Introduction
Breast cancer is the most common malignancy in
females worldwide (1,2). Despite great advances in diagnosis and
appropriate systemic therapy, the mortality of breast cancer
remains high, accounting for 14% of the total cancer-related deaths
(3). Identifying key molecules
contributing to the malignant properties of breast cancer cells is
essential for future development of new and effective anti-breast
cancer approaches.
Flotillins, also known as reggie proteins, are
markers of lipid rafts that contain two ubiquitously expressed and
highly conserved homologous isoforms, i.e., flotillin-1 (FLOT1) and
FLOT2 (4,5). Flotillin proteins play important roles
in various cellular processes, such as adhesion, actin cytoskeletal
reorganization, endocytosis, phagocytosis and transduction of
cellular signals (6,7). Apart from the functions of flotillins
in the cellular and organelle membranes, FLOT2 has been reported to
be involved in oncogenesis. Hazarika et al showed that
overexpression of FLOT2 was associated with human melanoma
progression and lymph node metastasis (8). In gastric cancer, FLOT2 was reported
as an independent prognostic factor (9), whereas in head and neck cancer FLOT2
overexpression was reported to show a strong predictive value for
the development of metastases (10). Yan et al showed that
upregulation of FLOT2 was associated with renal cell carcinoma
progression (11). In
nasopharyngeal carcinoma, the expression of FLOT2 was confirmed to
be an independent predicted factor for lymph node metastasis
(12). Recently, in breast cancer,
it was reported that FLOT2 was associated with cancer progression
and poor survival outcomes (13),
yet the precise mechanism of its oncogenic function remains
unclear.
In the present study, we found that knockdown of
FLOT2 inhibited proliferation of breast cancer cells. Mechanistic
basis for such an antiproliferative effect of FLOT2 depletion may
be linked to suppression of Akt phosphorylation and subsequent
activation of FOXOs, which consequently promote upregulation of CDK
inhibitors p21Cip1 and p27Kip1. Our findings
suggest that FLOT2 plays a role in the proliferation of human
breast cancer, indicating that FLOT2 may be a potential target for
human breast cancer treatment.
Materials and methods
Cell lines
Breast cancer MCF-7 and MDA-MB-231 cell lines were
grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(HyClone, Logan, UT, USA).
Vectors and gene transduction
Expression of FLOT2 was stably knocked down in MCF-7
and MDA-MB-231 cells using the pSUPER-retroviral vector and the
oligonucleotides for FLOT2 as previously described for HeLa cells
(14). Recombinant retroviral
vectors were produced by transient cotransfection as previously
described (15). Viral infection
was performed serially, and stable cell lines expressing
FLOT2-RNAis were selected with 0.5 μg/ml puromycin 48 h
after infection. After a 10-day selection, whole cell lysates were
fractionated on sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) to examine the level of FLOT2
protein.
Western blot analysis
Western blotting was performed according to standard
methods as previously described (16), using anti-p-Akt (ser473), anti-Akt,
anti-p-Rb (ser608), anti-Rb, anti-p-FOXO1 (ser256), anti-FOXO1,
anti-p-FOXO3a (ser253), anti-FOXO3a, anti-p-FOXO4 (Ser193),
anti-FOXO4, anti-cyclin A, anti-CDK4, anti-CDK6,
anti-p21Cip1 and anti-p27Kip1 (Cell Signaling
Technology, Danvers, MA, USA); and anti-FLOT2 and anti-α-tubulin
(Sigma-Aldrich, St. Louis, MO, USA).
Real-time PCR
Total RNA from cultured cells was extracted using
the RNeasy kit (Qiagen, Crawley, UK). Each cDNA template was made
from total RNA with a reverse transcriptase kit according to the
manufacturer’s instructions (Invitrogen). Amplification reactions
were performed using the SYBR Premix Ex Taq™ (Takara Shuzo, Kyoto,
Japan) in a 25 μl volume. The following cycling parameters
were used: 30 sec at 95°C for initial denaturing, 5 sec at 95°C for
denaturing and 30 sec at 60°C for annealing and extension for a
total of 40 cycles. The fold-change in mRNA was calculated by the
2−ΔΔCt method. All samples were normalized to GAPDH. The
primer sequences used were: p21Cip1-up,
5′-CGATGCCAACCTCCTCAACGA-3′ and p21Cip1-dn,
5′-TCGCAGACCTCCAGCATCCA-3′; p27Kip1-up,
5′-TGCAACCGACGATTCTTCTACTCAA-3′ and p27Kip1-dn,
5′-CAAGCAGTGATGTATCTGATAAACAAGGA-3′; GAPDH-up,
5′-ACCACAGTCCATGCCATCAC-3′ and GAPDH-dn,
5′-TCCACCACCCTGTTGCTGTA-3′.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay
MTT assay was performed as previously described
(17). Briefly, cells were seeded
in 96-well flat-bottom plates at a density of 0.2×104
cells/well. At each time point, cells were stained with 100
μl sterile MTT dye (0.5 mg/ml) for 4 h at 37°C, followed by
removal of the culture medium and addition of 150 μl of
dimethyl sulfoxide (DMSO) (both from Sigma-Aldrich). The absorbance
was measured at 570 nm, with 630 nm as the reference wavelength.
All experiments were performed in triplicates.
Colony formation assays
Cells were plated in 6-well plates (3×102
cells/well) and cultured for 10 days. The colonies were stained
with 1% crystal violet for 30 sec after fixation with 10%
formaldehyde for 5 min.
Bromodeoxyuridine (BrdUrd) labeling and
immunofluorescence
Cells were plated on coverslips (Fisher Scientific,
Pittsburgh, PA, USA). After 24 h, the cells were incubated with
BrdUrd for 1 h and stained with the anti-BrdUrd antibody (Upstate,
Temecula, CA, USA) according to the manufacturer’s instructions.
Gray level images were acquired under a laser scanning microscope
(Zeiss Axiovert 100M; Carl Zeiss, Germany).
Flow cytometry
Cells were harvested, washed with cold
phosphate-buffered saline (PBS) and processed for cell cycle
analysis using flow cytometry. Briefly, the cells were fixed in 75%
ethanol and stored at −20°C for later analysis. The fixed cells
were centrifuged at 1,000 rpm and washed with cold PBS twice. RNase
A (20 μg/ml final concentration) and propidium iodide (PI)
staining solution (50 μg/ml final concentration) was added
to the cells and incubated for 30 min at 37°C in the dark. Fifty
thousand cells were analyzed using a FACSCalibur instrument
(Becton-Dickinson, San Jose, CA, USA).
Statistical analysis
The data given in the text are expressed as means ±
standard deviations (SD). Comparisons between groups for
statistical significance were carried out with a two-tailed
Student’s t-test. In all cases, p<0.05 was considered to
indicate a statistically significant result.
Results
Downregulation of FLOT2 inhibits the
proliferation of breast cancer cells
To evaluate the biological function of FLOT2 in
breast cancer, we constructed FLOT2-knockdown cell models using two
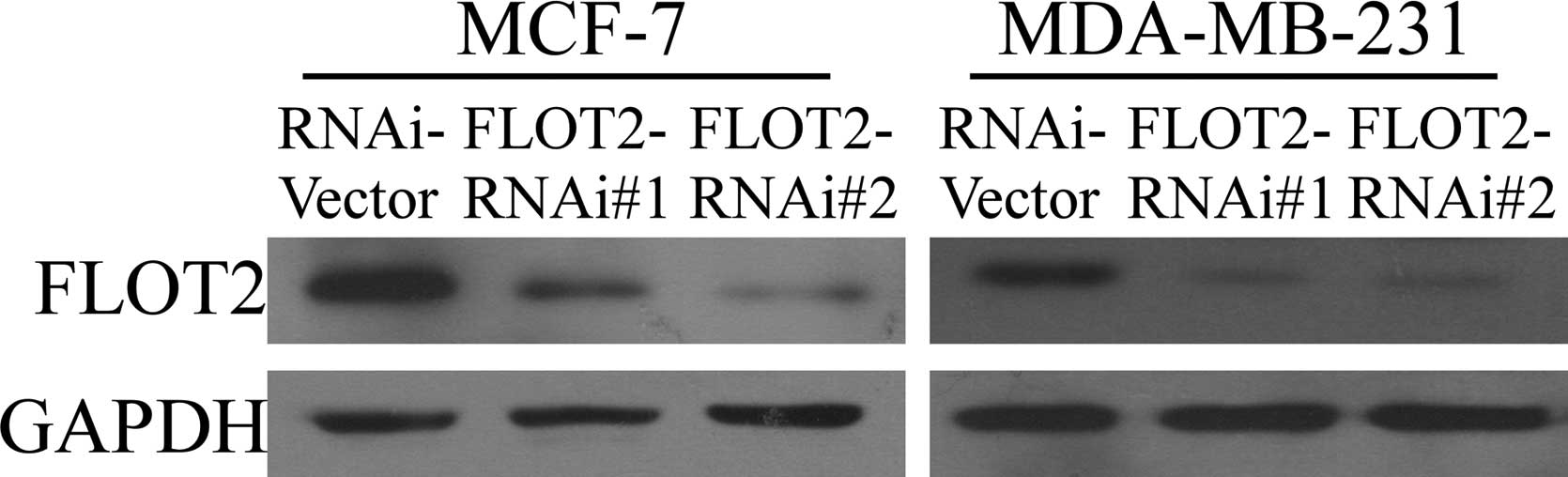
FLOT2-specific shRNAs. As shown in Fig.
1, both shRNAs effectively knocked down the expression of
endogenous FLOT2 protein in both the MCF-7 and MDA-MB-231 cells. An
MTT assay showed that depletion of FLOT2 expression caused a
significant reduction in viability of both MCF-7 and MDA-MB-231
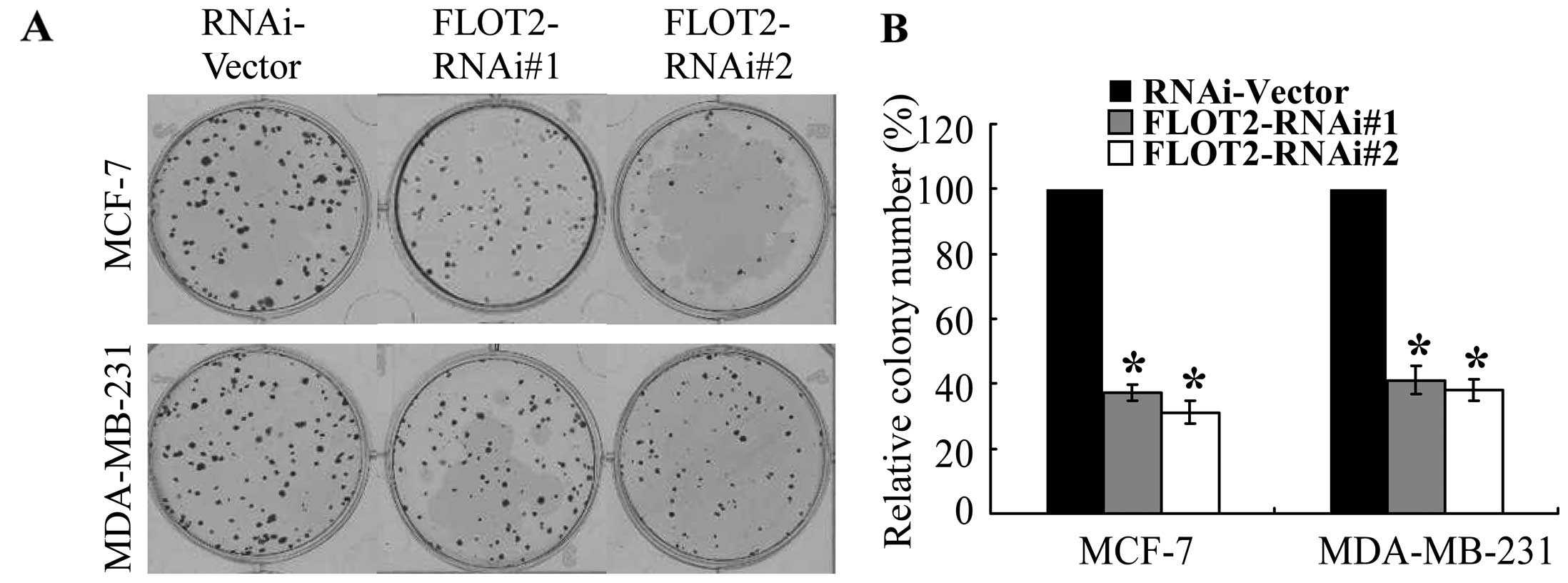
breast cancer cell lines (Fig. 2),
and these results were further confirmed by a colony formation
assay (Fig. 3). These data suggest
that FLOT2 may be involved in promoting the proliferation of breast
cancer cells.
Silencing of FLOT2 results in the G1-S
phase cell cycle arrest of breast cancer cells
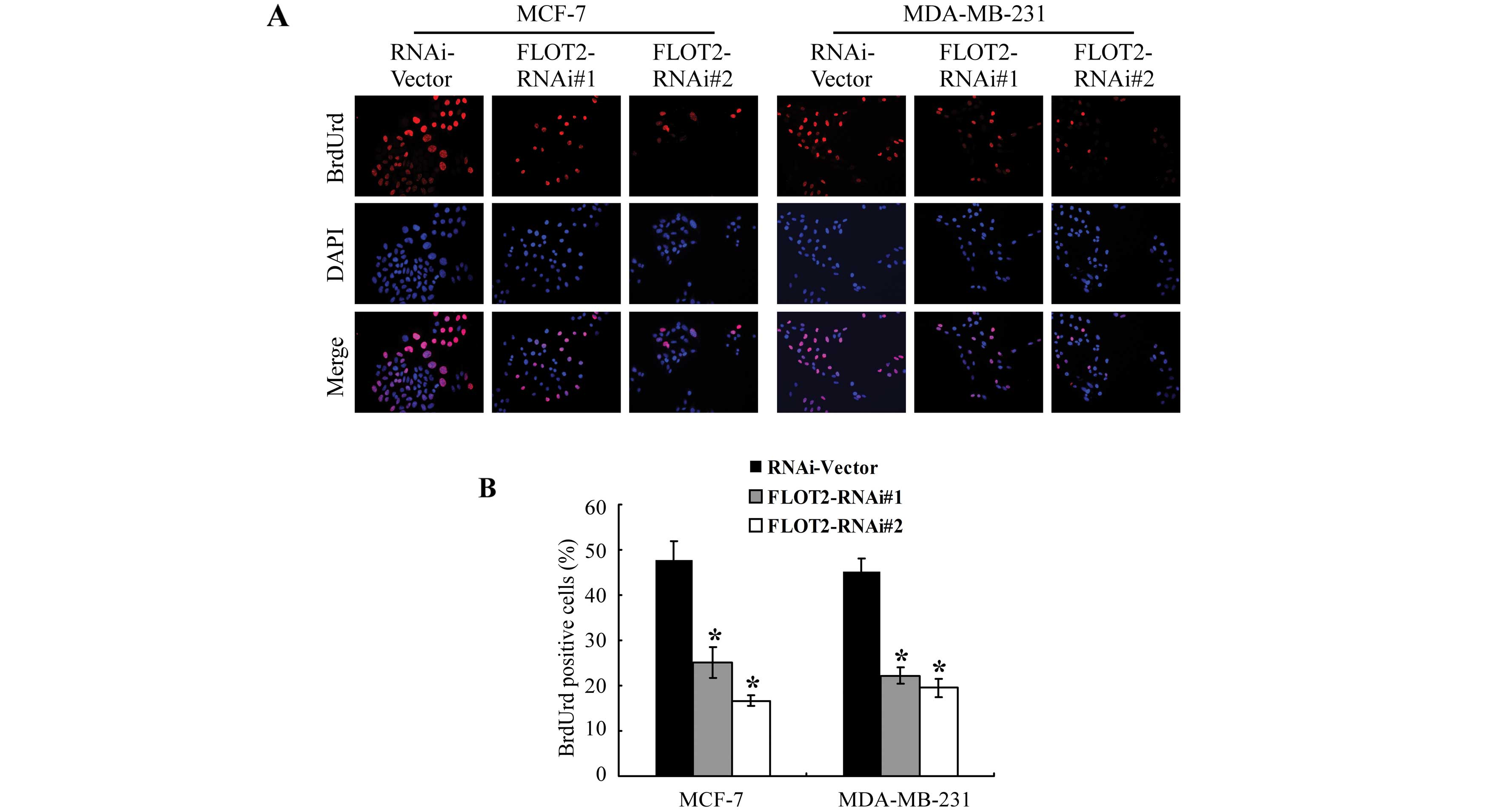
To investigate the mechanism that mediates the
proliferation-promoting function of FLOT2, a BrdUrd incorporation
assay was performed. As shown in Fig.
4, the silencing of FLOT2 in the MCF-7 and MDA-MB-231 cells
markedly decreased the percentages of cells with incorporated
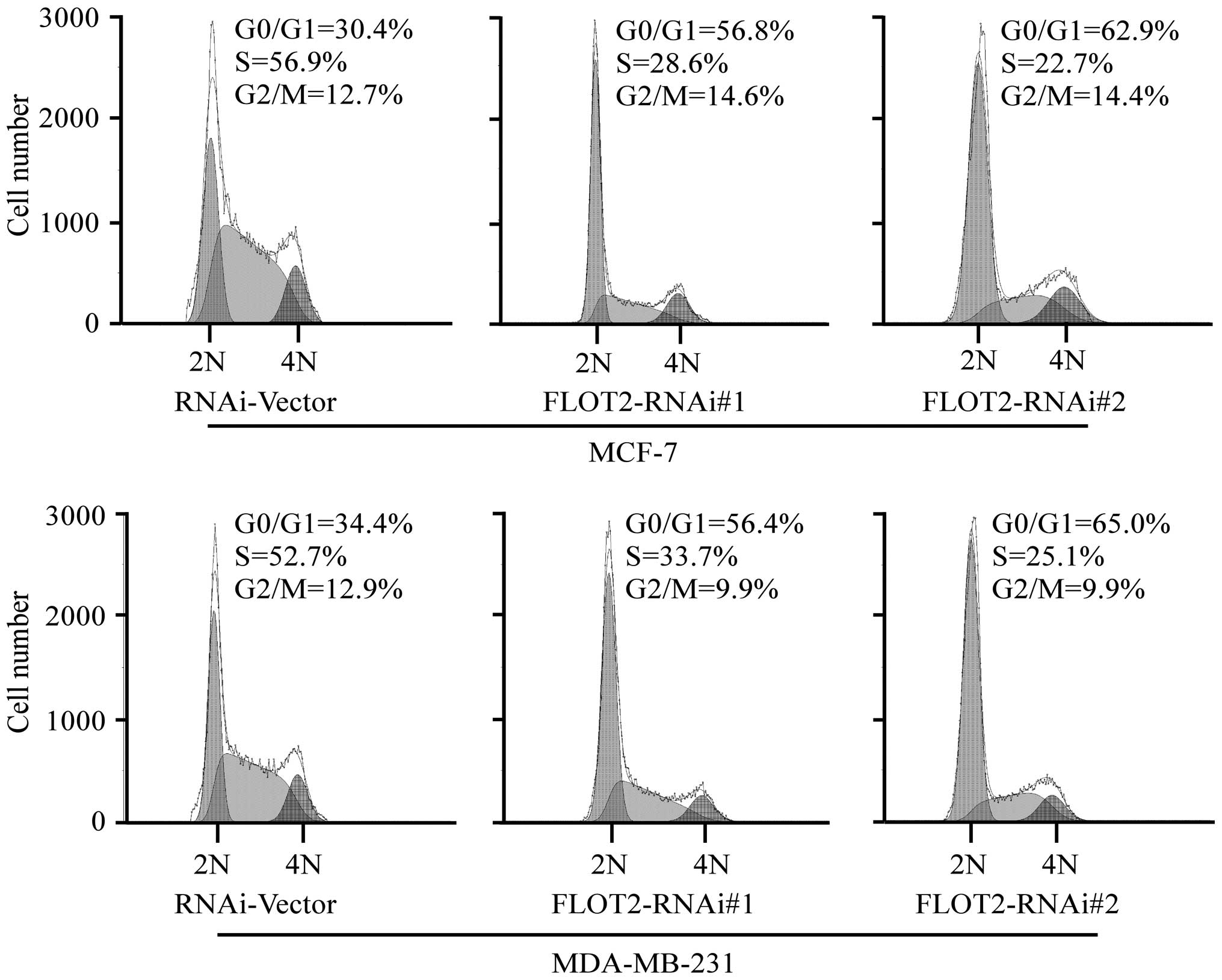
BrdUrd. Flow cytometric analysis showed that downregulation of
FLOT2 significantly increased the percentage of cells in the G0/G1
peak but decreased the percentage of cells in the S peak (Fig. 5). These results suggest that the
silencing of FLOT2 induces G1-S phase arrest of breast cancer
cells.
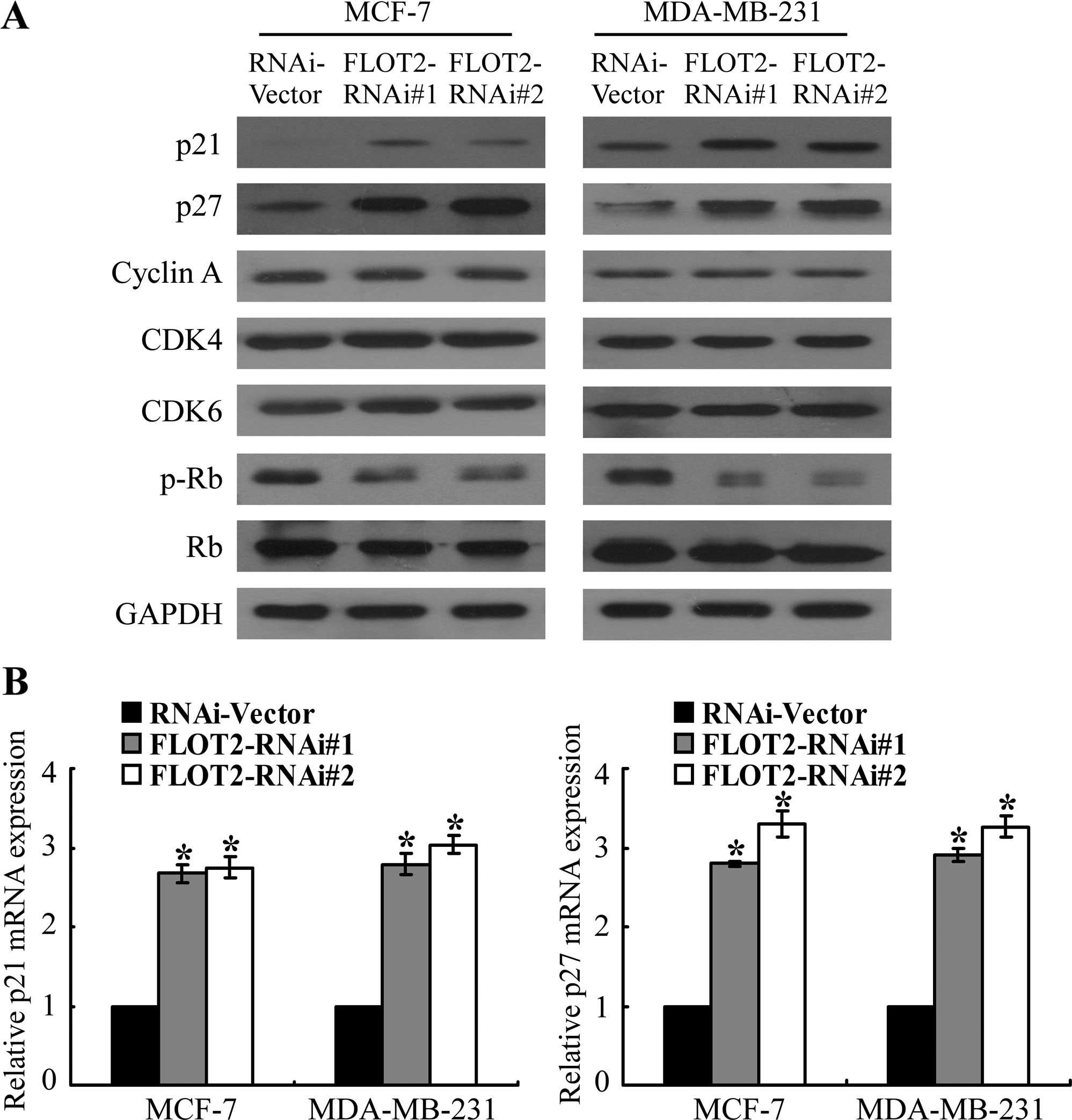
Silencing of FLOT2 upregulates cell cycle
inhibitors p21Cip1 and p27Kip1 in breast
cancer cells
The observed correlation of deregulated FLOT2
expression with the proliferation of breast cancer cells prompted
us to further investigate the possibility that cell cycle
regulators may mediate the modulation by FLOT2. As shown in
Fig. 6A, western blot analysis
revealed that the silencing of FLOT2 had no effect on the
expression of cyclin A, CDK4 and CDK6, all of which are cell cycle
promoters. Instead, the expression levels of two CDK inhibitors,
p21Cip1 and p27Kip1, were markedly
upregulated in the FLOT2 shRNA-transduced cells at both the protein
(Fig. 6A) and the mRNA level
(Fig. 6B). As expected, the
phosphorylation level of Rb, the downstream target protein of CDK,
was shown to be suppressed in the FLOT2-silenced cells (Fig. 6A), further supporting the notion
that FLOT2 is involved in the regulation of proliferation of breast
cancer cells.
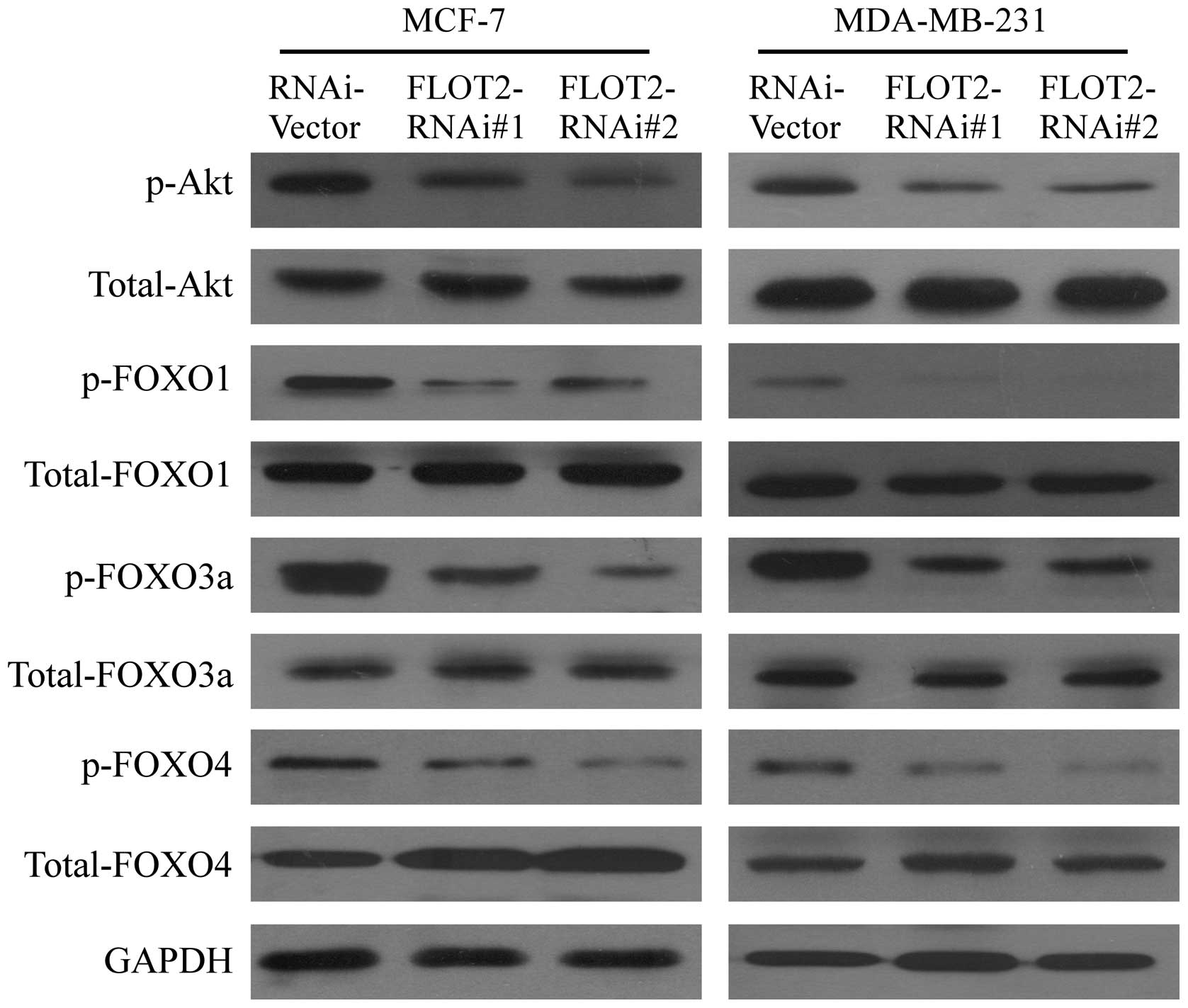
Silencing of FLOT2 enhances the
transcriptional activity of FOXO factors via inhibiting Akt
activation
The previous finding that the expression of
p21Cip1 and p27Kip1 could be
transcriptionally regulated by FOXO family transcriptional factors
(18,19) prompted us to test whether FLOT2
modulates the activity of FOXO factors. As shown in Fig. 7, western blot analysis revealed that
the phosphorylation levels of FOXO1, FOXO3a and FOXO4 were
decreased in FLOT2 shRNA-infected cells, compared with those in the
vector-control cells. It is known that phosphorylation of FOXOs is
mediated through activation of PI3K/Akt signaling (20–22).
Thus, we further examined whether FLOT2 activated the PI3K/Akt
pathway. As shown in Fig. 7,
downregulation of FLOT2 decreased the phosphorylation of Akt in
both the MCF-7 and MDA-MB-231 cells as compared with the
transduction control cells. Taken together, these data revealed
that the observed upregulation of cell cycle inhibitors
p21Cip1 and p27Kip1 caused by the silencing
of FLOT2 was associated with inhibition of Akt kinase activity and
subsequently enhanced transcriptional activity of FOXO factors.
Discussion
This study demonstrated that knockdown of endogenous
FLOT2, a lipid raft specific protein, inhibited the proliferation
of breast cancer cells. We showed that silencing of FLOT2 using
RNAi resulted in suppression of Akt phosphorylation and subsequent
activation of FOXOs, which led to the upregulation of CDK
inhibitors p21Cip1 and p27Kip1. These
findings provide new insights into the potential role of the
upregulation of FLOT2 in promoting oncogenesis and progression of
breast cancer.
Oncogenesis is a complex multi-step process,
characterized by uncontrolled cell growth and tumor formation.
During oncogenesis cells proliferate in a progressive, deregulated
manner. Uncontrolled cell growth is associated with various
alterations in genes or proteins related to regulation of
proliferation, cell death and genetic stability, such as
tumor-suppressor genes, oncogenes, growth factors and cell adhesion
molecules (23). Thus,
identification of genes and their products involved in the
molecular events leading to oncogenesis is the key to the
development of effective therapeutic strategies.
A correlation of FLOT2 with cancer development and
progression has recently been demonstrated by studies from several
groups, in which FLOT2 was found to be expressed at high levels in
various types of human cancers, including head and neck cancer,
melanoma, gastric cancer, nasopharyngeal carcinoma and breast
cancer (8–13). Consistent with these clinical
findings, at the cellular level we found that depletion of FLOT2
suppressed the proliferation of breast cancer cells, a key
biological event essential for cancer development and progression.
The experiments on the effects of FLOT2 depletion on cell
viability, colony-formation ability and BrdUrd incorporation
confirmed that FLOT2 is a proliferation promotor. Further
experiments showed that silencing of FLOT2 in breast cancer cells
enhanced G1-S phase arrest. Such a connection between FLOT2 and
G1/S phase transition was shown to be mechanistically mediated by
the cell cycle inhibitors p21Cip1 and
p27Kip1, which the present study demonstrated to be
upregulated by knocking down FLOT2 in the breast cancer cells.
FOXO proteins are a family of transcription factors
that play important roles in regulating the expression of genes
involved in a variety of biological processes, such as
proliferation, differentiation, stress response and cellular
apoptosis (24,25). It has been demonstrated that FOXO
proteins act as tumor suppressors as evidenced by its
transcriptional induction of CDK inhibitors, including
p21Cip1, p27Kip1and p57kip2. It
has been demonstrated that phosphorylation of FOXOs by Akt leads to
FOXO nuclear/cytoplasmic translocation and subsequent degradation
via the ubiquitin-proteasome system (26,27).
In the present study, decreased phosphorylation, and subsequent
increased transcriptional activity, of FOXO factors (FOXO1, FOXO3a
and FOXO4), which are known activators for p21Cip1 and
p27Kip1 transcription, was found when FLOT2 expression
was depleted and such an effect was likely to be mediated by
suppression of Akt phosphorylation.
In summary, our finding that knockdown of FLOT2, a
marker of lipid rafts, inhibited the proliferation of breast cancer
cells through modulation of the Akt/FOXO/p21/p27 pathway
illustrates a new mode of action in the molecular mechanism
underlying the oncogenesis of breast cancer. Understanding the
precise role played by FLOT2 in breast cancer progression will not
only increase our knowledge of the biology of breast cancer but may
also enable development of a novel therapeutic strategy via
suppression of FLOT2.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81101682), a
Science and Technology Planning Project of Guangzhou Municipal
Health Bureau (no. 201102A213045), and a Ph.D. Start-up Fund of
Guangzhou Women and Children’s Medical Center (no. 201012).
References
|
1
|
Friedenreich CM: Physical activity and
breast cancer: Review of the epidemiologic evidence and biologic
mechanisms. Recent Results Cancer Res. 188:125–139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng L, Zhou B, Meng X, Zhu W, Zuo A,
Wang X, Jiang R and Yu S: A model of spontaneous mouse mammary
tumor for human estrogen receptor- and progesterone
receptor-negative breast cancer. Int J Oncol. 45:2241–2249.
2014.PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guan Y, Song H, Zhang G and Ai X:
Overexpression of flotillin-1 is involved in proliferation and
recurrence of bladder transitional cell carcinoma. Oncol Rep.
32:748–754. 2014.PubMed/NCBI
|
|
5
|
Babuke T and Tikkanen R: Dissecting the
molecular function of reggie/flotillin proteins. Eur J Cell Biol.
86:525–532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin C, Wu Z, Lin X, Yu C, Shi T, Zeng Y,
Wang X, Li J and Song L: Knockdown of FLOT1 impairs cell
proliferation and tumorigenicity in breast cancer through
upregulation of FOXO3a. Clin Cancer Res. 17:3089–3099. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Langhorst MF, Reuter A and Stuermer CA:
Scaffolding microdomains and beyond: The function of
reggie/flotillin proteins. Cell Mol Life Sci. 62:2228–2240. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hazarika P, McCarty MF, Prieto VG, George
S, Babu D, Koul D, Bar-Eli M and Duvic M: Up-regulation of
Flotillin-2 is associated with melanoma progression and modulates
expression of the thrombin receptor protease activated receptor 1.
Cancer Res. 64:7361–7369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Z, Wang J, Sun Z, Sun X, Wang Z and Xu
H: Flotillin2 expression correlates with HER2 levels and poor
prognosis in gastric cancer. PLoS One. 8:e623652013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rickman DS, Millon R, De Reynies A, Thomas
E, Wasylyk C, Muller D, Abecassis J and Wasylyk B: Prediction of
future metastasis and molecular characterization of head and neck
squamous-cell carcinoma based on transcriptome and genome analysis
by microarrays. Oncogene. 27:6607–6622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan Y, Yang FQ, Zhang HM, Che J and Zheng
JH: Up-regulation of flotillin-2 is associated with renal cell
carcinoma progression. Tumour Biol. 35:10479–10486. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen Q, Li J, Wang W, et al: Increased
expression of flotillin-2 protein as a novel biomarker for lymph
node metastasis in nasopharyngeal carcinoma. PLoS One.
9:e1016762014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Yang Q, Guo L, Li XH, Zhao XH,
Song LB and Lin HX: Flotillin-2 is associated with breast cancer
progression and poor survival outcomes. J Transl Med. 11:1902013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pust S, Dyve AB, Torgersen ML, van Deurs B
and Sandvig K: Interplay between toxin transport and flotillin
localization. PLoS One. 5:e88442010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hahn WC, Dessain SK, Brooks MW, King JE,
Elenbaas B, Sabatini DM, DeCaprio JA and Weinberg RA: Enumeration
of the simian virus 40 early region elements necessary for human
cell transformation. Mol Cell Biol. 22:2111–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie G, Tang H, Wu S, Chen J, Liu J and
Liao C: The cyclin-dependent kinase inhibitor SNS-032 induces
apoptosis in breast cancer cells via depletion of Mcl-1 and
X-linked inhibitor of apoptosis protein and displays antitumor
activity in vivo. Int J Oncol. 45:804–812. 2014.PubMed/NCBI
|
|
17
|
Xie G, Zhu X, Li Q, et al: SZ-685C, a
marine anthraquinone, is a potent inducer of apoptosis with
anticancer activity by suppression of the Akt/FOXO pathway. Br J
Pharmacol. 159:689–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura N, Ramaswamy S, Vazquez F,
Signoretti S, Loda M and Sellers WR: Forkhead transcription factors
are critical effectors of cell death and cell cycle arrest
downstream of PTEN. Mol Cell Biol. 20:8969–8982. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cardozo T and Pagano M: The SCF ubiquitin
ligase: Insights into a molecular machine. Nat Rev Mol Cell Biol.
5:739–751. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burgering BM: A brief introduction to
FOXOlogy. Oncogene. 27:2258–2262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu W, Wang J, Ma L, Tang X, Qiao Y, Pan Q,
Yu Y and Sun F: CD166 plays a pro-carcinogenic role in liver cancer
cells via inhibition of FOXO proteins through AKT. Oncol Rep.
32:677–683. 2014.PubMed/NCBI
|
|
23
|
Doerfler W, Hohlweg U, Müller K, Remus R,
Heller H and Hertz J: Foreign DNA integration - perturbations of
the genome - oncogenesis. Ann NY Acad Sci. 945:276–288. 2001.
View Article : Google Scholar
|
|
24
|
Arden KC: FoxOs in tumor suppression and
stem cell maintenance. Cell. 128:235–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SH, Lee JH, Berek JS and Hu MC:
Auranofin displays anticancer activity against ovarian cancer cells
through FOXO3 activation independent of p53. Int J Oncol.
45:1691–1698. 2014.PubMed/NCBI
|
|
26
|
Huang H, Regan KM, Wang F, Wang D, Smith
DI, van Deursen JM and Tindall DJ: Skp2 inhibits FOXO1 in tumor
suppression through ubiquitin-mediated degradation. Proc Natl Acad
Sci USA. 102:1649–1654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seoane J, Le HV, Shen L, Anderson SA and
Massagué J: Integration of Smad and forkhead pathways in the
control of neuroepithelial and glioblastoma cell proliferation.
Cell. 117:211–223. 2004. View Article : Google Scholar : PubMed/NCBI
|