not uPAR. Simultaneously,
rNAPc2 reduced uPA protein release in a dose-dependent manner in
the supernatants of the A549 and H1299 cells (Fig. 4C). rNAPc2 at 3 μg/ml was sufficient
to inhibit uPA protein release in the cell supernatants after
rNAPc2 treatment for 24 h (Fig.
4C). Treatment with uPA partially rescued the inhibition of
cell invasion by rNAPc2 (P<0.05) (Fig. 4D). These results showed that rNAPc2
reduced cell invasion by inhibiting uPA expression in the NSCLC
cells.
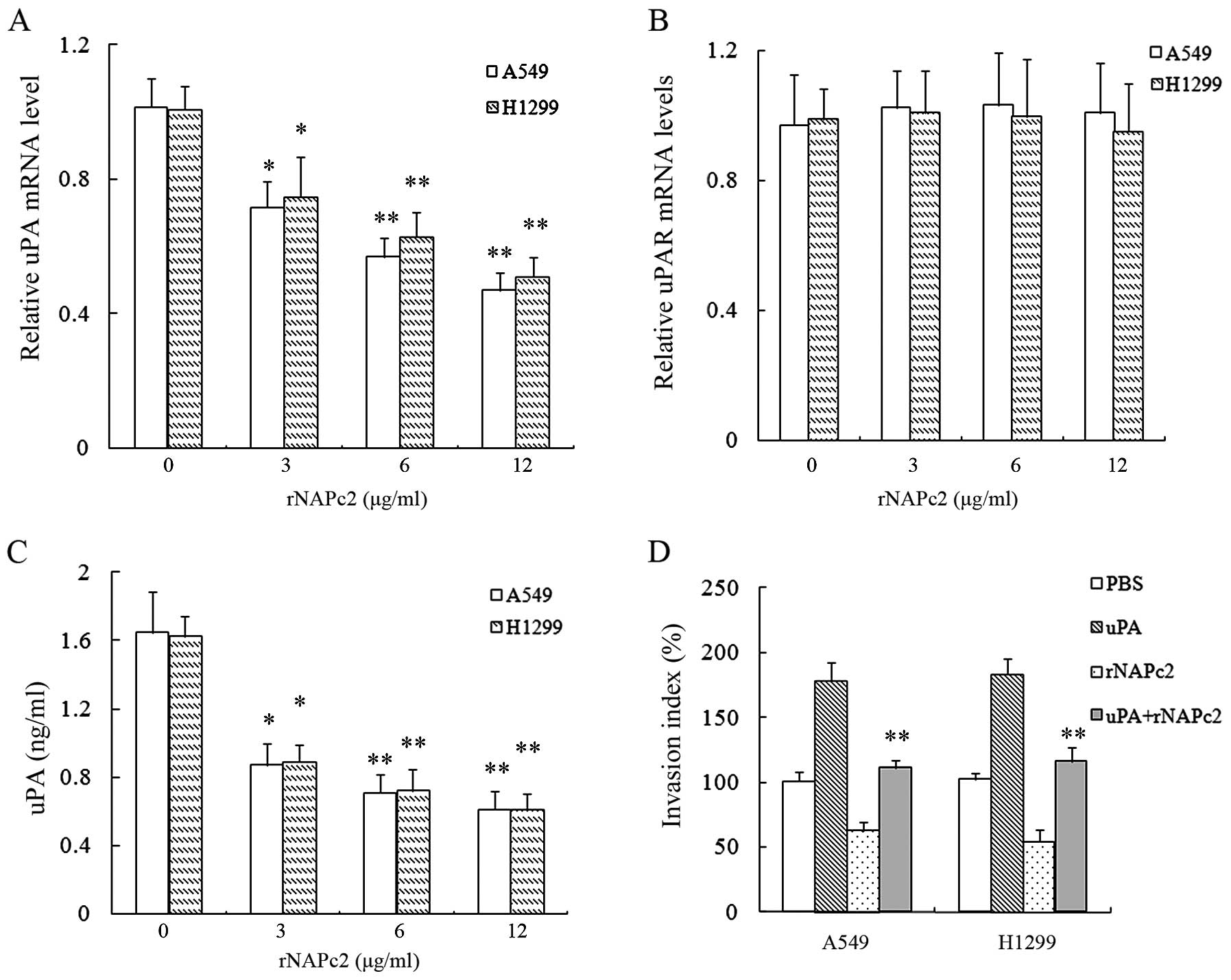 | Figure 4rNAPc2 reduces cell invasion by
inhibiting uPA expression in NSCLC cells. (A) rNAPc2 decreased the
expression level of uPA mRNA in NSCLC cells in a dose-dependent
manner. Cells were treated with various concentrations (0, 3, 6 and
12 μg/ml) of rNAPc2 for 24 h. The relative levels of uPA mRNA were
determined by real-time PCR. *P<0.05 and
**P<0.01. (B) Effect of rNAPc2 on the expression of
uPAR mRNA in the NSCLC cells. The cells were treated with various
concentrations (0, 3, 6 and 12 μg/ml) of rNAPc2 for 24 h. The
relative level of uPAR mRNA was determined by real-time PCR. The
expression level of uPAR mRNA was not significantly different
between the rNAPc2 treatment group and the control (P>0.05). (C)
rNAPc2 reduces uPA protein release in a dose-dependent manner in
the supernatants of the NSCLC cells. The cells were treated with
various concentrations (0, 3, 6 and 12 μg/ml) of rNAPc2 for 24 h.
The level of uPA in conditioned medium was measured by ELISA.
*P<0.05; **P<0.01. (D) Treatment with
uPA partially rescued the inhibition of cell invasion by rNAPc2.
rNAPc2 (6 μg/ml) was added into the upper chamber after the cells
were pre-incubated with the recombinant uPA (100 nmol/l) for 1 h.
The cells were allowed to migrate for 24 h at 37°C. Invasion index
was measured. Compared with control, the treatment with the uPA
increased the invasion index of the NSCLC cells.
*P<0.05 and **P<0.01. rNAPc2, nematode
anticoagulant protein c2; NSCLC, non-small cell lung cancer; uPA,
urokinase plasminogen activator; NSCLC, non-small cell lung cancer.
PBS, phosphate-buffered saline. |
rNAPc2 reduces expression and release of
uPA by inhibiting NF-κB activation in the NSCLC cell lines
NF-κB is present in the cytoplasm in a resting state
and translocates to the nucleus in an activated state. We found
that absorbance, which represents the transcriptional activation of
NF-κB, was decreased in the rNAPc2-treated cells (Fig. 5A). Incubation with 5 ng/ml TNF-α for
6 h led to nuclear translocation of P65, indicating activation of
NF-κB. However, translocation of NF-κB to the nucleus was
significantly abolished by rNAPc2 (Fig.
5B).
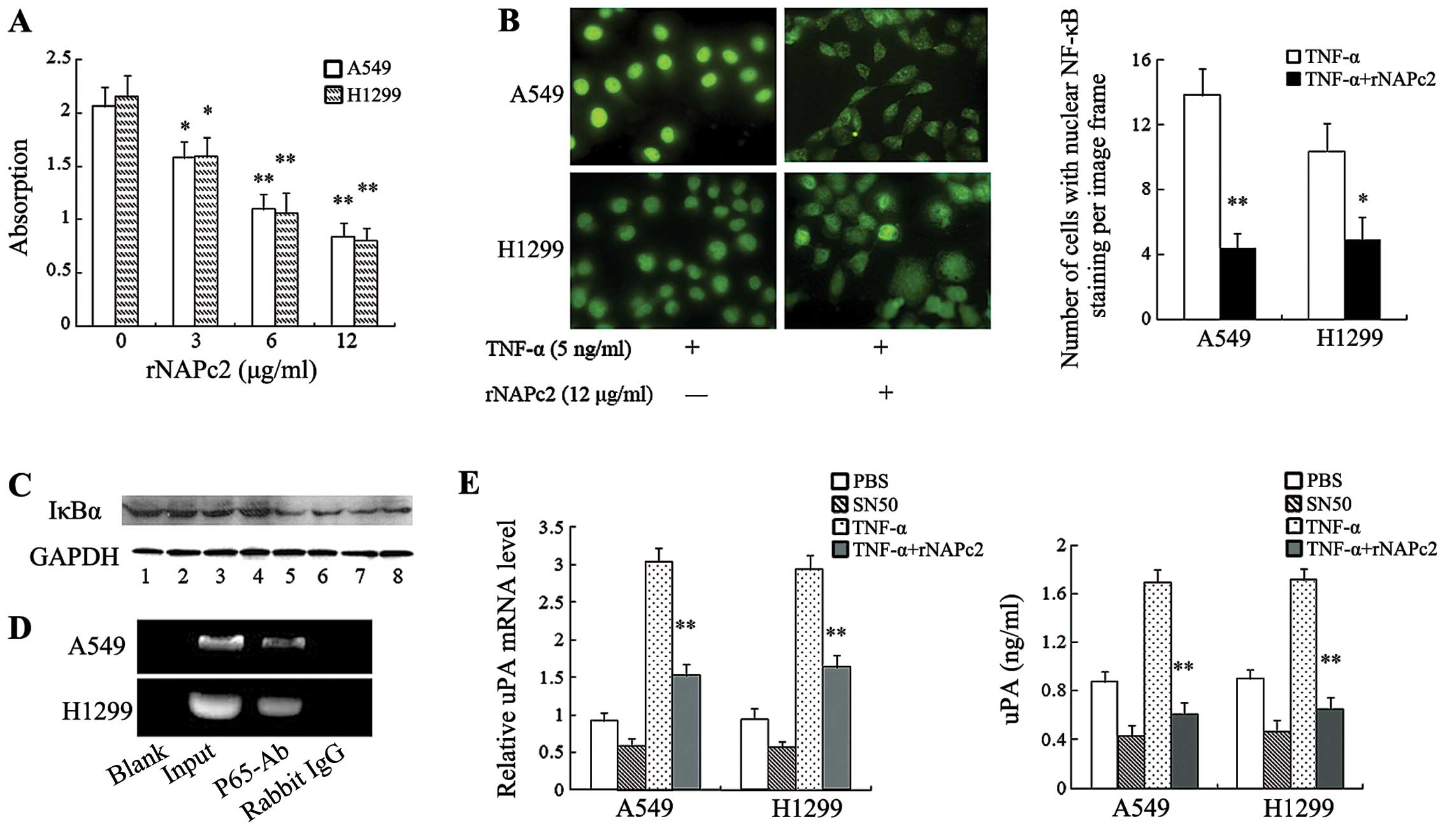 | Figure 5rNAPc2 reduces expression and release
of uPA by inhibiting NF-κB activation in NSCLC cells. (A) rNAPc2
inhibited TNF-α-induced NF-κB activation of NSCLC cells as
determined by ELISA. Cells were treated with 5 ng/ml TNF-α after
pre-incubation with various concentrations (0, 3, 6 and 12 μg/ml)
of rNAPc2 for 1 h. After TNF-α treatment for 6 h, NF-κB
transcription factor assay of nuclear extracts was performed. NF-κB
activation was decreased by rNAPc2. *P<0.05 and
**P<0.01. (B) rNAPc2 significantly abolished the
translocation of NF-κB to the nucleus induced by TNF-α
(magnification, ×400). Cells were treated with 5 ng/ml TNF-α after
pre-incubation with rNAPc2 (12 μg/ml) for 1 h. After TNF-α
treatment for 6 h, translocation of P65 to the nucleus was measured
by immunofluorescence. The number of cells with nuclear NF-κB
staining was quantified by per image frame. *P<0.05
and **P<0.01. (C) rNAPc2 decreased the IκBα
degradation in the NSCLC cell lines. Cytoplasmic IκBα degradation
was examined by western blot analysis. Lane 1 (A549) and lane 2
(H1299), controls; lane 3 (A549) and lane 4 (H1299), treated with 3
μg/ml rNAPc2; lane 5 (A549) and lane 6 (H1299), treated with 6
μg/ml rNAPc2; lane 7 (A549) and lane 8 (H1299), treated with 12
μg/ml rNAPc2. Equal protein loading was confirmed using the GAPDH
antibody. (D) NF-κB binds to the uPA promoter in the A549 and H1299
cells. ChIP assays were performed to identify uPA promoter
sequences containing the putative NF-κB binding sites from
chromatin complexes before (input) or after immunoprecipitation
(anti-p65 or IgG control). (E) Inhibition of NF-κB activation
decreased the level of mRNA expression and release of uPA in
conditioned medium from A549 and H1299 cells. Cells were treated
with TNF-α (5 ng/ml) after pre-incubation with SN50 (100 μmol/l)
for 1 h. The relative levels of uPAR mRNA were determined by
real-time PCR. Levels of uPA in conditioned medium were measured by
ELISA. SN50 reduced the level of mRNA expression and release of
uPA. *P<0.05; **P<0.01, compared with
the control (0 μg/ml rNAPc2). rNAPc2, nematode anticoagulant
protein c2; NSCLC, non-small cell lung cancer; uPA, urokinase
plasminogen activator; NF-κB, nuclear factor-κB; NSCLC, non-small
cell lung cancer. |
Phosphorylation and subsequent proteolytic
degradation of IκBα liberates NF-κB for nuclear translocation and
binding to consensus sequences on promoters and activates
NF-κB-regulated genes. We therefore examined cytoplasmic IκBα
degradation by western blot analysis. As shown in Fig. 5C, there was notable inhibition in
IκBα degradation in the cells following rNAPc2 treatment. We
performed an chromatin immunoprecipitation assay to identify that
NF-κB can directly bind to the uPA promoter (Fig. 5D).
Then, the level of uPA mRNA expression was analyzed
after inhibiting NF-κB activity of the cells. As shown in Fig. 5E, blockage of NF-κB activity reduced
the level of uPA mRNA expression in the NSCLC cells.
Correspondingly, ELISA analysis revealed that inhibition of NF-κB
activity decreased uPA release in the conditioned medium of the
NSCLC cells.
Discussion
Cancer patients are highly susceptible to
thrombosis, which accounts for a significant portion of the
morbidity and mortality of the disease. Antithrombotic drugs
including warfarin and low-molecular-weight heparin have been used
to prevent deep vein thrombosis and pulmonary embolism in cancer
patients (22,23). In addition to preventing venous
thromboembolism, antithrombotic therapy inhibits experimental
metastasis and prolongs survival in patients with solid tumor
malignancies (22). NAPc2 was
initially identified as a specific inhibitor of the tissue factor
(TF)/factor VIIa complex with novel antithrombotic activity
(8). Previous studies have shown
that NAPc2 can block angiogenesis, tumor growth and metastasis by
decreasing TF/FVIIa activity in experimental mouse models of
melanoma and colorectal cancer (8).
In contrast, rNAP5, a second nematode anticoagulant protein that
specifically inhibits fXa, does not exhibit antitumor activity.
Since the hemostatic activity of TF/fVIIa is mediated through
activation of fXa, these data suggest that proteolytic activity of
TF/fVIIa promotes tumor growth and angiogenesis through an
independent novel mechanism (9). In
the present study, we employed a novel system to generate
high-purity native NAPc2 in high yield by synthesizing the gene for
NAPc2 with overlapping oligonucleotides and the IMPACT-TWIN system
in E. coli. In fact, the generation of native rNAPc2 by
traditional expression and purification systems has been difficult.
Expression of recombinant protein in E. coli usually
contains a permanent methionine or another tag that interferes with
the bioactivity of the recombinant molecules. Expression of the
recombinant protein in yeast and other eukaryotic cells commonly
results in a low yield of products. The IMPACT™-TWIN protein fusion
and purification system utilizes the inducible self-cleavage
activity of engineered protein splicing elements for protein
purification and manipulation. The gene for rNAPc22 was fused with
the genes for the intein and CBD, allowing us to purify the
expressed rNAPc2 with a chitin column and pH-dependent
cleavage.
Therefore, we obtained a high yield of rNAPc2 and
observed that rNAPc2 obviously inhibited the invasive ability of
A549 and H1299 cells in a dose-dependent manner and impaired tumor
growth in a xenograft model of nude mice. Recent studies have
demonstrated that patients with lung cancer express high levels of
the uPA/uPAR system (13). The
uPA/uPAR system induces cancer cell proliferation and migration.
Inhibition of the uPA/uPAR system function with specific inhibitors
significantly decreases cancer invasion and metastasis (24). In fact, plasma levels of uPA were
elevated in patients with bladder carcinoma and were associated
with features of biologically aggressive disease (14). We showed that rNAPc2 downregulates
the expression of uPA mRNA and decreases uPA release.
Several transcription factors, such as NF-κB have
been implicated in the regulation of expression of uPA and uPAR
(25). NF-κB plays an important
role in carcinogenesis. NF-κB induces the expression of diverse
target genes that promote cell proliferation, regulate apoptosis,
facilitate angiogenesis and stimulate invasion and metastasis
(26,27). Furthermore, many cancer cells show
aberrant or constitutive NF-κB activation which mediates resistance
to chemotherapy and radiotherapy (28,29).
To study the mechanisms responsible for the observed low uPA
release and expression in NSCLC cells after rNAPc2 treatment, we
detected possible alterations of NF-κB activity. NF-κB is active in
the nucleus and is inhibited through its sequestration in the
cytoplasm by IκB. IκBs bind to NF-κB dimers and sterically block
the function of their nuclear localization sequences, thereby
causing their cytoplasmic retention. The NF-κB-IκB complex can also
shuttle between the cytoplasm and the nucleus in unstimulated cells
(30). We also observed that rNAPc2
caused inhibition of IκBα degradation in the cytoplasm in NSCLC
cells. The results showed that activation of NF-κB was
significantly abolished by rNAPc2. Furthermore, the results also
showed that the blockage of NF-κB activity reduced the level of uPA
mRNA expression and cell invasion in the NSCLC cells.
Simultaneously, chromatin immunoprecipitation further demonstrated
that NF-κB binds to the promoter of uPA. Many signaling pathways
activate transcription factors, such as ERK, hypoxia-inducible
factor 1α that act on the uPA promoter, driving uPA expression in
cancer (31,32). Here, we did not study whether rNAPc2
also modulates these signaling pathways and further research needs
to be conducted.
In conclusion, rNAPc2 inhibits cell invasion by
inhibiting uPA expression in NSCLC cells. Our results suggest that
rNAPc2 may be a potent agent for the prevention of NSCLC
progression. Additionally, the results demonstrated that NF-κB
plays an important role in NAPc2-induced downregulation of uPA
expression.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 30800633), the Sichuan
Province Science and Technology Foundation for Youths (no.
09ZQ026-034), the Sichuan Province Science and Technology
Foundation (no. 2012SZ0010), and the Program for Changjiang
Scholars and Innovative Research Team in the University (no. IRT
0935).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schulman S: Advantages and limitations of
the new anticoagulants. J Intern Med. 275:1–11. 2014. View Article : Google Scholar
|
|
4
|
Mungall D: rNAPc2. Nuvelo Curr Opin
Investig Drugs. 5:327–333. 2004.
|
|
5
|
Schulman S: Patient self-management of
anticoagulants reduced arterial thromboembolism and adverse
effects. ACP J Club. 143:82005.PubMed/NCBI
|
|
6
|
Vlasuk GP, Bradbury A, Lopez-Kinninger L,
Colón S, Bergum PW, Maki S and Rote WE: Pharmacokinetics and
anticoagulant properties of the factor VIIa-tissue factor inhibitor
recombinant Nematode Anticoagulant Protein c2 following
subcutaneous administration in man. Dependence on the
stoichiometric binding to circulating factor X. Thromb Haemost.
90:803–812. 2003.PubMed/NCBI
|
|
7
|
Lee AY and Vlasuk GP: Recombinant nematode
anticoagulant protein c2 and other inhibitors targeting blood
coagulation factor VIIa/tissue factor. J Intern Med. 254:313–321.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moons AH, Peters RJ, Bijsterveld NR, Piek
JJ, Prins MH, Vlasuk GP, Rote WE and Büller HR: Recombinant
nematode anticoagulant protein c2, an inhibitor of the tissue
factor/factor VIIa complex, in patients undergoing elective
coronary angioplasty. J Am Coll Cardiol. 41:2147–2153. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hembrough TA, Swartz GM, Papathanassiu A,
Vlasuk GP, Rote WE, Green SJ and Pribluda VS: Tissue factor/factor
VIIa inhibitors block angiogenesis and tumor growth through a
nonhemostatic mechanism. Cancer Res. 63:2997–3000. 2003.PubMed/NCBI
|
|
10
|
Zhao J, Aguilar G, Palencia S, Newton E
and Abo A: rNAPc2 inhibits colorectal cancer in mice through tissue
factor. Clin Cancer Res. 15:208–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raghu H, Nalla AK, Gondi CS, Gujrati M,
Dinh DH and Rao JS: uPA and uPAR shRNA inhibit angiogenesis via
enhanced secretion of SVEGFR1 independent of GM-CSF but dependent
on TIMP-1 in endothelial and glioblastoma cells. Mol Oncol.
6:33–47. 2012. View Article : Google Scholar :
|
|
12
|
Zhang Y, Kenny HA, Swindell EP, Mitra AK,
Hankins PL, Ahn RW, Gwin K, Mazar AP, O’Halloran TV and Lengyel E:
Urokinase plasminogen activator system-targeted delivery of
nanobins as a novel ovarian cancer therapy. Mol Cancer Ther.
12:2628–2639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Offersen BV, Pfeiffer P, Andreasen P and
Overgaard J: Urokinase plasminogen activator and plasminogen
activator inhibitor type-1 in nonsmall-cell lung cancer: Relation
to prognosis and angiogenesis. Lung Cancer. 56:43–50. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shariat SF, Monoski MA, Andrews B, Wheeler
TM, Lerner SP and Slawin KM: Association of plasma urokinase-type
plasminogen activator and its receptor with clinical outcome in
patients undergoing radical cystectomy for transitional cell
carcinoma of the bladder. Urology. 61:1053–1058. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nowicki TS, Moscatello AL, Shin E, Schantz
S, Tiwari RK and Geliebter J: The urokinase plasminogen activator
system in metastatic papillary thyroid carcinoma: A potential
therapeutic target. J Clin Endocrinol Metab. 96:3062–3064. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kenny HA, Leonhardt P, Ladanyi A, Yamada
SD, Montag A, Im HK, Jagadeeswaran S, Shaw DE, Mazar AP and Lengyel
E: Targeting the urokinase plasminogen activator receptor inhibits
ovarian cancer metastasis. Clin Cancer Res. 17:459–471. 2011.
View Article : Google Scholar :
|
|
17
|
Zhou H, Tang Y, Liang X, Yang X, Yang J,
Zhu G, Zheng M and Zhang C: RNAi targeting urokinase-type
plasminogen activator receptor inhibits metastasis and progression
of oral squamous cell carcinoma in vivo. Int J Cancer. 125:453–462.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gondi CS, Lakka SS, Dinh DH, Olivero WC,
Gujrati M and Rao JS: Intraperitoneal injection of a hairpin
RNA-expressing plasmid targeting urokinase-type plasminogen
activator (uPA) receptor and uPA retards angiogenesis and inhibits
intracranial tumor growth in nude mice. Clin Cancer Res.
13:4051–4060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tummalapalli P, Gondi CS, Dinh DH, Gujrati
M and Rao JS: RNA interference-mediated targeting of urokinase
plasminogen activator receptor and matrix metalloproteinase-9 gene
expression in the IOMM-lee malignant meningioma cell line inhibits
tumor growth, tumor cell invasion and angiogenesis. Int J Oncol.
31:5–17. 2007.PubMed/NCBI
|
|
20
|
Bauer TW, Liu W, Fan F, et al: Targeting
of urokinase plasminogen activator receptor in human pancreatic
carcinoma cells inhibits c-Met- and insulin-like growth factor-I
receptor-mediated migration and invasion and orthotopic tumor
growth in mice. Cancer Res. 65:7775–7781. 2005.PubMed/NCBI
|
|
21
|
Hatfield GW and Roth DA: Optimizing
scaleup yield for protein production: Computationally Optimized DNA
Assembly (CODA) and Translation Engineering. Biotechnol Annu Rev.
13:27–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stevenson JL, Choi SH and Varki A:
Differential metastasis inhibition by clinically relevant levels of
heparins - correlation with selectin inhibition, not antithrombotic
activity. Clin Cancer Res. 11:7003–7011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verso M and Agnelli G: New and old
anticoagulants in cancer. Thromb Res. 129(Suppl 1): S101–S105.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nalla AK, Asuthkar S, Bhoopathi P, Gujrati
M, Dinh DH and Rao JS: Suppression of uPAR retards
radiation-induced invasion and migration mediated by integrin
β1/FAK signaling in medulloblastoma. PLoS One. 5:e130062010.
View Article : Google Scholar
|
|
25
|
Moreau M, Mourah S and Dosquet C:
β-Catenin and NF-κB cooperate to regulate the uPA/uPAR system in
cancer cells. Int J Cancer. 128:1280–1292. 2011. View Article : Google Scholar
|
|
26
|
Gyrd-Hansen M and Meier P: IAPs: From
caspase inhibitors to modulators of NF-kappaB, inflammation and
cancer. Nat Rev Cancer. 10:561–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aurora AB, Biyashev D, Mirochnik Y,
Zaichuk TA, Sánchez-Martinez C, Renault MA, Losordo D and Volpert
OV: NF-kappaB balances vascular regression and angiogenesis via
chromatin remodeling and NFAT displacement. Blood. 116:475–484.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castro-Gamero AM, Borges KS, da Silva
Silveira V, Lira RC, de Paula Gomes Queiroz R, Valera FC, Scrideli
CA, Umezawa K and Tone LG: Inhibition of nuclear factor-κB by
dehydroxymethylepoxyquinomicin induces schedule-dependent
chemosensitivity to anticancer drugs and enhances chemoinduced
apoptosis in osteosarcoma cells. Anticancer Drugs. 23:638–650.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hunter JE, Willmore E, Irving JA,
Hostomsky Z, Veuger SJ and Durkacz BW: NF-κB mediates
radio-sensitization by the PARP-1 inhibitor, AG-014699. Oncogene.
31:251–264. 2012. View Article : Google Scholar
|
|
30
|
Truhlar SM, Torpey JW and Komives EA:
Regions of IkappaB alpha that are critical for its inhibition of
NF-kappaB. DNA interaction fold upon binding to NF-kappaB. Proc
Natl Acad Sci USA. 103:18951–18956. 2006. View Article : Google Scholar
|
|
31
|
Tacchini L, Matteucci E, De Ponti C and
Desiderio MA: Hepatocyte growth factor signaling regulates
transactivation of genes belonging to the plasminogen activation
system via hypoxia inducible factor-1. Exp Cell Res. 290:391–401.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Min HJ, Lee Y, Zhao XF, Park YK, Lee MK,
Lee JW and Kim S: TMPRSS4 upregulates uPA gene expression through
JNK signaling activation to induce cancer cell invasion. Cell
Signal. 26:398–408. 2014. View Article : Google Scholar
|