Introduction
Oral squamous cell carcinoma (OSCC) is one of the
most common malignancies arising in oral cavity. Remarkable
advancement in reconstructive surgery and diagnostic modalities has
improved the survival rate of OSCC patients. However, treatment
failure for OSCC patients has lethal potential due to locoregional
recurrence and distant metastasis. Unfortunately, advanced OSCC
remains refractory and lethal in >50% of the cases (1,2).
Preoperative chemoradiotherapy has become an
established part of the clinical management of locoregionally
advanced operable OSCC in order to control locoregional disease
(3–7). The beneficial effects of preoperative
chemoradiotherapy include downstaging of the primary tumor, an
increased resectability rate and the elimination of
micrometastases. Kirita et al (7) demonstrated that preoperative cisplatin
(CDDP)-based intravenous chemotherapy and concurrent radiotherapy
resulted in a clinical tumor response of 92.8% and a good
prognosis, with a 79.3% 5-year overall survival rate, in cases of
resectable advanced OSCC. Several other studies have also
demonstrated improved 5-year survival rates by using this treatment
in patients with advanced OSCC (4–8).
However, successful and satisfactory response to preoperative
chemoradiotherapy is seldom achieved in all patients with advanced
OSCC. It is thus crucial to elucidate the molecular mechanism of
the differential chemosensitivity and identify molecular markers to
distinguish between responders and non-responders (9–11).
It is well known that cancer cells are exacerbated
by chronic inflammation (11). The
primary role in this linkage is played by cytokines and chemokines
produced by cancer cells as well as activated immune cells
(12). Interleukin-6 (IL-6), a
pleiotropic cytokine with a variety of biological activities, is
secreted by different cell types including macrophages, T- and
B-lymphocytes, fibroblast, endothelial cells, keratinocytes and
cancer cells (13). Reportedly, an
increased expression of IL-6 has been investigated in different
types of cancers and high serum levels of IL-6 have been associated
with metastasis and unfavorable prognosis (13–17).
Furthermore, possible involvement of IL-6 signaling in the
resistance to chemotherapy and radiotherapy has been documented in
recent studies (18–20). In view of these findings, we
hypothesized that IL-6 signaling pathway could be responsible for
the progression and treatment resistance of OSCC. In the present
study, we therefore examined the association of IL-6 expression
with the clinical outcomes, especially with the histological
response by preoperative chemoradiotherapy in patients with
OSCC.
Materials and methods
Patient characteristics
We enrolled 78 patients with primary OSCC who were
treated in the Department of Oral and Maxillofacial Surgery at
Kyushu University Hospital from 2005 to 2011. The average age of
the patients was 65.7±13.2 years (range, 19–89). Fifty-two patients
were males and 26 were females. An informed consent was obtained
from all the participant patients before any procedure or treatment
was initiated (IRB no.: 25-227). Thirty-nine patients with
locoregionally advanced OSCC underwent chemoradiotherapy
preoperatively. Following the initial biopsy, all the specimens
were fixed in 4% buffered formalin solution and were embedded in
paraffin blocks. Subsequently, the paraffin-embedded specimens were
processed to 5 μm thick sections, stained with hematoxylin
and eosin (H&E) and examined by experienced oral pathologists
to confirm the diagnosis and histologic grade. The tumor stage was
classified according to the TNM classification of the International
Union Against Cancer (21). Tumor
histologic grade was defined according to the WHO classification
(22). The mode of the tumor
invasion was determined from H&E stained specimens according to
the Yamamoto-Kohama criteria as follows: grade 1 = well-defined
borderline; grade 2 = cords, less-marked borderline; grade 3 =
groups of cells, no distinct borderline; grade 4 = diffuse invasion
(4C = cord-like type; 4D = widespread type) (23). Patients and tumor characteristics
are shown in Table I.
 | Table IAssociation of IL-6 expression with
clinical characteristics in OSCC. |
Table I
Association of IL-6 expression with
clinical characteristics in OSCC.
| Clinical
factors | Cases (%) | IL-6 expression
| P-value |
|---|
| Negative | Low | High |
|---|
| Gender | | | | | N.S. |
| Male | 52 (66.7) | 16 | 12 | 24 | |
| Female | 26 (33.3) | 8 | 10 | 8 | |
| Primary site | | | | | N.S. |
| Tongue | 42 (53.9) | 14 | 15 | 13 | |
| Gingiva | 26 (33.3) | 7 | 5 | 14 | |
| Buccal mucosa | 7 (9.0) | 2 | 1 | 4 | |
| Oral floor | 3 (3.8) | 1 | 1 | 1 | |
| Clinical stage | | | | | P<0.05 |
| I and II | 42 (53.8) | 17 | 14 | 11 | |
| III and IV | 36 (46.2) | 7 | 8 | 21 | |
| Clinical T
stage | | | | | N.S. |
| T1/T2 | 50 (64.1) | 20 | 15 | 15 | |
| T3/T4 | 28 (35.9) | 4 | 7 | 17 | |
| Nodal
metastasis | | | | | P<0.05 |
| Yes | 35 (44.9) | 7 | 7 | 21 | |
| No | 43 (55.1) | 17 | 15 | 11 | |
| Local
recurrence | | | | | N.S. |
| Yes | 14 (17.9) | 3 | 2 | 9 | |
| No | 64 (82.1) | 21 | 20 | 23 | |
| Distant
metastasis | | | | | P<0.05 |
| Yes | 5 (6.4) | 0 | 0 | 5 | |
| No | 73 (93.6) | 24 | 22 | 27 | |
| Histologic
grade | | | | | N.S. |
| Grade 1 | 29 (37.2) | 7 | 9 | 13 | |
| Grade 2 | 49 (62.8) | 17 | 13 | 19 | |
| Mode of
invasion | | | | | N.S. |
| Grade 1/2/3 | 61 (78.2) | 19 | 17 | 25 | |
| Grade 4C/4D | 17 (21.8) | 5 | 5 | 7 | |
Preoperative chemoradiotherapy
The patients with locoregionally advanced OSCC
received external beam irradiation to the primary tumor and the
metastatic lymph nodes in daily fractions of 2 Gy, 5 times weekly,
for 3 weeks. S-1 (TS-1; Taiho Pharmaceutical, Tokyo, Japan), an
oral fluoropyrimidine preparation that consists of tegafur,
5-chloro-2,4-dihydroxypyridine (a dihydropyrimidine dehydrogenase
inhibitor) and potassium oxonate which inhibits orotate
phosphoribosyltransferase in the gastrointestinal tract, was used
as an anticancer drug in this regimen. Oral administration of S-1
started one week prior to the radiotherapy and was continued
throughout the radiotherapy period. Standard individual doses of
S-1 were calculated according to the body surface area (BSA): BSA
<1.25 m2, 80 mg; 1.25 ≤BSA <1.5 m2, 100
mg; BSA ≥1.5 m2, 120 mg. However, in patients with
reduced renal function (decreased creatinine clearance values), S-1
was administered at a lower dose, generally one step lower than the
standard dose. Radical surgery was carried out at 2–6 weeks
(average, 27.5±5.22 days) after the end of the preoperative
chemoradiotherapy.
Immunohistochemistry
Immunohistochemical staining was performed on 5
μm thick sections that were sliced serially from
paraffin-embedded blocks after formalin fixation of the excised
specimens. The sections were deparaffinized in xylene and
rehydrated in graded ethanol (100, 95, 90, 85 and 75%). For antigen
retrieval, the sections were immersed in Dako Target Retrieval
Solution (Dako Cytomation, Denmark) and autoclaved at 121°C for 5
min. The endogenous peroxide activities were then eliminated with
3% hydrogen peroxide for 5 min, and the sections were rinsed twice
for 10 min with phosphate-buffered saline (PBS) at pH 7.4.
Non-specific protein bindings were attenuated by incubation for 30
min with 10% goat serum and then the sections were incubated with
each primary antibody overnight at 4°C. The following antibodies
were used: anti-human monoclonal IL-6 antibody (Abcam, UK; diluted
1:50), anti-human monoclonal IL-6 receptor (IL-6R) antibody
(Invitrogen, CA, USA; diluted 1:50) and anti-human monoclonal
phospho-signal tranducer and activator of transcription 3 (p-STAT3)
antibody (Cell Signaling Technology, MA, USA; diluted 1:400). The
sections were rinsed twice for 10 min with PBS and incubated with
secondary antibody conjugated with peroxidase-labeled amino acid
polymer for 1 h at room temperature. After being rinsed with PBS
twice for 10 min, the immunoreactivity was visualized by immersing
the sections in 3,3′-diaminobenzidine and 0.6% hydrogen peroxide
(DAB substrate kit; Nichirei, Tokyo, Japan). Subsequently, the
sections were counterstained with Mayer’s hematoxylin, dehydrated
in graded ethanol (75, 85, 90, 95 and 100%), cleared with xylene
and finally mounted with permanent mounting medium (Malinol
mounting medium; Muto Pure Chemicals, Tokyo, Japan). Negative
controls were prepared by substituting PBS for each primary
antibody. To evaluate the expression of IL-6 in the OSCC,
positively stained cancer cells were counted in at least three
randomly selected areas at ×200 magnifications, and then each
percentage of these positive cancer cells was calculated as
labeling index (LI). The LI was computed by dividing the number of
the positively stained cells by that of all the cancer cells. The
patients with OSCC were divided into three groups as follows:
negative group = LI <5%; low IL-6 group = 5% ≤ LI <30%; high
IL-6 group = LI ≥30%.
Clinicopathological evaluation of
preoperative chemoradiotherapy
The classification of therapeutic efficacy
established by Shimosato et al (24) was used to evaluate the
histopathological response of the primary site tumors: grade 0, no
noticeable change; grade I, minimal cellular changes, but the
majority of the tumor cells appear viable; grade IIa, despite the
presence of the cellular changes and partial destruction of the
tumors, the tumor is still readily recognizable and many tumor
cells appear viable; grade IIb, tumor destruction is extensive, but
viable cell nests are present in small areas of the tumor (up to
one quarter of the tumor mass, excluding areas of coagulative
necrosis); grade III, only a few scattered, markedly altered and
presumably non-viable tumor cells are present, singly or in small
clusters, and few or no viable cells are seen; and grade IV, no
tumor cells remaining in any section. Cutting of resected specimens
was carried out by step-section method at intervals of 5 mm.
Statistical analyses
All statistical analyses in the present study were
performed by JMP software version 11 (SAS Institute, NC, USA).
Chi-squared test was used to assess the significant differences
between each group. Survival rates were also calculated by
Kaplan-Meier method and the P-value was calculated by Log-rank
test. A P-value of <0.05 was considered to be statistically
significant.
Results
Expression of IL-6 proteins in the OSCC
and adjacent nonmalignant oral mucosa
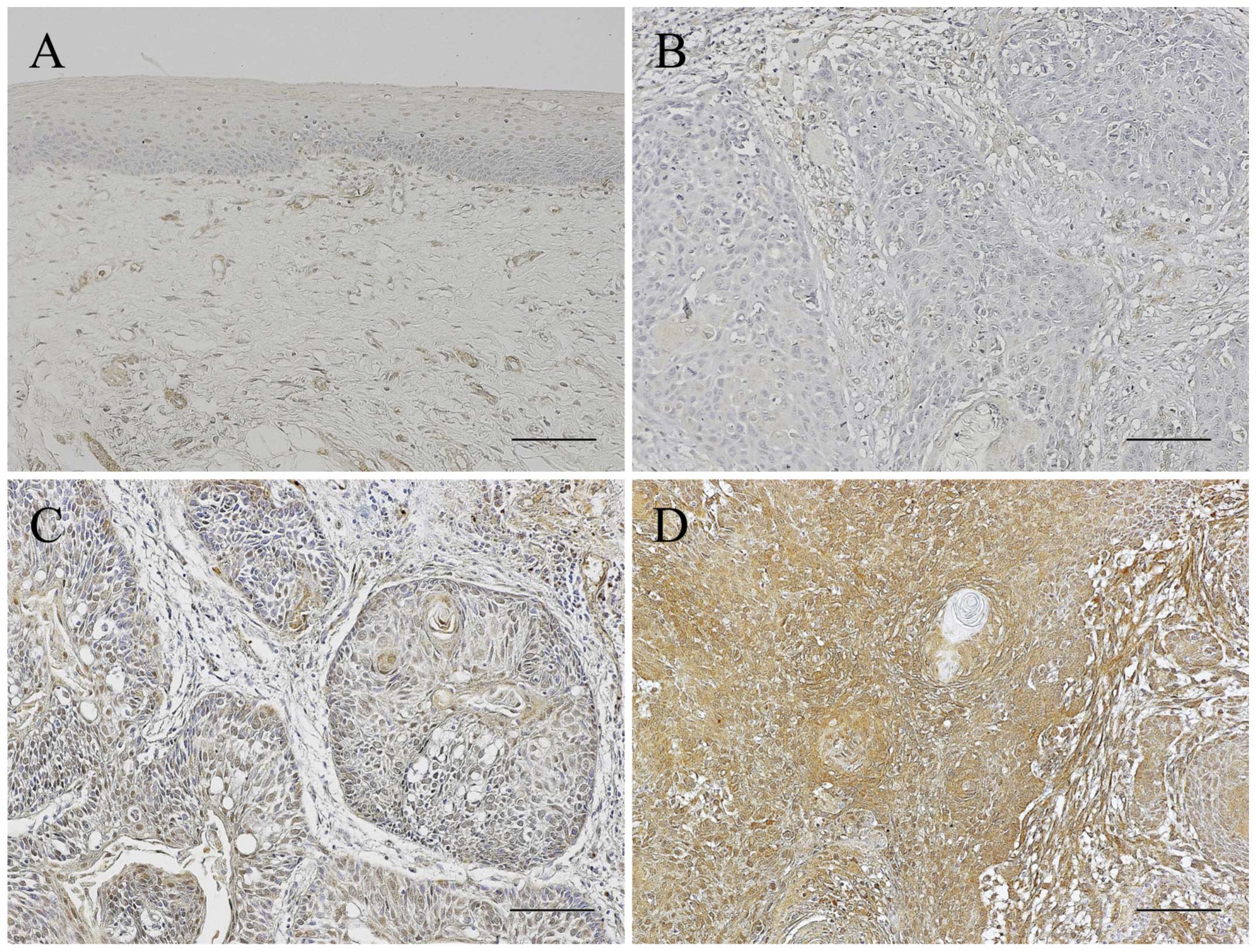
Immunoreactivity for IL-6 was almost not detected in
the adjacent non-malignant oral epithelia and weakly investigated
in the submucosal tissues. In the OSCC patients, the IL-6
expression pattern was different in the individual specimens. The
number of patients in the negative, low and high expression group
were 24, 22 and 32, respectively. The OSCC patients were thus
divided into three groups according to IL-6 positive rates as
follows: negative, low and high expression groups. In the negative
group, most cancer cells were negative for a IL-6 immunoreactivity
whereas stromal cells around the cancer nests expressed IL-6
slightly. IL-6 expression of the low expression group was scattered
in the cancer cells whereas almost all the cancer cells expressed
IL-6 in the high expression group. The stromal cells also strongly
expressed IL-6 in the high expression group (Fig. 1).
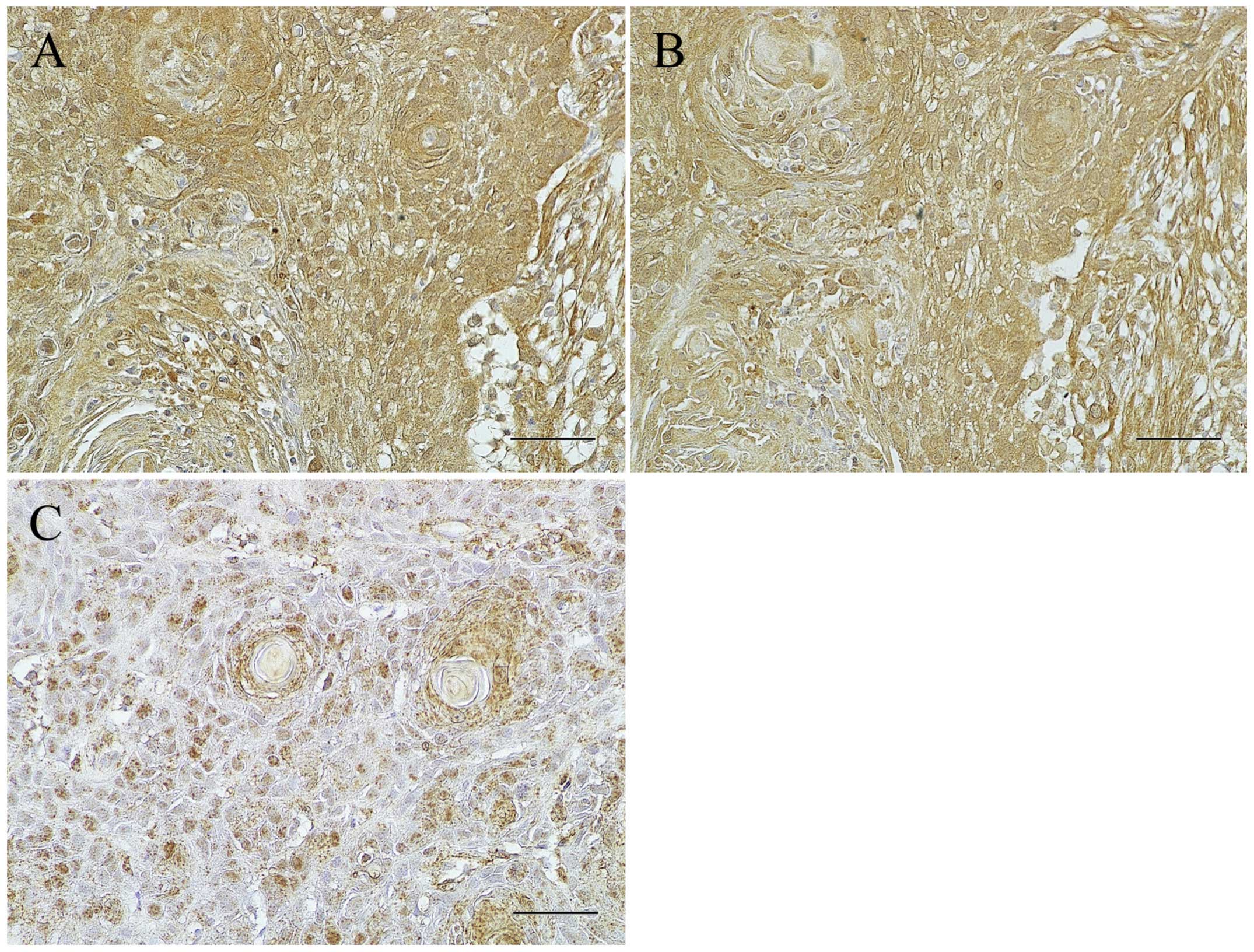
In order to examine whether IL-6 signaling is
activated in the OSCC cells, the expression of IL-6R and p-STAT3
was further examined immunohistochemically in the patients of the
high IL-6 expression group. IL-6R was detected in the cytoplasm and
plasma membrane of almost all the cancer cells and observed in
harmony with that of IL-6 in almost all the OSCC cells of the IL-6
high expression group (Fig. 2A and
B), but not or weakly in the negative and low expression group
(data not shown). p-STAT3 expression was localized in the nucleus
of the cancer cells and scattered widely in the cancer nests
(Fig. 2C).
Association of IL-6 expression with
clinicopathologic characteristics in OSCC patients
The associations of IL-6 expression with the
clinicopathologic factors of the OSCC patients were examined. The
patients in the high expression group had a more advanced clinical
stage than those in the negative and low expression groups.
Furthermore, the prevalence of the cervical lymph node or distant
metastasis in the high expression group was significantly higher
than those in the negative and low expression groups (P<0.05,
Chi-squared test). On the contrary, other clinical factors
including gender, primary site, clinical T classification, local
recurrence rate, histologic grade and mode of invasion did not show
significant differences among these groups (Table I).
Association of IL-6 expression with
histopathologic tumor response to preoperative chemoradiotherapy
for locally advanced OSCC
In the 39 patients who underwent preoperative
chemoradiotherapy for locally advanced OSCC, the association of
IL-6 expression in the cancer cells with histologic tumor response
in the resected specimens was further examined. Equal to or higher
than Grade IIb were judged to be histologically effective. All the
patients in the negative group were good responders, whereas only a
third of the patients had a histologically effective response in
the high expression group. Increased IL-6 expression in the cancer
cells was significantly associated with poor response to
preoperative chemoradiotherapy (*P<0.05,
**P<0.01, Chi-squared test Table II).
 | Table IIAssociation of IL-6 immunoreactivity
with histopathologic tumor response to preoperative
chemoradiotherapy. |
Table II
Association of IL-6 immunoreactivity
with histopathologic tumor response to preoperative
chemoradiotherapy.
| IL-6
expression | Pathologic response
|
|---|
| Effective
(IIb-IV) | Non-effective
(I-IIa) |
|---|
|
Negativea,b | 5 | 0 |
| Low | 11 | 5 |
| High | 6 | 12 |
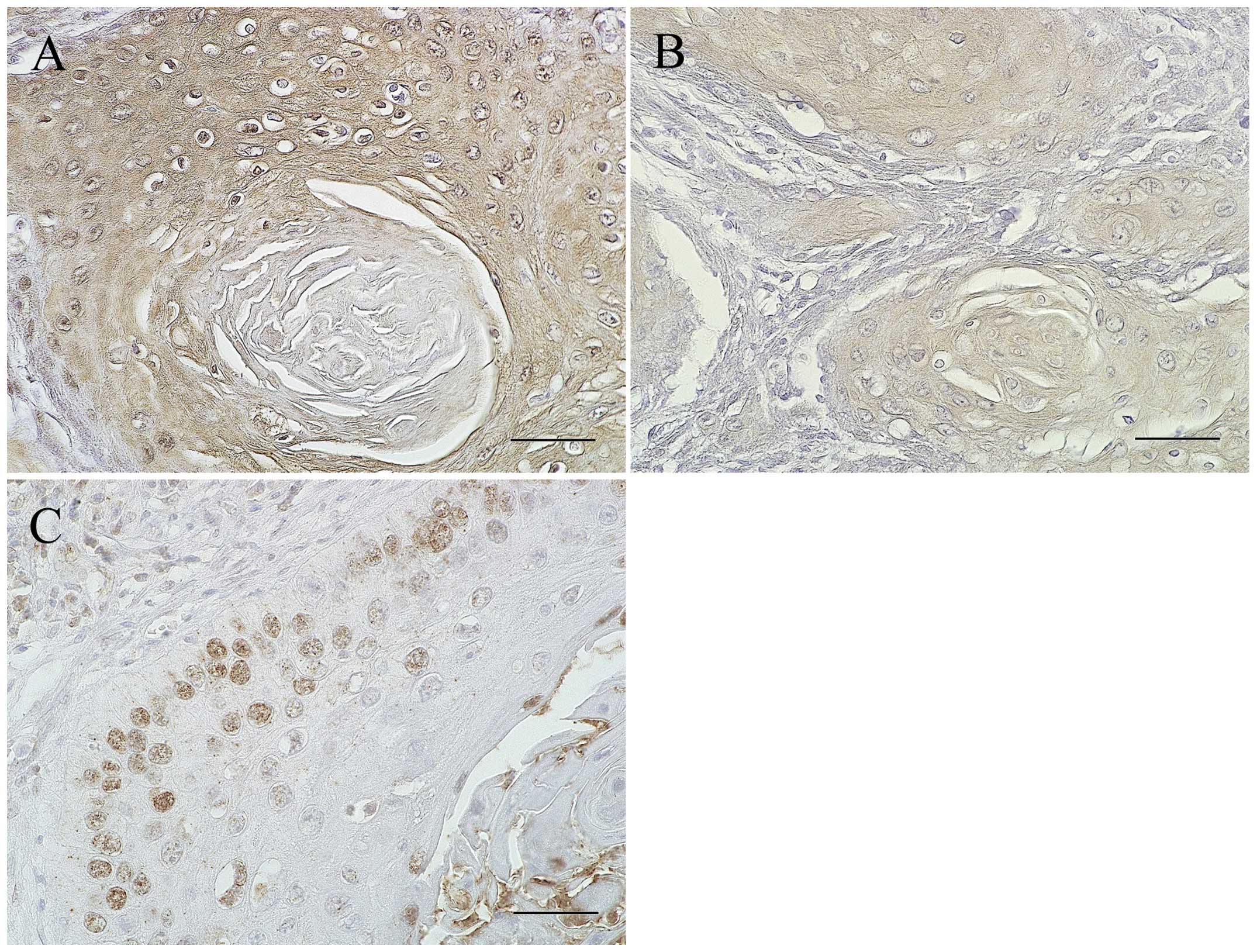
Expression of IL-6, IL-6R and p-STAT3 in
the residual cancer cells after preoperative chemoradiotherapy
In order to examine whether STAT3 signaling is also
activated in the residual cancer cells after chemoradiotherapy, the
expression of IL-6R and p-STAT3 was further examined
immunohistochemically in the 12 patients of the high expression
group with poor response to chemoradiotherapy. In all the cases,
IL-6R was detected in the cytoplasm and cytomembrane of almost all
residual cancer cells, while p-STAT3 was expressed only in the
outer layers of the cancer nest (Fig.
3).
Comparison of clinical outcomes and
prognosis among the groups with differential immunoreactivities for
IL-6
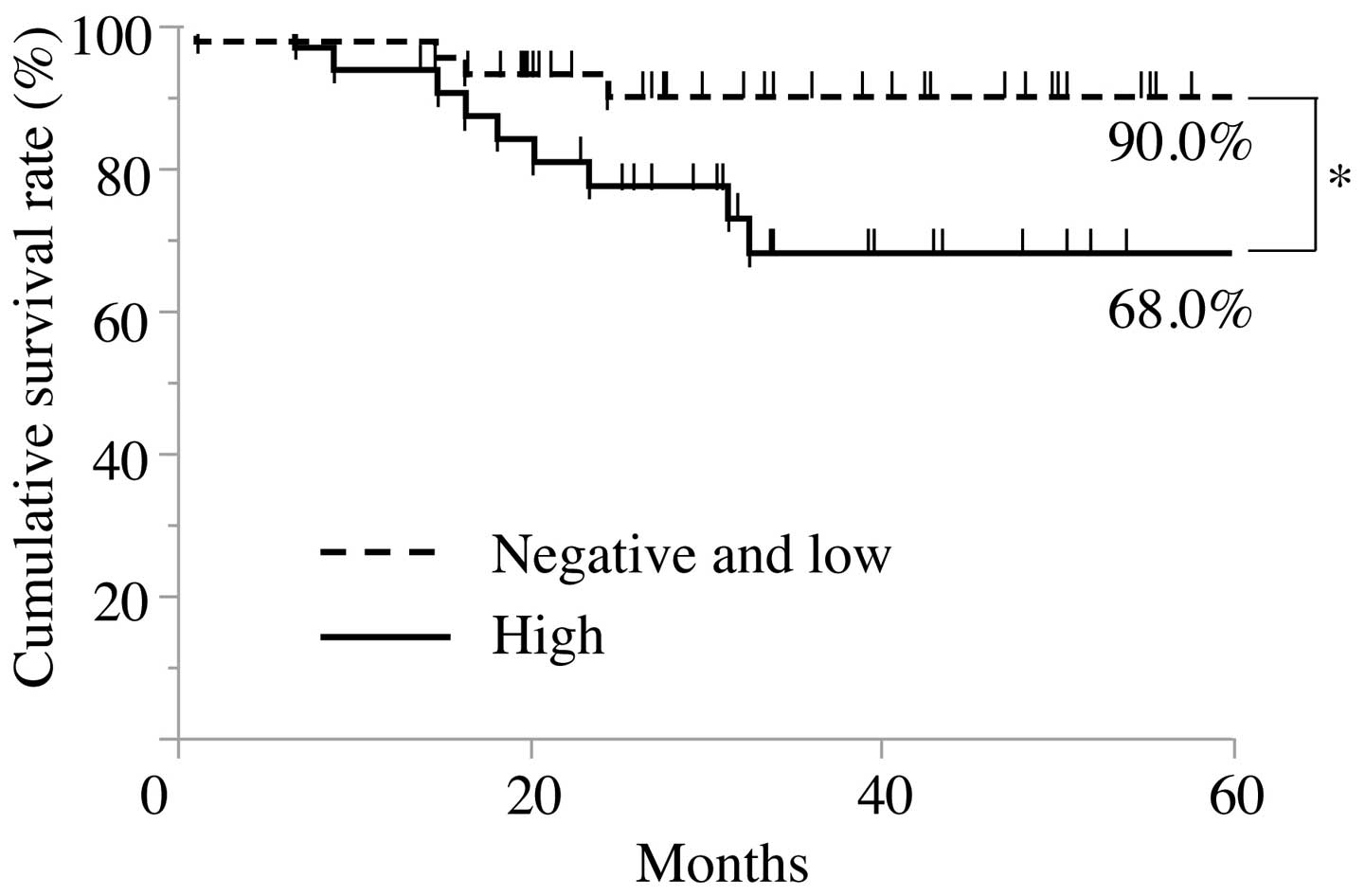
For the purpose of evaluating the correlation
between immunoreactivity for IL-6 in cancer cells and the clinical
outcomes of the patients with OSCC, the survival rates were
calculated by the Kaplan-Meier method. In the cause-specific
cumulative survival curves, the patients in the high expression
group had a significantly more unfavorable outcome than those in
the negative and low expression groups (Log-rank test, P<0.05;
Fig. 4). The cumulative survival
rate for 5 years in the high expression group was 68.0%, whereas
that in the negative and low expression groups was 90.0%.
Discussion
In the present study, we examined the association of
IL-6 expression in cancer cells with clinicopathological factors in
the OSCC patients by immunostaining. There have been several
studies on the correlation of the serum IL-6 concentration with the
prognosis in patients with OSCC, but not with the expression of
IL-6 proteins in the OSCC specimens (25–29).
Immunostaining for IL-6 in OSCC revealed that the increased
expression was more frequently observed in patients with an
advanced clinical stage, cervical lymph node metastasis or distant
metastasis. Shinriki et al (30), demonstrated by using tocilizumab,
humanized anti-IL-6R antibody, that IL-6 signaling stimulated
VEGF-C synthesis and lymphangiogenesis in OSCC. Recent studies
revealed that elevated serum levels of IL-6 were significantly
associated with the progression of malignancies, such as, ovarian
cancer, esophageal cancer, and head and neck cancer (26,31,32).
Furthermore, positive correlation between salivary IL-6 levels and
locoregional recurrence have been observed in the OSCC patients
(29), supporting our results, and
suggesting that IL-6 plays a key role in the progression of
OSCC.
Binding of IL-6 with its receptor initiates
homodimerization of gp130 and then triggers signaling cascades
through JAK-STAT, Ras-MAPK and PI3K-Akt pathways (13,33).
Notably, constitutive activation of STAT3 has been reported to
contribute to oncogenesis in a variety of malignancies (26,34–36).
It is well known that IL-6 induces the transient phosphorylation of
STAT3 and leads to its translocation into the nucleus. Expression
of IL-6R and p-STAT3 was thus examined in the present study. The
expression of IL-6R was observed in harmony with that of IL-6 in
almost all the OSCC cells of the IL-6 high expression group, but
not or weakly in the negative and low expression group.
Furthermore, the expression of p-STAT3 was investigated in the
nucleus of the cancer cells, suggesting that STAT3 signaling is
activated through autocrine loop stimulation between IL-6 and IL-6R
in OSCC cells.
Previous studies showed that IL-6 signaling pathway
could be responsible for acquirement of malignant transformation,
such as the ability to invade and metastasize, a migratory
capacity, and resistance to chemotherapy or radiotherapy (18–20,37–39).
Therefore, association of IL-6 expression with histopathologic
tumor response to chemoradiotherapy was examined in the patients
with locoregionally advanced OSCC treated by the preoperative
chemoradiotherapy with S-1. The results showed that increased IL-6
expression in the cancer cells was significantly associated with
poor response to preoperative chemoradiotherapy. Furthermore, the
residual cancer cells in the resected specimens expressed IL-6,
IL-6R and p-STAT3. Chen et al (18) demonstrated the significance of IL-6
signaling pathway in the resistance of pharyngeal cancer to
irradiation and the epidermal growth factor receptor inhibitor.
Sugimura et al (40) also
showed that Let-7, one of the microRNAs, modulates the
chemosensitivity to cisplatin through the regulation of IL-6/STAT3
signaling pathway in esophageal squamous cell carcinoma. In ovarian
cancer, wang et al (41)
reported that autocrine production of IL-6 confers resistance to
cisplatin and paclitaxel. Gilbert et al (42) also demonstrated that IL-6 secreted
from the endothelial cells after treatment with doxorubicin induced
chemoresistant niche and is associated with increased resistance to
DNA damaging agents in paracrine manner. Although the detail
mechanism in OSCC remains to be elucidated in the present study,
these results suggest that activation of IL-6/STAT3 signaling
pathway through autocrine and paracrine stimulation may be involved
in modulation of chemosensitivity to anticancer drugs. In addition,
it was also suggested that IL-6 expression in cancer cells could be
a useful predictive factor for tumor response to chemoradiotherapy
in OSCC.
In the present study, we also examined the
association of the IL-6 expression with the prognosis of patients
with OSCC. In the cause specific cumulative survival curves, the
patients in the high expression group had a significantly more
unfavorable outcome than that in the negative and low expression
groups. However, the association between the increased IL-6
expression and clinical prognosis for head and neck cancer patients
remains controversial. Chen et al (43) showed that high IL-6 expression in
tumor cells was significantly associated with poor prognosis in
OSCC patients. Duffy et al (26) also demonstrated that pretreatment
serum IL-6 levels could be a valuable biomarker for predicting
recurrence and overall survival among HNSCC patients. These results
were consistent with our results in the present study. On the
contrary, Wang et al (44)
reported that the patients with positive expression of IL-6 mRNA
transcripts in cancer cells had a significantly favorable survival
rate compared with those with the negative expression. This
contradictory conclusion may have resulted from the use of
different study populations or methodologies, or from different
etiologies including smoking, alcohol consumption and betel nut
chewing.
In conclusion, we showed that high expression of
IL-6 in cancer cells predict poor response to chemoradiotherapy and
unfavorable prognosis in patients with OSCC. Although further
studies are needed to understand the complex roles of IL-6
signaling, completely revealing the functions of IL-6 during tumor
progression will lead to new approaches for the treatment of
OSCC.
Acknowledgments
The present study was found in part by Grant-in-Aid
from the Ministry of education, Culture, Sports, Science and
Technology of Japan, no. 24249091 (to S.N.) and no. 26463014 (to
S.K.).
Abbreviations:
|
IL-6
|
interleukin-6
|
|
OSCC
|
oral squamous cell carcinoma
|
|
p-STAT3
|
phospho-signal tranducer and activator
of transcription 3
|
References
|
1
|
Funk GF, Karnell LH, Robinson RA, et al:
Presentation, treatment, and outcome of oral cavity cancer: A
National Cancer Data Base report. Head Neck. 24:165–180. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kirita T, Ohgi K, Shimooka H, Yamanaka Y,
Tatebayashi S, Yamamoto K, Mishima K and Sugimura M: Preoperative
concurrent chemoradiotherapy plus radical surgery for advanced
squamous cell carcinoma of the oral cavity: An analysis of
long-term results. Oral Oncol. 35:597–606. 1999. View Article : Google Scholar
|
|
4
|
Freier K, Engel M, Lindel K,
Flechtenmacher C, Mühling J, Hassfeld S and Hofele C: Neoadjuvant
concurrent radiochemotherapy followed by surgery in advanced oral
squamous cell carcinoma (OSCC): A retrospective analysis of 207
patients. Oral Oncol. 44:116–123. 2008. View Article : Google Scholar
|
|
5
|
Mohr C, Bohndorf W, Carstens J, et al:
Preoperative radiochemotherapy and radical surgery in comparison
with radical surgery alone. Int J Oral Maxillofac Surg. 23:140–148.
1994.PubMed/NCBI
|
|
6
|
Klug C, Berzaczy D, Voracek M and Millesi
W: Preoperative chemoradiotherapy in the management of oral cancer:
A review. J Craniomaxillofac Surg. 36:75–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kirita T, Yamanaka Y, Imai Y, Yamakawa N,
Aoki K, Nakagawa Y, Yagyuu T and Hasegawa M: Preoperative
concurrent chemoradiotherapy for stages II–IV oral squamous cell
carcinoma: A retrospective analysis and the future possibility of
this treatment strategy. Int J Oral Maxillofac Surg. 41:421–428.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawano S, Zheng Y, Oobu K, Matsubara R,
Goto Y, Chikui T, Yoshitake T, Kiyoshima K, Jinno T, Maruse Y,
Mitate E, Kitmura R, Tanaka H, Toyoshima T, Sugiura T and Nakamura
S: Clinicopathological evaluation of preoperative chemoradiotherapy
with S-1 for locally advanced oral squamous cell carcinoma. Oncol
Lett (In press);
|
|
9
|
Matsubara R, Kawano S, Kiyosue T, Goto Y,
Hirano M, Jinno T, Toyoshima T, Kitamura R, Oobu K and Nakamura S:
Increased ΔNp63 expression is predictive of malignant
transformation in oral epithelial dysplasia and poor prognosis in
oral squamous cell carcinoma. Int J Oncol. 39:1391–1399.
2011.PubMed/NCBI
|
|
10
|
Kiyosue T, Kawano S, Matsubara R, Goto Y,
Hirano M, Jinno T, Toyoshima T, Kitamura R, Oobu K and Nakamura S:
Immunohistochemical location of the p75 neurotrophin receptor
(p75NTR) in oral leukoplakia and oral squamous cell carcinoma. Int
J Clin Oncol. 18:154–163. 2013. View Article : Google Scholar
|
|
11
|
Goto Y, Kawano S, Matsubara R, et al:
Possible involvement of ΔNp63 downregulation in the invasion and
metastasis of oral squamous cell carcinoma via induction of a
mesenchymal phenotype. Clin Exp Metastasis. 31:293–306. 2014.
View Article : Google Scholar
|
|
12
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kishimoto T: Interleukin-6: From basic
science to medicine - 40 years in immunology. Annu Rev Immunol.
23:1–21. 2005. View Article : Google Scholar
|
|
14
|
Kurzrock R, Redman J, Cabanillas F, Jones
D, Rothberg J and Talpaz M: Serum interleukin 6 levels are elevated
in lymphoma patients and correlate with survival in advanced
Hodgkin’s disease and with B symptoms. Cancer Res. 53:2118–2122.
1993.PubMed/NCBI
|
|
15
|
Schafer ZT and Brugge JS: IL-6 involvement
in epithelial cancers. J Clin Invest. 117:3660–3663. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin MT, Lin BR, Chang CC, Chu CY, Su HJ,
Chen ST, Jeng YM and Kuo ML: IL-6 induces AGS gastric cancer cell
invasion via activation of the c-Src/RhoA/ROCK signaling pathway.
Int J Cancer. 120:2600–2608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong VW, Yu J, Cheng AS, Wong GL, Chan HY,
Chu ES, Ng EK, Chan FK, Sung JJ and Chan HL: High serum
interleukin-6 level predicts future hepatocellular carcinoma
development in patients with chronic hepatitis B. Int J Cancer.
124:2766–2770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen CC, Chen WC, Lu CH, Wang WH, Lin PY,
Lee KD and Chen MF: Significance of interleukin-6 signaling in the
resistance of pharyngeal cancer to irradiation and the epidermal
growth factor receptor inhibitor. Int J Radiat Oncol Biol Phys.
76:1214–1224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han Z, Feng J, Hong Z, Chen L, Li W, Liao
S, Wang X, Ji T, Wang S, Ma D, Chen G and Gao Q: Silencing of the
STAT3 signaling pathway reverses the inherent and induced
chemoresistance of human ovarian cancer cells. Biochem Biophys Res
Commun. 435:188–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan HQ, Huang XB, Ke SZ, Jiang YN, Zhang
YH, Wang YN, Li J and Gao FG: Interleukin 6 augments lung cancer
chemotherapeutic resistance via ataxia-telangiectasia
mutated/NF-kappaB pathway activation. Cancer Sci. 105:1220–1227.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH, Witte Sobin LH and Wittekind CH:
TNM Classification of Malignant Tumors. 6th edition. Wiley-Liss;
New York, NY: 2002
|
|
22
|
Wahi PN, Cohen B, Luthra UK, et al:
Histological consideration. Histological Typing of Oral and
Orpharyngeal Tumors. Wahi PN, Cohen B, Luthra UK, et al: World
Health Organization; Geneva: pp. 15–19. 1977
|
|
23
|
Yamamoto E, Miyakawa A and Kohama G: Mode
of invasion and lymph node metastasis in squamous cell carcinoma of
the oral cavity. Head Neck Surg. 6:938–947. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shimosato Y, Oboshi S and Baba K:
Histological evaluation of effects of radiotherapy and chemotherapy
for carcinomas. Jpn J Clin Oncol. 1:19–35. 1971.
|
|
25
|
Riedel F, Zaiss I, Herzog D, Götte K, Naim
R and Hörmann K: Serum levels of interleukin-6 in patients with
primary head and neck squamous cell carcinoma. Anticancer Res.
25:2761–2765. 2005.PubMed/NCBI
|
|
26
|
Duffy SA, Taylor JM, Terrell JE, Islam M,
Li Y, Fowler KE, Wolf GT and Teknos TN: Interleukin-6 predicts
recurrence and survival among head and neck cancer patients.
Cancer. 113:750–757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meyer F, Samson E, Douville P, Duchesne T,
Liu G and Bairati I: Serum prognostic markers in head and neck
cancer. Clin Cancer Res. 16:1008–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mojtahedi Z, Khademi B, Hashemi SB, Abtahi
SM, Ghasemi MA, Fattahi MJ and Ghaderi A: Serum interleukin-6
concentration, but not interleukin-18, is associated with head and
neck squamous cell carcinoma progression. Pathol Oncol Res.
17:7–10. 2011. View Article : Google Scholar
|
|
29
|
Sato J, Ohuchi M, Abe K, Satoh T, Abe T,
Yamazaki Y, Satoh A, Notani K and Kitagawa Y: Correlation between
salivary interleukin-6 levels and early locoregional recurrence in
patients with oral squamous cell carcinoma: preliminary study. Head
Neck. 35:889–894. 2013. View Article : Google Scholar
|
|
30
|
Shinriki S, Jono H, Ueda M, Ota K, Ota T,
Sueyoshi T, Oike Y, Ibusuki M, Hiraki A, Nakayama H, Shinohara M
and Ando Y: Interleukin-6 signalling regulates vascular endothelial
growth factor-C synthesis and lymphangiogenesis in human oral
squamous cell carcinoma. J Pathol. 225:142–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lane D, Matte I, Rancourt C and Piché A:
Prognostic significance of IL-6 and IL-8 ascites levels in ovarian
cancer patients. BMC Cancer. 11:2102011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen MF, Chen PT, Lu MS, Lin PY, Chen WC
and Lee KD: IL-6 expression predicts treatment response and outcome
in squamous cell carcinoma of the esophagus. Mol Cancer. 12:262013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirano T, Nakajima K and Hibi M: Signaling
mechanisms through gp130: A model of the cytokine system. Cytokine
Growth Factor Rev. 8:241–252. 1997. View Article : Google Scholar
|
|
34
|
Chakravarti N, Myers JN and Aggarwal BB:
Targeting constitutive and interleukin-6-inducible signal
transducers and activators of transcription 3 pathway in head and
neck squamous cell carcinoma cells by curcumin (diferuloylmethane).
Int J Cancer. 119:1268–1275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Calò V, Migliavacca M, Bazan V, Macaluso
M, Buscemi M, Gebbia N and Russo A: STAT proteins: From normal
control of cellular events to tumorigenesis. J Cell Physiol.
197:157–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsu CP and Chung YC: Influence of
interleukin-6 on the invasiveness of human colorectal carcinoma.
Anticancer Res. 26:4607–4614. 2006.
|
|
38
|
Ara T and Declerck YA: Interleukin-6 in
bone metastasis and cancer progression. Eur J Cancer. 46:1223–1231.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jimi E, Furuta H, Matsuo K, Tominaga K,
Takahashi T and Nakanishi O: The cellular and molecular mechanisms
of bone invasion by oral squamous cell carcinoma. Oral Dis.
17:462–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sugimura K, Miyata H, Tanaka K, Hamano R,
Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori
M and Doki Y: Let-7 expression is a significant determinant of
response to chemotherapy through the regulation of IL-6/STAT3
pathway in esophageal squamous cell carcinoma. Clin Cancer Res.
18:5144–5153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ
and Li LZ: Autocrine production of interleukin-6 confers cisplatin
and paclitaxel resistance in ovarian cancer cells. Cancer Lett.
295:110–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gilbert LA and Hemann MT: DNA
damage-mediated induction of a chemoresistant niche. Cell.
143:355–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen CJ, Sung WW, Lin YM, Chen MK, Lee CH,
Lee H, Yeh KT and Ko JL: Gender difference in the prognostic role
of interleukin 6 in oral squamous cell carcinoma. PLoS One.
7:e501042012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang YF, Chang SY, Tai SK, Li WY and Wang
LS: Clinical significance of interleukin-6 and interleukin-6
receptor expressions in oral squamous cell carcinoma. Head Neck.
24:850–858. 2002. View Article : Google Scholar : PubMed/NCBI
|