Introduction
Ovarian cancer is the fifth leading cause of
mortality in females, with epithelial ovarian cancer being the most
common pathological type (1). In
developed countries, it is the most lethal gynecologic cancer since
>70% of women were diagnosed with advanced stage and cure rates
at this stage are <30% (2).
Identification of molecular markers or pathways may be useful in
determining the potential therapeutic targets or novel therapeutic
methods.
The cystic fibrosis transmembrane conductance
regulator (CFTR), an ~170 kDa glycosylated protein, is known as a
cAMP-dependent chloride (Cl-) anion conducting channel (3). CFTR is found in epithelial cells of
human tissues, including the female reproductive tract (4). By controlling ion and protein
transport, CFTR is thought to function in most human cells to
assist in the maintenance of cell homeostasis (5). CFTR belongs to the ATP-binding
cassette (ABC) transporter family, members of which utilize ATPase
activity to transport substrates across cell membranes and which
are involved in various types of cancer (6). The CFTR gene mutation, the
major of which is the deletion of phenylalanine at position 508,
may lead to dysfunction at the plasma membrane (7). Such mutations were associated with an
increased risk of digestive tract cancers (8), for example, pancreatic cancer
(9). However, it has been
demonstrated that, CFTR mutation plays a protective role for the
risk of prostate cancer (10),
malignant melanoma (7) and lung
cancer (11).
In the absence of the CFTR gene mutation,
CFTR plays a tumor-suppressing role in regulation of prostate
cancer development through miR-193b targeting urokinase plasminogen
activator (uPA) (12). Another
study proposed that CFTR expression was significantly downregulated
in breast cancer, which promotes epithelial-to-mesenchymal
transition and is associated with poor prognosis (13). CFTR is also known to be involved in
modulating signaling pathways in cell inflammation and apoptosis
(14,15). Although previous studies suggested a
role for CFTR in various types of cancer, the relationship between
CFTR and ovarian cancer remains to be determined.
Therefore, in the present study, to identify the
potential role of CFTR in the pathogenesis of ovarian cancer, CFTR
expression was evaluated in human epithelial ovarian cancer, benign
epithelial ovarian tumor and normal ovarian tissues using
immunohistochemical staining. The role of CFTR in the malignancy of
ovarian cancer was observed in CFTR-RNAi SKOV3 and A2780 cells
in vitro and in vivo. The present study demonstrated
that CFTR expression of ovarian cancer, which was associated with
clinical features, was significantly higher than that in benign
ovarian tumor and normal ovaries. CFTR knockdown suppressed the
malignant behavior of ovarian cancer cells, including cell
invasion, motility and proliferation.
Materials and methods
Patients and specimens
There were 112 paraffin-embedded tissue samples,
including 83 epithelial ovarian cancer, 18 benign epithelial tumor
and 11 normal ovarian tissues (resected for non-ovarian diseases)
that were collected from the Department of Pathology, the Second
Affiliated Hospital of Chongqing Medical University between 2010
and 2013. All the ovarian cancer patients underwent surgical
exploration and cytoreduction as the initial treatment. The
clinicopathological characteristics are presented in Table I. The patients were staged according
to the International Federation of Gynecology and obstetrics
(FIGO). The tissue blocks were re-evaluated by two senior
pathologists for histological type and histopathologic grading.
Tissue samples were obtained following informed consent by the
patients. The study protocol was approved by the Ethics Committee
of the Second Affiliated Hospital of Chongqing Medical
University.
 | Table IAssociation of CFTR expression with
clinicopathological characteristics in 83 cases of human epithelial
ovarian cancer. |
Table I
Association of CFTR expression with
clinicopathological characteristics in 83 cases of human epithelial
ovarian cancer.
| Characteristics | No. of pts
(n=83) | CFTR expression
| P-value |
|---|
Low no.
(%) | High no.
(%) |
|---|
| Age (years) |
| <51.0 | 47 | 16 (34) | 31 (66) | 0.654 |
| >51.0 | 36 | 14 (39) | 22 (61) | |
| Serum Ca-125 level
(U/ml) |
| <35 | 17 | 11 (65) | 6 (35) | 0.010a |
| >35 | 66 | 19 (29) | 47 (71) | |
| FIGO stage |
| I/II | 31 | 18 (58) | 13 (42) | 0.002a |
| III/IV | 52 | 12 (23) | 40 (77) | |
| Histological
grade |
| 1 | 18 | 14 (78) | 4 (22) | 0.000a |
| 2 | 29 | 9 (31) | 20 (69) | |
| 3 | 36 | 7 (19) | 29 (81) | |
| Grades 2–3 vs.
1 |
| Ascites (ml) | | | | |
| <100 | 15 | 7 (47) | 8 (53) | 0.384 |
| >100 | 68 | 23 (34) | 45 (66) | |
| Tumor type |
| Serous | 54 | 12 (22) | 42 (78) | |
| Mucinous | 8 | 6 (75) | 2 (25) | 0.006a |
| Clear cell | 14 | 4 (29) | 10 (71) | 0.726 |
| Endometrioid
compared with serous type | 7 | 5 (71) | 2 (29) | 0.015a |
| Tumor size
(cm) |
| <5 | 13 | 2 (15) | 11 (85) | 0.121 |
| >5 | 70 | 28 (40) | 42 (60) | |
| Serum HE4 level
(pm) |
| <70 | 22 | 11 (50) | 11 (50) | |
| >70 | 61 | 19 (31) | 42 (69) | 0.129 |
Immunohistochemistry
The tissue slides were deparaffinized in xylene and
rehydrated through graded ethanol. Antigen retrieval was performed
in 10 mmol/l boiling sodium citrate buffer at pH 6.0 for 15 min by
microwave irradiation. The slides were then incubated with 3%
hydrogen peroxide (H2O2) for 10 min at room
temperature. After rinsing, the non-specific binding site was
blocked with 10% normal goat serum for 15 min at room temperature.
A mouse monoclonal anti-human CFTR antibody (diluted 1:200, ab2784;
Abcam, Cambridge, Uk) was applied to slides in a moist chamber at
4°C overnight. After washing with phosphate-buffered saline (PBS),
the slides were incubated with biotinylated secondary antibody for
15 min at room temperature (diluted 1:1,000, cat. no. SP-9002;
Zhongshan Golden Bridge Inc., China). The slides were treated with
ABC reagent for 15 min at room temperature, and stained with
3,3′-diaminobenzidine (DAB), followed by counterstaining with
hematoxylin.
Cells with brown staining for the membrane and/or
cytoplasm were considered positive. A semi-quantitative scoring
system was applied to assess protein level based on intensity (0–3:
0, absence of staining; 1, weakly stained; 2, moderately stained;
and 3, strongly stained) and percentage of positive tumor cells
(0–3: absence of positive cells; 1, 0.1–33% of cells positive; 2,
33.1–66% of cells positive; 3, >66.1% of cells positive) and
generated a score ranging from 0 to 9 when multiplied (16). Low expression was defined as a
staining score of 0–4, and high expression was defined as a
staining score of 5–9.
Cell lines and culture conditions
The human A2780 and SKOV3 epithelial ovarian cancer
cell lines were obtained from the Laboratory of Obsterics and
Gynecology, the Second Affiliated Hospital of Chongqing Medical
University. The cells were cultured in Dulbecco’s modified Eagle’s
medium supplemented with 10% fetal bovine serum and antibiotics
(100 U/ml penicillin and 100 μg/ml streptomycin) (all from
Invitrogen, Carlsbad, CA, USA). The cells were cultured in an
incubator with 5% CO2 at 37°C.
Western blotting
Total proteins were extracted using lysis buffer
(Beyotime, Jiangsu, China) consisting of 50 mM Tris (pH 7.4), 1%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS and a protease
inhibitor mixture supplemented with 1 mM phenylmethanesulfonyl
fluoride (PMSF) according to the manufacturer’s instructions. The
protein concentration was measured using a BCA protein assay kit
(Beyotime). Total proteins (100 μg) of each sample were
separated on 8% SDS-PAGE gel by electrophoresis and transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore Corporation,
Billerica, MA, USA). The membranes were then blocked in 5% skim
milk at 37°C for 1 h. Subsequently, the membranes were incubated,
respectively, in mouse monoclonal anti-human CFTR antibody (diluted
1:1,000, ab2784) and rabbit monoclonal anti-β-actin antibody
(diluted 1:1,000, ab133626) (both from Abcam) at 4°C overnight.
After washing with TBST, the membranes were incubated with
HRP-conjugated secondary antibodies for 1 h at room temperature.
Proteins were visualized with the ECL system (GE Healthcare,
Pittsburgh, PA, USA) using the ChemiDoc XRS system (Bio-Rad,
Philadelphia, PA, USA).
Gene knockdown
To knockdown CFTR in the ovarian cells, four human
CFTR-specific small hairpin RNA (shRNA) expression vectors in
pGFP-V-RS plasmid were purchased from OriGene (TG313958; OriGene
Technologies, Inc., Rockville, MD, USA). Cells transfected with
non-effective shRNA cassette in pGFP-V-RS plasmid were used as a
negative control. Vector DNA (1 μg) was transfected into
A2780 and SKOV3 cells by PolyJet™ transfection reagent (SignaGen
Laboratories, Ijamsville, MD, USA). At two days after transfection,
2.5×106 cells were digested and replanted into five
10-cm dishes in full medium containing puromycin at concentration
of 0.9 μg/ml for A2780 and 0.5 μg/ml for SKOV3 cells,
and selected for 2–3 weeks. Puromycin-resistant and GFP-positive
cell colonies were separated and verified by western blotting. The
stably transfected cell lines were then cultured in medium
containing 0.5 μg/ml of puromycin for A2780 and 0.25
μg/ml for SKOV3 cells for subsequent studies.
Cell invasion and migration assays
For cell invasion and migration assays, a cell
invasion assay kit (8-μm pore size; Cell Biolabs Inc., San
Diego, CA, USA) was used according to the manufacturer’s
instructions. Approximately 1×105 cells were placed into
the Transwell insert with serum-free media. After 24–48 h, the
cells on the inner layer of the Transwell insert were wiped away,
then inserts were fixed and stained, and photographed with a light
microscope under high magnification objective. Each insert was then
transferred to an empty well, and extracted with extraction
solution for 10 min on an orbital shaker. Extraction solution (100
μl) from each sample was transferred to a 96-well microtiter
plate and OD 560 nm was measured using a microplate reader (Thermo
Scientific, waltham, MA, USA). The procedure was repeated
independently three times with triplicate inserts for each
group.
Plate colony formation assay
We seeded 200 cells into 6-well culture plates in
triplicate. The cells were collected after 14 days and then stained
with Giemsa stain. The colony containing >50 cells was counted
under the microscope.
Cell adhesion assay
We pre-coated 96-well plates with 100 μl
Matrigel at concentration of 0.04 μg/ml (BD Matrigel,
Franklin Lakes, NJ, USA). Prior to cell seeding, the plates were
incubated in serum-free medium at 37°C for 30 min for rehydration.
Cells (3×104) were seeded in 100 μl growth medium
in triplicate and incubated for 1 h. After rinsing, the adherent
cells were fixed using 4% formaldehyde for 10 min, and stained by
crystal violet. Adherent cell staining was extracted by acetic acid
and quantified using a microplate reader (Thermo Scientific) at OD
490 nm.
Cell proliferation assay
For the proliferation assay, 500 cells were seeded
in 96-well plates in triplicate and cultured for days 1, 2, 3, 4
and 5. The ST-8 Cell Counting Kit (KeyGen Biotech, Nanjing, China)
and a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay method, were applied to estimate the
proliferation of viable cells. The cells were collected at specific
time-points (6, 24, 48, 72, 96 and 120 h) and incubated for an
additional 2 h in 10% WST-8 solution at 37°C. Absorbance was
measured at 450 nm using a microplate reader (Thermo Scientific).
The procedure was repeated independently three times.
Tumor formation in nude mice
To investigate tumorigenicity, 5-week-old athymic
female nude mice were provided by the Institution Animal Care
Committee at Chongqing Medical University. Approximately
4×106 cells in 100 μl suspension of SKOV3-CFTRi
cells (7 implants) with PBS and SKOV3-NC cells (cells transfected
with non-effective shRNA cassette) as a control (7 implants) were
injected subcutaneously. The tumor size was measured on a weekly
basis and monitored over 5 weeks. At the end of the experiment, the
mice were euthanized and the xenografts collected for subsequent
analysis. The tumor size was calculated as: 0.5234 × [long diameter
(short diameter)2]. The experimental procedure was
approved by the Institution Animal Care Committee at Chongqing
Medical University.
Statistics analysis
Independent Student’s t-test was used to make a
comparison of the data between two groups. Relevance analysis
between CFTR immunostaining and clinicopathological characteristics
was performed by Pearson’s χ2 or Fisher’s exact tests.
Statistical analysis was carried out by SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 (two-sided) was considered to
indicate a statistically significant result.
Results
CFTR exhibits a high expression in
ovarian cancer and is correlated with clinicopathological
characteristics
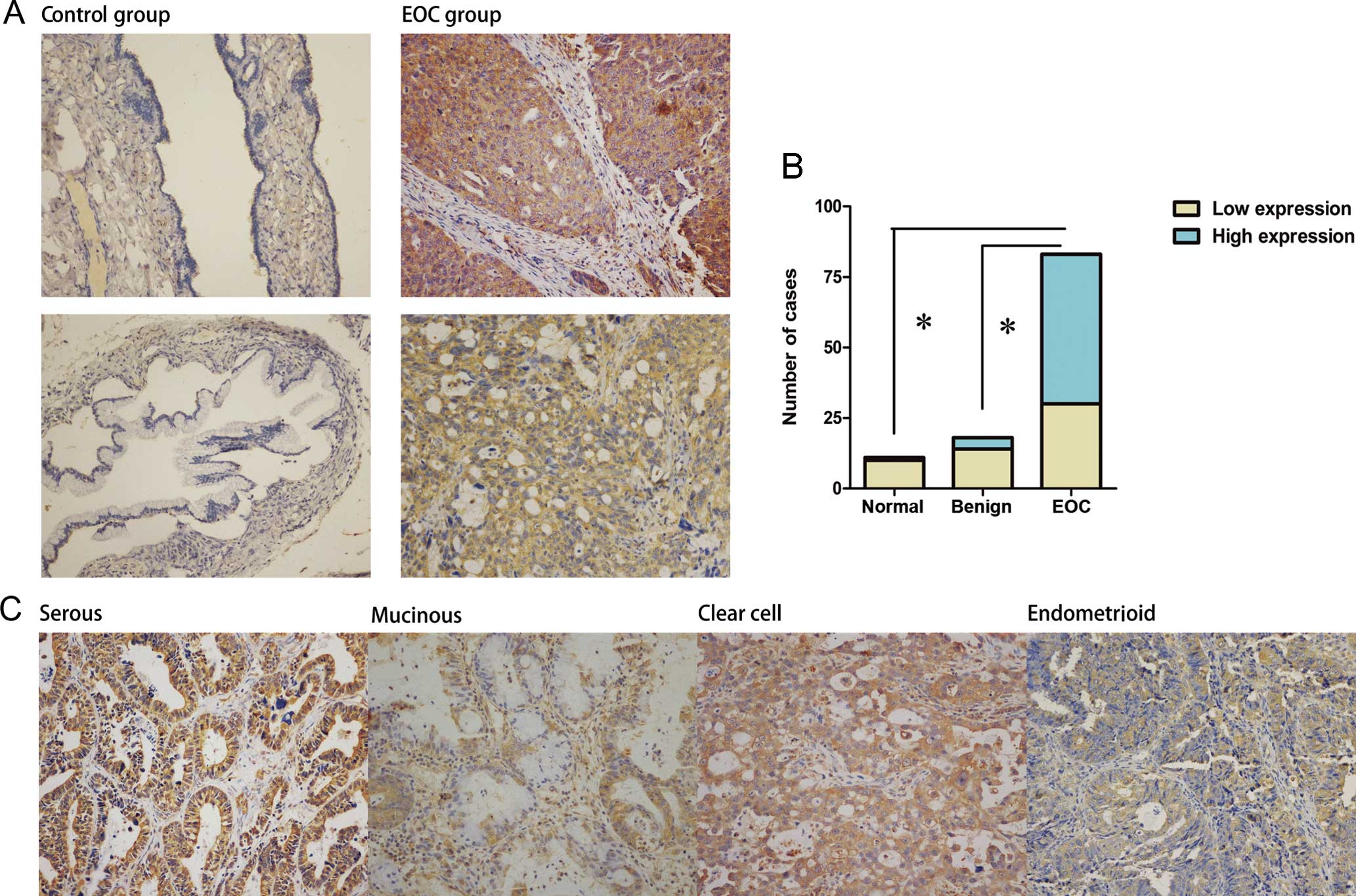
CFTR staining was predominantly detected in the cell
membrane and cytoplasm (Fig. 1A).
The expression of CFTR in ovarian cancer specimens was
significantly higher than that in benign (P<0.05) and normal
ovaries (P<0.05, Fig. 1B).
Moreover, in a majority of benign tumors (77.8%) and normal ovaries
(90.1%), it was difficult to detect CFTR, which was in contrast to
the cancer group (Fig. 1B).
Regarding the clinicopathological characteristics, the statistical
analysis revealed that the CFTR protein level was well-related to
advanced clinical stages (stage III/IV vs. I/II, P<0.05), poor
histological grade (grade 2–3 vs. 1, p<0.05), and a higher serum
Ca-125 level (P<0.05, Table I).
Furthermore, we observed that CFTR staining was stronger in the
serous type and clear cell type as compared to the remaining types
(P<0.05, Table I).
Silencing of CFTR expression by RNA
interference
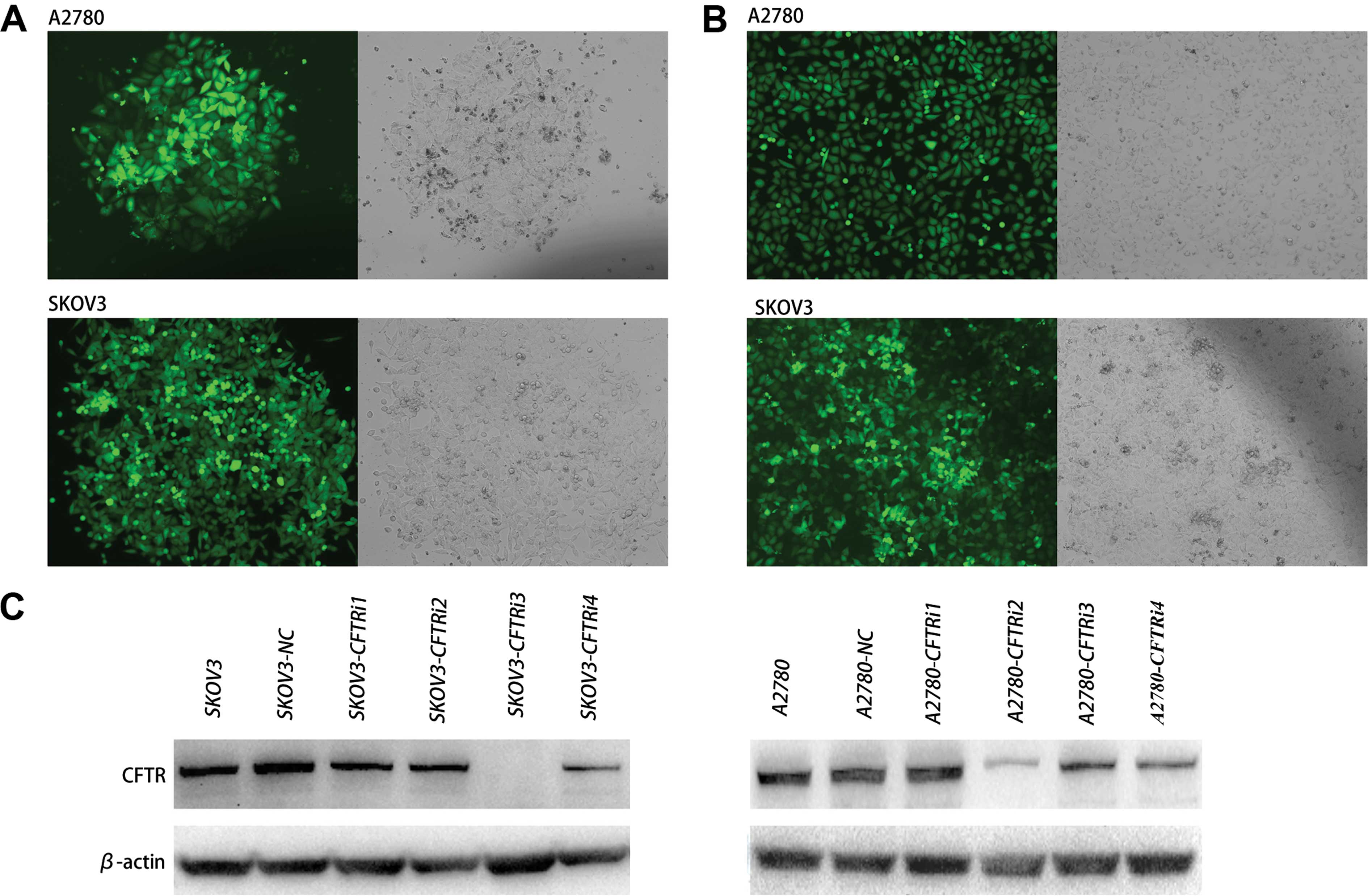
To examine the role of CFTR in serous ovarian cancer
cells, we knocked down CFTR in SKOV3 and A2780 cells. The cells
were transfected with CFTR-specific shRNA expression pGFP-V-RS
vectors and the stably transfected cells were selected (referred to
as CFTRi in Fig. 2A and B).
Non-effective shRNA-infected cells were designated as a negative
control, referred to as SKOV3-NC and A2780-NC. Western blot results
revealed that the CFTR protein level of CFTRi cells was
significantly reduced compared with that of its corresponding
control cells (Fig. 2C). Thus,
SKOV3-CFTRi3 and A2780-CFTRi2 were used in the subsequent
experiments.
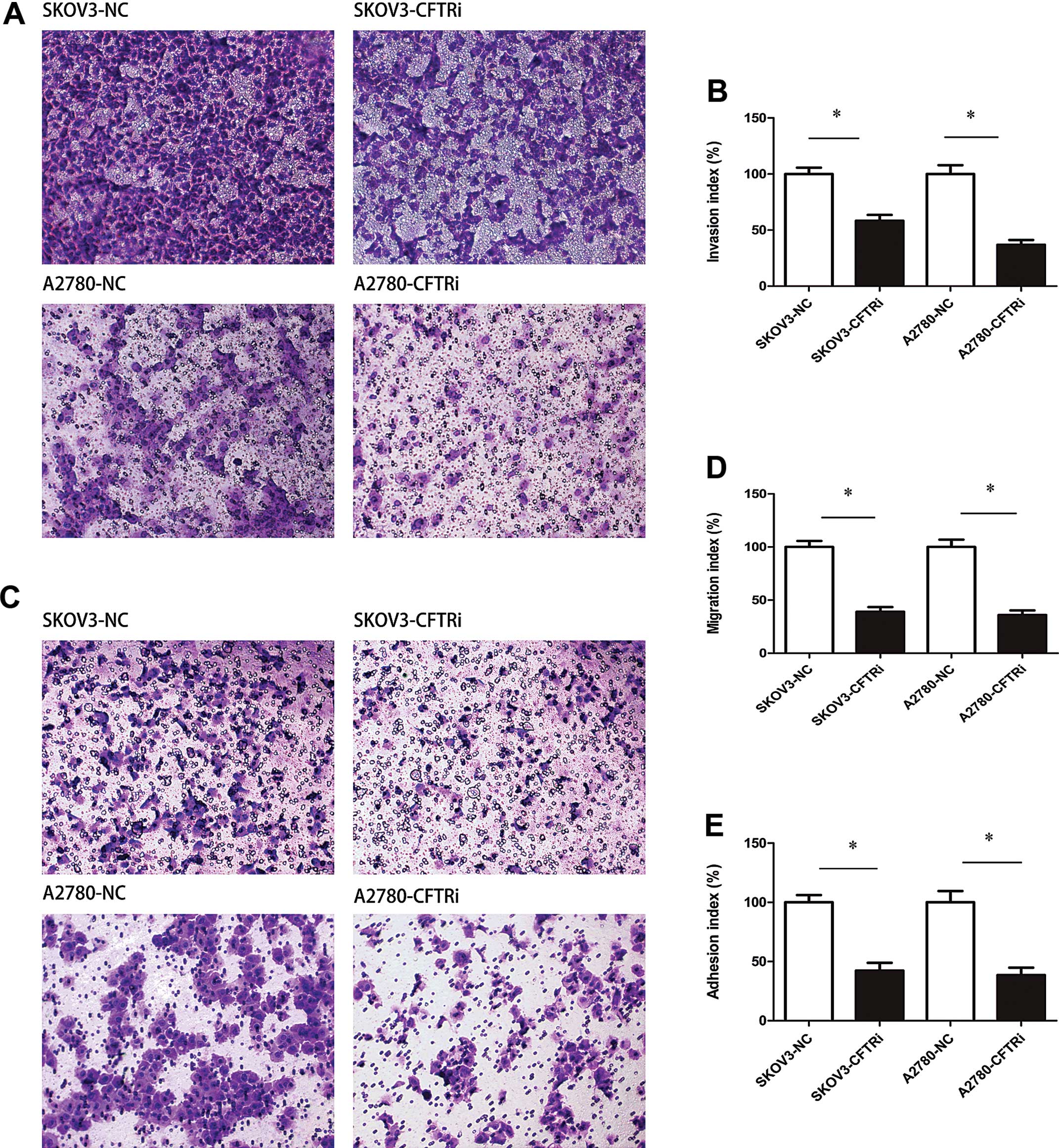
Knockdown of CFTR inhibits cell motility
and invasion in vitro
SKOV3-CFTRi3 cells exhibited an impaired migration
capacity of 60.89% compared to SKOV3-NC cells (P<0.05) (Fig. 3A and B). Similarly, A2780-CFTRi2
cells had a reduced migration capacity of 63.93% compared to
A2780-NC cells (P<0.05). For SKOV3-CFTRi3 and A2780-CFTRi2, an
~41.72 and 63.11% reduction in the number of migratory cells was
observed compared with SKOV3-NC and A2780-NC cells (P<0.05 for
both, Fig. 3C and D).
Knockdown of CFTR inhibits cell adhesion
in vitro
As shown in Fig. 3E,
there was a 42.31 and 38.52% decreased percentage of optical
density (OD) value in SKOV3-CFTRi3 and A2780-CFTRi2 cells compared
to the control cells (P<0.05 for both).
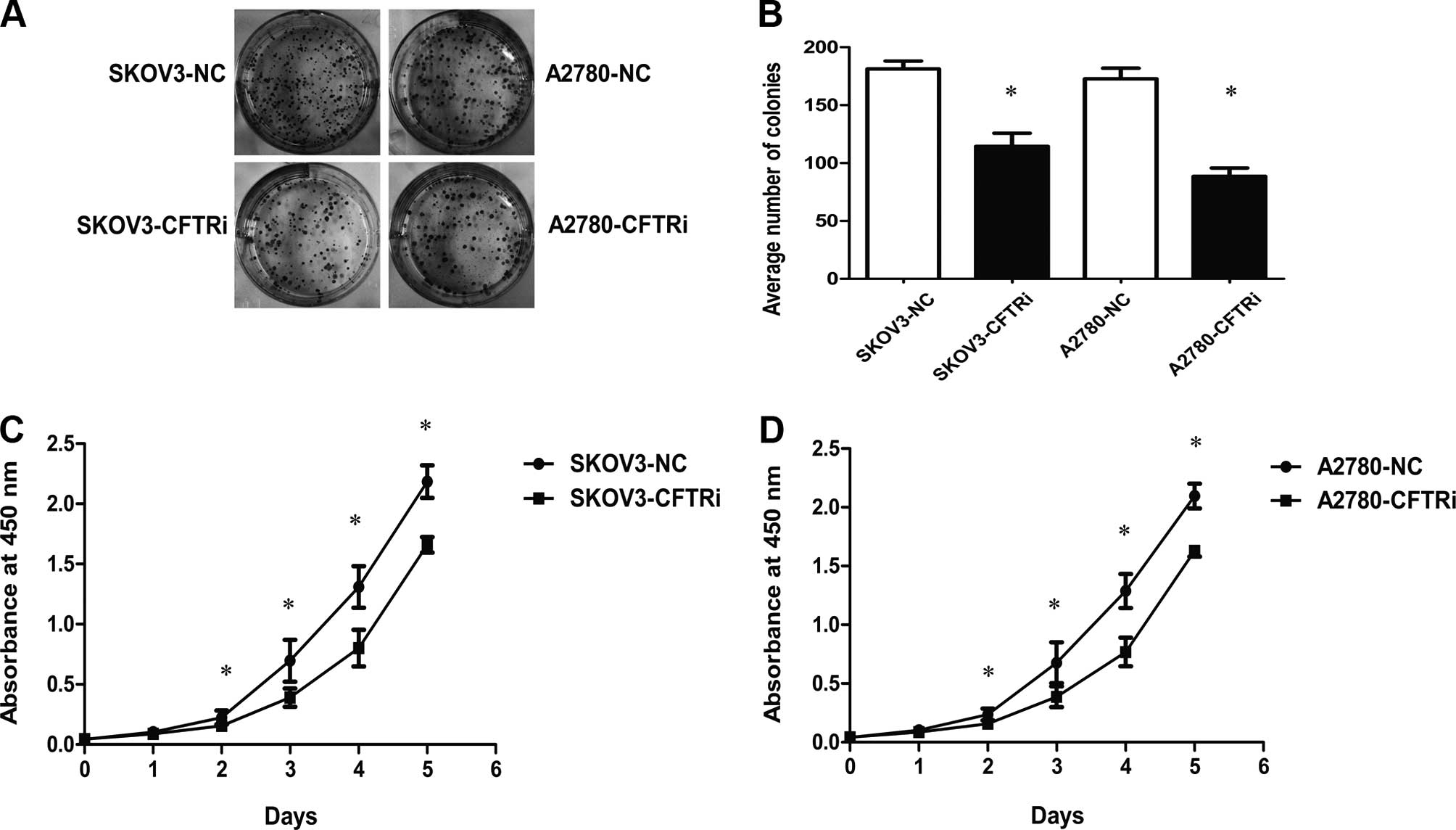
Knockdown of CFTR inhibits proliferation
and colony formation in vitro
The results of the WST-8 assay (Fig. 4C and D) showed that the growth
ability of SKOV3-CFTRi3 and A2780-CFTRi2 cells decreased compared
with the corresponding control cells. Moreover, CFTR knockdown
cells also had a low colony formation ability as the number of
colonies of CFTRi cells were decreased when compared with the
corresponding control cells (Fig. 4A
and B).
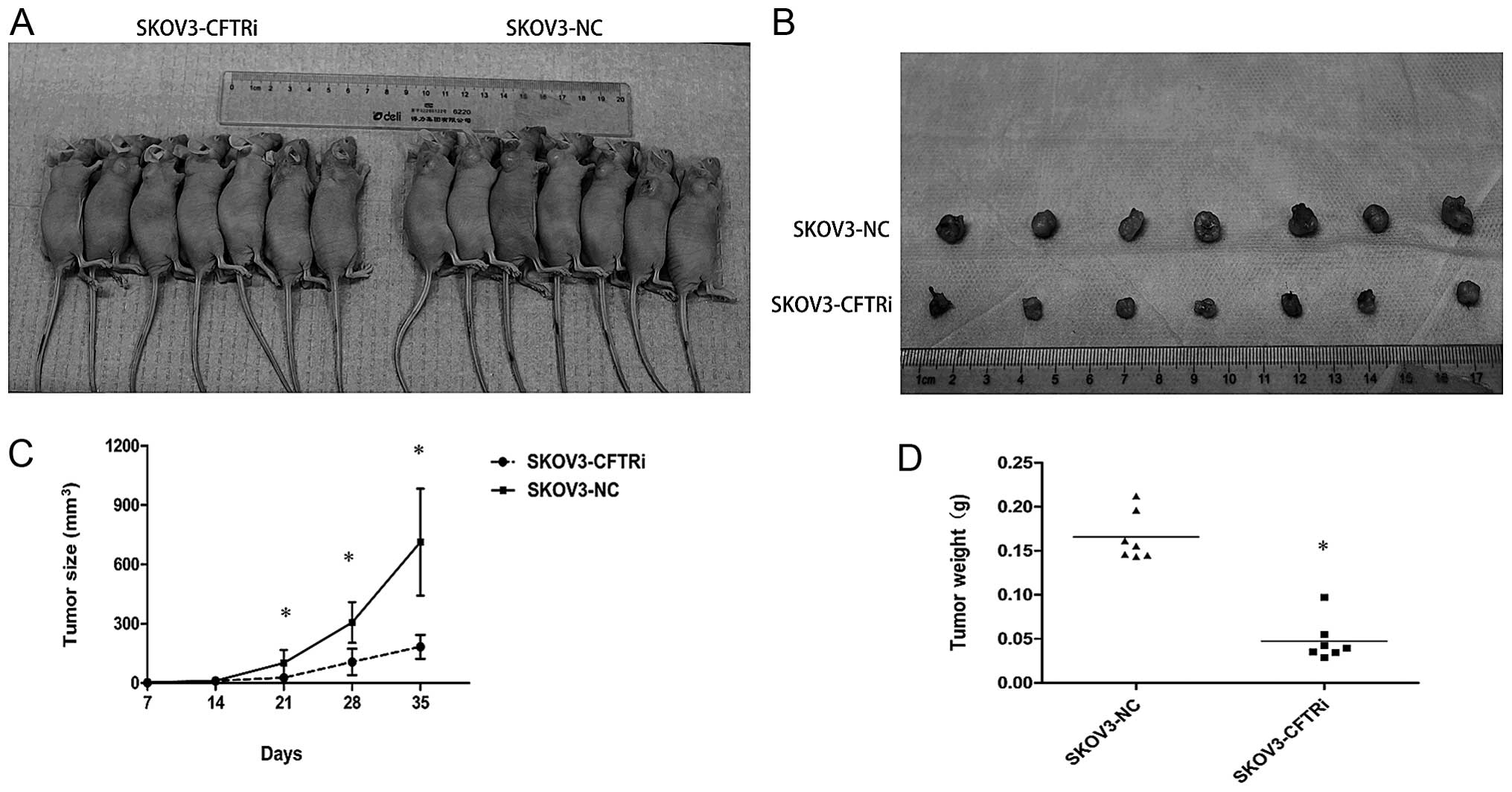
CFTR knockdown inhibits xenograft tumor
formation
Cells were subcutaneously injected into the flanks
to form xenograft tumors in nude mice. We measured the size of
xenograft tumors once a week for a total of five weeks. We observed
that the tumor size of the SKOV3-CFTRi3 group was statistically
significantly smaller than that of the SKOV3-NC group from the
third week until the end of the experiment (Fig. 5A). The size of xenograft tumors of
SKOV3-CFTRi3 (183±61 cm3) was significantly smaller than
that of the control (713±270 cm3) at the end of
experiment (Fig. 5B and C). At the
same time, the average tumor weight of SKOV3-CFTRi3 group was lower
than that of the control group (Fig.
5D). Notably, these results showed that CFTR knockdown affected
the tumor size significantly rather than the incidence of tumor
formation, suggesting that CFTR knockdown suppresses tumor
progression rather than tumor initiation in vivo.
Discussion
CFTR is a membrane of the ATP-binding cassette (ABC)
transporter family, which is an ancient family of transmembrane
proteins. The ABCC subfamily of ABC transporters includes CFTR and
the MRPs, which were active drug exporters (6). In this study, we initially
investigated the expression and localization of CFTR in ovarian
cancer, benign tumors and normal ovaries by immunohistochemical
analysis. The results showed that CFTR expression was significantly
increased in ovarian cancer compared with the other tissue types.
Moreover, we focused on the association between the CFTR and
clinicopathological characteristics to determine the potential
clinical significance in ovarian cancer. Of those characteristics,
the high expression of CFTR was well-associated with poor clinical
stage, advanced histological grade and an increased Ca-125 level.
These results indicated that the expression of CFTR had a positive
correlation with the progression state and malignancy degree of
ovarian cancer, suggesting that CFTR is a potential biomarker in
progression of ovarian cancer. In subsequent experiments, we
investigated the expression of CFTR in metastasis outside the
pelvis or retroperitoneal or inguinal node of patients, and
measured the conditions of prognosis and survival data of patients
to evaluate the prognostic value of CFTR. Notably, we found that
CFTR expression was significantly higher in the serous carcinoma
and clear cell carcinoma as compared to the remaining carcinomas
used in our study. Serous ovarian cancer is the most common
histotype and accounts for more than half of epithelial ovarian
cancer patients (17). Therefore,
we selected the serous ovarian cancer cell lines for further
study.
In our experiments, the expression of CFTR in SKOV3
and A2780 cells was knocked down by shRNA-mediated gene silencing.
To enhance the efficiency of transfection, we generated and
isolated stable transfectants proved important in the investigation
of the function of CFTR in malignant behaviors in vivo. It
was observed that CFTR-RNAi cells showed decreased cell motility,
invasion, adhersion, colony formation, and proliferation,
particularly migration and invasion. We have demonstrated the
effect of CFTR knockdown on the inhibition of malignant behaviors,
indicating that CFTR is a potential therapeutic target to ovarian
cancer treatment.
We also investigated the function of CFTR in
malignant behaviors in vivo. The results revealed xenograft
tumors existing in CFTR expressed larger tumor size and rapid tumor
growth. The present study has therefore demonstrated that
inhibition of CFTR suppressed xenograft tumor development in
vivo.
Accumulating evidence suggests that CFTR plays a key
role in the progression and metastasis of cancer. Genetic
variations in CFTR may be associated with increased or decreased
risk for developing cancers, suggesting that CFTR is a tumor
suppressor or promoter, according to the type of tumor (7,9–11),
MUC4, an important protein suppressing the progression and
metastasis of pancreatic cancer cells, has proven to be
downregulated by CFTR (18). In the
female reproductive system, it has been reported that the
overexpression of CFTR in cervical cancer is concerned with cancer
development, aggressive biological behaviors and poor prognosis of
patients (19).
Previous studies have showed that CFTR binds to
ezrin-radixin-moesin-binding (ERM) phosphoprotein 50 (EBP50), an
apical membrane PDZ domain-containing protein. EBP50 is essential
in the apical polarization of CFTR in epithelial cells (20) and is known to interact via its
C-terminal domain with the ERM-family proteins, which in turn bind
to the actin cytoskeleton (21). We
hypothesized that the CFTR affects the cell adhersion, invasion and
migration abilities due to connecting membrane rafts to the actin
cytoskeleton.
However, EBP50 is identified as a specific
Cbp-binding partner, and Cbp binds the protein tyrosine kinase Csk
(21). The Cbp-Csk complex
suppresses the activity of membrane-associated Src-family kinases
which play an important role in the regulation of essential cell
functions such as growth and receptor signaling (22–24).
Therefore, we suggest that the overexpression of CFTR in ovarian
cancer progression may be associated with the activation of c-src
signaling pathway. Thus, further investigation is needed to
identify the mechanisms of CFTR in ovarian cancer development and
progression.
In conclusion, to the best of our knowledge, our
data have provided clinical and laboratorial evidence for the first
time that CFTR expression is well correlated with ovarian cancer
progression and aggressive behaviors, indicating CFTR may be a
novel tumor marker for ovarian cancer, particularly for serous
carcinoma. However, a greater number of cases should be included to
make the prospective studies valid. Nevertheless, our result
supports the important role of CFTR in ovarian cancer.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81172492), the Key Project
of Chongqing Science and Technology Commission (grant no. CSTC
2012JJB10030), and the Key Project of Chongqing Municipal Health
Bureau (grant no. 2011-1-056).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rauh-Hain JA, Krivak TC, Del Carmen MG and
Olawaiye AB: Ovarian cancer screening and early detection in the
general population. Rev Obstet Gynecol. 4:15–21. 2011.PubMed/NCBI
|
|
3
|
Bear CE, Li CH, Kartner N, et al:
Purification and functional reconstitution of the cystic fibrosis
transmembrane conductance regulator (CFTR). Cell. 68:809–818. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tizzano EF, Silver MM, Chitayat D,
Benichou JC and Buchwald M: Differential cellular expression of
cystic fibrosis transmembrane regulator in human reproductive
tissues. Clues for the infertility in patients with cystic
fibrosis. Am J Pathol. 144:906–914. 1994.PubMed/NCBI
|
|
5
|
Schwiebert EM, Benos DJ, Egan ME, Stutts
MJ and Guggino WB: CFTR is a conductance regulator as well as a
chloride channel. Physiol Rev. 79(Suppl 1): S145–S166.
1999.PubMed/NCBI
|
|
6
|
Higgins CF: ABC transporters: from
microorganisms to man. Annu Rev Cell Biol. 8:67–113. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Padua RA, Warren N, Grimshaw D, et al: The
cystic fibrosis ΔF508 gene mutation and cancer. Hum Mutat.
10:45–48. 1997. View Article : Google Scholar
|
|
8
|
Neglia JP, FitzSimmons SC, Maisonneuve P,
et al: The risk of cancer among patients with cystic fibrosis.
Cystic Fibrosis and Cancer Study Group. N Engl J Med. 332:494–499.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mcwilliams RR, Petersen GM, Rabe KG, et
al: Cystic fibrosis transmembrane conductance regulator (CFTR) gene
mutations and risk for pancreatic adenocarcinoma. Cancer.
116:203–209. 2010.
|
|
10
|
Qiao D, Yi L, Hua L, et al: Cystic
fibrosis transmembrane conductance regulator (CFTR) gene 5T allele
may protect against prostate cancer: a case-control study in
Chinese Han population. J Cyst Fibros. 7:210–214. 2008. View Article : Google Scholar
|
|
11
|
Li Y, Sun Z, Wu Y, et al: Cystic fibrosis
transmembrane conductance regulator gene mutation and lung cancer
risk. Lung Cancer. 70:14–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie C, Jiang XH, Zhang JT, et al: CFTR
suppresses tumor progression through miR-193b targeting urokinase
plasminogen activator (uPA) in prostate cancer. Oncogene.
32:2282–2291. 2291.e1–2291.e7. 2013. View Article : Google Scholar
|
|
13
|
Zhang JT, Jiang XH, Xie C, et al:
Downregulation of CFTR promotes epithelial-to-mesenchymal
transition and is associated with poor prognosis of breast cancer.
Biochim Biophys Acta. 1833:2961–2969. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Z, Peng X, Li J, Zhang Y and Hu L:
Constitutive activation of nuclear factor κB contributes to cystic
fibrosis transmembrane conductance regulator expression and
promotes human cervical cancer progression and poor prognosis. Int
J Gynecol Cancer. 23:906–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jacquot J, Tabary O, Le Rouzic P and
Clement A: Airway epithelial cell inflammatory signalling in cystic
fibrosis. Int J Biochem Cell Biol. 40:1703–1715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Au CW, Siu MK, Liao X, et al: Tyrosine
kinase B receptor and BDNF expression in ovarian cancers - effect
on cell migration, angiogenesis and clinical outcome. Cancer Lett.
281:151–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Dai JM, Che YL, et al: Elmo1 helps
dock180 to regulate Rac1 activity and cell migration of ovarian
cancer. Int J Gynecol Cancer. 24:844–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh AP, Chauhan SC, Andrianifahanana M,
et al: MUC4 expression is regulated by cystic fibrosis
transmembrane conductance regulator in pancreatic adenocarcinoma
cells via transcriptional and post-translational mechanisms.
Oncogene. 26:30–41. 2007. View Article : Google Scholar
|
|
19
|
Peng X, Wu Z, Yu L, et al: Overexpression
of cystic fibrosis transmembrane conductance regulator (CFTR) is
associated with human cervical cancer malignancy, progression and
prognosis. Gynecol Oncol. 125:470–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moyer BD, Denton J, Karlson KH, et al: A
PDZ-interacting domain in CFTR is an apical membrane polarization
signal. J Clin Invest. 104:1353–1361. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brdicková N, Brdicka T, Andera L, et al:
Interaction between two adapter proteins, PAG and EBP50: a possible
link between membrane rafts and actin cytoskeleton. FEBS Lett.
507:133–136. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wiener JR, Windham TC, Estrella VC, et al:
Activated Src protein tyrosine kinase is overexpressed in
late-stage human ovarian cancers. Gynecol Oncol. 88:73–79. 2003.
View Article : Google Scholar
|
|
23
|
Wiener JR, Nakano K, Kruzelock RP, Bucana
CD, Bast RC Jr and Gallick GE: Decreased Src tyrosine kinase
activity inhibits malignant human ovarian cancer tumor growth in a
nude mouse model. Clin Cancer Res. 5:2164–2170. 1999.PubMed/NCBI
|
|
24
|
Kim HS, Han HD, Armaiz-Pena GN, et al:
Functional roles of Src and Fgr in ovarian carcinoma. Clin Cancer
Res. 17:1713–1721. 2011. View Article : Google Scholar : PubMed/NCBI
|