Introduction
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a cytokine, a member of the tumor necrosis factor
family and a selective inducer of apoptosis in a range of tumor
cells (1). TRAIL binding to its
receptors results in activating the death receptors (DR) including
DR4 or DR5 to induce the receptor trimerization and recruit the
Fas-associated death domain protein, which leads to the activation
of the caspase cascade (caspase−8, −9, −10, and −3) to transmit the
cell death signal (2,3). TRAIL is a promising anticancer agent
with the ability to selectively induce apoptosis in various tumors,
but not in most normal cells (4).
However, A549 cells show resistance to the apoptotic effects of
TRAIL (5).
The biochemical and pharmacological properties of
gingerol, a natural ginger component, include antioxidant,
anti-inflammatory, and anti-tumorigenic activities (6,7).
Gingerol treatment results in mitochondrial damage and inhibits
cell survival pathways in colorectal cancer cells (8). Gingerol has an anti-tumorigenic effect
by inducing apoptosis in tumor cells (9–11).
However, the exact apoptosis-inducing molecular mechanism of
gingerol remains unknown.
Autophagy is a self-degradation mechanism
responsible for cell death and survival (12). Autophagy flux is the complete
autophagy process, starting with formation of autophagosomes around
the cargo, fusion of the autophagosome with lysosomes, and
degradation and recycling of the cargo (13,14).
Microtubule-associated protein 1 light chain 3 β (also called
ATG8), which is a ubiquitin-like protein, is necessary for
autophagosome formation and is widely used as an autophagy marker
(15,16). p62/SQSTM1, also known as a
ubiquitin-binding protein, integrates into the autophagosome by
directly attaching to LC3-II and is degraded by autophagy (17). Inhibiting autophagy increases p62
levels (18). Many anticancer drugs
upregulate autophagy, leading to uncontrolled tumors (19–21).
Recent studies have shown that pharmacologically or genetically
inhibiting autophagy increases the effects of radiotherapy and
chemotherapy (22–24), suggesting that inhibiting autophagy
is a favorable cancer treatment strategy. Anti-malarial and
anti-rheumatoid arthritis drugs (chloroquine) act as autophagy
inhibitors during cancer therapy (25,26).
Chloroquine prevents lysosome acidification and fuses with
autophagosomes as a result of degradation of the metabolic stress
products, thereby inducing apoptosis (27–30).
Our study determined that gingerol sensitized
TRAIL-induced apoptosis in TRAIL-resistant A549 cells by inhibiting
the autophagy flux. Treatment with gingerol or TRAIL alone did not
influence cell death; thus, we examined whether co-treatment of
gingerol with TRAIL had more of an effect on A549 lung
adenocarcinoma cells. We investigated the sensitizing effect of
gingerol on TRAIL-induced apoptosis in A549 lung adenocarcinoma
cells by inhibiting the autophagy flux.
Materials and methods
Cell culture
Cancer cells originating from lung (A549) tumors
were obtained from the American Type Culture Collection (Global
Bioresource Center, Manassas, VA, USA). The cells were cultured in
RPMI-1640 medium (Gibco-BRL, Grand Island, NY, USA) supplemented
with 10% (v/v) fetal bovine serum and antibiotics (100 μg/ml
penicillin-streptomycin). The cell cultures were incubated in an
atmosphere containing 5% CO2 at 37°C.
Reagents
Recombinant gingerol was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). TRAIL (100 ng/ml) was
purchased from Abfrontier (Geumcheon-gu, Seoul, South Korea) and
chloroquine (10 μM) was purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Cell viability test
The A549 cells were plated at a density of 1.0×10
4 cells in 12-well plates and were incubated at 37°C for
24 h. The A549 cells were pretreated with gingerol in a
dose-dependent manner (0, 10, 20, and 40 μM). After the 12-h
gingerol pretreatment, the recombinant TRAIL (100 ng/ml) protein
was added and co-incubated for 2 h. The cells were also pretreated
with chloroquine (10 μM) for 1 h followed by gingerol
treatment. The cell morphology was photographed under a microscope
(inverted microscope; Nikon, Japan) and the cell viability was
determined using the crystal violet staining method. The cells were
stained with a staining solution (0.5% crystal violet in 30%
ethanol and 3% formaldehyde) for 10 min at room temperature, washed
4 times with PBS and dried. Then, the cells were lysed with a 1%
SDS solution and measured at an absorbance of 550 nm. The cell
viability was calculated from relative dye intensity and it was
compared with the control.
Western blot assay
The A549 cell lysates were prepared by harvesting
the cells, washing them in cold PBS, resuspending in a lysis buffer
[25 mM HEPES (pH 7.4), 100 mM EDTA, 5 mM MgCl2, 0.1 mM
DTT, and a protease inhibitor mixture], and sonicating the lysate.
The proteins (35 μg) were separated on a 10–15% SDS gel and
transferred to a nitrocellulose membrane. After 1 h of incubation
with a 1:1,000 primary antibody dilution buffer (1% milk with
PBS-Tween-20), the membranes were developed by enhanced
chemiluminescence using a secondary antibody. The antibodies used
for immunoblotting were LC3 (Novus Biologicals, Littleton, CO,
USA), anti-p62 (Millipore Corp., Milford, MA, USA), cleaved
caspase-3 (Cell Signaling Technology, Danvers, MA, USA), and
β-actin (Sigma-Aldrich). Images were examined using a Fusion-FX7
imaging system (Vilber Lourmat, Marne-la-Vallée, France).
Immunocytochemistry (ICC)
The A549 cell lines were cultured on a glass
coverslip and treated with gingerol, chloroquine and/or TRAIL,
washed with PBS and fixed with 3–4% paraformaldehyde in PBS for 15
min at room temperature. The cells were then washed twice in ice
cold PBS, the samples were incubated for 10 min in PBS containing
0.25% Triton X-100 and the cells were washed with PBS 3 times for 5
min. The cells were blocked with 1% BSA in PBST for 30 min,
incubated with the primary antibodies (LC3 and anti-p62, diluted in
1% BSA in PBST) in a humidified chamber for 1 h at room temperature
or overnight at 4°C, the solution was decanted and then the cells
were washed with PBS 3 times for 5 min. The cells were incubated
again with the secondary antibody in 1% BSA for 1 h at room
temperature in the dark and the secondary antibody solution was
decanted and washed with PBS 3 times for 5 min. The cells were
incubated with DAPI for 1 min and rinsed with PBS. Finally, the
cells were mounted with a fluorescent mounting medium and
visualized via a fluorescence microscope.
Statistical analysis
The unpaired t-test or the Welch’s correction were
used for comparison between two groups. For multiple comparison,
the one-way ANOVA followed by the Tukey-Kramer’s test was used. All
statistical analysis was performed using GraphPad Prism software.
Results were considered to be statistically significant for values
*P<0.05, **P< 0. 01.
Results
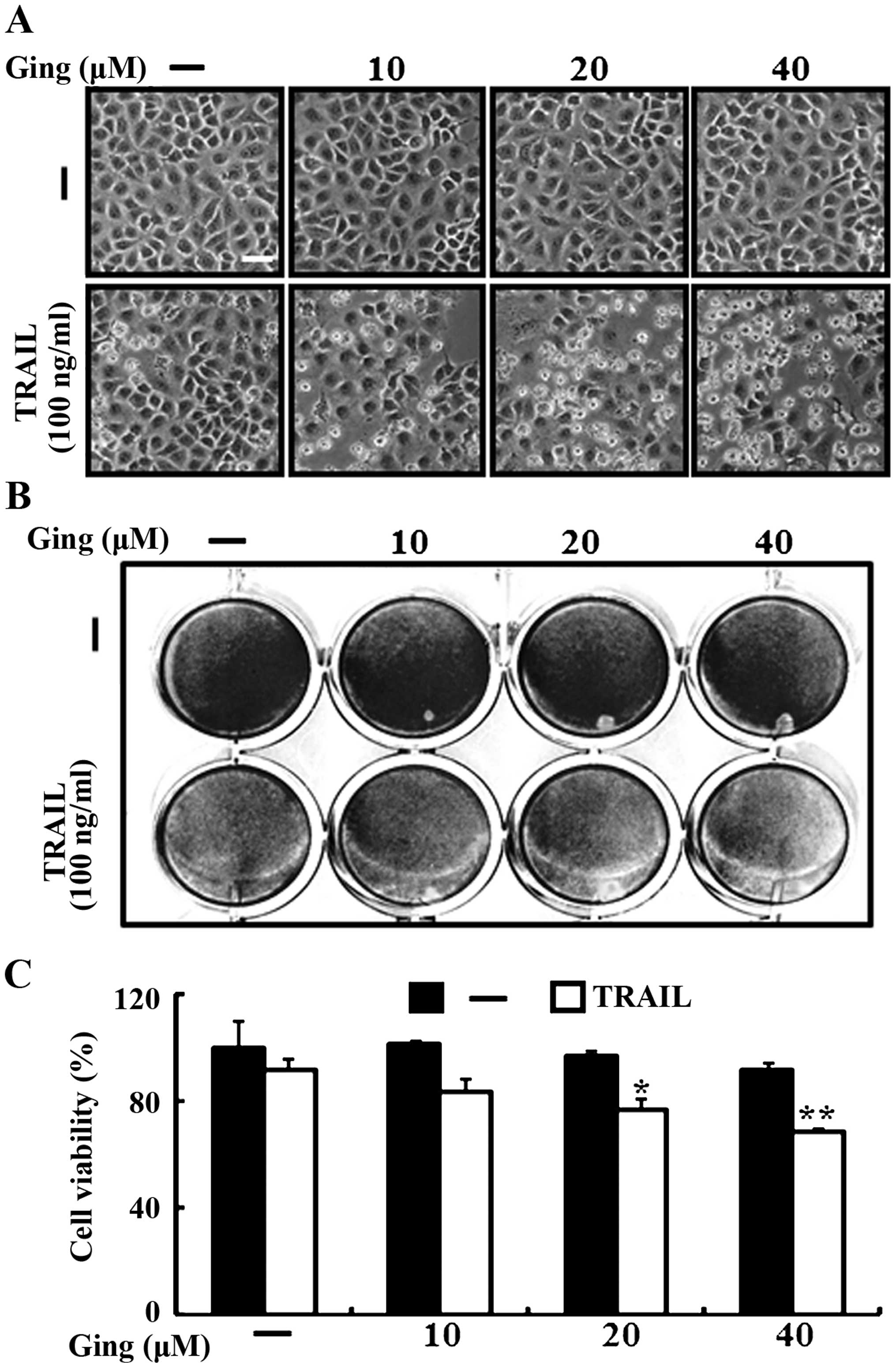
Gingerol enhances TRAIL-induced apoptosis
in A549 cells
We investigated the effects of gingerol co-treatment
on TRAIL-induced apoptosis in A549 lung adenocarcinoma cells. The
cells were pretreated with the indicated doses of gingerol for 12 h
and were treated with the TRAIL protein for an additional 2-h. We
photographed the cells to investigate the cell morphological
changes using a light microscope and the crystal violet assay. As
shown in Fig. 1, TRAIL treatment
alone either did not or only slightly induced cell death,
indicating that the A549 cells are highly resistant to
TRAIL-induced apoptosis, but gingerol enhanced TRAIL-induced
apoptosis in a dose-dependent manner. The cell morphology also
supported this enhanced effect of gingerol, showing that the
combination of TRAIL and gingerol enhanced the number of the
apoptotic cells compared with that of gingerol or TRAIL alone
(Fig. 1A). The TRAIL and gingerol
co-treatment reduced cell viability and significantly increased
apoptosis in the A549 cells (Fig.
1B and C). These results indicate that gingerol increased
TRAIL-induced apoptosis in TRAIL-resistant A549 cells.
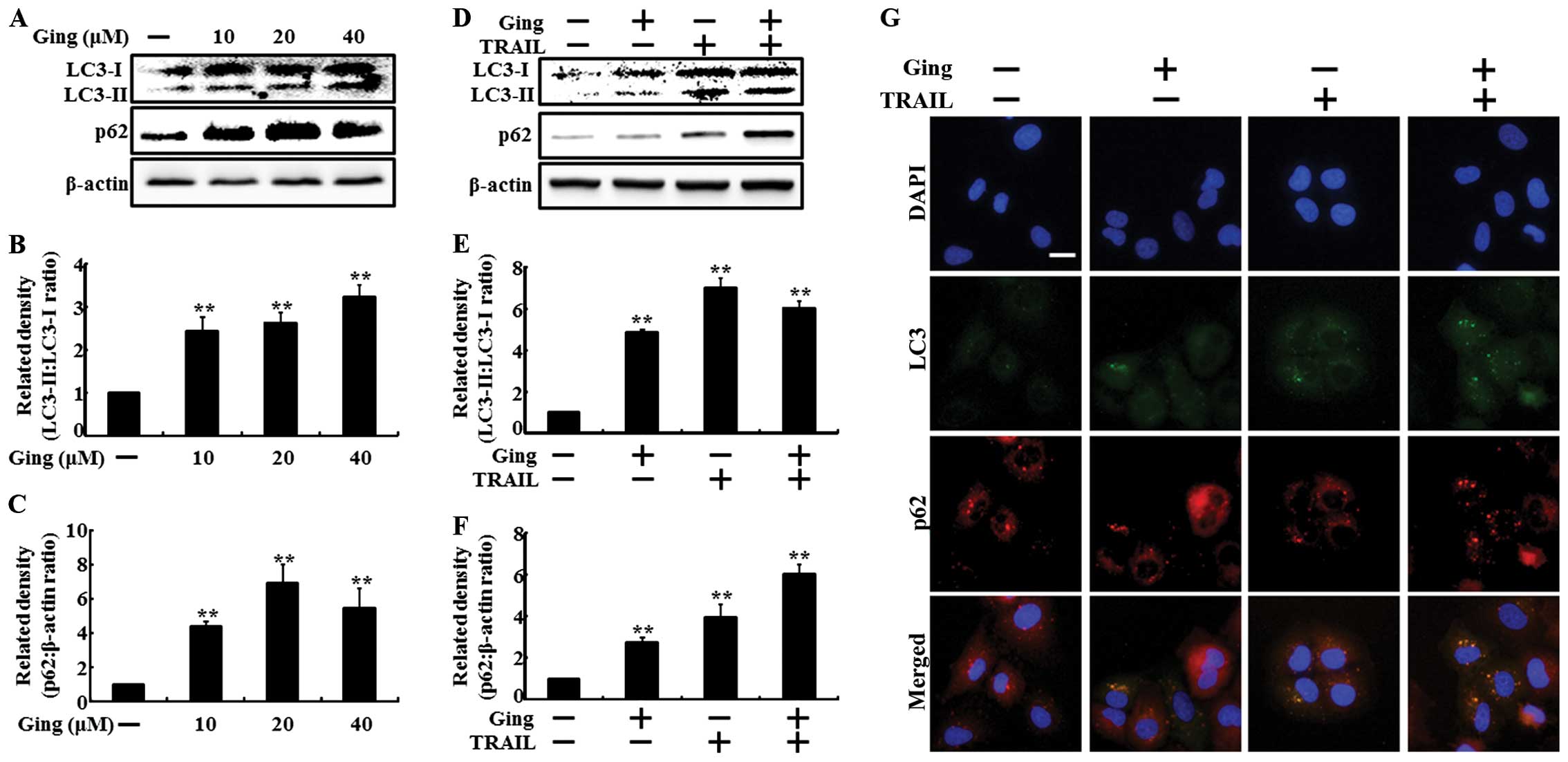
Gingerol inhibits autophagy flux in lung
adenocarcinoma cells
To investigate the effect of gingerol on autophagy
flux, we treated the A549 cells with gingerol in a dose-dependent
manner for 12 h and with TRAIL for an additional 2-h. All the cell
lysates were subjected to western blot analysis to determine
changes in LC3-II and p62. LC3-II expression is a marker for
complete autophagosomes. p62 is a ubiquitin-binding protein
involved in lysosome- or proteasome-dependent protein degradation.
Treatment with increasing gingerol concentrations for 12 h resulted
in a dose-dependent increase in LC3-II and p62 levels in the A549
cells (Fig. 2A). The density graphs
of the protein levels in LC3-II and p62 were also dose-dependently
changed (Fig. 2B and C). The
combined TRAIL and gingerol treatment enhanced the LC3-II and p62
levels compared with those of gingerol or TRAIL alone (Fig. 2D). The density graphs of the protein
levels in LC3-II and p62 were also increased in combined TRAIL and
gingerol treatment (Fig. 2E and F).
The ICC results showed that co-treatment of gingerol with TRAIL
enhanced LC3-II and p62 protein levels compared to those of
gingerol or TRAIL treatment alone (Fig.
2G).
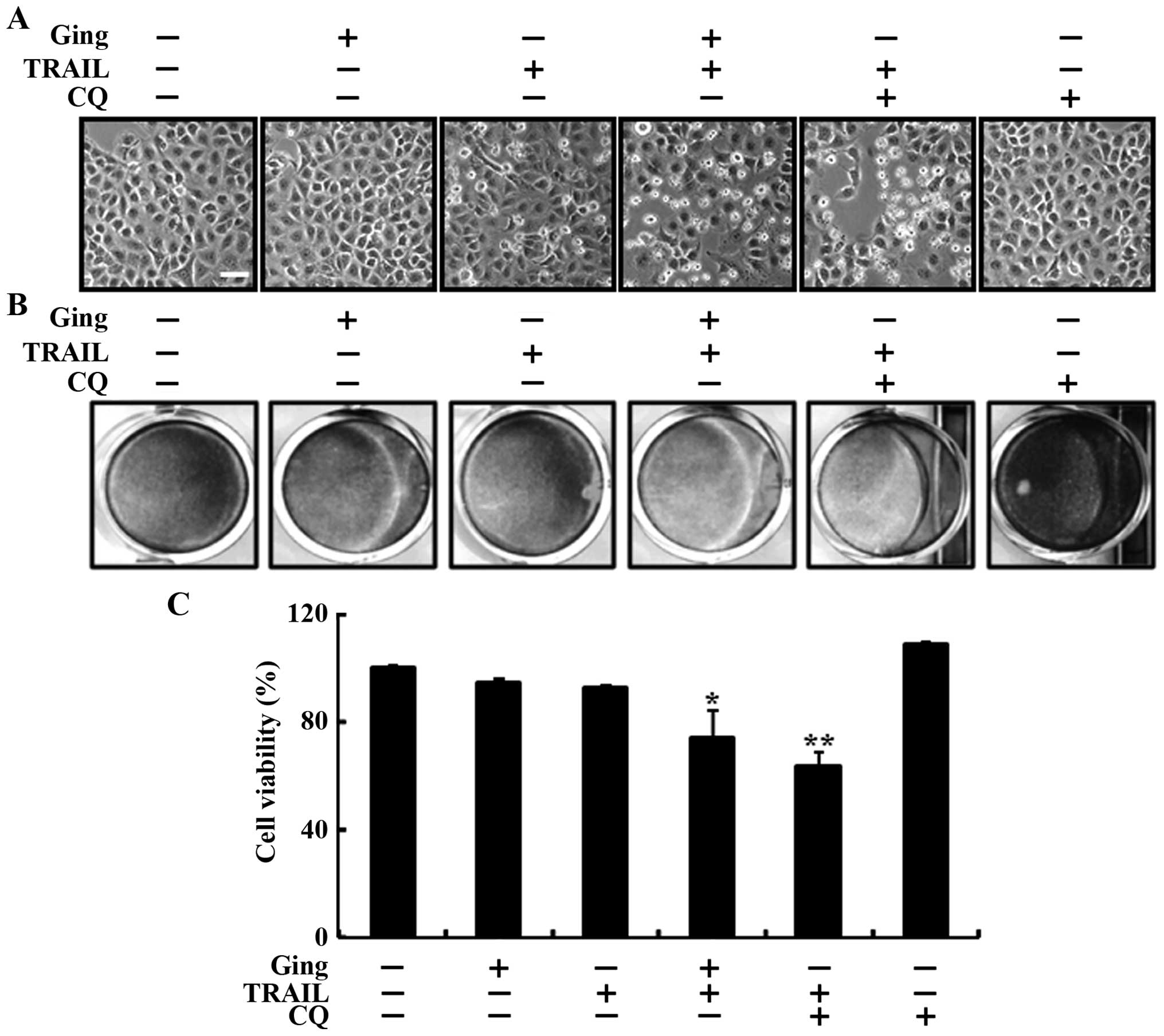
Gingerol-mediated enhancement of
TRAIL-induced cell death by inhibiting autophagy flux
We used chloroquine to investigate the effect of
gingerol-mediated enhancement of TRAIL-induced cell death in A549
lung adenocarcinoma cells. The cells were pretreated with the
indicated gingerol doses for 12 h and then treated with the TRAIL
protein for an additional 2-h. The cells were also pretreated with
chloroquine for 1 h followed by gingerol treatment. We photographed
the cells to investigate the morphological changes using a light
microscope and the crystal violet assay. As shown in Fig. 3, treating the A549 cells with either
TRAIL or gingerol alone slightly enhanced cell death, but combined
treatment with TRAIL and chloroquine strongly enhanced cell death.
The cell morphology results also supported this enhanced cell death
effect by TRAIL and chloroquine compared to that of gingerol or
TRAIL alone (Fig. 3A). The combined
treatment of chloroquine and TRAIL reduced cell viability and
significantly increased cell death in the A549 lung cancer cells
(Fig. 3B and C). These results
indicate that gingerol-mediated enhanced TRAIL-induced cell death
by inhibiting the autophagy flux.
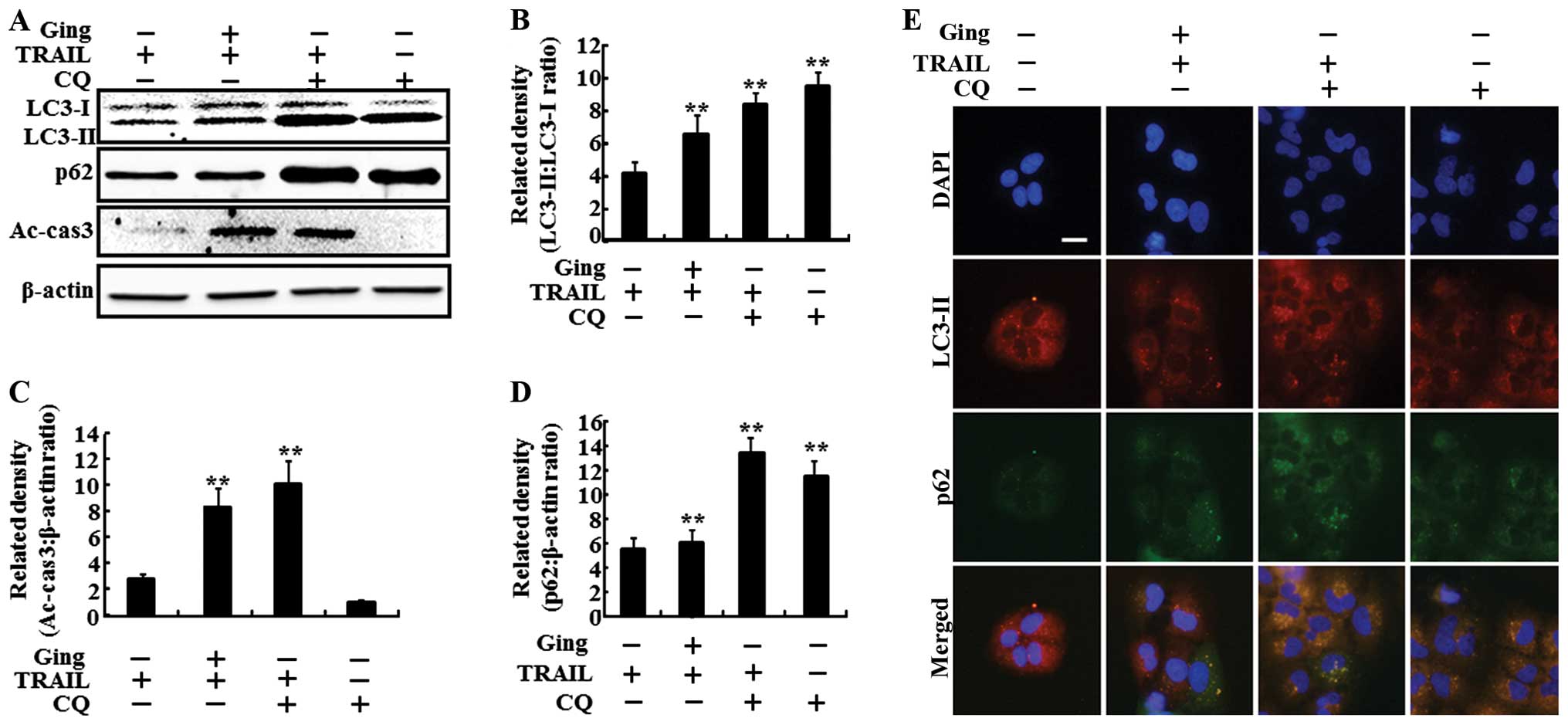
Gingerol-mediated enhancement of the
TRAIL-induced apoptotic pathway by inhibiting the autophagy
flux
We investigated the effect of gingerol-mediated
enhancement of the TRAIL-induced apoptotic pathway by inhibiting
the autophagy flux with chloroquine. The cells were pretreated with
the indicated doses of gingerol for 12 h and were treated with the
TRAIL protein for an additional 2-h. The cells were also pretreated
with chloroquine (10 μM) for 1 h followed by gingerol
treatment. As shown in Fig. 4A, the
gingerol and TRAIL co-treatment increased the LC3-II level.
Treatment with chloroquine alone also greatly increased the LC3-II
level. The combined treatment of TRAIL and chloroquine resulted in
more LC3-II compared to that of gingerol or TRAIL alone. The
gingerol and TRAIL co-treatment increased the p62 level.
Furthermore, the combined treatment of TRAIL and chloroquine
increased the p62 levels more than that of chloroquine or TRAIL
alone, confirming that gingerol inhibits the autophagy flux in A549
cells. The gingerol and TRAIL co-treatment inhibited cell viability
and significantly enhanced apoptosis in the A549 cells. These
results were confirmed by the intracellular apoptosis indicator
cleaved caspase-3. The density graphs of the protein levels in
LC3-II, caspase-3 and p62 were also changed in combined TRAIL and
chloroquine treatment (Fig. 4B, C
and D). The ICC results showed that the TRAIL and chloroquine
combined treatment increased the LC3-II and p62 levels compared to
those of chloroquine or gingerol alone (Fig. 4E).
Discussion
The purpose of the present study was to investigate
the function of gingerol and gingerol and TRAIL co-treatment on the
inhibition of autophagy flux and the regulation of TRAIL-mediated
sensitivity by gingerol in A549 human adenocarcinoma cells. The
results suggest that gingerol sensitizes TRAIL-induced apoptosis in
A549 lung adenocarcinoma cells by inhibiting autophagy flux.
TRAIL is a promising anticancer agent with the
ability to selectively trigger apoptosis in various tumors, but not
in most normal cells. The therapeutic potential of TRAIL is being
tested in various clinical trials (31). Induction of apoptosis requires
additional treatment with other chemotherapeutic agents, which
however, damage the normal cells. Gingerol has antiemetic,
antipyretic, anticancer and anti-inflammatory activities (32,33).
Furthermore, it is not significantly toxic to normal cells, but it
reduces rat liver tumor growth and induces apoptosis via a
TNF-α-mediated pathway (34).
Autophagy is a self-degradation mechanism responsible for cell
death or survival. Autophagy flux is the complete process of
autophagy, and A549 cell proliferation was impaired by chloroquine,
which is an autophagy inhibitor, suggesting that A549 cells may be
dependent on the autophagy pathway.
treatment enhanced the LC3-II and p62 levels
compared to that in the control by inhibiting the autophagy flux (F
Jin et al (5) showed that
the A549 cells are resistant to the apoptotic effects of TRAIL. We
observed that gingerol or TRAIL alone either did not or only
slightly induced death of the A549 adenocarcinoma cells. However,
the combined treatment of gingerol and TRAIL strongly induced death
in the A549 cells (Fig. 1). Some
studies have shown that TRAIL induces autophagy in various types of
cancer cells (35,36). However, our results indicate that
treatment with increasing concentrations of gingerol or TRAIL alone
resulted in a dose-dependent increase in the LC3-II and p62 levels
in the A549 adenocarcinoma cells. The combined TRAIL and gingerol
ig. 2). Gingerol promotes TRAIL-induced apoptosis in gastric cancer
cells (37). Pharmacological or
genetic inhibition of autophagy induces death in various cancer
cells, and we investigated the sensitivity of A549 cell
proliferation to pharmacological inhibition of autophagy with
chloroquine. Co-treatment with gingerol and TRAIL increased the
LC3-II and p62 levels. Furthermore, TRAIL and chloroquine combined
treatment increased the LC3-II and p62 levels compared to those of
chloroquine or gingerol alone. These results were confirmed by the
intracellular apoptosis indicator cleaved caspase-3 (Figs. 3 and 4).
In conclusion, gingerol induces apoptosis in A549
cells by inhibiting the autophagy flux. The combination of gingerol
and TRAIL strongly enhanced apoptosis in TRAIL-resistant A549
cells, suggesting that gingerol sensitizes TRAIL-induced apoptosis
in TRAIL-resistant A549 adenocarcinoma cells by inhibiting the
autophagy flux.
Acknowledgments
This study was supported by a grant from the
National Research Foundation of Korea (NRF), funded by the Korean
Government (MISP) (no. 2013R1A4A1069486).
References
|
1
|
Gonzalvez F and Ashkenazi A: New insights
into apoptosis signaling by Apo2L/TRAIL. Oncogene. 29:4752–4765.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aggarwal BB: Signalling pathways of the
TNF superfamily: A double-edged sword. Nat Rev Immunol. 3:745–756.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellail AC, Tse MC, Song JH, Phuphanich S,
Olson JJ, Sun SY and Hao C: DR5-mediated DISC controls caspase-8
cleavage and initiation of apoptosis in human glioblastomas. J Cell
Mol Med. 14:1303–1317. 2010. View Article : Google Scholar :
|
|
4
|
Bellail AC, Qi L, Mulligan P, Chhabra V
and Hao C: TRAIL agonists on clinical trials for cancer therapy:
The promises and the challenges. Rev Recent Clin Trials. 4:34–41.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin CY, Park C, Hwang HJ, Kim GY, Choi BT,
Kim WJ and Choi YH: Naringenin up-regulates the expression of death
receptor 5 and enhances TRAIL-induced apoptosis in human lung
cancer A549 cells. Mol Nutr Food Res. 55:300–309. 2011. View Article : Google Scholar
|
|
6
|
Oyagbemi AA, Saba AB and Azeez OI:
Molecular targets of [6]-gingerol: Its potential roles in cancer
chemoprevention. Biofactors. 36:169–178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shukla Y and Singh M: Cancer preventive
properties of ginger: A brief review. Food Chem Toxicol.
45:683–690. 2007. View Article : Google Scholar
|
|
8
|
Lee TY, Lee KC, Chen SY and Chang HH:
6-gingerol inhibits ROS and iNOS through the suppression of
PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated
mouse macrophages. Biochem Biophys Res Commun. 382:134–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bode AM, Ma WY, Surh YJ and Dong Z:
Inhibition of epidermal growth factor-induced cell transformation
and activator protein 1 activation by [6]-gingerol. Cancer Res.
61:850–853. 2001.PubMed/NCBI
|
|
10
|
Chakraborty D, Bishayee K, Ghosh S, Biswas
R, Mandal SK and Khuda-Bukhsh AR: [6]-gingerol induces caspase
3-dependent apoptosis and autophagy in cancer cells: Drug-DNA
interaction and expression of certain signal genes in HeLa cells.
Eur J Pharmacol. 694:20–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee E and Surh YJ: Induction of apoptosis
in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol.
Cancer Lett. 134:163–168. 1998. View Article : Google Scholar
|
|
12
|
Kroemer G, Mariño G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizushima N: Autophagy: process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klionsky DJ, Abeliovich H, Agostinis P,
Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA,
Ballabio A, et al: Guidelines for the use and interpretation of
assays for monitoring autophagy in higher eukaryotes. Autophagy.
4:151–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakatogawa H, Ichimura Y and Ohsumi Y:
Atg8, a ubiquitin-like protein required for autophagosome
formation, mediates membrane tethering and hemifusion. Cell.
130:165–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Z, Nair U and Klionsky DJ: Atg8
controls phagophore expansion during autophagosome formation. Mol
Biol Cell. 19:3290–3298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bjørkøy G, Lamark T, Brech A, Outzen H,
Perander M, Overvatn A, Stenmark H and Johansen T: p62/SQSTM1 forms
protein aggregates degraded by autophagy and has a protective
effect on huntingtin-induced cell death. J Cell Biol. 171:603–614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizushima N and Yoshimori T: How to
interpret LC3 immuno-blotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livesey KM, Tang D, Zeh HJ and Lotze MT:
Autophagy inhibition in combination cancer treatment. Curr Opin
Investig Drugs. 10:1269–1279. 2009.PubMed/NCBI
|
|
20
|
Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu
Y, Xie M, Yin X, Livesey KM, Lotze MT, et al: HMGB1-induced
autophagy promotes chemotherapy resistance in leukemia cells.
Leukemia. 25:23–31. 2011. View Article : Google Scholar
|
|
21
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carew JS, Espitia CM, Esquivel JA II,
Mahalingam D, Kelly KR, Reddy G, Giles FJ and Nawrocki ST:
Lucanthone is a novel inhibitor of autophagy that induces cathepsin
D-mediated apoptosis. J Biol Chem. 286:6602–6613. 2011. View Article : Google Scholar :
|
|
24
|
Boya P, González-Polo RA, Casares N,
Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D,
Souquere S, Yoshimori T, et al: Inhibition of macroautophagy
triggers apoptosis. Mol Cell Biol. 25:1025–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sotelo J, Briceño E and López-González MA:
Adding chloroquine to conventional treatment for glioblastoma
multiforme: A randomized, double-blind, placebo-controlled trial.
Ann Intern Med. 144:337–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goldberg SB, Supko JG, Neal JW, Muzikansky
A, Digumarthy S, Fidias P, Temel JS, Heist RS, Shaw AT, McCarthy
PO, et al: A phase I study of erlotinib and hydroxychloroquine in
advanced non-small-cell lung cancer. J Thorac Oncol. 7:1602–1608.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Poole B and Ohkuma S: Effect of weak bases
on the intralysosomal pH in mouse peritoneal macrophages. J Cell
Biol. 90:665–669. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fan C, Wang W, Zhao B, Zhang S and Miao J:
Chloroquine inhibits cell growth and induces cell death in A549
lung cancer cells. Bioorg Med Chem. 14:3218–3222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang PD, Zhao YL, Deng XQ, Mao YQ, Shi W,
Tang QQ, Li ZG, Zheng YZ, Yang SY and Wei YQ: Antitumor and
antimetastatic activities of chloroquine diphosphate in a murine
model of breast cancer. Biomed Pharmacother. 64:609–614. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoon YH, Cho KS, Hwang JJ, Lee SJ, Choi JA
and Koh JY: Induction of lysosomal dilatation, arrested autophagy,
and cell death by chloroquine in cultured ARPE-19 cells. Invest
Ophthalmol Vis Sci. 51:6030–6037. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: Are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshikawa M, Hatakeyama S, Chatani N,
Nishino Y and Yamahara J: Qualitative and quantitative analysis of
bioactive principles in Zingiberis Rhizoma by means of high
performance liquid chromatography and gas liquid chromatography. On
the evaluation of Zingiberis Rhizoma and chemical change of
constituents during Zingiberis Rhizoma processing. Yakugaku Zasshi.
113:307–315. 1993.In Japanese. PubMed/NCBI
|
|
33
|
Nakazawa T and Ohsawa K: Metabolism of
[6]-gingerol in rats. Life Sci. 70:2165–2175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Habib SH, Makpol S, Abdul Hamid NA, Das S,
Ngah WZ and Yusof YA: Ginger extract (Zingiber officinale) has
anti-cancer and anti-inflammatory effects on ethionine-induced
hepatoma rats. Clinics (São Paulo). 63:807–813. 2008. View Article : Google Scholar
|
|
35
|
Singh K, Sharma A, Mir MC, Drazba JA,
Heston WD, Magi-Galluzzi C, Hansel D, Rubin BP, Klein EA and
Almasan A: Autophagic flux determines cell death and survival in
response to Apo2L/TRAIL (dulanermin). Mol Cancer. 13:702014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park KJ, Lee SH, Kim TI, Lee HW, Lee CH,
Kim EH, Jang JY, Choi KS, Kwon MH and Kim YS: A human scFv antibody
against TRAIL receptor 2 induces autophagic cell death in both
TRAIL-sensitive and TRAIL-resistant cancer cells. Cancer Res.
67:7327–7334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishiguro K, Ando T, Maeda O, Ohmiya N,
Niwa Y, Kadomatsu K and Goto H: Ginger ingredients reduce viability
of gastric cancer cells via distinct mechanisms. Biochem Biophys
Res Commun. 362:218–223. 2007. View Article : Google Scholar : PubMed/NCBI
|