Introduction
Nasopharyngeal carcinoma (NPC) is a tumor arising
from the epithelial cells in the nasopharynx. Although NPC is rare
in Western countries, its incidence remains high in Southern China
and Southeast Asia (1). Although
the etiology of NPC remains unclear, many factors such as
Epstein-Barr virus infection, environmental factors and genetic
alterations are associated with the development of NPC (2–5). More
importantly, patients with early-stage NPC are difficult to
diagnose and without intervention, NPC can easily metastasize into
local lymph nodes, leading to poor prognosis. Currently, patients
with NPC can be treated with chemoradiotherapy and sequential
radiotherapy and chemotherapy (1).
However, the efficacy of these therapeutic strategies is limited by
their undesirable side effects, local recurrence and distant
metastasis (6). Therefore,
understanding the molecular mechanisms underlying the metastasis of
NPC may reveal new targets for the design of new therapies for
patients with NPC (7).
Forkhead box M1 (FoxM1) is a member of the forkhead
family of transcription factors and is ubiquitously expressed in
proliferating and regenerating mammalian cells (8,9). FoxM1
is a key regulator of both G1-S and G2-M phases of the cell cycle
and mitotic spindle integrity (10). High levels of FoxM1 expression are
associated with the development of various cancers, including
hepatocellular, prostate, lung, glioma, cervical and gastric
cancers (11–15). Furthermore, FoxM1 overexpression can
promote epithelial-to-mesenchymal transition (EMT) and enhance the
invasion and migration of several types of cancers (16–19).
Hence, FoxM1 may be a potential therapeutic target for the
development of anticancer treatments (20–22).
However, little is known concerning the role of FoxM1 expression in
the proliferation, migration and invasion of NPC cells.
In the present study, we examined the impact of
treatment with FoxM1 inhibitor or FoxM1 silencing on the survival,
anchorage-independent proliferation, migration and invasion of
human NPC cells in vitro and in vivo. Furthermore, we
determined the potential effects of treatment with a FoxM1
inhibitor or FoxM1 silencing on the EMT process in NPC cells. Our
findings indicate that FoxM1 positively regulates the
proliferation, migration and invasion of NPC by enhancing the EMT
process.
Materials and methods
Cell lines and reagents
Human nasopharyngeal cancer cell lines, CNE-1 and
CNE-2, purchased from the Chinese Academy of Sciences Cell Bank
(Shanghai, China) were maintained in RPMI-1640 medium supplemented
with 10% newborn calf serum (NBCS), 100 U/ml penicillin and 100
μg/ml streptomycin at 37°C in a humidified atmosphere of 5%
CO2 and 95% air. The 293T cell line was obtained from
Invitrogen (Carlsbad, CA, USA) and cultured in Dulbecco’s modified
Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS;
Invitrogen), 100 U/ml penicillin and 100 μg/ml
streptomycin.
Thiostrepton is a cycling polypeptide antibiotic
with selective inhibitory activity against FoxM1 (23) and was purchased from Tocris Cookson
(Ellisville, MO, USA). Antibodies against FoxM1, E-cadherin, zinc
finger E-box binding homeobox 2 (ZEB2), zinc finger protein Snail2
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were all
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Growth inhibition assay
The impact of inhibition of FoxM1 on the
proliferation of NPC cells was determined by a growth inhibition
assay using the Cell Counting Kit-8 (CCK-8; Beyotime, China),
according to the manufacturer’s instructions. Briefly, CNE-1 and
CNE-2 cells at a density of 3,000 cells/well were cultured in
96-well plates overnight and were treated in pentaplicate with
thiostrepton at various concentrations (0, 1, 2, 4, 8 and 16
μmol/l) for 48 or 72 h. During the last 2 h of culture, the
cells were exposed to a water-soluble tetrazolium salt of WST-8 (10
μl/well), and the levels of the resulting WST-8 formazan in
individual wells were measured at 450 nm using a microplate reader
(Biotek, Winooski, VT, USA).
RNA isolation, reverse transcription and
qRT-PCR
Total RNA was extracted from individual groups of
NPC cell lines using TRIzol (Takara, Shiga, Japan), and the
resulting RNA (5 μg/sample) was reversely transcribed into
cDNA using the First Strand cDNA kit (Bio-Rad, Hercules, CA, USA),
according to the manufacturer’s instructions. The relative levels
of target gene mRNA transcripts to control GAPDH in individual
samples were determined using the specific primers and SYBR-Green
system according to the manufacturer’s instructions (Takara). The
sequences of primers were: sense, 5′-GCT TGC CAG AGT CCT TTT TGC-3′
and antisense, 5′-CCA CCT GAG TTC TCG TCA ATG C-3′ for human FoxM1
(123 bp); sense, 5′-CGG GAA TGC AGT TGA GGA TC-3′ and antisense,
5′-AGG ATG GTG TAAGCG ATG GC-3′ for human E-cadherin (154 bp);
sense, 5′-GAT GCC GCG CTC CTTCCT GG-3′ and antisense, 5′-GGG GGA
CTC ACT CGC CCC AA-3′ for human Snail2 (168 bp); sense, 5′-TGC CAC
CAG TCC AGA CCA GTA T-3′ and antisense, 5′-GCT CCA GTT GGG TAG GTG
TAG G-3′ for human ZEB2 (173 bp); sense, 5′-GAA GGT CGG AGT CAA CGG
ATT T-3′ and antisense, 5′-CGC TCC TGG AAG ATG GTG A-3′ for human
GAPDH (119 bp). The PCR amplification was performed in triplicate
at 94°C for 3 min and subjected to 35 cycles of 94°C for 40 sec and
61°C for 45 sec, followed by one cycle of extension at 72°C for 10
min. The relative levels of gene mRNA transcripts to control GAPDH
were calculated by normalizing to the control and using the
2−ΔΔCt method.
Lentivirus construction and
infection
Individual lentivirus plasmid vectors for expressing
small interfering RNA (siRNA) specific for FoxM1 or control siRNA
were constructed. After sequencing, the generated plasmids,
together with two packaging plasmids (pVSVG and 48.91; Cambridge,
MA, USA) were co-transfected into 293T cells by calcium phosphate
transfection, respectively. Another type of lentivirus was
established for expressing firefly luciferase. Forty-eight hours
after transfection, the media of the cultured 293T cells were
collected and after centrifugation, the titers of the generated
individual types of lentiviruses were measured by flow cytometry.
CNE-2 cells (5×105/well) were infected with the
FoxM1-siRNA or control siRNA expressing lentivirus combined with
the lentivirus for expressing firefly luciferase at an MOI of 50 to
generate firefly luciferase and FoxM1 stable silenced CNE-2
cells.
Gene silencing using siRNA
FoxM1 siRNA and scrambled control siRNA were
purchased from Invitrogen. CNE-1 and CNE-2 cells were transfected
with either FoxM1-specific or control siRNA by Lipofectamine 2000
reagent (Invitrogen) as described previously (24).
In vitro cell migration and invasion
assays
CNE-1 and CNE-2 cells at a density of
8×104 cells/well were cultured in the presence or
absence of 8 μmol/l thiostrepton in the top wells of 24-well
Transwell plates (Corning Inc., Corning, NY, USA) that had been
coated with fibronectin. After the cells were cultured for 24 h,
the cells on the bottom surface of the top well were stained with
Giemsa and examined under a microscope in a blinded manner. In
addition, CNE-1 and CNE-2 cells were transfected with
FoxM1-specific or control siRNA for 48 h and these cells were
harvested for assessment of their migration in the presence or
absence of 8 μmol/l thiostrepton.
To determine the impact of FoxM1 inhibition on NPC
invasion, CNE-1 and CNE-2 cells at a density of
8×104cells/well were cultured in the presence or absence
of 8 μmol/l thiostrepton in the top wells that had been
pre-coated with 24 mg/ml Matrigel (R&D Systems, Minneapolis,
MN, USA). Moreover, the FoxM1-specific or control siRNA-transfected
CNE-1 and CNE-2 cells were tested for their invasion in the
presence or absence of 8 μmol/l thiostrepton.
Colony formation assay
The impact of FoxM1 inhibition on
anchorage-independent proliferation of NPC cells was assessed.
CNE-1 and CNE-2 cells at 2×103 cells/well were mixed
with 1 ml of culture medium containing 0.3% (w/v) agar, 20% FBS and
layered over a basal layer of 1 ml of culture medium containing
0.6% (w/v) agar and 20% FBS in 6-well plates in the presence or
absence of 8 μmol/l of thiostrepton. The cells were exposed
to fresh medium containing the drug weekly and cultured for 2–3
weeks. The numbers of individual colonies containing >50 cells
were counted under a microscope in a blinded manner. The colony
formation efficiency of individual groups of cells was calculated
as: (the numbers of colonies/numbers of cells inoculated) ×
100%.
In addition, the generated FoxM1 shRNA stably
expressing CNE-2 and control CNE-2 cells (2×103
cells/well) were also tested for their anchorage-independent
proliferation and the formed colonies were examined under a
fluorescence microscope.
Western blotting
CNE-1 and CNE-2 cells were treated with, or without,
8 μmol/l of thiostrepton for 48 h. Similarly, the CNE-1 and
CNE-2 cells were transfected with control or FoxM1-specific siRNA
for 24 h and treated with, or without, thiostrepton for 48 h. The
cells were harvested and lysed using a nuclear protein extraction
kit (Takara). After quantification of the protein concentrations,
the cell lysates (30 μg/lane) were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto Hybond-enhanced chemiluminescence nitrocellulose
membranes (GE Healthcare, Piscataway, NJ, USA). After being blocked
with 5% fat-free dry milk, the membranes were probed with
polyclonal antibodies against FoxM1 (C-20), E-cadherin, Slug, ZEB-2
and monoclonal anti-GAPDH antibody (all from Santa Cruz
Biotechnology). After being washed with TBST, the bound antibodies
were detected with horseradish peroxidase (HRP)-conjugated
secondary antibodies and visualized using enhanced
chemiluminescence (ECL; GE Healthcare). The relative levels of
target proteins to the control GAPDH expression were determined by
densitometric scanning and calculated using ImageJ software.
Animal experiments
Male BALB/C nude mice at 4–5 weeks of age were
purchased from the Shanghai Institute of Material Medicine, Chinese
Academy of Science (Shanghai, China) and housed in a specific
pathogen-free (SPF) animal facility in our campus. The experimental
protocol was established in accordance with institutional
guidelines, and was approved by the Animal Use and Care Committee
of Chongqing Medical University.
The control CNE-2 cells stably expressing firefly
luciferase and CNE-2 cells stably expressing the FoxM1-specific
shRNA were harvested, washed with PBS, adjusted to
2×106/ml in PBS. To induce tumors in vivo,
individual mice were injected subcutaneously with 2×106
control CNE-2 or FoxM1-specific shRNA stably expressing CNE-2 cells
into the flank (n=10 per group). Some of the mice were monitored
for the growth of implanted NPC cells weekly up to 30 days post
inoculation. The volume of the formed tumor in individual mice was
calculated according to the following formula: tumor volume =
(length × width2)/2. At the end of this experiment, the
mice were anesthetized and sacrificed. Their subcutaneous tumors
were dissected out and the tumor volumes were measured. In
addition, the remaining mice were monitored for lung metastasis up
to 60 days post inoculation. Their lungs were dissected and
examined for the numbers of metastatic tumors in the lungs.
Briefly, the lung tissues were imaged, fixed with 10% formalin and
paraffin-embedded. The lung tissue sections (4 μm) were
stained with hematoxylin and eosin (H&E) and every 100th
section was examined under a light microscope.
Immunohistochemistry
The dissected subcutaneous tumors were fixed in 10%
formalin and paraffin-embedded. The tumor tissue sections
(4-μm) were deparaffinized, rehydrated, and treated with 3%
H2O2 in methanol, followed by antigen
retrieval with citrate buffer (pH 6.0) in a high pressure steamer.
After being blocked with 5% fat-free dry milk in TBST, the sections
were incubated with polyclonal antibodies against FoxM1,
E-cadherin, ZEB2 and Snail2, respectively. The bound antibodies
were detected with HRP-conjugated secondary antibodies and
visualized with DBA staining, followed by microimaging under a
microscope.
Statistical analysis
Data are expressed as representative images or the
means ± SD. The difference among groups was determined by the
Student’s t-test or repeated one-way ANOVA using SPSS 17.0
software. A P-value of <0.05 was considered to indicate
statistical significance.
Results
Targeting of FoxM1 expression reduces the
viability of NPC cells
FoxM1 is crucial for the proliferation and
differentiation of tumor cells and its expression is associated
with the development of different types of cancers (14). Thiostrepton is a specific inhibitor
of FoxM1 (25) and can inhibit
proteasomal properties (26). To
explore the role of FoxM1 in regulating NPC cell proliferation, we
first tested the impact of FoxM1 inhibition on the viability of NPC
cells in vitro. Human NPC CNE-1 and CNE-2 cells were treated
with the indicated concentrations of thiostrepton for 48 and 72 h,
and their cell viability was determined using the Cell Counting
Kit-8. As shown in Fig. 1A,
treatment with different doses of thiostrepton for 48 h reduced the
relative viability of the CNE-1 cells in a dose-dependent manner,
and treatment with thiostrepton for 72 h further reduced the
relative viability of the CNE-1 cells. A similar pattern of
thiostrepton toxicity against CNE-2 cells was observed. At a
concentration of 8 μM, thiostrepton reduced the relative
viability of both cell lines by ~58 and 54%, respectively. Hence,
thiostrepton reduced the relative viability of NPC cells in a dose-
and time-dependent manner.
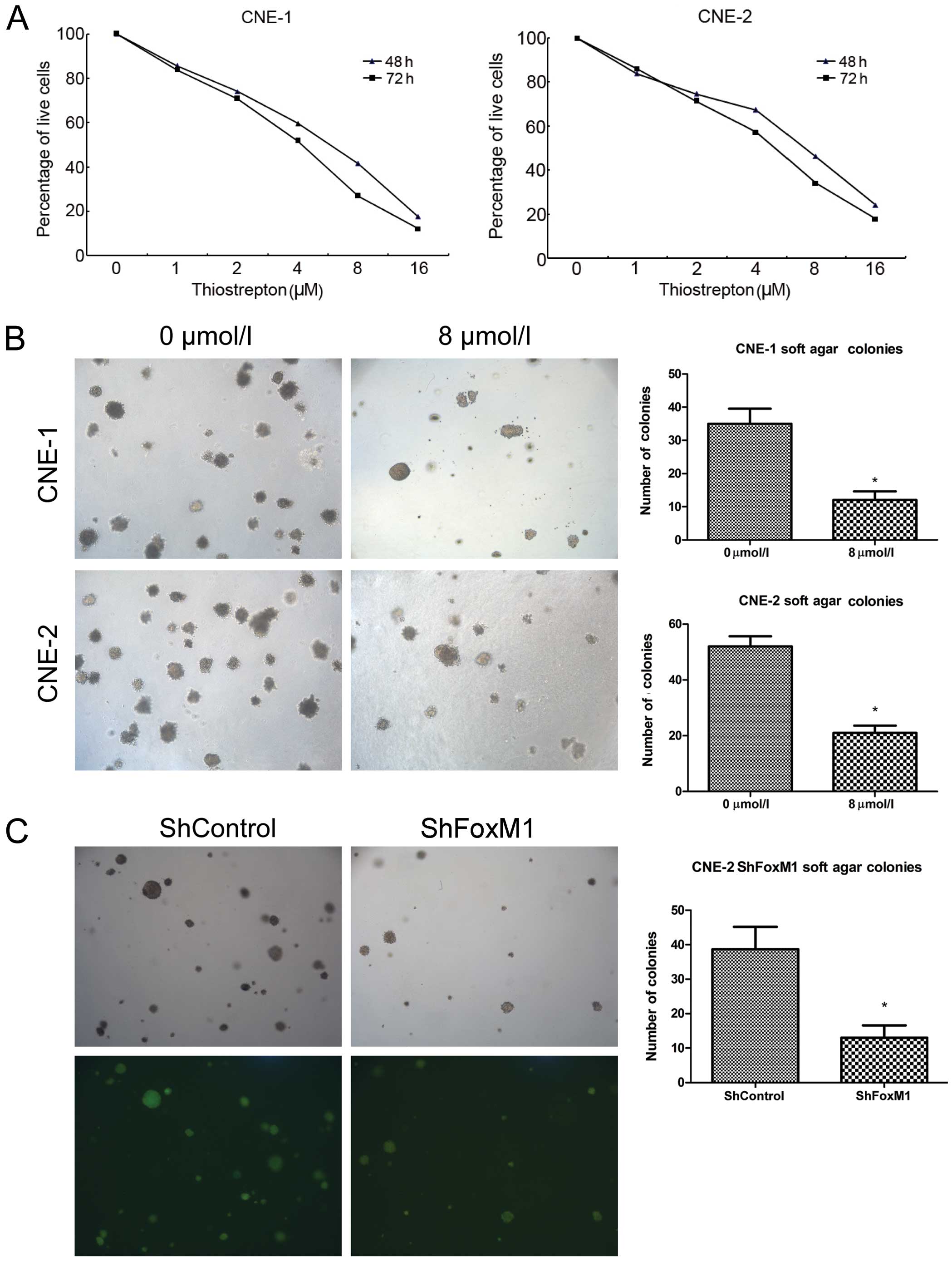 | Figure 1Thiostrepton has a potent cytotoxic
effect on NPC cells in vitro. (A) CNE-1 and CNE-2 cells were
treated with thiostrepton (0, 1, 2, 4, 8 and 16 μM) for 48
or 72 h, and the survival of cells was determined using the CCK-8
assay. Data are the means (%) related to the controls (100%) from
three separate experiments. (B) Thiostrepton inhibited the colony
formation of NPC cells. CNE-1 and CNE-2 cells were treated with, or
without, 8 μM thiostrepton and cultured in soft-agar to
assess the colony formation. (C) Knockdown of FoxM1 inhibited the
colony formation of the CNE-2 cells in vitro. The
FoxM1-specific shRNA stably expressing shFoxM1 and control
shControl CNE-2 cell lines were established, and the colony
formation was determined by soft-agar cultures. Data are
representative images or expressed as the means ± SD of individual
groups of cells from three separate experiments.
*P<0.05 vs. the controls. NPC, nasopharyngeal
carcinoma; FoxM1, forkhead box M1. |
Next, we examined the effect of thiostrepton on
anchorage-independent proliferation of NPC cells by colony
formation assays. We found that treatment with 8 μM
thiostrepton significantly inhibited the numbers of formed CNE-1
and CNE-2 colonies in vitro (P<0.05 for both cells,
Fig. 1B). To further investigate
the effect of FoxM1 inhibition on anchorage-independent
proliferation of NPC cells, we generated FoxM1-specific shRNA
expressing or control lentiviruses and infected CNE-2 cells to
establish FoxM1-specific shRNA stably expressing CNE-2 cell lines.
Subsequently, we tested the impact of FoxM1 silencing on
anchorage-independent proliferation of CNE-2 cells in vitro.
We found that stable knockdown of FoxM1 expression significantly
reduced the numbers of formed CNE-2 colonies in vitro
(P<0.05, Fig. 1C). These three
lines of evidence indicated that targeting of FoxM1 inhibited the
proliferation of NPC cells and reduced the relative viability of
NPC cells in vitro.
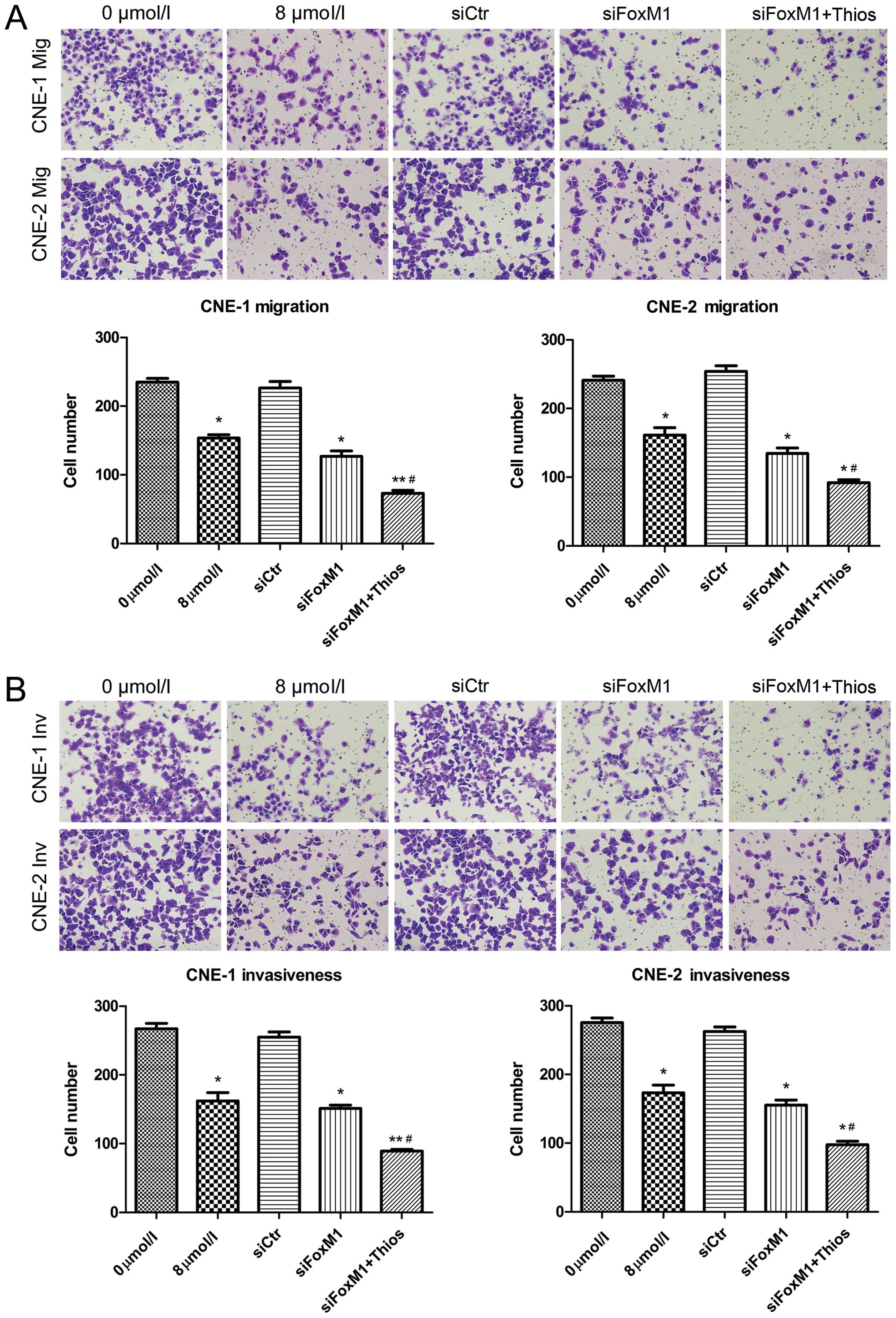
Targeting of FoxM1 inhibits the migration
and invasion of NPC cells in vitro
FoxM1 can modulate MMP production and regulate cell
migration and invasion (10,17)
Accordingly, we tested the effect of thiostrepton on the migration
of NPC cells in vitro by a Transwell migration assay. We
found that treatment with 8 μM thiostrepton significantly
reduced the numbers of migrated CNE-1 and CNE-2 cells (P<0.05
for both cell lines, Fig. 2A).
Similarly, in comparison with that of the unmanipulated cells,
transfection of CNE-1 and CNE-2 cells with control siRNA did not
change the numbers of migrated NPC cells while transfection with
FoxM1-specific siRNA significantly reduced the numbers of migrated
CNE-1 and CNE-2 cells (P<0.05 for both cell lines). More
importantly, the numbers of migrated CNE-1 and CNE-2 cells that had
been transfected with control siRNA and treated with 8 μM
were similar to that of the CNE-1 and CNE-2 treated with 8
μM thiostrepton (data not shown) while treatment with the
same dose of thiostrepton further reduced the numbers of migrated
CNE-1 and CNE-2 cells that had been transfected with FoxM1-specific
siRNA. These data clearly indicated that treatment with
thiostrepton and FoxM1 silencing synergistically inhibited the
migration of CNE-1 and CNE-2 cells in vitro. A similar
pattern of FoxM1 inhibition on the invasiveness of both CNE-1 and
CNE-2 cells was observed (Fig. 2B).
These data clearly demonstrated that targeting of FoxM1 inhibited
the migration and invasion of the NPC cells in vitro.
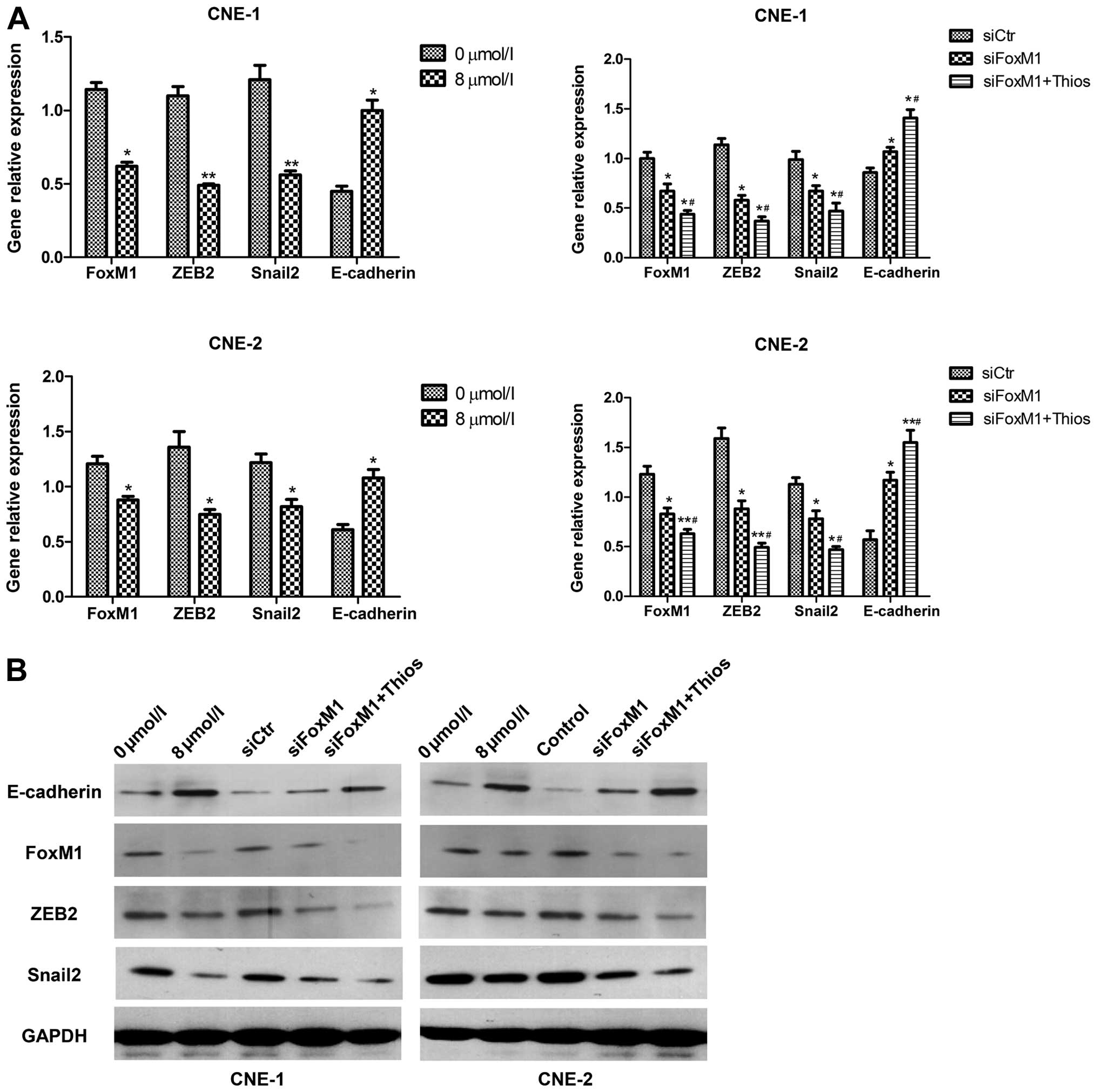
Targeting of FoxM1 inhibits the EMT
process in NPC cells
The EMT process is crucial for the migration and
invasion of cancer cells and during the EMT process, downregulation
of E-cadherin and upregulation of ZEB2 and Slug expression occur in
cancer cells. To understand the potential mechanisms underlying the
regulatory effect of FoxM1 inhibition on the migration and invasion
of NPC cells, we tested the impact of FoxM1 inhibition on the
expression of E-cadherin, ZEB2 and Slug in the CNE-1 and CNE-2
cells by quantitative RT-PCR and western blot assays. As shown in
Fig. 3A, in comparison with that in
the unmanipulated control NPC cells, treatment with thiostrepton
significantly reduced the relative levels of FoxM1, ZEB2 and Slug
mRNA transcripts in the CNE-1 and CNE-2 cells (P<0.05). In
contrast, treatment with thiostrepton significantly upregulated the
relative levels of E-cadherin mRNA transcripts in both NPC cell
lines. Similarly, transfection with FoxM1-specific siRNA
significantly reduced the relative levels of FoxM1 mRNA
transcripts, as compared to that in the control siRNA-transfected
NPC cells, demonstrating effective knockdown of FoxM1 expression in
both NPC cell lines. Furthermore, knockdown of FoxM1 expression
significantly reduced the relative levels of ZEB2 and Slug mRNA
transcripts in both NPC cell lines, but significantly upregulated
the relative levels of E-cadherin mRNA transcripts in both cell
lines. In addition, treatment with thiostrepton further
significantly reduced the relative levels of FoxM1, ZEB2 and Slug
mRNA transcripts, but elevated the relative levels of E-cadherin
mRNA transcripts in the FoxM1-silencing CNE-1 and CNE-2 cells.
Western blot analyses revealed that the targeting of
FoxM1 by treatment with thiostrepton or transfection of
FoxM1-specific siRNA not only significantly reduced the relative
levels of FoxM1, ZEB2 and Slug expression, but also significantly
enhanced the relative levels of E-cadherin expression in CNE-1 and
CNE-2 cells (P<0.05, Fig. 3B).
Targeting of FoxM1 by both treatment with thiostrepton and
siRNA-based FoxM1 silencing synergistically inhibited the FoxM1,
ZEB2 and Slug expression, but enhanced the E-cadherin expression in
both NPC cell lines in vitro. Collectively, these data
suggest that targeting of FoxM1 expression significantly inhibits
the EMT process, which may inhibit the migration and invasion of
NPC cells.
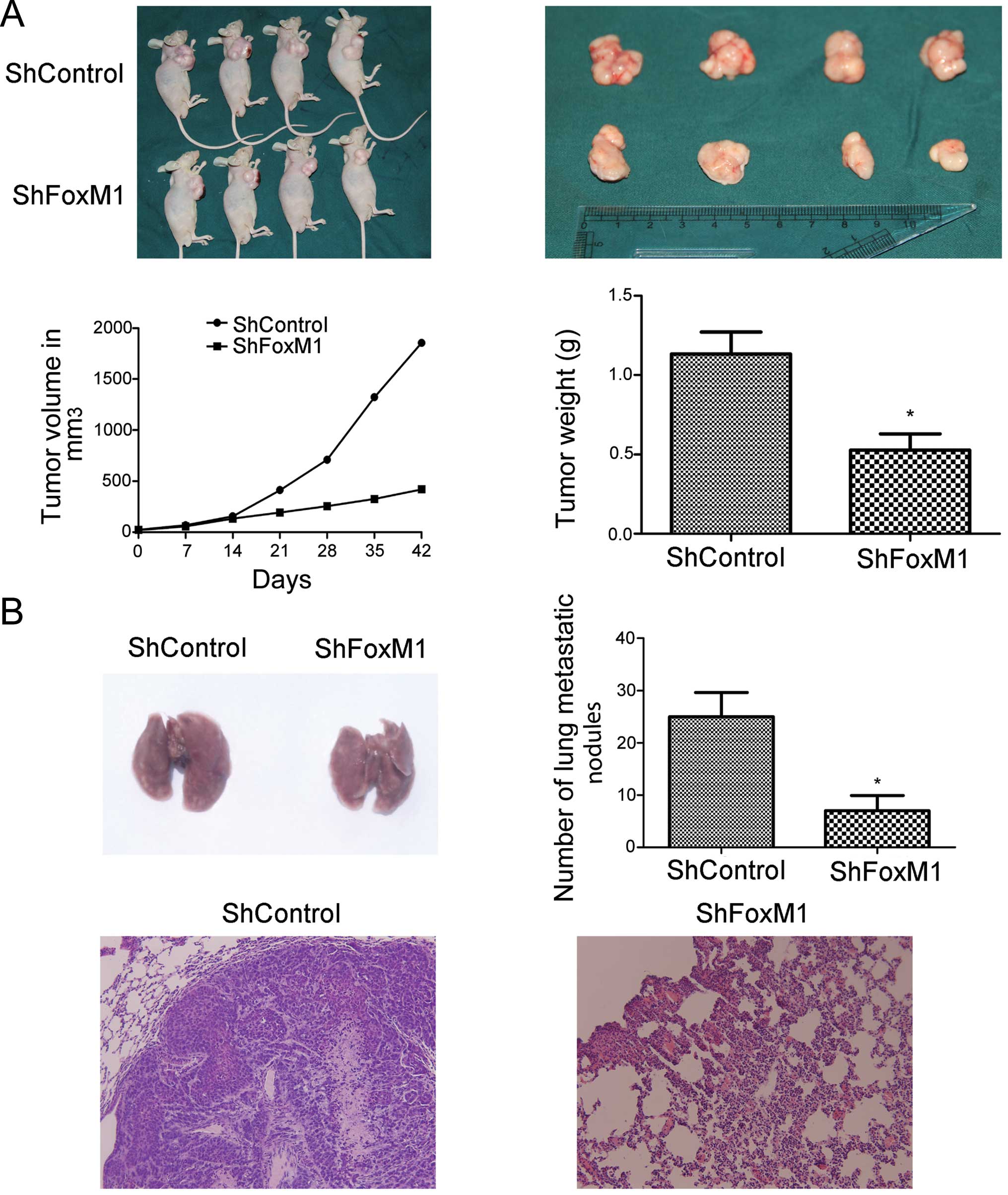
Targeting of FoxM1 expression
significantly inhibits the growth and migration of implanted NPC in
vivo
To determine the effect of FoxM1 silencing on the
growth and migration of NPC tumors in vivo, we implanted the
FoxM1 shRNA stably expressing or control CNE-2 cells into nude
mice. We found that while the tumors induced by control CNE-2 cells
rapidly grew with time, the tumors induced by the FoxM1 shRNA
stably expressing CNE-2 cells grew slowly. As a result, the volume
of the tumors derived from the FoxM1 shRNA-expressing CNE-2 cells
was significantly less than that of the tumors derived from the
control CNE-2 cells (P<0.05, Fig.
4A). Further analyses indicated that the mean weight of the
tumors derived from the FoxM1 shRNA stably expressing CNE-2 cells
was significantly less than that of the tumors derived from the
control CNE-2 cells (P<0.05, Fig.
4A). More importantly, the number of metastatic nodules in the
lungs of the FoxM1 shRNA-expressing tumors were significantly less
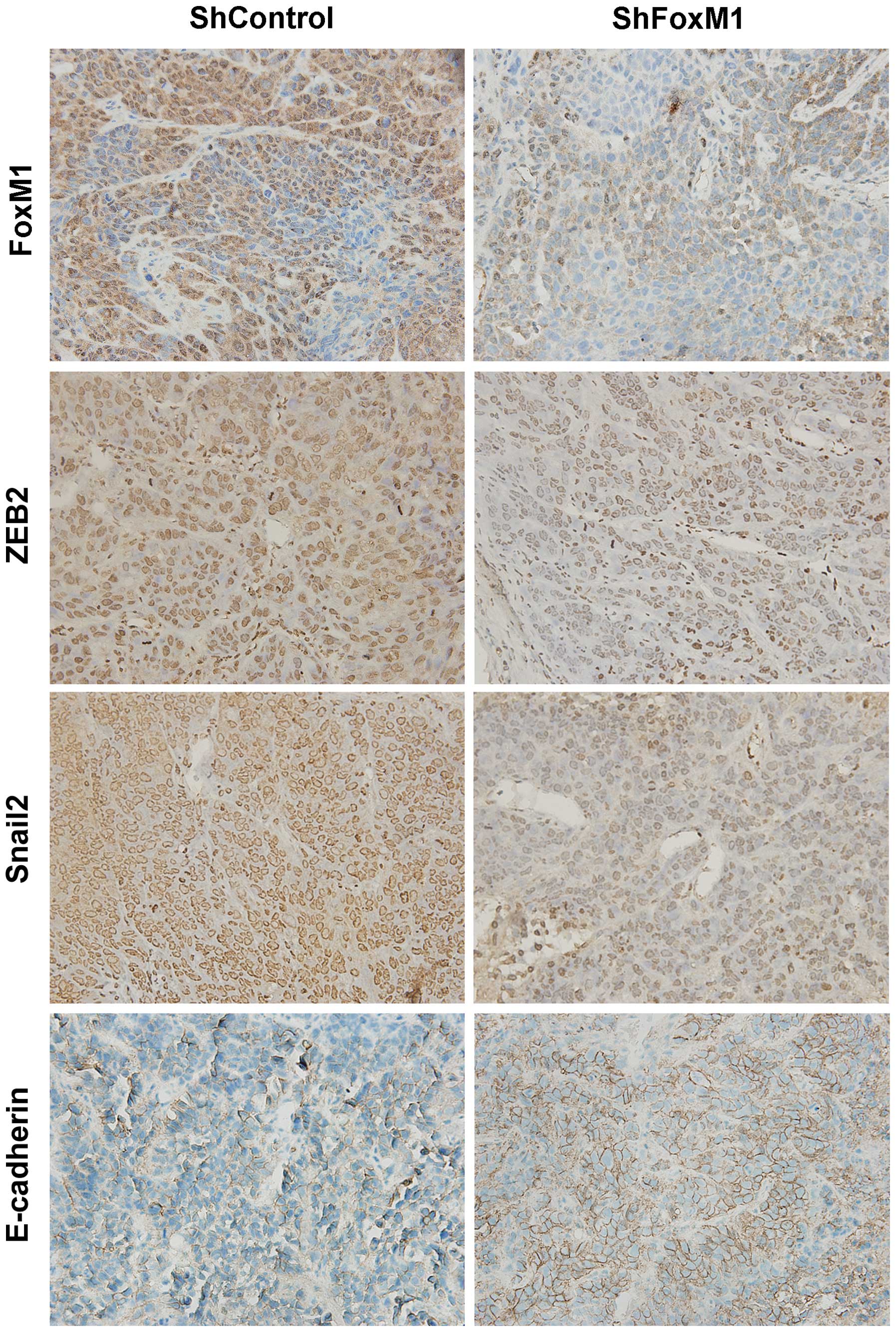
than that in the control tumors (P<0.05, Fig. 4B). Immunohistochemical analyses
revealed that the intensity of anti-FoxM1, anti-ZEB2 and anti-Slug
staining in the tumors of the FoxM1 shRNA stably expressing tumors
was obviously less than that in the control tumors (Fig. 5). In contrast, the intensity of
anti-E-cadherin staining in the tumors of the FoxM1-shRNA stably
expressing tumors was markedly stronger than that in the control
tumors. Collectively, targeting of FoxM1 expression significantly
inhibited the growth and lung metastasis of implanted NPC cells
in vivo, associated with inhibition of the EMT process in
NPC cells.
Discussion
FoxM1 overexpression has been found in a variety of
aggressive human carcinomas (11,20,27–29)
and is associated with the early steps of metastasis of pancreatic
and prostate cancers (17,30,31).
In the present study, we examined the role of FoxM1 in the
proliferation, migration and invasion of human NPC cells. We found
that inhibition or knockdown of FoxM1 significantly reduced the
survival of NPC cells and inhibited the anchorage-independent
proliferation, migration and invasion of NPC cells. Furthermore,
FoxM1 silencing inhibited the growth and lung metastasis of
implanted NPC tumors in mice. Inhibition of FoxM1 or knockdown of
FoxM1 expression increased the relative levels of E-cadherin, but
reduced ZEB2 and Snail2 expression in the NPC cells and tumors.
More importantly, treatment of FoxM1-silenced CNE-2 cells with the
FoxM1 inhibitor synergistically enhanced the inhibitory effects.
These novel data clearly indicate that knockdown of FoxM1 inhibits
the growth and metastasis of human NPC.
Cancer metastasis involves a series of complex
steps, including decreased adhesion, increased motility, cell
attachment, matrix dissolution and migration (18). During tumor progression, cancer
cells undergo dramatic changes in cytoskeletal organization to
adopt an invasive phenotype and eventually metastasize to other
organs (16). The EMT process has
been thought to be crucial for the migration and metastasis of
cancer (32,33). During the EMT process, cancer cells
usually lose E-cadherin expression and gain ZEB2, Snail2, vimentin
and N-cadherin expression (34–38).
In the present study, we found that treatment with the FoxM1
inhibitor or knockdown of FoxM1 expression significantly increased
the relative levels of E-cadherin expression, but decreased the
ZEB2 and Snail2 expression in NPC cells and related NPC tumors.
Previous studies have shown that FoxM1 can stimulate the expression
of MMP-2, MMP-9 and vascular endothelial growth factor (VEGF) in
pancreatic cancers and promotes the metastasis of prostate cancer
(17,30,31).
More importantly, we found that inhibition or knockdown of FoxM1
expression inhibited the migration of NPC cells in vitro and
the lung metastasis of implanted NPC in vivo. Our findings
extended these findings and support the notion that FoxM1
positively regulates the EMT process in cancer cells. Our findings
also provide a new explanation why high levels of FoxM1 expression
are associated with a poor prognosis of NPC (21). Hence, FoxM1 may be a potential
target for the intervention of NPC.
Previous studies have shown that FoxM1 is a
regulator of cell cycling and is important for the maintenance of
genomic stability and chromosomal integrity (8–10).
Furthermore, FoxM1 has been shown to stimulate Snail2 expression in
pancreatic cancer cells (17). We
found that knockdown of FoxM1 expression not only reduced the
relative levels of Snail2 and ZEB2 expression, but also upregulated
E-cadherin expression in NPC cells. These data suggest that FoxM1
may directly or indirectly stimulate ZEB2 expression in NPC cells.
Although FoxM1 has been recognized as a tumorigenesis-promoting
transcription factor, FoxM1c transactivates the expression of mouse
and human E-cadherin, a tumor suppressor (38). Indeed, endothelial cell-specific
FoxM1 knockout promoted urethane-induced lung tumors in mice. It is
possible that FoxM1 has dual functions in regulating tumorigenesis.
We found that knockdown of FoxM1 inhibited the EMT process,
proliferation and migration of NPC cells, suggesting that FoxM1 may
act as a tumorigenesis- and metastasis-promoting transcription
factor in NPC.
In summary, our data indicated that treatment with a
FoxM1 inhibitor or knockdown of FoxM1 expression reduced the
survival of NPC cells and inhibited the anchorage-independent
proliferation, migration and invasion of NPC cells. Knockdown of
FoxM1 inhibited the tumor growth and lung metastasis of NPC cells
in mice. The inhibition of FoxM1 downregulated ZEB2 and Snail2
expression, but upregulated E-cadherin expression in the NPC cells
and related tumors, indicating that knockdown of FoxM1 inhibited
the EMT process in NPC. Collectively, our data suggest that FoxM1
may be a positive regulator of NPC metastasis and a potential
target for prognosis and therapy. Therefore, our findings may
provide new insights into molecular regulation by FoxM1 of the
metastasis of NPC and may aid in the design of new therapies for
intervention of NPC in the clinic.
Acknowledgments
This study was supported by a grant from the
National Natural Science Fund Committee Project (no. X1550).
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
FoxM1
|
forkhead box M1
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
Thios
|
thiostrepton
|
|
siCtr
|
siControl
|
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Z, Luo W, Zhou Y, et al: Potential
tumor suppressor NESG1 as an unfavorable prognosis factor in
nasopharyngeal carcinoma. PLoS One. 6:e278872011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alajez NM, Shi W, Hui AB, Bruce J,
Lenarduzzi M, Ito E, Yue S, O’Sullivan B and Liu FF: Enhancer of
zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal
carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell
Death Dis. 1:e852010. View Article : Google Scholar
|
|
4
|
Wang S and Fang W: Increased expression of
hepatoma-derived growth factor correlates with poor prognosis in
human nasopharyngeal carcinoma. Histopathology. 58:217–224. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang W, Li X, Jiang Q, et al:
Transcriptional patterns, biomarkers and pathways characterizing
nasopharyngeal carcinoma of Southern China. J Transl Med. 6:322008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chua DT, Ma J, Sham JS, Mai HQ, Choy DT,
Hong MH, Lu TX and Min HQ: Long-term survival after cisplatin-based
induction chemotherapy and radiotherapy for nasopharyngeal
carcinoma: a pooled data analysis of two phase III trials. J Clin
Oncol. 23:1118–1124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Razak AR, Siu LL, Liu FF, Ito E,
O’Sullivan B and Chan K: Nasopharyngeal carcinoma: the next
challenges. Eur J Cancer. 46:1967–1978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Costa RH, Kalinichenko VV, Holterman AX
and Wang X: Transcription factors in liver development,
differentiation, and regeneration. Hepatology. 38:1331–1347. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye H, Kelly TF, Samadani U, Lim L, Rubio
S, Overdier DG, Roebuck KA and Costa RH: Hepatocyte nuclear factor
3/fork head homolog 11 is expressed in proliferating epithelial and
mesenchymal cells of embryonic and adult tissues. Mol Cell Biol.
17:1626–1641. 1997.PubMed/NCBI
|
|
10
|
Wang IC, Chen YJ, Hughes D, Petrovic V,
Major ML, Park HJ, Tan Y, Ackerson T and Costa RH: Forkhead box M1
regulates the transcriptional network of genes essential for
mitotic progression and genes encoding the SCF (Skp2-Cks1)
ubiquitin ligase. Mol Cell Biol. 25:10875–10894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalin TV, Wang IC, Ackerson TJ, Major ML,
Detrisac CJ, Kalinichenko VV, Lyubimov A and Costa RH: Increased
levels of the FoxM1 transcription factor accelerate development and
progression of prostate carcinomas in both TRAMP and LADY
transgenic mice. Cancer Res. 66:1712–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim IM, Ackerson T, Ramakrishna S, et al:
The forkhead box M1 transcription factor stimulates the
proliferation of tumor cells during development of lung cancer.
Cancer Res. 66:2153–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu M, Dai B, Kang SH, et al: FoxM1B is
overexpressed in human glioblastomas and critically regulates the
tumorigenicity of glioma cells. Cancer Res. 66:3593–3602. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan DW, Yu SY, Chiu PM, Yao KM, Liu VW,
Cheung AN and Ngan HY: Over-expression of FOXM1 transcription
factor is associated with cervical cancer progression and
pathogenesis. J Pathol. 215:245–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D,
Huang S, Tan D and Xie K: Critical role and regulation of
transcription factor FoxM1 in human gastric cancer angiogenesis and
progression. Cancer Res. 69:3501–3509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Banerjee S, Kong D, Li Y and
Sarkar FH: Down-regulation of forkhead box M1 transcription factor
leads to the inhibition of invasion and angiogenesis of pancreatic
cancer cells. Cancer Res. 67:8293–8300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lynch TP, Ferrer CM, Jackson SR, Shahriari
KS, Vosseller K and Reginato MJ: Critical role of O-Linked
β-N-acetylglucosamine transferase in prostate cancer invasion,
angiogenesis, and metastasis. J Biol Chem. 287:11070–11081. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue YJ, Xiao RH, Long DZ, et al:
Overexpression of FoxM1 is associated with tumor progression in
patients with clear cell renal cell carcinoma. J Transl Med.
10:2002012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D
and Sarkar FH: Forkhead box M1 transcription factor: A novel target
for cancer therapy. Cancer Treat Rev. 36:151–156. 2010. View Article : Google Scholar :
|
|
21
|
Chen H, Yang C, Yu L, Xie L, Hu J, Zeng L
and Tan Y: Adenovirus-mediated RNA interference targeting FOXM1
transcription factor suppresses cell proliferation and tumor growth
of nasopharyngeal carcinoma. J Gene Med. 14:231–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang C, Chen H, Yu L, Shan L, Xie L, Hu J,
Chen T and Tan Y: Inhibition of FOXM1 transcription factor
suppresses cell proliferation and tumor growth of breast cancer.
Cancer Gene Ther. 20:117–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwok JM, Myatt SS, Marson CM, Coombes RC,
Constantinidou D and Lam EW: Thiostrepton selectively targets
breast cancer cells through inhibition of forkhead box M1
expression. Mol Cancer Ther. 7:2022–2032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hussain AR, Al-Jomah NA, Siraj AK,
Manogaran P, Al-Hussein K, Abubaker J, Platanias LC, Al-Kuraya KS
and Uddin S: Sanguinarine-dependent induction of apoptosis in
primary effusion lymphoma cells. Cancer Res. 67:3888–3897. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uddin S, Bavi P, Siraj AK, et al: Leptin-R
and its association with PI3K/AKT signaling pathway in papillary
thyroid carcinoma. Endocr Relat Cancer. 17:191–202. 2010.
View Article : Google Scholar
|
|
26
|
Bhat UG, Halasi M and Gartel AL: FoxM1 is
a general target for proteasome inhibitors. PLoS One. 4:e65932009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pilarsky C, Wenzig M, Specht T, Saeger HD
and Grützmann R: Identification and validation of commonly
overexpressed genes in solid tumors by comparison of microarray
data. Neoplasia. 6:744–750. 2004. View Article : Google Scholar
|
|
28
|
Bektas N, Haaf A, Veeck J, Wild PJ,
Lüscher-Firzlaff J, Hartmann A, Knüchel R and Dahl E: Tight
correlation between expression of the forkhead transcription factor
FOXM1 and HER2 in human breast cancer. BMC Cancer. 8:422008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katoh M and Katoh M: Human FOX gene family
(Review). Int J Oncol. 25:1495–1500. 2004.PubMed/NCBI
|
|
30
|
Chandran UR, Ma C, Dhir R, Bisceglia M,
Lyons-Weiler M, Liang W, Michalopoulos G, Becich M and Monzon FA:
Gene expression profiles of prostate cancer reveal involvement of
multiple molecular pathways in the metastatic process. BMC Cancer.
7:642007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai B, Kang SH, Gong W, Liu M, Aldape KD,
Sawaya R and Huang S: Aberrant FoxM1B expression increases matrix
metal-loproteinase-2 transcription and enhances the invasion of
glioma cells. Oncogene. 26:6212–6219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kraljevic Pavelic S, Sedic M, Bosnjak H,
Spaventi S and Pavelic K: Metastasis: new perspectives on an old
problem. Mol Cancer. 10:222011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gavert N and Ben-Ze’ev A:
Epithelial-mesenchymal transition and the invasive potential of
tumors. Trends Mol Med. 14:199–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: new insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Araki K, Shimura T, Suzuki H, Tsutsumi S,
Wada W, Yajima T, Kobayahi T, Kubo N and Kuwano H: E/N-cadherin
switch mediates cancer progression via TGF-β-induced
epithelial-to-mesenchymal transition in extrahepatic
cholangio-carcinoma. Br J Cancer. 105:1885–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakajima S, Doi R, Toyoda E, et al:
N-cadherin expression and epithelial-mesenchymal transition in
pancreatic carcinoma. Clin Cancer Res. 10:4125–4133. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mandal M, Myers JN, Lippman SM, et al:
Epithelial to mesen-chymal transition in head and neck squamous
carcinoma: association of Src activation with E-cadherin
down-regulation, vimentin expression, and aggressive tumor
features. Cancer. 112:2088–2100. 2008. View Article : Google Scholar : PubMed/NCBI
|