Introduction
Salivary adenoid cystic carcinomas (SACCs) are
malignant tumors of the head and neck and are characterized by
unique clinical features and behaviors. SACCs occur in the major
and minor salivary glands and disperse to the oral and
oropharyngeal mucosa, tracheobronchial tree and the esophagus. The
biological properties of this dispersal include slow and indolent
growth, a low probability of regional nodal metastasis, a high
propensity for perineural migration and distant metastasis, and a
high incidence of recurrence. It has been reported that 40–60% of
SACC patients develop distant metastases in the lungs, bone and
soft tissues (1–3). Distant metastases lead to poor patient
survival. Therefore, the search for molecules that are relevant to
SACC migration and metastasis and the study of the corresponding
molecular mechanisms will introduce a new experimental foundation
and provide possible molecular targets for the early diagnosis,
therapy and prognostic analysis of SACC (4).
The Wnt signal represents a path that has been
highly versatile in the process of biological evolution (5) and consists of numerous signaling
proteins that include extracellular factors (Wnt), transmembrane
receptors (frizzled and FZD), a cytoplasmic protein (β-catenin),
nuclear transcription factors (TCF and LEF) and others. These
signaling proteins are primarily activated in the embryonic
development process and play significant roles in embryo
development and tissues differentiation. Increasing numbers of
studies have indicated that tumorigenesis and tumor metastasis are
related to the abnormal activation of the Wnt signaling pathway
(6–8). The FZD gene family contains the genes
for the receptors of the Wnt signaling pathway, and the receptor
proteins (frizzled proteins) encoded by FZD can combine with Wnt
ligands to activate the Wnt signaling pathway (9–11). It
has been reported that FZD accelerates the occurrence and
development of carcinomas (9,12,13),
but FZD has also been reported to be tumor suppressor gene in other
studies (14,15). At present, the roles of FZD2 in SACC
remain unclear.
In this study, we aimed to explore the differences
in the expression of FZD2 between ACC-83 and ACC-LM cells using
quantitative real-time PCR. We also analyzed the expression of FZD2
in clinical SACC samples with and without metastasis using
immunohistochemistry to examine the correlation between FZD2
expression and SACC metastasis. We silenced FZD2 via small
interfering RNA (siRNA) and overexpressed FZD2 using plasmid to
elucidate the effects of FZD2 on SACC growth and migration. We
explored the expression of E-cadherin, vimentin, MET and PAI-1
after FZD2 was reduced in SACC cells.
Materials and methods
Cell culture and clinical samples
The highly metastatic cell line SACC-83 and the
minimally metastatic cell line SACC-LM were provided by the Peking
University School of Stomatology. The cells were maintained in 1640
with 10% fetal bovine serum (FBS) (both from Gibco, USA), and
incubated in a humidified atmosphere of 95% air and 5%
CO2 at 37°C. Pathologically diagnosed tissue samples
were obtained from the First Affiliated Hospital of Fujian Medical
University and were collected between 1997 and 2008. Forty
paraffin-embedded samples, including 19 cases with metastasis or
recurrence and 21 cases without metastasis or recurrence, had been
kept on-file by the Pathology Department. This study was approved
by the Institutional Review Board of Fujian Medical University, and
written informed consent was obtained from each participant.
Quantitative real-time PCR analysis
Total RNA was extracted from the SACC cells using
TRIzol reagent (#15596018; Invitrogen, Carlsbad, CA, USA), and RNA
purities and concentrations were detected by ultraviolet
spectrometry. The RNAs were separately diluted to the same
concentration after being measured and then reverse-transcribed
into cDNA with the PrimeScript RT reagent kit (#RR037A; Takara,
Japan). PCR was performed in triplicate using the primers listed in
Table I and SYBR Premix Ex Taq™
(#RR420A; Takara) according to the manufacturer's instructions. The
fluorescence values from the 12 cycles were used as the background
signal, and the threshold value was set at 10 times the standard
deviation of the fluorescence signals of cycles 4–12. The
expression levels were normalized to β-actin mRNA levels for each
sample obtained from parallel assays and analyzed according to
Livak and Schmittgen (16).
 | Table ISequences of the primers used in this
study. |
Table I
Sequences of the primers used in this
study.
| Gene | Access no. | Sequence |
|---|
| FZD2-F | NM001466 |
AGTTCTATCCGCTGGTGAAGGT |
| FZD2-R | NM001466 |
GCCCAGAAACTTGTAGCTGAGA |
| ACTB-F | NM001101 |
CCTGGCACCCAGCACAAT |
| ACTB-R | NM001101 |
GGGCCGGACTCGTCATACT |
| Vimentin-F | NM001292 |
TGGACCAGCTAACCAACGACAA |
| Vimentin-R | NM001292 |
GTTCAAGGTCAAGACGTGCCAG |
| MET-F | NM001127 |
GCTGACTTCTCCACTGGTTCCT |
| MET-R | NM001127 |
ACCAAGGTAAACAGGAGCACGA |
| PAI-1-F | NM000602 |
GCCAAGAGCGCTGTCAAGAAG |
| PAI-1-R | NM000602 |
TTCACCAAAGACAAGGGCCAGG |
| E-cadherin-F | NM004360 |
GCTTCCCTCTTTCATCTCCTGA |
| E-cadherin-R | NM004360 |
GCCACATTTTCTTCTTGCTCCT |
Immunohistochemical staining assay
The immunohistochemical SP three-step approach was
used to stain and analyze the SACC pathological tissues using
ovarian carcinomas as a positive control group, and
phosphate-buffered saline (PBS) was used in place of the primary
antibodies in the negative control groups. After deparaffinization
in xylene, the sections were rehydrated in a decreasing gradient of
ethanol and washed for 10 min in PBS (pH 7.2). Endogenous
peroxidase activity was inhibited by incubation in methanol
containing 3% H2O2 for 10 min. After several
washes in PBS, the sections were blocked with a universal blocking
reagent (Maxin, USA) for 10 min at room temperature and then
incubated with the primary antibody against FZD2 (1:5,000 dilution;
GR76263-10; Abcam Cambridge, UK) for 1 h at room temperature. After
several washes in PBS, the sections were incubated with a
biotin-conjugated secondary antibody (Maxin) for 10 min at room
temperature. After several washes in PBS, the sections were
incubated with streptavidin-peroxidase (Maxin) for 10 min at room
temperature. The sections were rinsed with PBS, and the antibody
complexes were visualized by incubation with diaminobenzidine
tetrahydrochloride (DAB) chromogen (Maxin). The sections were then
counterstained with hematoxylin (Dako, Denmark), dehydrated and
examined by light microscopy. All slides were reviewed
independently by two pathologists who were blinded to each other's
readings. The staining results were assessed on the following
three-tier scale: negative indicated no staining, 1+ indicated weak
staining and 2+ indicated strong staining. The immunohistochemical
results were graded of 1 of 3 different scores as follows: negative
indicated no staining or 1+ staining in ≤30% of the cells, positive
indicated 1+ staining in >30% of the cells or 2+ staining in
<50% of cells, and strong positive indicated 2+ staining in
>50% of the cells (17).
RNAi and plasmid transfection
Twenty-four hours before transfection, SACC-83 cells
in the exponential phase of growth were digested, counted and
plated into 6-well plates at 3×105 cells/well. The cells
were then transfected with siRNAs (18–20)
(GenePharma, Shanghai, China) (the sequences are indicated in
Table II) using Lipofectamine
RNAiMAX (1044526) or plasmid (a kind gift from Changgong Li,
Department of Pediatrics, University of Southern California) using
Lipofectamine 3000 (1713234) (both from Invitrogen) according to
the manufacturer's instructions.
 | Table IIThe sequences of siRNAs used in this
study. |
Table II
The sequences of siRNAs used in this
study.
| siRNA | Sequence
(5′-3′) |
|---|
| FZD2-605 |
5′-GCGAAGCCCUCAUGAACAATT-3′
5′-UUGUUCAUGAGGGCUUCGCTT-3′ |
| FZD2-931 |
5′-CCCGAUGGUUCCAUGUUCUTT-3′
5′-AGAACAUGGAACCAUCGGGTT-3′ |
| FZD2-1792 |
5′-CCGACUUCACGGUCUACAUTT-3′
5′-AUGUAGACCGUGAAGUCGGTT-3′ |
| Negative
control |
5′-UUCUCCGAACGUGUCACGUTT-3′
5′-ACGUGACACGUUCGGAGAATT-3′ |
Western blotting
Total cell proteins were extracted, and protein
assays were examined with a BCA kit and an ELISA reader. The total
proteins were separated by 8% SDS-PAGE and transferred onto PVDF
membranes (Amersham, USA). Subsequently, the membranes were
immunoblotted with primary antibodies against FZD2 (1:5,000
dilution; GR76263-10; Abcam) or β-actin (1:2,000; 1014F; CWBio,
Beijing, China) in 5% bovine serum albumin overnight, washed three
times with tris-buffered saline with 0.1% Tween-20, and incubated
with secondary antibody (1:2,000 dilution; Abcam). The
immunoreactive protein bands were visualized via CDP-Star reagent
(Roche, USA), and the signals were scanned with a densitometer for
semi-quantification of the signal intensities.
Cell viability assay
Cell proliferation was measured by counting the
cells in the logarithmic phase with a Cell Counting Kit-8 (#CK04;
Dojindo Kumamoto, Japan). The cells were first transfected with
siRNA or plasmid and then plated into a 96-well plate. Cells from
each group were plated in 3-wells, and each well had
2×103 cells. The absorbance of each well was measured
with a microplate reader at the same time over 5 consecutive days.
This process was repeated in triplicate for the statistical
analyses and to draw the corresponding curves.
Colony formation assay
Twenty-four hours after siRNA or plasmid
transfection, the cells were plated into 6-well plates (500
cells/well) and cultured for 2 weeks. Colonies were fixed with cold
methanol for 10 min and stained with 1% crystal violet for 30
min.
In vitro cell migration assay
The cell migration assays were performed in 24-well
Transwell chambers (#353097; BD Biosciences, USA). Twenty-four
hours after siRNA or plasmid transfection, the cells were serum
starved for 24 h and then collected with 1640 with 0.1% FBS. The
cells were plated into the upper chamber at a density of
1.0×105 cells/well, and 700 µl of 1640 containing
10% FBS was added to the lower chamber. Forty-eight hours later,
the cells in the upper chamber were removed with cotton swabs and
stained with 1% crystal violet for 10 min. The cells of five random
microscopic fields (×200) were counted and photographed.
Statistical analyses
The data were analyzed with the SPSS 22.0 statistics
software package. Rank-sum tests were used to compare the rates
between the two groups of immunohistochemistry data, and
multi-sample average one-way ANOVA tests were used for between
group comparisons. α=0.05 was taken to indicate statistical
significance.
Results
Expression of FZD2 differed across tissue
types
Both overexpression and downregulation of FZD2 have
been observed in human cancers relative to expression in normal
samples. In this study, we first explored the expression of FZD2 in
different human cancers from the Oncomine database. According to
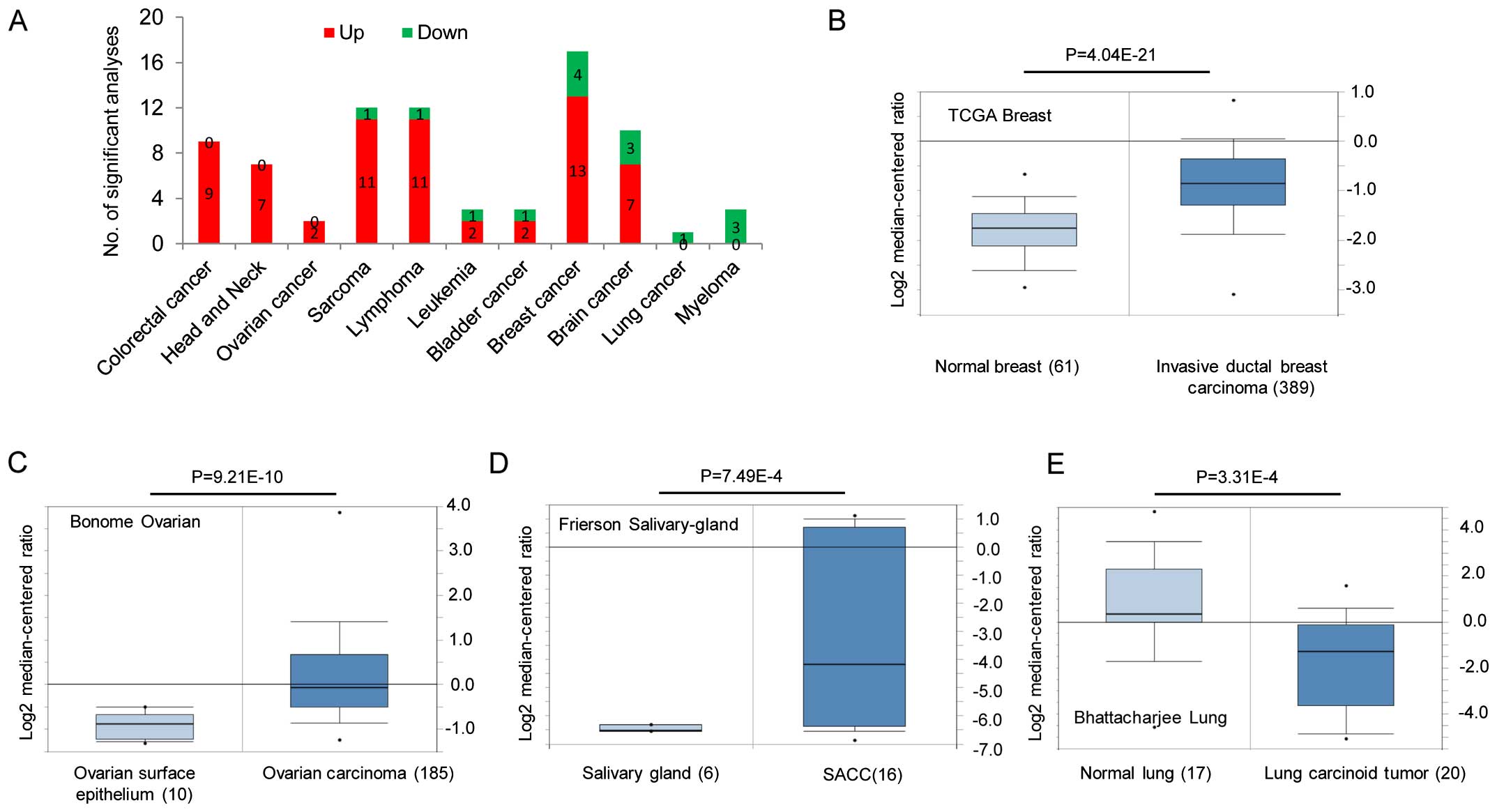
this publicly available database, FZD2 is primarily overexpressed
in colorectal, head and neck, sarcoma, lymphoma, leukemia, bladder,
breast and brain cancers (Fig.
1A–D), and is downregulated in lung cancer and myeloma
(Fig. 1A and E). Gene expression
data for these studies are available from the Oncomine database as
are the studies describing these results (http://www.oncomine.org). We also conducted an
unbiased bioinformatic analysis of the gene-expression profiles of
1,609 patients with breast cancer, 1,436 patients with ovarian
cancer and 1,432 patients with lung cancer using Kaplan-Meier (KM)
plotter, which is a meta-analysis-based biomarker assessment tool.
This analysis tool utilizes Affymetrix gene-expression profiling
data and includes multiple probe sets for most genes (21–23).
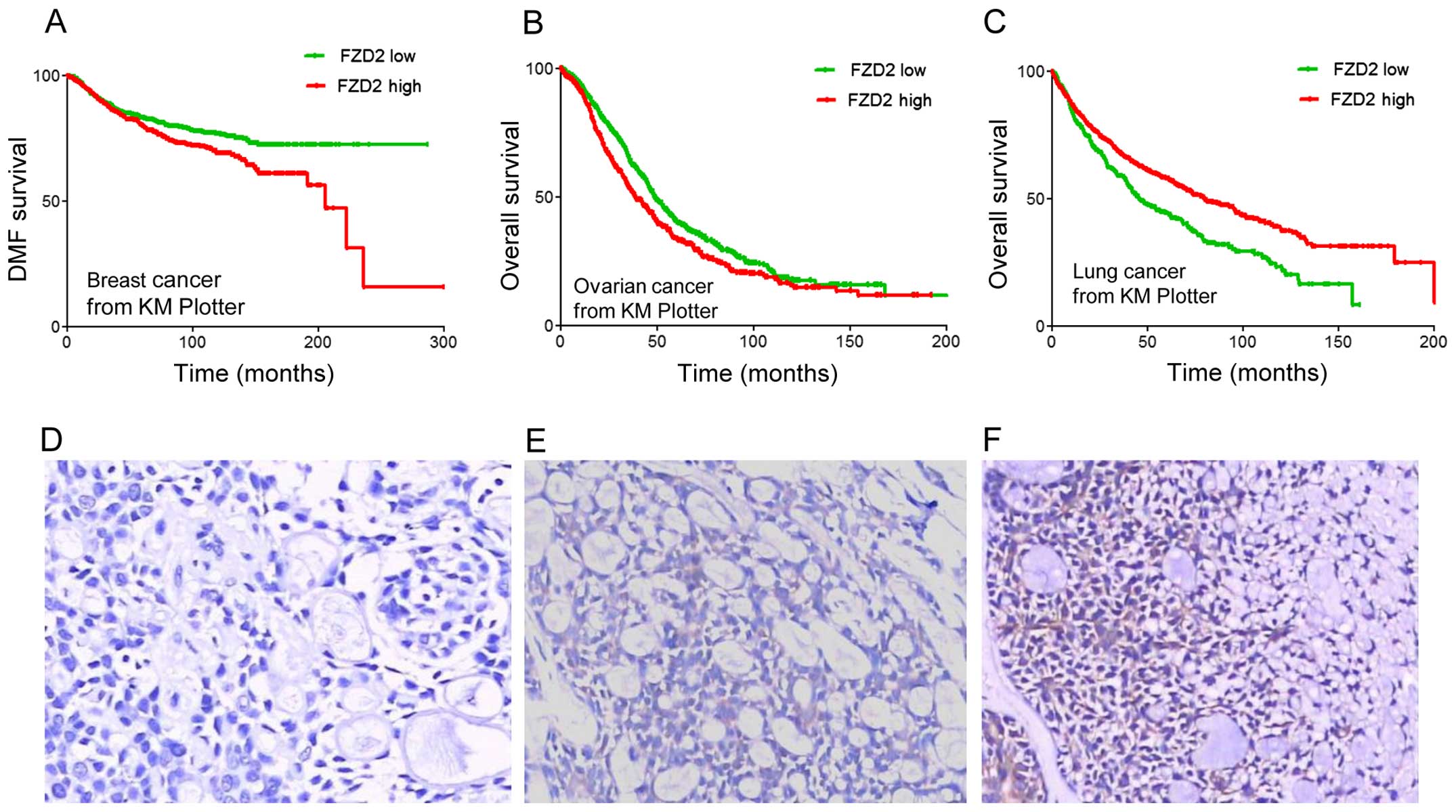
As displayed in Fig. 2A–C, higher
expressions of FZD2 (the best cut-off was auto-selected) were
associated with poor prognosis and shorter relapse-free survival
among breast cancer (P=3.7×10−3, Fig. 2A) and ovarian cancer patients
(P=4.6×10−4, Fig. 2B)
but better prognosis and longer survival (P=1.3×10−6,
Fig. 2C) among lung cancer
patients. In this study, we investigated the expression of FZD2 in
SACCs using immunohistochemistry. As shown in Table III and Fig. 2D–F, the expression of FZD2 was
downregulated in the SACC samples with metastasis and recurrence
when compared to the samples without metastasis (P<0.05). These
results imply that FZD2 may have a negative effect on the
progression of adenoid cystic carcinoma.
 | Table IIIExpression of FZD2 in the SACC
samples with and without metastasis and recurrence. |
Table III
Expression of FZD2 in the SACC
samples with and without metastasis and recurrence.
| Metastasis and
recurrence | n | Negative | Positive | Strong
positive | P-value |
|---|
| Yes | 19 | 13 | 5 | 1 | 0.032 |
| No | 21 | 8 | 7 | 6 | |
FZD2 inhibits cell proliferation in
vitro
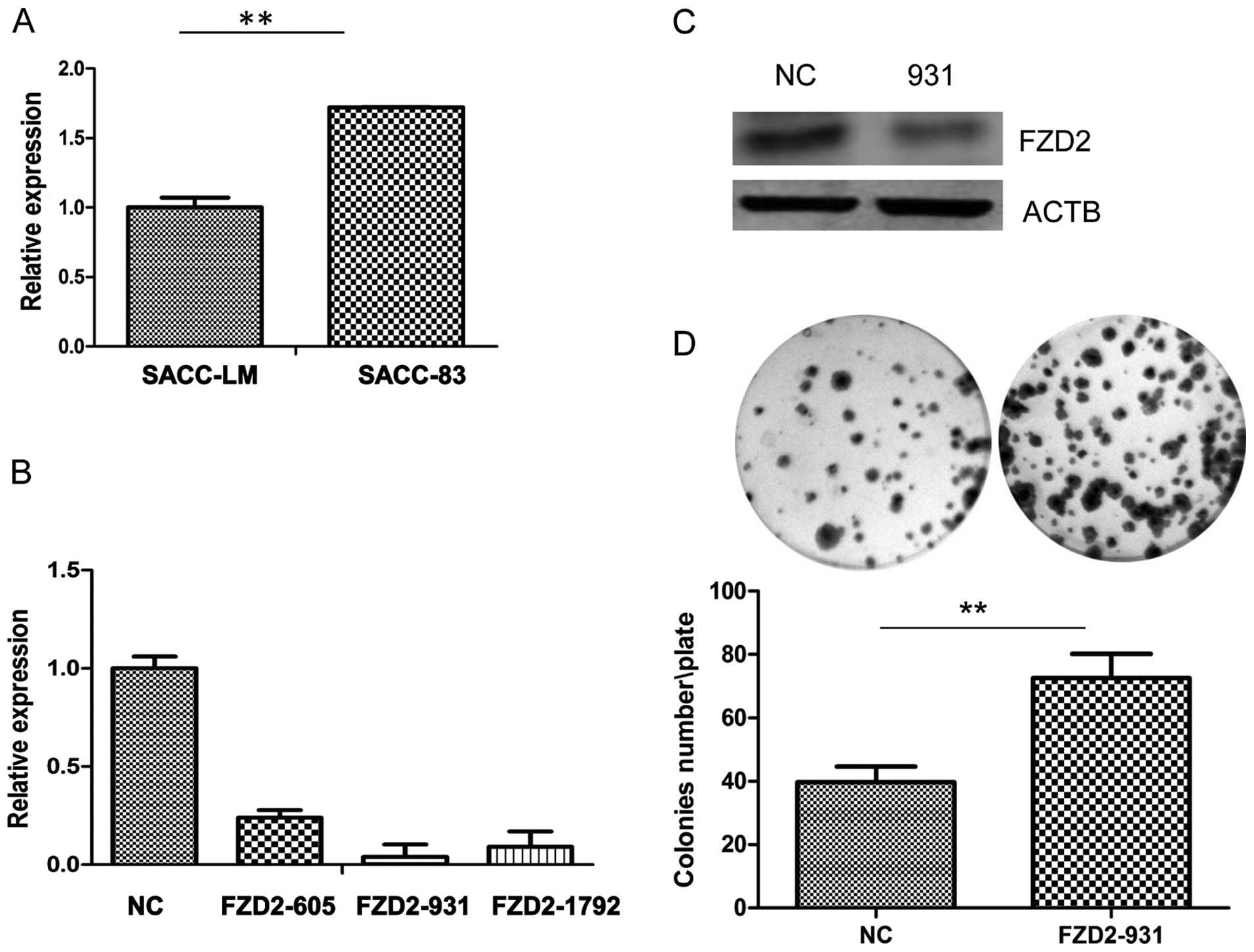
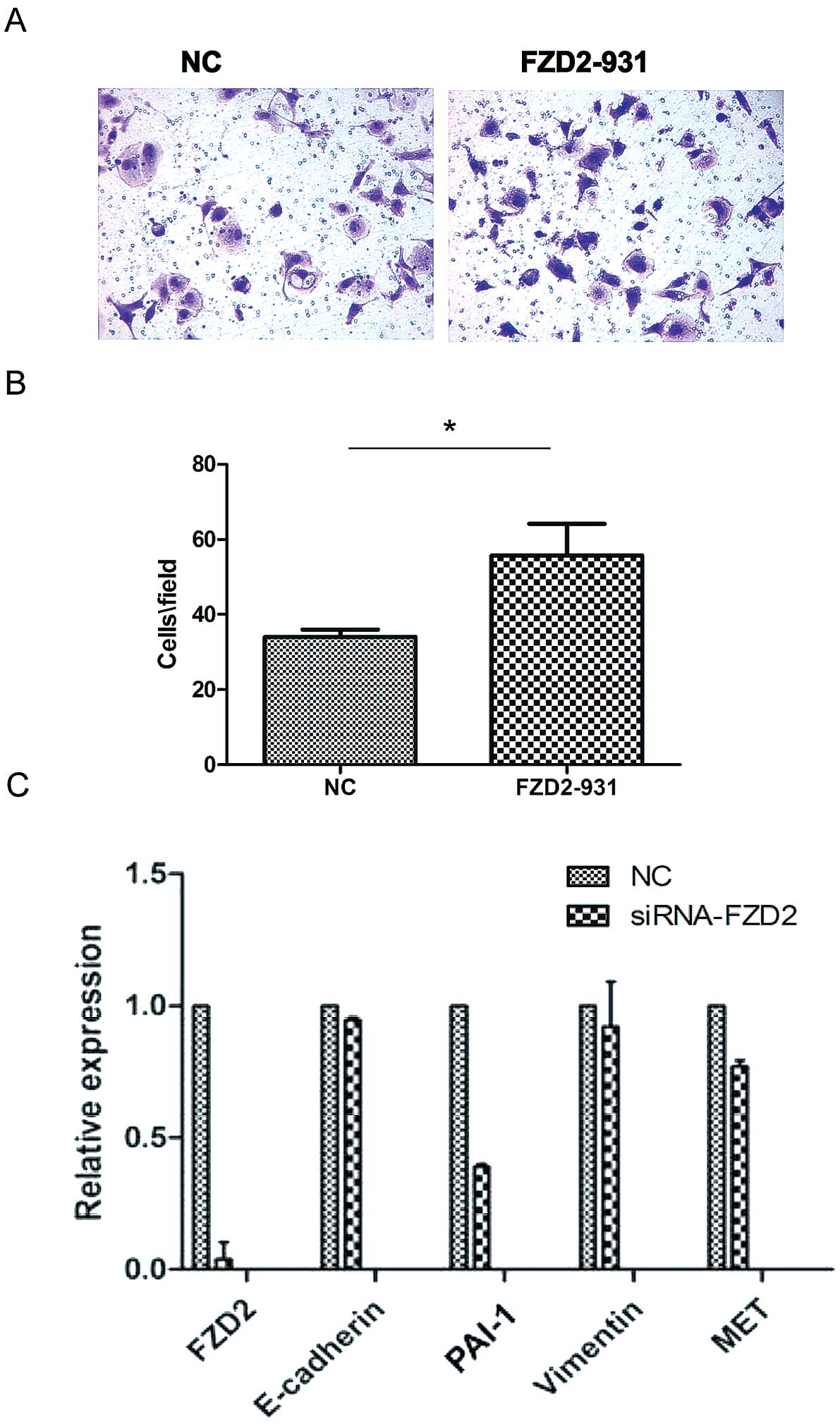
Our real-time PCR results (Fig. 3A) revealed that FZD2 was reduced in
the ACC-LM highly metastatic adenoid cystic carcinoma cell line
compared to the minimally metastatic ACC-83 cell line, which
suggests that FZD2 may act as an inhibitor of the metastasis of
adenoid cystic carcinomas. To investigate the effects of FZD2 on
the proliferation of cancer cells, siRNA-mediated knockdown of
ACC-83 was employed in ACC-83 cells. As shown by the real-time PCR
(Fig. 3B), three siRNAs targeting
FZD2 (siRNA-931) efficiently reduced FZD2 expression in the cells
compared to the negative contros (NCs), but siRNA-931 has the
highest efficiency in mRNA level and protein level (Fig. 3C). So we used siRNA-931 for further
study. Knockdown of FZD2 did not increase the cell proliferation in
the short term by CCK8 assay (data not shown) but it significantly
promoted growth of the ACC-83 cells in the long term (2 weeks) as
measured by colony formation assay (Fig. 3D; P<0.01). To further confirm the
results of this loss-of-function study, the ACC-83 cells were
transfected with an plasmid carrying the correct coding sequence of
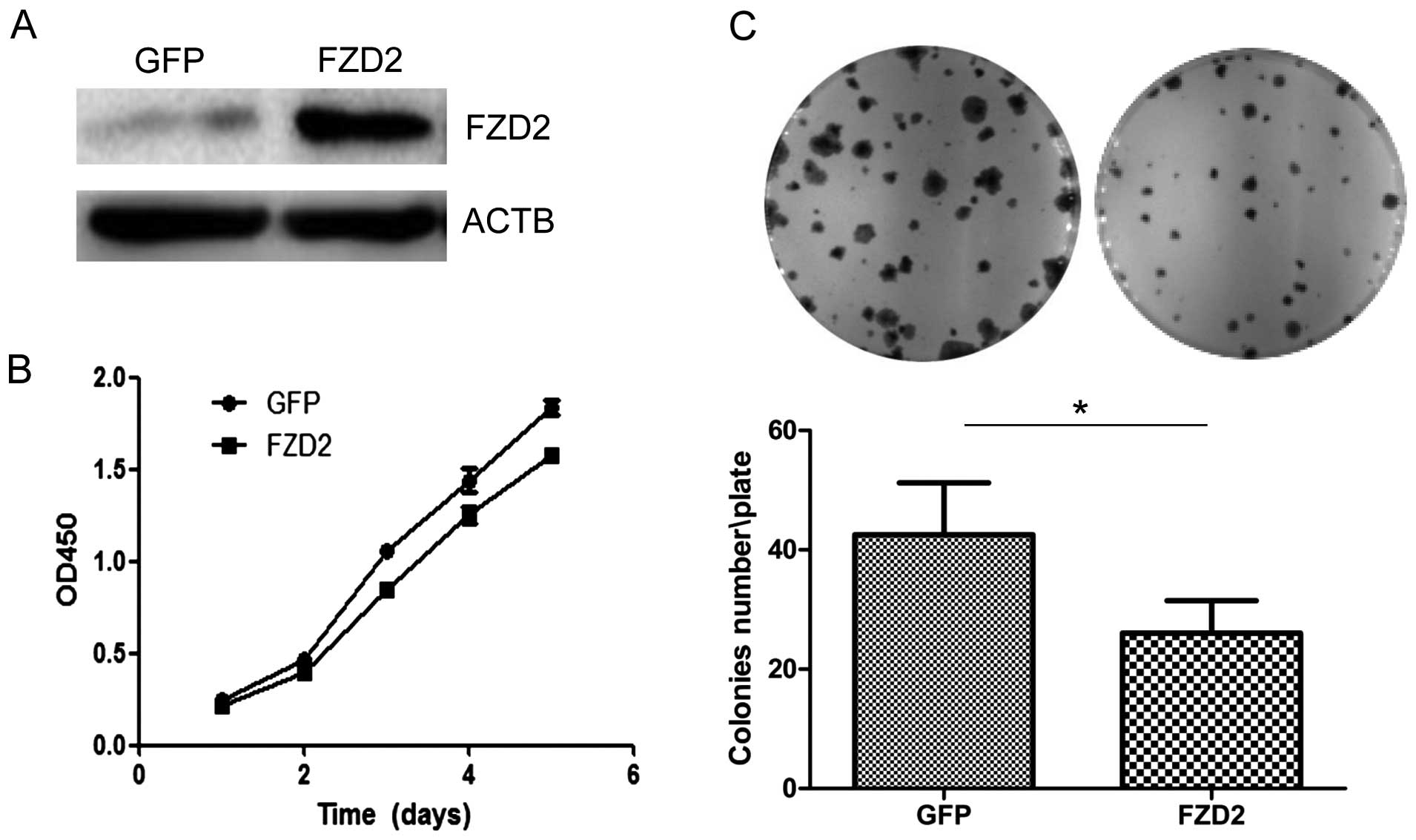
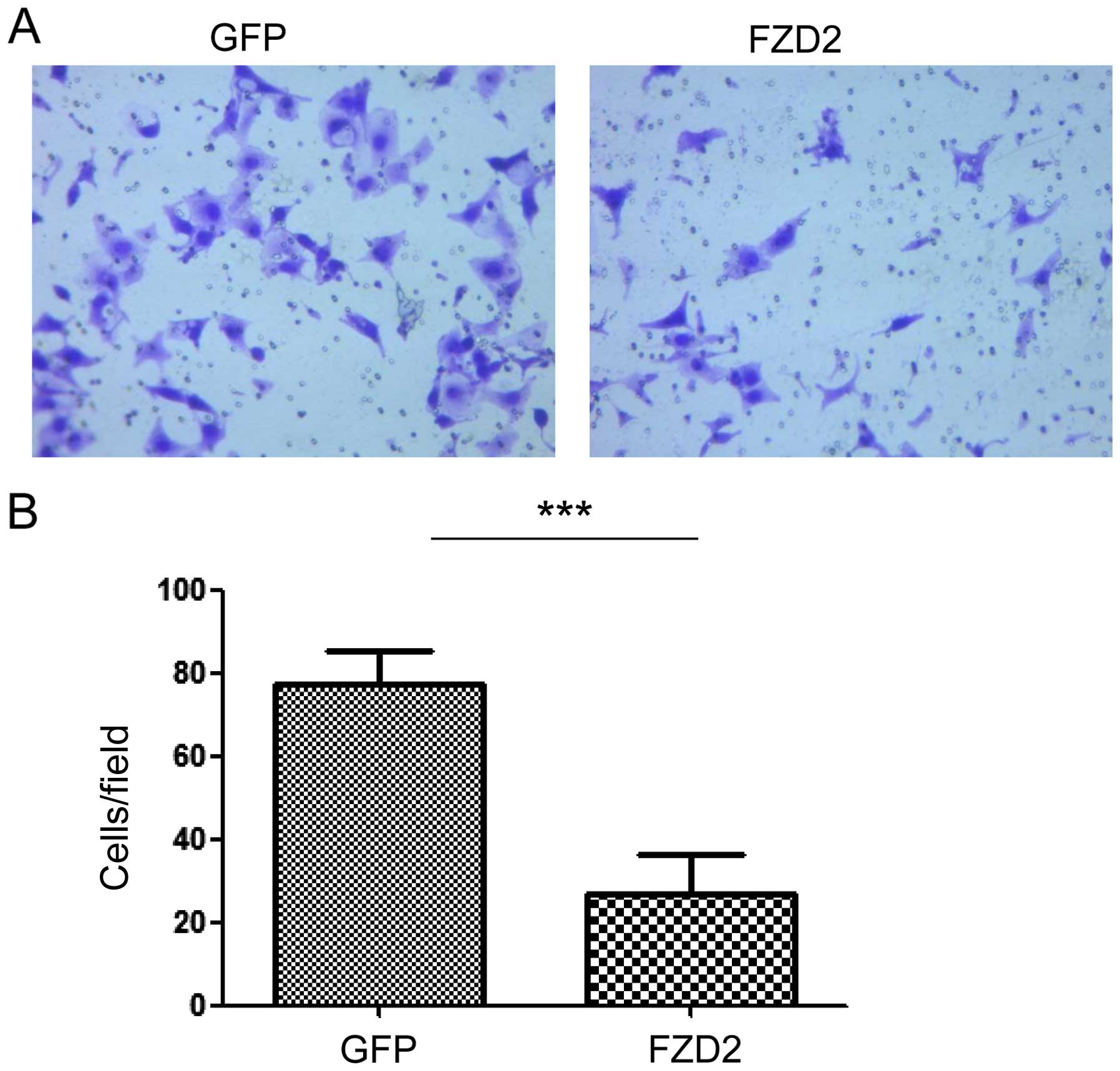
the intracellular cytoplasmic domain of FZD2. Overexpression of
FZD2 in ACC-83 cells (Fig. 4A)
inhibited cell proliferation over the short term as detected by the
CCK8 assay (Fig. 4B; P<0.05 at
days 3 and 4, and P<0.01 at day 5) and colony formation assay
(Fig. 4C; P<0.05). Collectively,
these data indicate that the overexpression of FZD2 restrained SACC
proliferation in vitro, which supports the notion that FZD2
is a tumor suppressor gene in SACCs.
FZD2 inhibits cell migration in
vitro
Next, we examined the role of FZD2 in SACC
migration. As shown in Fig. 5,
knockdown of FZD2 in ACC-83 cells significantly increased cell
migration (Fig. 5A and B;
P<0.05, n=3). A recent study showed that FZD2 can drive
epithelial-mesenchymal transition (EMT) and cell migration through
a previously unrecognized, non-canonical pathway that includes Fyn
and Stat3 (24). To explore the
potential mechanism of FZD2, the expression of the EMT-related
genes was measured by real-time PCR after transfection of FZD2
siRNA. Compared with control, the expression of PAI-1 was
downregulated after FZD2 knockdown, while the other genes like
E-cadherin, vimentin and MET were not changed (Fig. 5C) in ACC-83 cells. In contrast, cell
motility was decreased when FZD2 was overexpressed in ACC-83 cells
and as indicated by the results from the Transwell assay (Fig. 6A and B; P<0.001, n=3). These
results demonstrate that FZD2 is a tumor suppressor gene in SACC
that may inhibit the migration of ACC-83 cells.
Discussion
The Wnt signaling pathway plays multiple roles in
biological development and tumorigenesis. When Wnt combines with a
transmembrane protein (FZD) receptor of the Frizzled family on the
cytoplasmic membrane, three intracellular signaling paths are
activated (25,26). In Wnt signaling activation, Wnt
proteins can combine with FZD receptors and receptor complexes
S(FZD/LRP) consisting of low-density lipoprotein
receptor-associated proteins on the cell surface [LDL receptor
protein (LRP)] to block β-catenin via phosphorylation and
ubiquitin-mediated degradation, which produces large amounts of
free β-catenin that accumulate in the cytoplasm and are then moved
into the nucleus to activate the transcription and expression
tumorigenesis and metastasis of target genes (27). The primary effects of Wnt-5a or
Wnt-11 binding to FZD receptors are mediated by Ca2+,
which activates the Ca2+ trimeric G protein, which in
turn affects calmodulin protein-dependent kinase (CaMKII) and
protein kinase C (PKC) to induce T cell nuclear factor excitation
and ultimately the expression of target genes. Ca2+
channels are principally activated by low-conversion type Wnt
proteins such as Wnt5a. This pathway is involved in gastrulation
and also inhibits the β-catenin pathway (28). The Fizzled protein that is coded by
FZD2 is one of the receptor proteins in the Wnt signaling pathway
and is strongly expressed in fetal kidney, craniofacial and lung
tissues and in cancers such as gastric, colon and ovarian
carcinomas. Königshoff et al provided evidence that FZD2 may
contribute to congenital pulmonary fibrosis based on biological
analysis and qPCR identification (29). Prakash et al reported that
FZD2 is involved in gastrointestinal stromal tumors in children and
adolescents since FZD2 is upregulated in the younger group
(30). Li et al found that
the FZD2 receptor protein molecule can associate with the Wnt3a
stimulated by Ror2, and this association enables the classical Wnt
signaling pathway to positively regulate lung cancer (31). Kirikoshi et al utilized
cDNA-PCR analysis and identification to suggest that FZD2 and FZD9
play vital roles in the occurrence of gastric cancer (32). Many studies have implied that FZD2
is closely related to the occurrence and development of numerous
tumors.
Our qRT-PCR data demonstrated reduced expression of
FZD2 in SACC-LM cells compared to SACC-83 cells, which is
presumably related to the biological behavior of SACCs. Through
immunohistochemical analyses of the protein encoded by FZD2, we
found greater levels of FZD2 expression in the non-metastatic
adenoid cystic carcinoma specimens than in the highly metastatic
samples, which suggests that FZD2 may act as a tumor suppressor.
Further investigations are warranted to clarify the increases in
cell growth and migration in the SACC cells following the silencing
of FZD2 and the declines following the overexpression of FZD2.
Based on the above results, we speculate FZD2 is suppressive in
SACCs, and the results of the present study suggest that FZD2 may
have potential therapeutic applications in the treatment of SACC
patients by inhibition of cell growth and metastasis.
In this study, we examined some target genes of Wnt
signaling pathway in SACC cells following the study by Gujral et
al (24) and found that PAI-1
was downregulated by silenced FZD2, but there were no significant
changes in the expression of E-cadherin, vimentin and MET.
Stefansson and Lawrence reported that PAI-1 could inhibit VSMC
migration (33), while Degryse
et al reported that PAI-1 could promote VSMC migration
(34). The experiments carried out
by Garg et al indicated PAI-1 and VN regulate each other's
functions and may play key roles in VSMC migration and intimal
hyperplasia (35). Wu et al
provided evidence from wild-type mice that PAI-1 successfully
inhibited the proliferation of SMCs (36). Brandal et al showed that
PAI-1 inhibited the proliferation of both SMCs and ECs in
proliferation assays (37). Our
study uncovered that the role of tumor suppression of FZD2 in SACC
may be mediated by PAI-1.
Acknowledgments
This study was supported by the National Natural
Sciences Foundation of China (no. 81172583), the Natural Sciences
Foundation of Fujian (project nos. 2010J01157 and 2011J01167), and
the Key Project of Science and Technology Foundation of Fujian
Province of China (no. 2011Y0025). The authors thank Changgong Li
for providing FZD2 plasmid.
References
|
1
|
Matsuba HM, Simpson JR, Mauney M and
Thawley SE: Adenoid cystic salivary gland carcinoma: A
clinicopathologic correlation. Head Neck Surg. 8:200–204. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rapidis AD, Givalos N, Gakiopoulou H,
Faratzis G, Stavrianos SD, Vilos GA, Douzinas EE and Patsouris E:
Adenoid cystic carcinoma of the head and neck. Clinicopathological
analysis of 23 patients and review of the literature. Oral Oncol.
41:328–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ampil FL and Misra RP: Factors influencing
survival of patients with adenoid cystic carcinoma of the salivary
glands. J Oral Maxillofac Surg. 45:1005–1010. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimoda M, Sugiura T, Imajyo I, Ishii K,
Chigita S, Seki K, Kobayashi Y and Shirasuna K: The T-box
transcription factor Brachyury regulates epithelial-mesenchymal
transition in association with cancer stem-like cells in adenoid
cystic carcinoma cells. BMC Cancer. 12:3772012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simonetti M, Agarwal N, Stösser S, Bali
KK, Karaulanov E, Kamble R, Pospisilova B, Kurejova M, Birchmeier
W, Niehrs C, et al: Wnt-Fzd signaling sensitizes peripheral sensory
neurons via distinct noncanonical pathways. Neuron. 83:104–121.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agarwal JP, Jain S, Gupta T, Tiwari M,
Laskar SG, Dinshaw KA, Chaturvedi P, D'cruz AK and Shrivastava SK:
Intraoral adenoid cystic carcinoma: Prognostic factors and outcome.
Oral Oncol. 44:986–993. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng D, Decker KF, Zhou T, Chen J, Qi Z,
Jacobs K, Weilbaecher KN, Corey E, Long F and Jia L: Role of
WNT7B-induced noncanonical pathway in advanced prostate cancer. Mol
Cancer Res. 11:482–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
García Campelo MR, Alonso Curbera G,
Aparicio Gallego G, Grande Pulido E and Antón Aparicio LM: Stem
cell and lung cancer development: Blaming the Wnt, Hh and Notch
signalling pathway. Clin Transl Oncol. 13:77–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pez F, Lopez A, Kim M, Wands JR, Caron de
Fromentel C and Merle P: Wnt signaling and hepatocarcinogenesis:
Molecular targets for the development of innovative anticancer
drugs. J Hepatol. 59:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dijksterhuis JP, Petersen J and Schulte G:
WNT/Frizzled signalling: receptor-ligand selectivity with focus on
FZD-G protein signalling and its physiological relevance: IUPHAR
Review 3. Br J Pharmacol. 171:1195–1209. 2014. View Article : Google Scholar :
|
|
12
|
Wang Y and Zheng T: Screening of hub genes
and pathways in colorectal cancer with microarray technology.
Pathol Oncol Res. 20:611–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Milovanovic T, Planutis K, Nguyen A, Marsh
JL, Lin F, Hope C and Holcombe RF: Expression of Wnt genes and
frizzled 1 and 2 receptors in normal breast epithelium and
infiltrating breast carcinoma. Int J Oncol. 25:1337–1342.
2004.PubMed/NCBI
|
|
14
|
Goksel G, Bilir A, Uslu R, Akbulut H,
Guven U and Oktem G: WNT1 gene expression alters in heterogeneous
population of prostate cancer cells; decreased expression pattern
observed in CD133+/CD44+ prostate cancer stem
cell spheroids. J BUON. 19:207–214. 2014.PubMed/NCBI
|
|
15
|
Ulivieri A, Lavra L, Dominici R,
Giacomelli L, Brunetti E, Sciacca L, Trovato M, Barresi G, Foukakis
T, Jia-Jing L, et al: Frizzled-1 is down-regulated in follicular
thyroid tumours and modulates growth and invasiveness. J Pathol.
215:87–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Su BH, Qu J, Song M, Huang XY, Hu XM, Xie
J, Zhao Y, Ding LC, She L, Chen J, et al: NOTCH1 signaling
contributes to cell growth, anti-apoptosis and metastasis in
salivary adenoid cystic carcinoma. Oncotarget. 5:6885–6895. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jinek M and Doudna JA: A three-dimensional
view of the molecular machinery of RNA interference. Nature.
457:405–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Snead NM and Rossi JJ: RNA interference
trigger variants: Getting the most out of RNA for RNA
interference-based therapeutics. Nucleic Acid Ther. 22:139–146.
2012.PubMed/NCBI
|
|
20
|
Kanasty RL, Whitehead KA, Vegas AJ and
Anderson DG: Action and reaction: The biological response to siRNA
and its delivery vehicles. Mol Ther. 20:513–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar
|
|
22
|
Gyorffy B, Lánczky A and Szállási Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sriuranpong V, Borges MW, Ravi RK, Arnold
DR, Nelkin BD, Baylin SB and Ball DW: Notch signaling induces cell
cycle arrest in small cell lung cancer cells. Cancer Res.
61:3200–3205. 2001.PubMed/NCBI
|
|
24
|
Gujral TS, Chan M, Peshkin L, Sorger PK,
Kirschner MW and MacBeath G: A noncanonical Frizzled2 pathway
regulates epithelial-mesenchymal transition and metastasis. Cell.
159:844–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
MacDonald BT, Hien A, Zhang X, Iranloye O,
Virshup DM, Waterman ML and He X: Disulfide bond requirements for
active Wnt ligands. J Biol Chem. 289:18122–18136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kahn M: Can we safely target the WNT
pathway? Nat Rev Drug Discov. 13:513–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Daniels DL and Weis WI: Beta-catenin
directly displaces Groucho/TLE repressors from Tcf/Lef in
Wnt-mediated transcription activation. Nat Struct Mol Biol.
12:364–371. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
van de Schans VA, Smits JF and
Blankesteijn WM: The Wnt/frizzled pathway in cardiovascular
development and disease: Friend or foe? Eur J Pharmacol.
585:338–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Königshoff M, Balsara N, Pfaff EM, Kramer
M, Chrobak I, Seeger W and Eickelberg O: Functional Wnt signaling
is increased in idiopathic pulmonary fibrosis. PLoS One.
3:e21422008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prakash S, Sarran L, Socci N, DeMatteo RP,
Eisenstat J, Greco AM, Maki RG, Wexler LH, LaQuaglia MP, Besmer P,
et al: Gastrointestinal stromal tumors in children and young
adults: A clinicopathologic, molecular, and genomic study of 15
cases and review of the literature. J Pediatr Hematol Oncol.
27:179–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li C, Chen H, Hu L, Xing Y, Sasaki T,
Villosis MF, Li J, Nishita M, Minami Y and Minoo P: Ror2 modulates
the canonical Wnt signaling in lung epithelial cells through
cooperation with Fzd2. BMC Mol Biol. 9:11–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kirikoshi H, Sekihara H and Katoh M:
Expression profiles of 10 members of Frizzled gene family in human
gastric cancer. Int J Oncol. 19:767–771. 2001.PubMed/NCBI
|
|
33
|
Stefansson S and Lawrence DA: The serpin
PAI-1 inhibits cell migration by blocking integrin alpha V beta 3
binding to vitronectin. Nature. 383:441–443. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Degryse B, Neels JG, Czekay RP, Aertgeerts
K, Kamikubo Y and Loskutoff DJ: The low density lipoprotein
receptor-related protein is a motogenic receptor for plasminogen
activator inhibitor-1. J Biol Chem. 279:22595–22604. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garg N, Goyal N, Strawn TL, Wu J, Mann KM,
Lawrence DA and Fay WP: Plasminogen activator inhibitor-1 and
vitronectin expression level and stoichiometry regulate vascular
smooth muscle cell migration through physiological collagen
matrices. J Thromb Haemost. 8:1847–1854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu J, Peng L, McMahon GA, Lawrence DA and
Fay WP: Recombinant plasminogen activator inhibitor-1 inhibits
intimal hyperplasia. Arterioscler Thromb Vasc Biol. 29:1565–1570.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brandal S, Blake CM, Sullenger BA and
Fortenberry YM: Effects of plasminogen activator
inhibitor-1-specific RNA aptamers on cell adhesion, motility, and
tube formation. Nucleic Acid Ther. 21:373–381. 2011. View Article : Google Scholar : PubMed/NCBI
|