Introduction
The formation of new blood vessels that supply the
tumor mass, or tumor angiogenesis, is a characteristic of cancer
and is important in tumor outgrowth, progression and metastasis,
due to the proliferation and metastasis of malignant tumors
depending on the sufficient nutrition supplied by the new blood
vessels (1–5). Molecules have been demonstrated as
angiogenesis-promoting factors (3,6–8), such
as vascular endothelial growth factor (VEGF), epidermal growth
factor (EGF), fibroblast growth factor (FGF), placental growth
factor (PlGF), transforming growth factor-α/β (TGF-α/β),
angiogenin, CD105 (endoglin), the anthrax-toxin-receptor (ATR,
TEM8), prostate-specific membrane antigen (PSMA), connective tissue
growth factor (CTGF), urokinase plasminogen activator (uPA) and the
up-to-date reported galactin-1 (Gal1) (also known as galectin, a
member of a conserved family of animal lectins). It has been
demonstrated, however, that VEGF-mediated signaling through
vascular endothelial growth factor receptor 2 (VEGFR2) is the
critical rate-limiting step in tumor angiogenesis, and is crucial
in angiogenesis or neovascularization (4,9).
VEGFR2, also known as kinase insert
domain-containing receptor (KDR) in human and fetal liver kinase 1
(flk1) in mouse, is involved as a therapeutic target of
anti-angiogenic therapy for various types of cancer because VEGFR2
is strictly expressed on endothelial cells and upregulated when
these cells proliferate during angiogenesis in the tumor
vasculature. A number of approaches have been previously used to
block the VEGFR2-mediated signaling in anti-angiogenic therapy for
various types of cancer, including neutralization of VEGF by
monoclonal antibodies (10–12), therapy with T4 phage displaying
VEGFR2 (13), administration of
small-molecule receptor tyrosine kinase inhibitors (RTKIs)
(6,14), and vaccination with endothelial
cells (15), VEGFR2 cDNA
transfected-bacteria (16,17) or VEGFR2 protein (18) pulsed- or mRNA- (19) and cDNA- (20) transfected-dendritic cells (DC).
Furthermore, it was identified that there were three
H-2Db-restricted CD8+ T-cell epitopes in
mouse VEGFR2, designated as KDR1, KDR2 and KDR3, respectively
(21). KDR1 was derived from the
intracellular domain of VEGFR2, while KDR2 and KDR3 were derived
from the extracellular domain (21). Immunization with KDR2 or KDR3, but
not KDR1 peptides, was able to break self-tolerance and induce a
specific CD8+ cytotoxic T-lymphocyte (CTL) immune
response in C57BL/6 mice, thereby inhibiting angiogenesis and tumor
growth in a mouse model (21). In
addition, it was found that KDR2 was more efficiently processed by
proteasomes and/or transported to the endoplasmic reticulum by
transporter-associated with antigen processing (TAP) rather than
KDR3, offsetting the higher affinity of KDR3 (21). Therefore, KDR2 is an attractive
candidate of CD8+ T-cell epitopes suitable for active
immunotherapy targeting tumor blood vessels in which the main aim
is to break self-tolerance of the molecules that promote
angiogenesis.
It has been demonstrated that the single-chain
trimer (SCT) composed of major histocompatibility complex (MHC)
class I heavy chain, β2-microglobulin (β2m), and antigen peptide
was a particularly powerful strategy used to increase the potency
of DNA vaccine against tumor (22–26)
and infection (22,27–31).
In the present study, we constructed a DNA vaccine encoding an SCT
composed of KDR2, β2m and H-2Db
[pcDNA3.1(+)-KDR2-β2m-H-2Db, or SCT-KDR2] and tested its
ability to induce specific CTL response to VEGFR2 and its capacity
to inhibit tumor-induced angiogenesis and metastasis in mouse
models.
Materials and methods
Mice and cells
Female C57BL/6 mice (H-2b), 6–8 weeks of
age, were purchased from SLAC Laboratory Animal Co., Ltd.,
(Shanghai, China) and were housed in specific pathogen-free
conditions. All the animal procedures were performed according to
approved protocols and in accordance with recommendations for the
proper use and care of laboratory animals by the Institutional
Committee. B16 (a mouse melanoma cell line of C57BL/6 origin,
H-2b), EL-4 (a lymphoma cell line, H-2b),
A293 (a human embryonic kidney cell line), and 3LL (a Lewis lung
carcinoma cell line, H-2b) were obtained from the
American Type Culture Collection (Manassas, VA, USA). H5V
(H-2b), a mouse endothelial cell line expressing VEGFR2,
was a kind gift from Dr A. Vecchi (Instituto Mario Negri, Via
Eritrea, Milan, Italy) as indicated in our previous report
(20). 3LL-sflk1 (3LL Lewis lung
carcinoma cells stably expressing extracellular domain of VEGFR2)
was established in our laboratory as previously described (20).
Plasmid DNA constructs and DNA
preparation
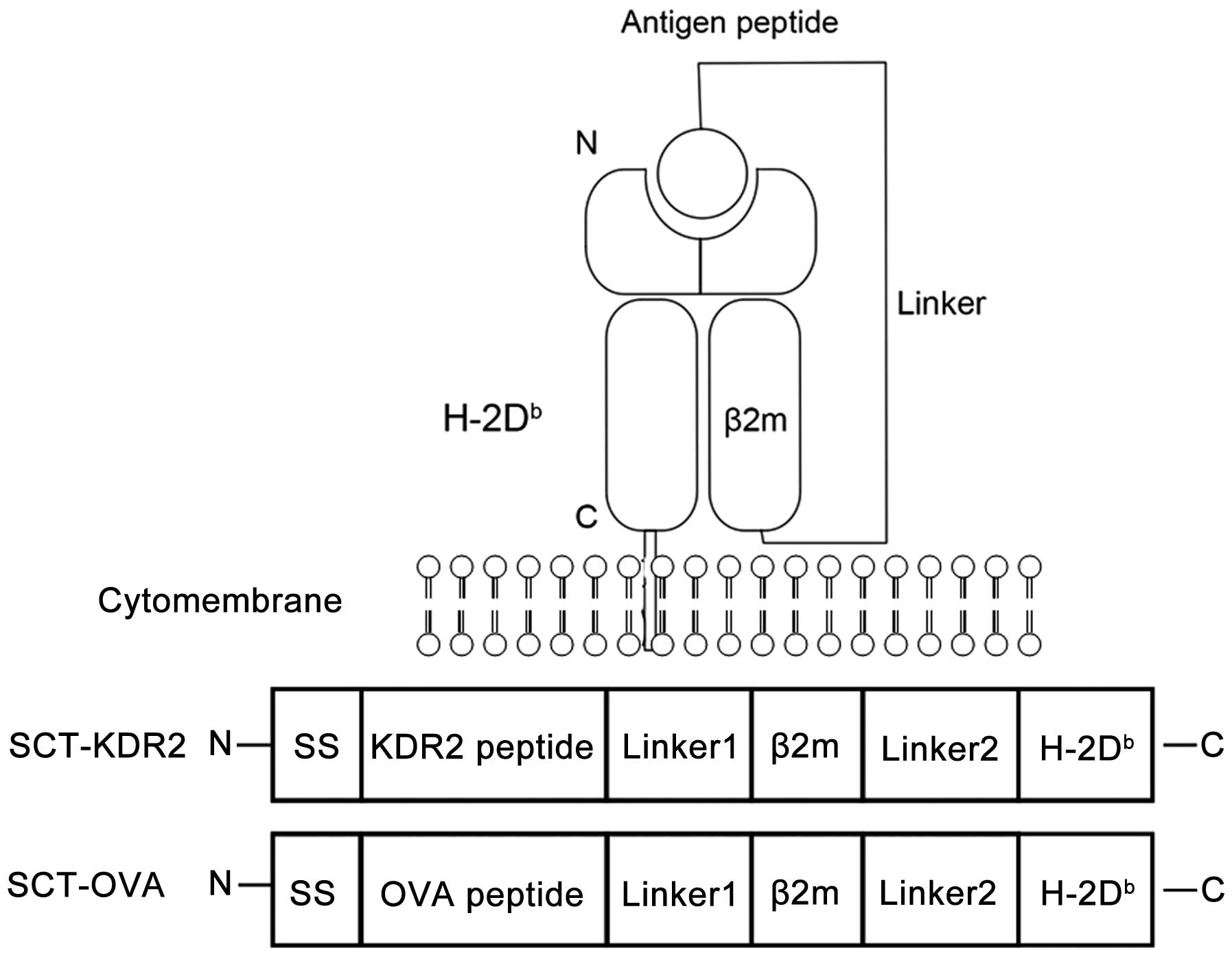
The construction of pcDNA3.1(+)-
KDR2-β2m-H-2Db (SCT-KDR2) and
pcDNA3.1(+)-OVA-β2m-H-2Db (SCT-OVA) is shown in Fig. 1. To construct SCT-KDR2, an insert
containing the mouse β2m signal peptide plus
H-2Db-restricted immunodominant KDR2 (aa400-408) epitope
and flanking NheI/SpeI restriction enzyme sites was
created by annealing two single-stranded oligo-nucleotides [sense,
5′-CATGCTAGCATGGCTCGC TCGGTGACCCTAGTCTTTCTGGTGCTTGTCTCACTGA
CCGGCTTGTATGCTGTCATCCTCACCAACCCCATTTC AATGACTAGTGGTG and antisense,
5′-CACCACTAGTCA TTGAAATGGGGTTGGTGAGGATGACAGCATACAAGC
CGGTCAGTGAGACAAGCACCAGAAAGACTAGGGTCA CCGAGCGAGCCATGCTAGCATG-3′,
Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China]. The
generated cDNA was linked, through the SpeI restriction
site, with Linker 1 which was generated by annealing two
single-stranded oligonucleotides flanking the SpeI and
HindIII restriction sites as follows:
5′-GACTAGTGGTGGCGGAGGTTCTGGTGGCGG
AGGTTCTGGTGGCGGAGGTTCTGGTGGCGGAGGTTC TAAGCTTGGG-3′ (sense) and
5′-CCCAAGCTTAGAACCT CCGCCACCAGAACCTCCGCCACCAGAACCTCCGCCA
CCAGAACCTCCGCCACCACTAGTC-3′ (antisense). The yielded cDNA was
subcloned into pcDNA3.1(+) (Invitrogen, Carlsbad, CA, USA) between
the NheI and HindIII restriction sites. cDNA encoding
H2-Db and β2m were cloned by RT-PCR from the spleen
cells of C57/B6 mouse with a Superscript One-Step RT-PCR system
(Invitrogen). Primers used for H-2Db were:
5′-TTGCGGCCGCGGCCCACACTCGATGC GGT-3′ (sense) and
5′-GCTCTAGATCACGCTTTACAATCT CGGAG-3′ (antisense). Full-length
H-2Db cDNA was then inserted into the above constructed
recombinant vector between the NotI and XbaI
restriction sites. Primers used for β2m were:
5′-CCCAAGCTTATCCAGAAAACCCCTCAAA TTC-3′ (sense) and
5′-CGGGATCCCATGTCTCGATCCCAG TAGAC-3′ (antisense). The β2m cDNA was
subcloned into the recombinant plasmid between the HindIII
and BamHI restriction sites. Linker 2 was produced by
annealing the following two single-stranded oligo-nucleotides
flanking the BamHI and NotI restriction sites:
5′-CGCGGATCCGGTGGCGGAGGT TCTGGTGGCGGAGGTTCTGGTGGCGGAGGTTCTGGT
GGCGGAGGTTCT-3′ (sense) and 5′-TTGCGGCCGCAAGA
ACCTCCGCCACCAGAACCTCCGCCACCAGAACCTCC GCCACCAGAACCTCCGCCACC-3′
(antisense) (Sangon Biotech). cDNA encoding Linker 2 was
subsequently inserted into the above generated recombinant vector
to form the SCT-KDR2. To generate SCT-OVA, β2m signal peptide plus
immunodominant KDR2 (aa400-408) epitope was replaced by β2m signal
peptide plus H-2Db-restricted immunodominant ovalbumin
(OVA) epitope (aa257-265, ESIINFEKL) which was generated by
annealing the synthesized oligo-nucleotides (sense,
GCTAGCATGGCTCGCTCGGTGACCCTAGTCT
TTCTGGTGCTTGTCTCACTGACCGGCTTGTATGCTTA
GTTGTACAGTTCAGCTCTTTATAATACTAGTGGTG-3′ and antisense,
5′-CACCACTAGTATTATAAAGAGCTGAA CTGTACAACTAAGCATACAAGCCGGTCAGTGAGACA
AGCACCAGAAAGACTAGGGTCACCGAGCGAGCCATG CTAGCATG-3′). The SCT DNA was
amplified in E. coli DH5α and prepared using an
endotoxin-free DNA Preparation kit (Qiagen, Valencia, CA, USA)
according to the manufacturer’s instructions.
Detection of SCT expression by flow
cytometric analysis
A293 cells were transfected with the corresponding
recombinant plasmid DNA by Lipofectamine™ 2000 (Invitrogen)
according to the manufacturer’s instructions. After 36 h, the
transfected cells were collected and stained with FITC-conjugated
rat anti-mouse H-2Db monoclonal antibody (BD Pharmingen,
San Diego, CA, USA). Flow cytometric analysis was performed using
Becton-Dickinson’s FACSCan.
Immunization of mice with DNA
vaccines
C57BL/6 mice were intradermally immunized three
times, at 7-day intervals, with 50 μg of
pcDNA3.1(+)-KDR2-β2m-H-2Db (SCT-KDR2),
pcDNA3.1(+)-OVA-β2m-H-2Db (SCT-OVA), or the control DNA
pcDNA3.1(+) (vector), respectively. Ten days after the last
immunization, the immunized mice were used for evaluation of CTL
response to VEGFR2, analyses of tumor cell-induced angiogenesis, or
observation of the anti-metastatic effects in murine-metastatic
models of B16 melanoma and 3LL Lewis lung carcinoma.
Detection of VEGFR2-specific CTL
cytotoxicity
CTL responses were detected as previously described
(20,32) with minor modifications. Briefly, 10
days after the last immunization, erythrocyte-depleted splenocytes
from mice (n=3–5 per group) were prepared and restimulated for 7
days with mitomycin-inactivated VEGFR2-expressing H5V cell line (at
a responder to stimulator ratio of 10:1) in the presence of 50 U/ml
murine IL-2 (PeproTech, UK) in RPMI-1640 with 10% fetal calf serum.
CTL activity was determined by a lactate dehydrogenase (LDH)
release assay with CytoTox 96 Non-Radioactive Cytotoxicity Assay
kit (Promega, Madison, WI, USA) at an effector:target ratio of
12.5:1, 25:1 and 50:1, respectively. The VEGFR2+ H5V and
3LL-sflk1 cell lines, and VEGFR2− EL-4 and 3LL cell
lines served as the targets, respectively. Specific cytotoxic
activity was calculated according to the following: % specific
lysis = 100× (experimental-effector spontaneous-target
spontaneous)/(target maximum-target spontaneous).
Alginate bead analysis of tumor
cell-induced angiogenesis
Alginate bead analysis of tumor cell-induced in
vivo angiogenesis was performed as previously described
(20). Briefly, 3LL Lewis lung
carcinoma cells were suspended in 1.5% sodium alginate and added
drop by drop into a swirling 37°C solution of 250 mM calcium
chloride. Alginate beads (each containing 5×104 tumor
cells) were prepared. Four beads were implanted subcutaneously into
each mouse through an incision made on the dorsal side under
anesthetization. After 12 days, 100 μl FITC-dextran solution
(20 mg/ml) were intravenously injected into each mouse. The mice
were sacrificed 20 min later and alginate beads in each mouse were
collected and incubated overnight at room temperature in 1 ml
Tris-HCl (1 mM, Ph 8.0). The beads were ground with a hand-held
mixer and an additional 1 ml Tris-HCl (1 mM, pH 8.0) was added.
Samples were vortexed and centrifuged at 250 × g for 5 min.
Fluorescence of FITC-dextran in the sample supernatants was
measured on a fluorescence spectrophotometer by excitation at 492
nm and emission at 515 nm. Concentrations of FITC-dextran were
extrapolated from a standard curve and data were expressed as
ng/bead.
Immunohistochemical analysis of
tumor-induced angiogenesis
The C57BL/6 mice (5 per group) were intradermally
vaccinated three times, at 7-day intervals, with SCT-KDR2, SCT-OVA,
or the vector. Ten days after the last immunization, each of the
immunized mice was inoculated subcutaneously with 1×105
B16 melanoma cells in the rear leg. After 10 days, tumors were
removed and fixed in 10% formalin. Tumors were embedded in paraffin
wax, and 5-μm sections were cut and used in the
immunohistochemical analysis of the factor VIII-related antigen by
streptoavidin biotin-peroxidase complex (SABC) method to evaluate
tumor-induced angiogenesis as previously described (33), with minor modifications. Briefly,
after deparaffinization, the tissue sections were incubated in 0.3%
H2O2 in methanol for 30 min to inactivate
endogenous peroxidase, and washed with PBS (0.01 M, pH 7.4). The
slides then were incubated at 4°C overnight with rabbit polyclonal
antibody against factor VIII-related antigen (1:200; Cell Marque,
Rocklin, CA, USA). After washing with TBST (pH7.6), biotinylated
goat anti-rabbit IgG (1:1,000; Jackson ImmunoResearch, PA, USA)
were applied to the sections for 30 min at room temperature. The
sections were then incubated with streptavidin-HRP (Sigma-Aldrich,
St. Louis, MO, USA) for 30 min at room temperature. HRP activity,
which resulted in a brown staining of the reaction sites, was
visualized by incubation for 10 min in Tris-buffered saline
containing 0.05% diaminobenzidine and 0.01%
H2O2. Hematoxylin was used for the
counterstaining.
B16 melanoma metastasis mouse model
C57BL/6 mice (10 per group) were intradermally
vaccinated three times, at 7-day intervals, with 50 μg
SCT-KDR2, SCT-OVA, or vector, respectively. Ten days after the last
immunization, the mice were intravenously injected with
1×106 B16 melanoma cells and were sacrificed 28 days
later. Tumor load was evaluated by counting tumor nodules on the
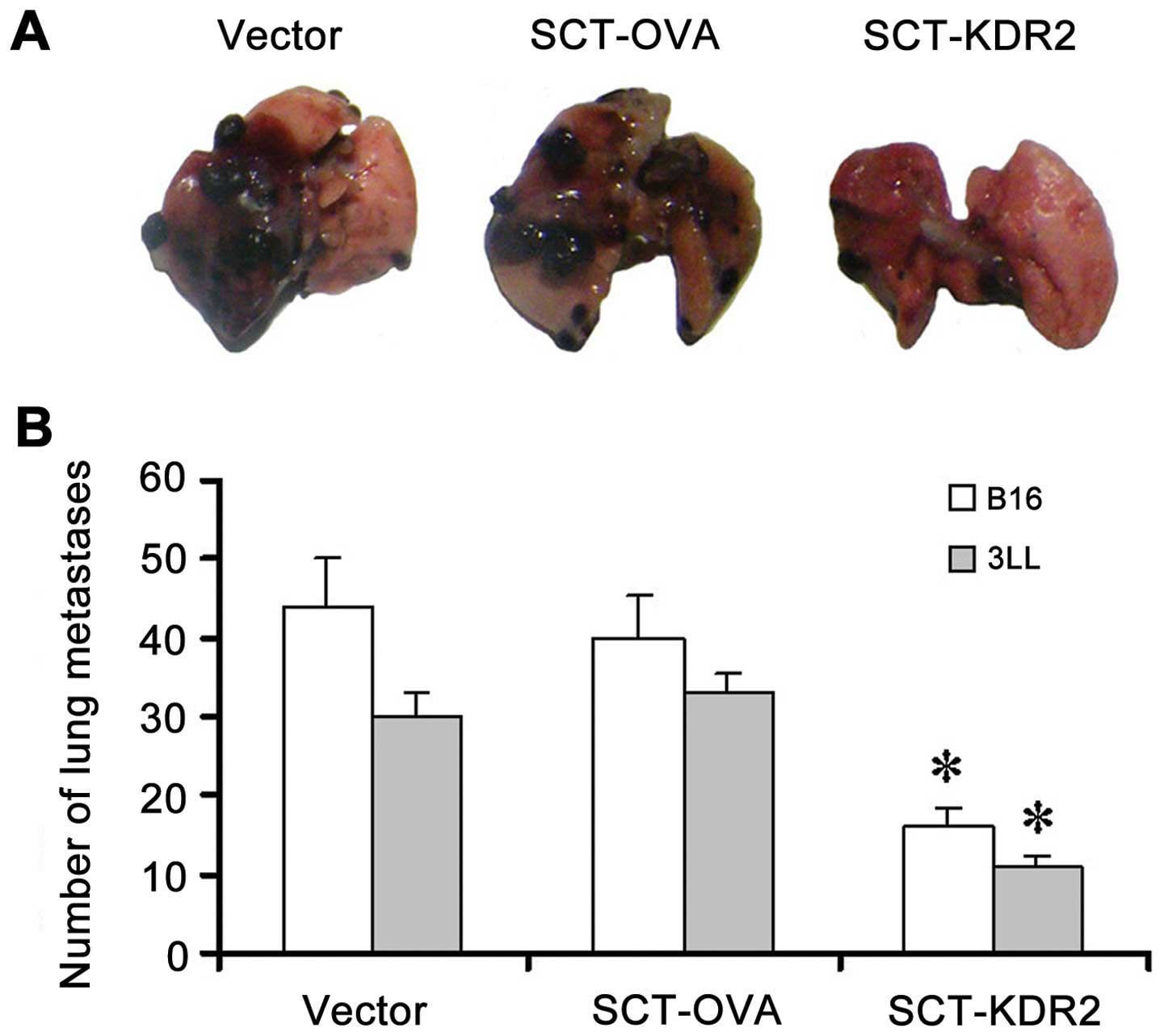
lung surfaces as previously described (20). Images of one representative
tumor-bearing lung in each group were captured.
3LL Lewis lung carcinoma metastasis mouse
model
C57BL/6 mice (10 per group) were intradermally
vaccinated three times, at 7-day intervals, with 50 μg
SCT-KDR2, SCT-OVA, or vector, respectively. Ten days after the last
immunization, the mice received an intrafootpad injection with
2×105 3LL tumor cells. When the tumor reached ~6 mm in
diameter, the tumor-bearing leg was amputated surgically. The mice
were sacrificed based on the metastatic death in the control
groups. Tumor load was evaluated by counting the tumor nodules on
the lung surfaces.
Statistical analyses
Statistical analyses were performed using the
Student’s t-test. P<0.05 was considered statistically
significant.
Results
Expression of KDR2-β2m-H-2Db
and OVA-β2m-H-2Db SCT
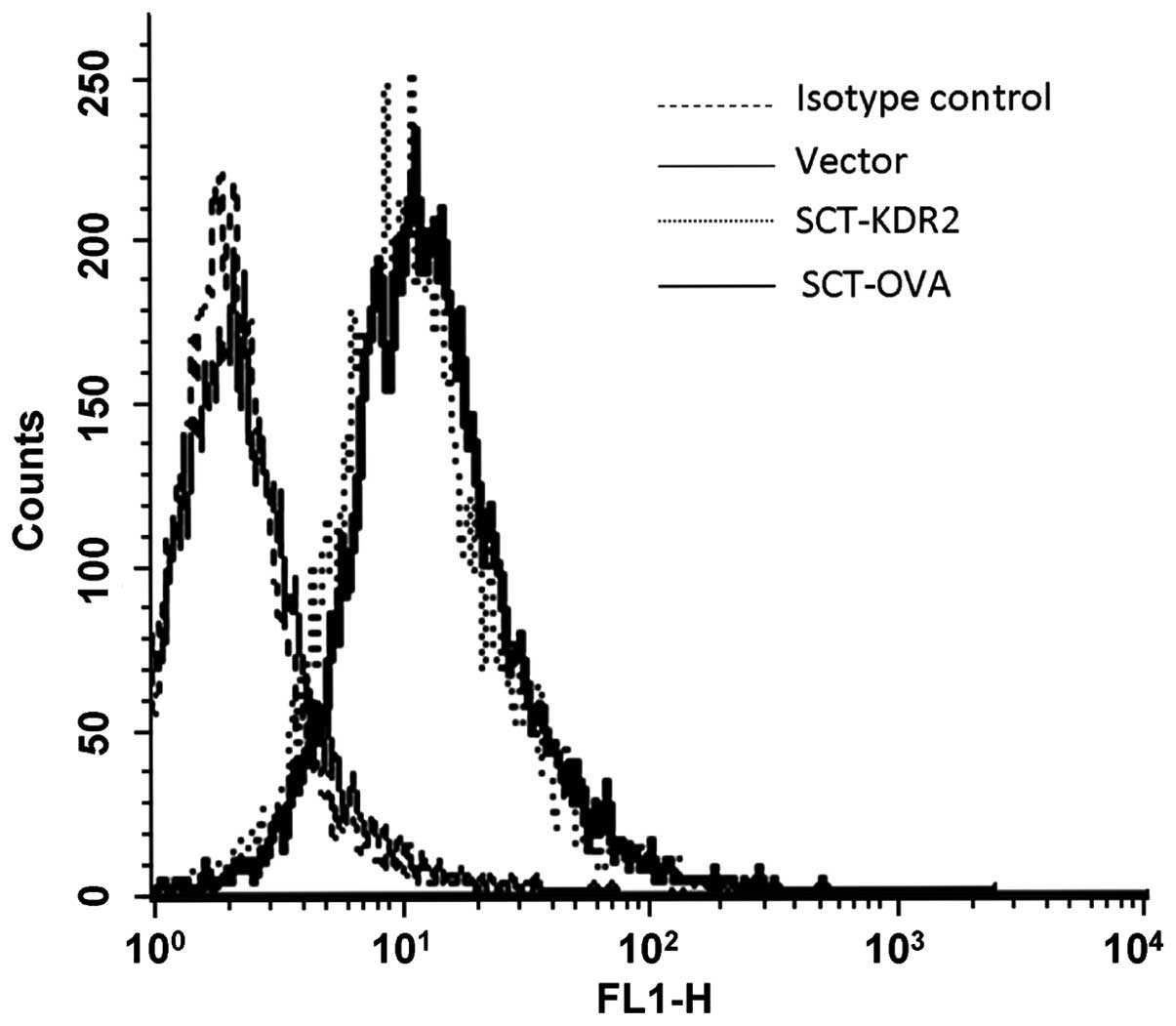
To characterize the SCT protein expression, human
A293 cells were transfected with vector, SCT-KDR2 and SCT-OVA
constructs, respectively. SCT expression on transfected cells was
examined by flow cytometry with FITC-conjugated rat anti-mouse
H2-Db monoclonal antibody. As shown in Fig. 2, A293 cells transfected with
SCT-KDR2 or SCT-OVA expressed mouse H-2Db, whereas A293
cells transfected with vector did not express mouse
H-2Db. These results confirmed that the SCT-encoded
recombinant plasmids pcDNA3.1(+)-KDR2-β2m-H-2Db and
pcDNA3.1(+)-OVA-β2m-H-2Db could be expressed
successfully in eukaryotic cells.
Immunization of mice with DNA vaccine
encoding KDR2-β2m-H-2Db SCT induces CTL response to VEGFR2
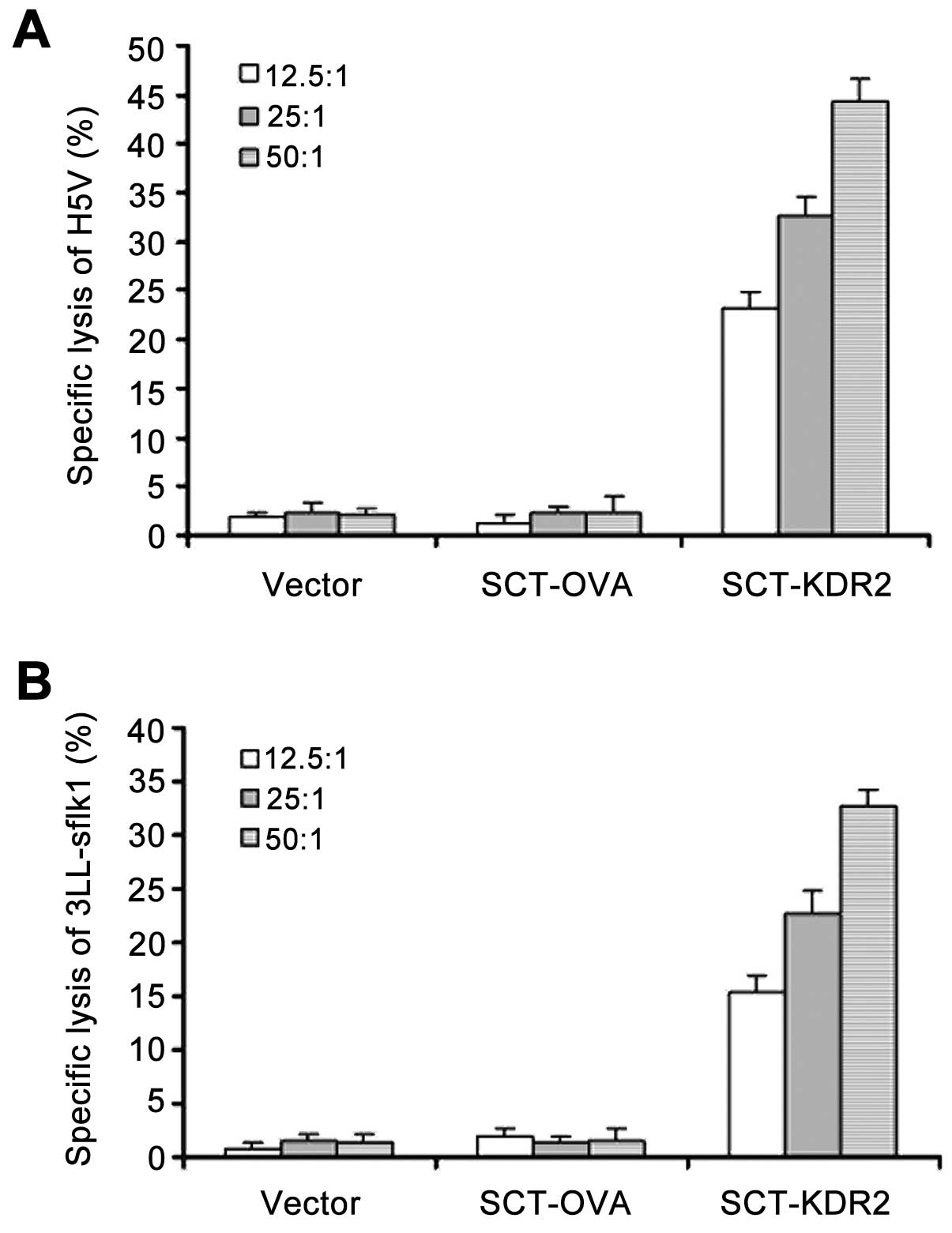
To examine whether vaccination with SCT-KDR2 was
able to break self-tolerance and induce CTL response to VEGFR2,
mice were intradermally immunized three times, at 7-day intervals,
with vector, SCT-OVA and SCT-KDR2. Ten days after the last
immunization, splenocytes were prepared and restimulated in
vitro for 7 days with the inactivated VEGFR2+
endothelial H5V cell line. CTL activity against H5V (Fig. 3A) and 3LL-sflk1 (Fig. 3B) was determined by LDH release
assay. As shown in Fig. 3,
vaccination of mice with SCT-KDR2 induced CTL responses to VEGFR2
significantly, while, vaccination with vector or SCT-OVA did not
induce such responses. Moreover, when the syngeneic
VEGFR2− 3LL and EL-4 cell lines were used as targets, no
significant cytotoxicity was detected (data not shown), suggesting
that this CTL response was VEGFR2-specific. Therefore, our data
suggested that vaccination of mice with SCT-KDR2 was able to break
self-tolerance and induce a CTL response to VEGFR2.
Immunization of mice with DNA vaccine
encoding KDR2-β2m-H-2Db SCT inhibits tumor-induced
angiogenesis
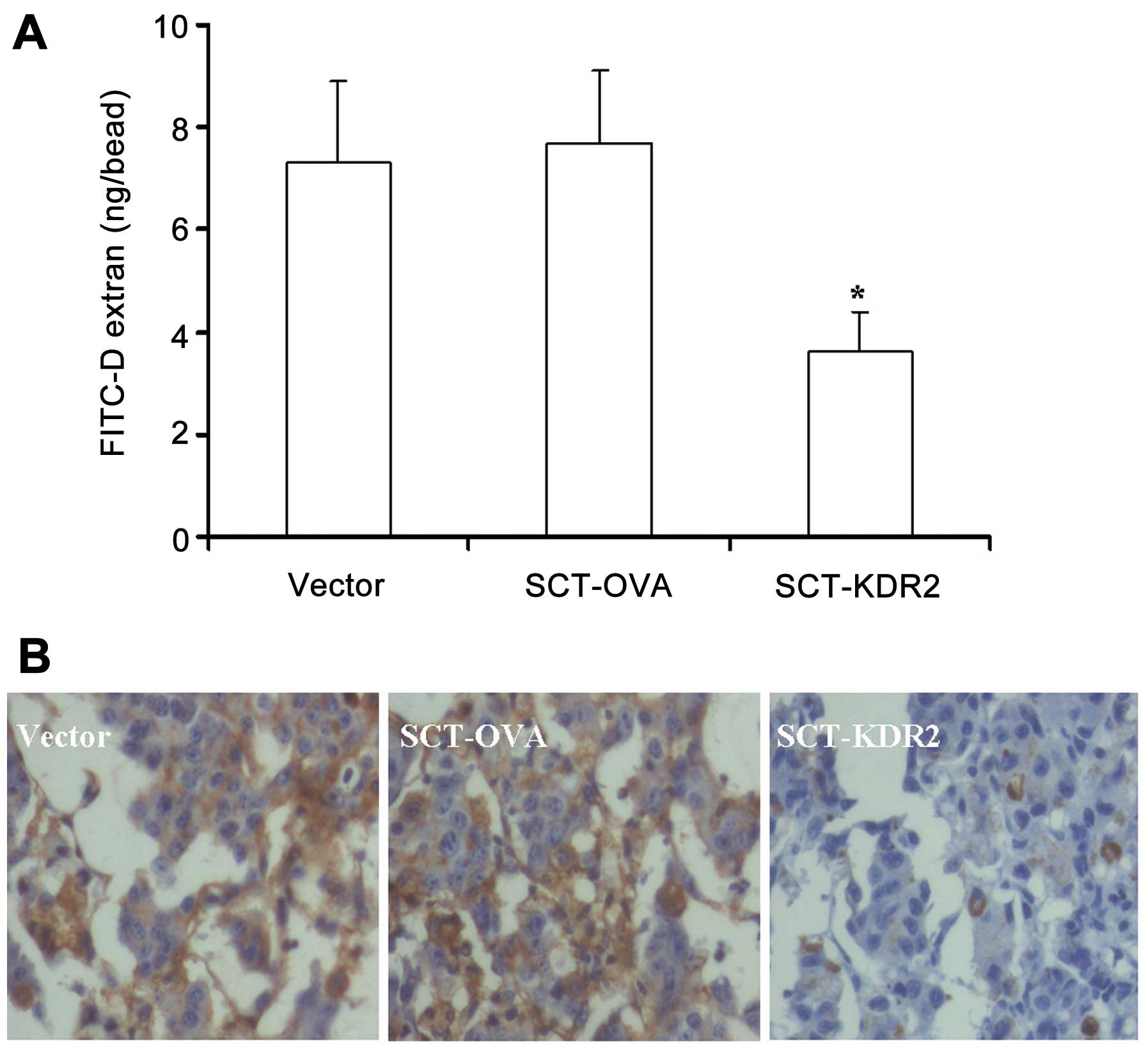
To examine whether the CTL response to VEGFR2 in
mice vaccinated with SCT-KDR2 construct inhibited tumor-induced
neovascularization, alginate bead and immunohistochemical analyses
of tumor cell-induced angiogenesis were performed. As shown in
Fig. 4A, FITC-dextran concentration
in alginate beads derived from mice immunized with SCT-KDR2 was
significantly reduced when compared with that in beads derived from
mice vaccinated with the vector or SCT-OVA (P<0.01). This
inhibition reached 53.2% when compared with the group of SCT-OVA
(3.6±0.8 vs. 7.7±1.4 ng/bead). Furthermore, the immunohistochemical
analysis of factor VIII-related antigen in B16 melanoma showed
again that vaccination of mice with SCT-KDR2 inhibited
tumor-induced vascularization. As shown in Fig. 4B, tumors in the SCT-KDR group showed
markedly decreased factor VIII-related antigen-positive cells
compared with those in the vector and SCT-OVA groups. Taken
together, our data demonstrated that vaccination of the mice with
SCT-KDR2 significantly inhibited tumor cell-induced
angiogenesis.
KDR2-β2m-H-2Db SCT DNA
vaccination inhibits tumor metastasis
To examine the anti-metastatic effects of SCT-KDR2
vaccination, a murine metastatic model with B16 melanoma was
initially employed. As shown in Fig.
5A, mice vaccinated with vector, or SCT-OVA developed extensive
pulmonary metastases. However, a marked reduction in pulmonary
metastases was observed in mice vaccinated with SCT-KDR2. The
number of tumor nodules on lung surfaces decreased by 63.6 and 60%
in mice vaccinated with SCT-KDR2 when compared with that in mice
vaccinated with vector, or SCT-OVA, respectively. Moreover, it was
obvious that the volumes of the tumor nodules on the lung surfaces
from the mice immunized with SCT-KDR2 were significantly reduced in
size as compared to those from the mice vaccinated with vector or
SCT-OVA (Fig. 5A), which is in
agreement with the results of tumor-induced neovascularization. To
confirm this finding, anti-metastatic effects using other tumor
models, such as the mouse 3LL Lewis lung carcinoma metastatic
model, was employed. As shown in Fig.
5B, our data showed again that vaccination of mice with
SCT-KDR2 markedly inhibited pulmonary metastasis.
Discussion
Anti-angiogenic therapy for various types of cancer
has attracted much attention in recent years. Blockade of VEGF-A
signaling with bevacizumab, a humanized anti-VEGF monoclonal
antibody, or RTKIs has improved progression-free survival and, in
some cases overall survival, across many types of cancer, including
metastatic colorectal and non-small cell lung cancer, renal cell
carcinoma, metastatic breast cancer, and hepatocarcinoma (6,34).
However, the clinical benefit of these therapies is variable, and
tumors from some treated patients eventually reinitiate growth due
to the induction of compensatory angiogenic pathways (35). In a recent study by Croci et
al found that glycosylation-dependent lectin-receptor
interactions compensate for the absence of cognate ligand and
preserve angiogenesis in response to VEGF blockade (7). Remodeling of glycome on the surfaces
of endothelial cells regulated the selective binding of
Gal1, which following the recognition of complex N-glycans
on VEGFR2, activated VEGF-like signaling (7). These findings demonstrated again that
VEGFR2-mediated signaling is the rate-limiting step in angiogenesis
and VEGFR2 is the most important target for anti-angiogenic
therapy. Moreover, in comparison with the administration of
angiogenic inhibitors and anti-angiogenic antibodies,
anti-angiogenic active immunotherapy has obvious advantages. If a
break of immunological tolerance to angiogenesis-promoting
regulators is successfully induced, the long-lasting immune
response to angiogenesis-related molecule may be present in the
body, thereby providing long-lasting inhibitory effects on
angiogenesis. Therefore, it is expected to be the more
cost-effective strategy as compared to angiogenic inhibitor or
anti-angiogenic antibody therapy where continuous use of the drugs
is required (2).
DNA vaccines have become potentially important
therapeutic agents for combating various types of cancer, including
anti-angiogenic immunotherapy for cancers, because of their
stability, safety, and simplicity (24). Unlike viral or bacterial vectors,
DNA vaccines may be administered repeatedly without eliciting a
neutralizing antibody response against the vectors themselves.
Although DNA vaccines have these advantages, one major drawback is
their limited potency, because naked DNA does not have the inherent
ability to replicate in vivo. Therefore, attempts have been
made to improve the potency of DNA vaccines (24).
It has been proven that a particularly powerful
strategy to enhance the potency of DNA vaccines involves bypassing
the antigen-processing pathway in DNA-transfected
antigen-presenting cells (APCs) by expressing an SCT consisting of
an immunogenic peptide, β2m and MHC class I heavy chain (22,27,36,37).
The SCT localizes to the plasma membrane of APCs and stably
displays an antigenic peptide for recognition by CD8+
CTLs (22). Therefore, in the
present study, we have introduced the SCT platform into our DNA
vaccine that consists of KDR2, β2m and H-2Db and
examined its ability to induce CTL response to VEGFR2 and its
capacity to inhibit tumor-induced angiogenesis and metastasis in
mouse models.
The results show that the constructed SCT DNA
vaccine was efficiently expressed in eukaryotic cells. Human A293
kidney embryonic cells were transfected with SCT DNA, and
expression of the SCT protein on the cell surface was examined by
flow cytometry with FITC-conjugated rat anti-mouse H2-Db
monoclonal antibody. As A293 cells do not express mouse MHC class I
molecules H-2Db themselves, the presence of
H-2Db on A293 cells demonstrates that SCT DNA could be
successfully expressed. As the main purpose of anti-angiogenic
active immunotherapy is to break self-immunological tolerance to
angiogenesis-promoting regulators and VEGFR2 is the most important
therapeutic target in anti-angiogenic therapy for cancers (2), it is critical to determine whether
SCT-KDR2 immunization could induce CTL response against VEGFR2. Our
results show that CTLs derived from mice vaccinated with SCT-KDR2
were able to kill VEGFR2+ H5V and 3LL-sflk1, while CTLs
from mice vaccinated with vector or SCT-OVA did not kill
VEGFR2+ H5V and 3LL-sflk1. Furthermore, when EL-4 and
3LL, which are syngeneic VEGFR2− cell lines were used as
targets, no significant cytotoxicity was observed, demonstrating
that this CTL response was VEGFR2-specific. Therefore, our findings
show that vaccination of mice with SCT-KDR2 was able to break
self-tolerance and induce CTL response to VEGFR2. We also examined
the effects of SCT-KDR2 vaccination on tumor-induced angiogenesis.
In accordance with the results in CTL activities, the alginate bead
and immunohistochemical analyses showed that vaccination of mice
with SCT-KDR2 markedly inhibited tumor-induced angiogenesis. In the
alginate bead analysis this inhibition reached 53.2% when compared
with the group vaccinated with SCT-OVA. With regard to the
anti-metastatic effects, the results show that the number of
metastatic tumors on the lung surfaces in the B16 melanoma and 3LL
Lewis lung carcinoma metastatic mouse models vaccinated with
SCT-KDR2 markedly decreased when compared with those in mouse
models vaccinated with vector or SCT-OVA. As VEGFR2 is strictly
expressed in proliferating endothelial cells (2,38), but
not in 3LL Lewis lung carcinoma and B16 melanoma cells (20), the anti-metastatic effects observed
were considered to be caused indirectly by interference with the
tumor cell-induced angiogenesis, which is also supported by the
results of the CTL response to VEGFR2 and tumor cell-induced
angiogenesis in vivo. Furthermore, the volumes of tumor
nodules on the lung surfaces from mice vaccinated with SCT-KDR2
were markedly reduced in size compared to those in mice vaccinated
with vector or SCT-OVA, which supports the hypothesis that
angiogenesis is critical for tumor outgrowth, progression and
metastasis.
Taken together, we have successfully shown that
vaccination with SCT-KDR2 DNA was able to break self-immunological
tolerance and induce a robust CTL response to VEGFR2, leading to
the significant inhibition of metastasis through inhibition of
tumor cell-induced angiogenesis in mouse B16 melanoma and 3LL Lewis
lung carcinoma metastatic models. Our data show that
VEGFR2-targeted SCT vaccination is an effective modality in
anti-angiogenic active immunotherapy for various types of cancer
and it is important to evaluate this modality further in
translational medicine.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 30872325) and from the
Science and Technology Bureau of Hangzhou, Zhejiang Province, P.R.
China (no. 20120633B30).
References
|
1
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan J, Jin P, Yan J and Kabelitz D:
Anti-angiogenic active immunotherapy: a new approach to cancer
treatment. Cancer Immunol Immunother. 57:1105–1114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung AS and Ferrara N: Developmental and
pathological angiogenesis. Annu Rev Cell Dev Biol. 27:563–584.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Zhang XF, Lu X, Jia HL, Liang L,
Dong QZ, Ye QH and Qin LX: MicroRNA-26a suppresses angiogenesis in
human hepatocellular carcinoma by targeting hepatocyte growth
factor-cMet pathway. Hepatology. 59:1874–1885. 2014. View Article : Google Scholar
|
|
6
|
Shojaei F: Anti-angiogenesis therapy in
cancer: Current challenges and future perspectives. Cancer Lett.
320:130–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Croci DO, Cerliani JP, Dalotto-Moreno T,
et al: Glycosylation-dependent lectin-receptor interactions
preserve angiogenesis in anti-VEGF refractory tumors. Cell.
156:744–758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Astorgues-Xerri L, Riveiro ME,
Tijeras-Raballand A, Serova M, Neuzillet C, Albert S, Raymond E and
Faivre S: Unraveling galectin-1 as a novel therapeutic target for
cancer. Cancer Treat Rev. 40:307–319. 2014. View Article : Google Scholar
|
|
9
|
Cross MJ and Claesson-Welsh L: FGF and
VEGF function in angiogenesis: signalling pathways, biological
responses and therapeutic inhibition. Trends Pharmacol Sci.
22:201–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkier T, Cella D and Davidson NE:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren S, Fengyu, Zuo S, Zhao M, Wang X, Wang
X, Chen Y, Wu Z and Ren Z: Inhibition of tumor angiogenesis in lung
cancer by T4 phage surface displaying mVEGFR2 vaccine. Vaccine.
29:5802–5811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bracarda S, Caserta C, Sordini L, Rossi M,
Hamzay A and Crinò L: Protein kinase inhibitors in the treatment of
renal cell carcinoma: Sorafenib. Ann Oncol. 18(Suppl 6): vi22–vi25.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei YQ, Wang QR, Zhao X, et al:
Immunotherapy of tumors with xenogeneic endothelial cells as a
vaccine. Nat Med. 6:1160–1166. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seavey MM, Maciag PC, Al-Rawi N, Sewell D
and Paterson Y: An anti-vascular endothelial growth factor receptor
2/fetal liver kinase-1 Listeria monocytogenes anti-angiogenesis
cancer vaccine for the treatment of primary and metastatic
Her-2/neu+ breast tumors in a mouse model. J Immunol.
182:5537–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zuo SG, Chen Y, Wu ZP, et al: Orally
administered DNA vaccine delivery by attenuated Salmonella
typhimurium targeting fetal liver kinase 1 inhibits murine Lewis
lung carcinoma growth and metastasis. Biol Pharm Bull. 33:174–182.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Wang MN, Li H, King KD, Bassi R, Sun
H, Santiago A, Hooper AT, Bohlen P and Hicklin DJ: Active
immunization against the vascular endothelial growth factor
receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp
Med. 195:1575–1584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nair S, Boczkowski D, Moeller B, Dewhirst
M, Vieweg J and Gilboa E: Synergy between tumor immunotherapy and
antiangiogenic therapy. Blood. 102:964–971. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan J, Heiser A, Marget M, Steinmann J and
Kabelitz D: Enhanced antimetastatic effect of fetal liver kinase 1
extracellular domain and interferon-gamma fusion gene-modified
dendritic cell vaccination. Gene Ther. 12:742–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong Y, Qian J, Ibrahim R, Berzofsky JA
and Khleif SN: Identification of H-2Db-specific CD8+ T-cell
epitopes from mouse VEGFR2 that can inhibit angiogenesis and tumor
growth. J Immunother. 29:32–40. 2006. View Article : Google Scholar
|
|
22
|
Hansen T, Yu YY and Fremont DH:
Preparation of stable single-chain trimers engineered with peptide,
beta2 microglobulin, and MHC heavy chain. Curr Protoc Immunol
Chapter. 17:Unit 17. 152009.
|
|
23
|
Huang CH, Peng S, He L, Tsai YC, Boyd DA,
Hansen TH, Wu TC and Hung CF: Cancer immunotherapy using a DNA
vaccine encoding a single-chain trimer of MHC class I linked to an
HPV-16 E6 immunodominant CTL epitope. Gene Ther. 12:1180–1186.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang TH, Mao CP, La V, Chen A, Hung CF and
Wu TC: Innovative DNA vaccine to break immune tolerance against
tumor self-antigen. Hum Gene Ther. 24:181–188. 2013. View Article : Google Scholar :
|
|
25
|
Hung CF, Calizo R, Tsai YC, He L and Wu
TC: A DNA vaccine encoding a single-chain trimer of HLA-A2 linked
to human mesothelin peptide generates anti-tumor effects against
human mesothelin-expressing tumors. Vaccine. 25:127–135. 2007.
View Article : Google Scholar
|
|
26
|
Huang B, Mao CP, Peng S, He L, Hung CF and
Wu TC: Intradermal administration of DNA vaccines combining a
strategy to bypass antigen processing with a strategy to prolong
dendritic cell survival enhances DNA vaccine potency. Vaccine.
25:7824–7831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim S, Zuiani A, Carrero JA and Hansen TH:
Single chain MHC I trimer-based DNA vaccines for protection against
Listeria monocytogenes infection. Vaccine. 30:2178–2186. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim S, Li L, McMurtrey CP, Hildebrand WH,
Weidanz JA, Gillanders WE, Diamond MS and Hansen TH: Single-chain
HLA-A2 MHC trimers that incorporate an immundominant peptide elicit
protective T cell immunity against lethal West Nile virus
infection. J Immunol. 184:4423–4430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheung YK, Cheng SC, Ke Y and Xie Y: Two
novel HLA-A*0201 T-cell epitopes in avian H5N1 viral nucleoprotein
induced specific immune responses in HHD mice. Vet Res. 41:242010.
View Article : Google Scholar
|
|
30
|
Cheung YK, Cheng SC, Sin FW, Chan KT and
Xie Y: Investigation of immunogenic T-cell epitopes in SARS virus
nucleocapsid protein and their role in the prevention and treatment
of SARS infection. Hong Kong Med J. 14(Suppl 4): 27–30.
2008.PubMed/NCBI
|
|
31
|
Cheung YK, Cheng SC, Sin FW, Chan KT and
Xie Y: Induction of T-cell response by a DNA vaccine encoding a
novel HLA-A*0201 severe acute respiratory syndrome coronavirus
epitope. Vaccine. 25:6070–6077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu YX, Li M, Jia XH, Du QX, Miao FT, Yao L
and Shen JD: HPV16 CTL epitope peptide-activated dendritic cell and
natural killer co-culture for therapy of cervical cancer in an
animal model. Asian Pac J Cancer Prev. 14:7335–7338. 2013.
View Article : Google Scholar
|
|
33
|
Wang D, Stockard CR, Harkins L, et al:
Immunohistochemistry in the evaluation of neovascularization in
tumor xenografts. Biotech Histochem. 83:179–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer.
8:579–591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ebos JM, Lee CR and Kerbel RS: Tumor and
host-mediated pathways of resistance and disease progression in
response to antiangiogenic therapy. Clin Cancer Res. 15:5020–5025.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu YY, Netuschil N, Lybarger L, Connolly
JM and Hansen TH: Cutting edge: Single-chain trimers of MHC class I
molecules form stable structures that potently stimulate
antigen-specific T cells and B cells. J Immunol. 168:3145–3149.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lybarger L, Yu YY, Miley MJ, Fremont DH,
Myers N, Primeau T, Truscott SM, Connolly JM and Hansen TH:
Enhanced immune presentation of a single-chain major
histocompatibility complex class I molecule engineered to optimize
linkage of a C-terminally extended peptide. J Biol Chem.
278:27105–27111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kanagawa N, Yanagawa T, Nakagawa T, Okada
N and Nakagawa S: Tumor vessel-injuring ability improves antitumor
effect of cytotoxic T lymphocytes in adoptive immunotherapy. Cancer
Gene Ther. 20:57–64. 2013. View Article : Google Scholar :
|