Introduction
Worldwide, colorectal cancer is the third most
common cancer (9.7%) and it was the fourth leading cause of
cancer-related mortality in 2012 (1). For the treatment of unresectable
metastatic colorectal cancer, systemic chemotherapeutic agents such
as fluoropyrimidines, irinotecan (CPT-11), oxaliplatin, and
targeted agents such as bevacizumab (an anti-VEGF monoclonal
antibody) and cetuximab, or panitumumab (anti-EGFR monoclonal
antibodies) are currently used, while the survival of patients with
unresectable metastatic colorectal cancer has improved (2–5). Even
if these standard therapies are initially effective, many patients
relapse due to the onset of drug resistance and are subsequently
placed on salvage chemotherapy. The multikinase inhibitor
regorafenib was reported to prolong the overall survival compared
to placebo for the treatment of unresectable refractory colorectal
cancer (6).
TAS-102 is a combination of an antineoplastic
thymidine-based nucleoside analogue, trifluridine (FTD) and a
thymidine phosphorylase inhibitor, tipiracil hydrochloride (TPI) at
a molecular ratio of 1:0.5. FTD is the active antitumor component
of TAS-102; its monophosphate form inhibits thymidylate synthase,
and its triphosphate form is incorporated into the DNA in tumor
cells. The inhibition of thymidylate synthase caused by oral FTD
rapidly disappears after the drug elimination, but the
incorporation of FTD into the DNA is known to have prolonged
antitumor effects (7–9).
When FTD is administered orally, it is rapidly
degraded to its inactive form in the intestines and the liver
(first-pass effect) (8), but the
combination with TPI helps to maintain adequate FTD plasma
concentrations (10). TPI thus,
potentiates the antitumor activity of FTD (10), and the optimal molecular ratio of
FTD to TPI has been proven to be 1:0.5 (11). In preclinical studies, both FTD and
TAS-102 were found to exhibit some unique antitumor effects, such
as their efficacy against 5-FU-resistant colorectal tumor cells not
only in vitro but also in vivo (12–14),
and a continued effect persisted after the end of drug
administration (9,15).
In a randomized phase II trial, the overall survival
period of patients receiving TAS-102 with the best supportive care
(9 months) was significantly longer than that of a placebo with the
best supportive care group (6.6 months, P=0.0011) in patients with
metastatic colorectal cancer, who were refractory to or intolerant
of standard chemotherapies (16).
TAS-102 showed a significant improvement in overall and progression
free survival and a favorable safety profile in comparison to
placebo in patients with metastatic colorectal cancer refractory to
standard chemotherapies in an international multicenter randomized
double-blind phase III study (RECOURSE), patients received in both
arms the best supportive care (17). TAS-102 was approved for clinical use
in Japan in March 2014. Bevacizumab and cetuximab or panitumumab
are key drugs in colorectal cancer treatment, used either alone or
in combination with other chemotherapies (3–5,18–21).
In the present study, we evaluated the antitumor
effects of TAS-102 in combination with bevacizumab and cetuximab or
panitumumab using a nude mouse xenograft model of colorectal
cancer.
Materials and methods
Reagents
FTD, F3TMP ammonium salt,
F3TDP, F3TTP and TPI were obtained from Taiho
Pharmaceutical (Tokyo, Japan). Bevacizumab and cetuximab or
panitumumab were purchased from Roche (Basel, Switzerland), Merck
Serono (Darmstadt, Germany), and Amgen (Thousand Oaks, CA, USA),
respectively. Hydroxypropyl methylcellulose (HPMC) was purchased
from Shin-Etsu Chemical (Tokyo, Japan).
Cancer cell lines
The human colon cancer cell lines SW48 and HCT116
were purchased from the American Type Culture Collection (ATCC;
Rockville, MD, USA), and Dainippon Pharma (Osaka, Japan),
respectively. SW48 and HCT116 cells were maintained by implantation
into the right axilla of nude mice at 3-week intervals. The
KRAS mutation status of SW48 and HCT116 are wild-type and
mutant, respectively (22).
Animals
Male nude mice were purchased from CLEA Japan
(Tokyo, Japan) and were housed under specific pathogen-free
conditions, with food and water provided ad libitum. All the
animal studies were performed according to the instructions and
with the approval of the Institutional Animal Care and use
Committee of Taiho Pharmaceutical Co. (approval nos. 14Tb04,
M01-2008-0004, 03-12-008 and AM003-14-016).
Antitumor activity in vivo
After the animals had been in quarantine for 1 week,
they were implanted subcutaneously with a solid human tumor, the
volume of which was ~8 mm3 (23). In order to evaluate the antitumor
activity, the mice were randomized on day 0 according to tumor
volume, once the mean tumor volume had reached ~100–200
mm3. Each group consisted of 6 or 7 mice.
TAS-102 was prepared by mixing FTD and TPI in a
molecular ratio of 1:0.5 in 0.5% HPMC solution. The dose of TAS-102
was expressed on the basis of the amount of FTD, and was
administered orally from day 1 to 14, twice a day at ~6-h intervals
at the reported effective dose (150 mg/kg/day) (7,11). For
the control group, vehicle (0.5% HPMC solution) was administered at
10 ml/kg in a similar manner. Bevacizumab was administered
intraperitoneally in a dose of 5 mg/kg on days 1, 4, 8 and 11.
Cetuximab and panitumumab were administered intraperitoneally in a
dose of 4.4 and 3 mg/kg, respectively, on days 1, 5, 8 and 12.
Tumor diameters were measured twice a week, and the
tumor volume was estimated as 0.5 × length × width2. The
relative tumor volume (RTV) was calculated using the following
formula: RTV = (tumor volume on measured day)/(tumor volume on day
0). On day 29, the tumor growth inhibition ratio (TGI, %) was
calculated using the following formula: TGI (%) = [1 − (RTV of the
treated group)/(RTV of the control group)] × 100 (%).
Antitumor activity was evaluated on the basis of the
time taken for the relative tumor volume to increase five-fold
(RTV5). In order to assess RTV5, the RTV change of each mouse was
plotted and the date when RTV5 was reached was estimated using
linear regression based on the dates on either side of this event
(24).
To evaluate toxicity, body weight was measured twice
a week and body weight change (BWC) was calculated using the
following formula: BWC (%) = [(body weight on the last day) − (body
weight on day 0)]/(body weight on day 0) × 100 (%). Toxicity was
defined as a BWC of <−20%, or toxic mortality.
Extraction and quantification of tumor
FTD and its phosphorylated forms
FTD and its phosphorylated forms were determined by
liquid chromatograph-mass spectrometry (LCMS-8040; Shimadzu, kyoto,
Japan). TAS-102 was administered orally from day 1 to 3 twice a day
(150 mg/kg) and bevacizumab was administered on day 1 (5 mg/kg)
into nude mice bearing SW48 and HCT116 xenografts. Each group
consisted of 5 mice. Two hours after the last TAS-102
administration, mice were sacrificed and tumors were collected and
frozen quickly by using liquid nitrogen.
For extraction of FTD and its metabolite, the tumors
were homogenized in 0.48 N perchloric acid solution with a
Multi-Beads Shocker (Yasui Kikai, Osaka, Japan), and centrifuged at
20,000 × g for 5 min at 4°C. The aqueous phase was recovered and,
twice the volume of the mixture of 0.5 N tri-n-octylamine and
dichloromethane (1:3) was added to the acid soluble fractions and
mixed by vortexing. Then samples were centrifuged at 20,000 × g for
5 min at 4°C. The aqueous phases were collected and used as samples
for the next mass spectrometric analysis. Samples (5 μl)
were analyzed on a triple quadruple mass spectrometer (LCMS-8040;
Shimadzu), with a Mastro C18 column (3 μm particle size,
length 150 mm and inner diameter 2.1 mm; Shimadzu GLC, Tokyo,
Japan). Samples from xenografts which were not administrated
TAS-102 were used as blank samples. FTD, F3TMP ammonium
salt, F3TDP and F3TTP were mixed at an
equally molecular ratio and a standard solution was prepared at the
concentration of 10, 3, 1, 0.3, 0.1 0.03 and 0.01 μM for
each compound. The mobile phase consisted of a linear gradient of
0.5 mM dibutylammonium acetate in distilled water (A) 100% methanol
(B): 0–4 min, 1–60% B (v/v); 4–10 min, 60–60% B; 10–10.1 min, 60–1%
B; 10.1–21 min, 1–1% B. The flow rate was 0.2 ml/min. The effluent
from the column was measured by mass spectrometry using
electrospray ionization (ESI). ESI parameters were as follows:
interface temperature 350°C, gas flow 3 l/min, heat-block
temperature 400°C, and drying gas flow 15 l/min. The mass
spectrometer was operated in the negative ion mode using
LabSolution software version 5.60 SP2 (Shimadzu) in a multiple
reaction monitoring mode. The monitored transitions were m/z
295.05>179.25 for FTD, m/z 375.05>179.20 for
F3dTMP, m/z 454.95>275.05 for F3dTDP, and
m/z 534.95>159.10 for F3dTTP. The lower limit of
quantification (LLOQ) was set up as a signal to noise ratio of 3 by
analyzing the standard tumor lysate. The LLOQs of FTD,
F3dTMP, F3dTDP and F3dTTP were
0.18, 0.06, 0.06, and 1.8 nmol/g tissue in SW48 lysate, and 0.06,
0.06, 0.06, and 0.6 nmol/g tissue in HCT116 lysate, respectively.
Values of FTD phosphates were calculated by taking the sum of
F3dTMP, F3dTDP and F3dTTP for each
mouse.
Statistical analysis
The significance of the differences in the mean RTV
between the treated and the control groups on day 29 was analyzed
by using the Aspin-Welch two-sided t-test. The combinational
antitumor effect of TAS-102 and bevacizumab, cetuximab or
panitumumab was analyzed according to a closed-testing procedure
using the Aspin-Welch two-tailed t-test (25). The statistical analysis of RTV5 was
evaluated using the log-rank test according to the reported method
(26). In cases where the RTV of
the treated animal was not reached, the data were censored and the
RTV5 was designated as 28 or 29. Differences with an associated
P-value of <0.05 were considered significant. P-values were
calculated using Exsus, version 8.1 (Arm Systex, Osaka, Japan).
The significance of increased FTD,
F3dTMP, F3dTDP, and F3dTTP in the
treated groups compared to the control groups was evaluated by
using the Student’s one-sided t-test with statistical software
JMP®, version 9.0.2 (SAS Institute, Cary, NC, USA).
Results
Bevacizumab increases the antitumor
efficacy of TAS-102
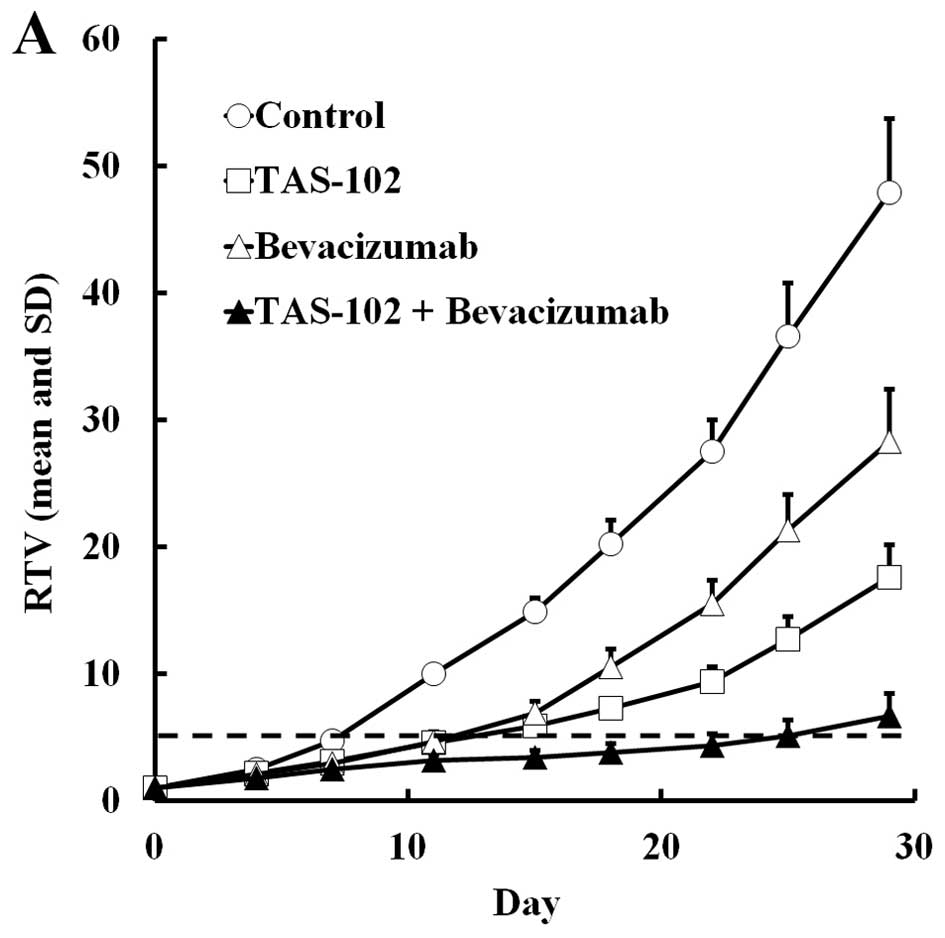
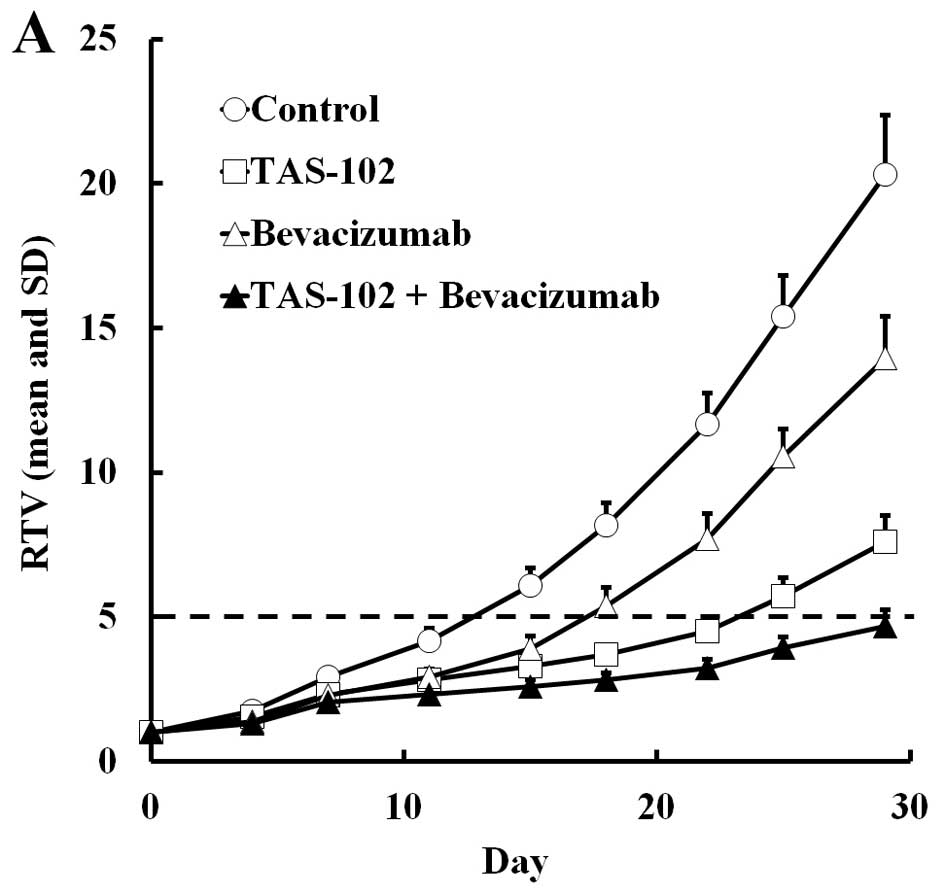
TAS-102 and bevacizumab either alone or in
combination, were administered to mice bearing SW48 or HCT116
colorectal tumors. The RTV change and BWC in SW48 and HCT116 are
shown in Figs. 1 and 2, respectively. In both experiments,
TAS-102 and bevacizumab alone inhibited tumor growth. Moreover,
combined TAS-102 and bevacizumab treatment had superior antitumor
activity compared to either drug alone, and had no significant
effect on the body weight compared to TAS-102 monotherapy.
We also evaluated the RTV5 of tumors. TAS-102 or
bevacizumab alone significantly extended the RTV5 (P<0.01), but
combined TAS-102 and bevacizumab extended the RTV5 still further
relative to either monotherapy in both SW48 and HCT116 xenografts
(Tables I and II). For SW48 tumors, the RTV5 of the
combination group was more than twice as long as the bevacizumab
monotherapy group, and for the HCT116 tumors, 4 of 6 mice treated
with combination therapy did not reach RTV5 by day 29.
 | Table IAntitumor activity and body weight
changes in mice implanted with human colorectal tumor SW48 after
treatment with TAS-102 and bevacizumab. |
Table I
Antitumor activity and body weight
changes in mice implanted with human colorectal tumor SW48 after
treatment with TAS-102 and bevacizumab.
| Group | Dose (mg/kg) | Schedule | RTVa (mean ± SD) | TGIb (%) | RTV5c (days) | BWCd
|
|---|
| (Mean ± SD, g) | (%) |
|---|
| Control | – | – | 47.94±5.78 | 0 | 7.23±0.23 | 2.0±2.0- | 7.8 |
| TAS-102 | 150 | Day 1–14
(b.i.d.) | 17.56±4.12e | 63.4 | 12.49±2.66g | 0.4±2.9 NS | 1.5 |
| Bevacizumab | 5 | Day 1, 4, 8,
11 | 28.27±2.61e | 41.0 | 11.61±1.07g | 2.1±1.5 NS | 8.1 |
| Combination | 150+5 | | 6.66±1.75e,f | 86.1 | 24.72±4.24g,h | 0.1±1.7 NS | 0.4 |
 | Table IIAntitumor activity and body weight
changes in mice implanted with human colorectal tumor HCT116 after
treatment with TAS-102 and bevacizumab. |
Table II
Antitumor activity and body weight
changes in mice implanted with human colorectal tumor HCT116 after
treatment with TAS-102 and bevacizumab.
| Group | Dose (mg/kg) | Schedule | RTVa (mean ± SD) | TGIb (%) | RTV5c (days) | BWCd
|
|---|
| (Mean ± SD, g) | (%) |
|---|
| Control | – | – | 20.32±2.04 | 0 | 12.81±1.06 | 0.6±1.9- | 2.2 |
| TAS-102 | 150 | Day 1–14
(b.i.d.) | 7.60±0.90e | 62.6 | 23.24±1.41g | −1.4±2.2 NS | −5.6 |
| Bevacizumab | 5 | Day 1, 4, 8,
11 | 13.97±1.43e | 31.3 | 17.32±1.17g | 1.3±0.5 NS | 4.9 |
| Combination | 150+5 | | 4.66±0.58e,f | 77.1 | >28.57g,h | −0.2±1.6 NS | −0.8 |
Increased FTD and FTD phosphate tumor
levels after being combined with bevacizumab and TAS-102
treatment
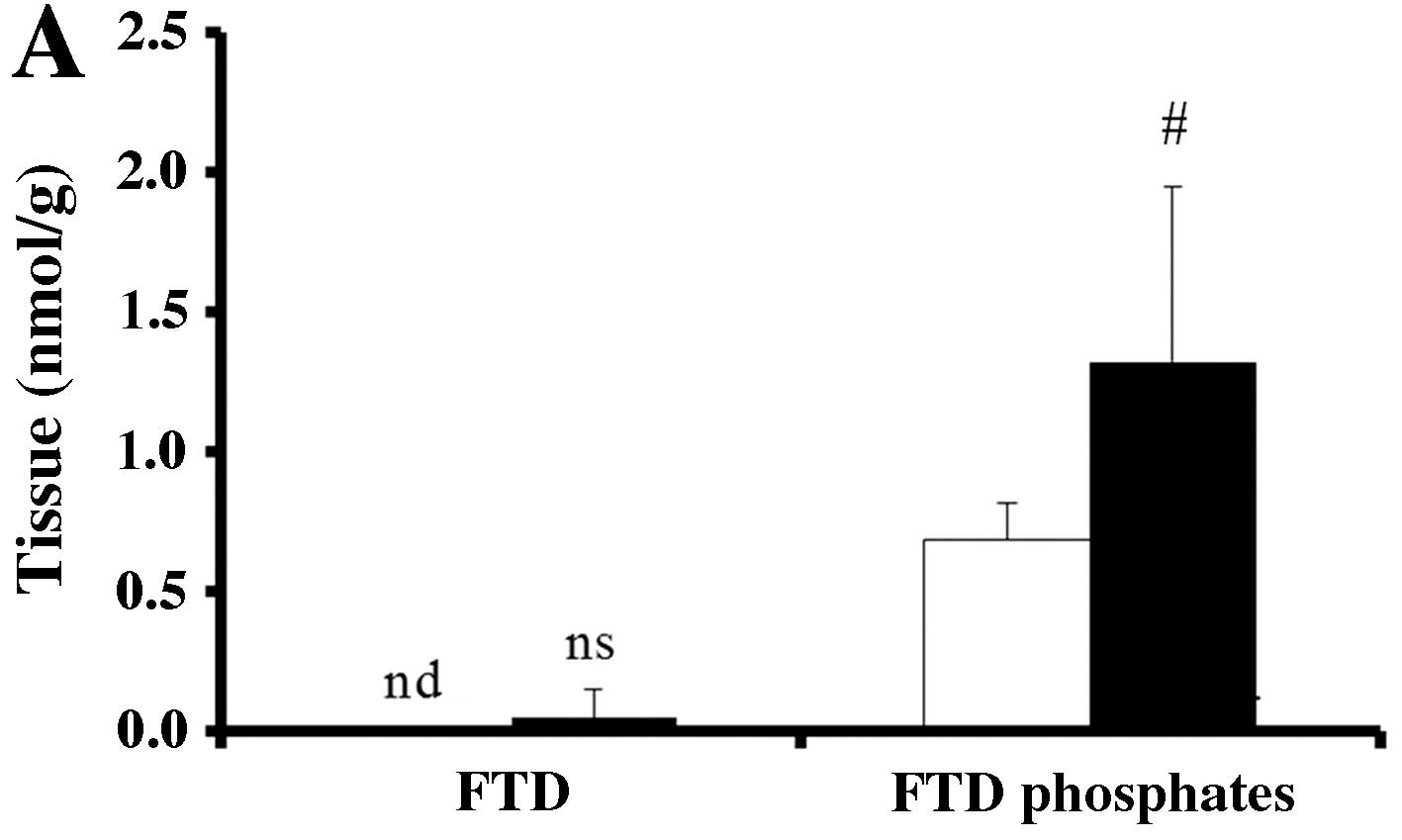
To investigate why bevacizumab improves the
antitumor effect of TAS-102, we measured the concentration of FTD
and its phosphates (F3dTMP, F3dTDP and
F3dTTP) in SW48 and HCT116 tumors. Very little FTD was
detected in SW48 tumors. FTD phosphates level was significantly
higher in the TAS-102 and bevacizumab combination group in SW48
tumors compared to that from mice treated with TAS-102 monotherapy
(P<0.05, Fig. 3A).
In HCT116 tumors, FTD was detected. Although it was
not significant, FTD and FTD phosphates tended to increase after
combined TAS-102 and bevacizumab treatment compared to TAS-102
monotherapy (Fig. 3b).
Cetuximab and panitumumab increase the
antitumor efficacy of TAS-102
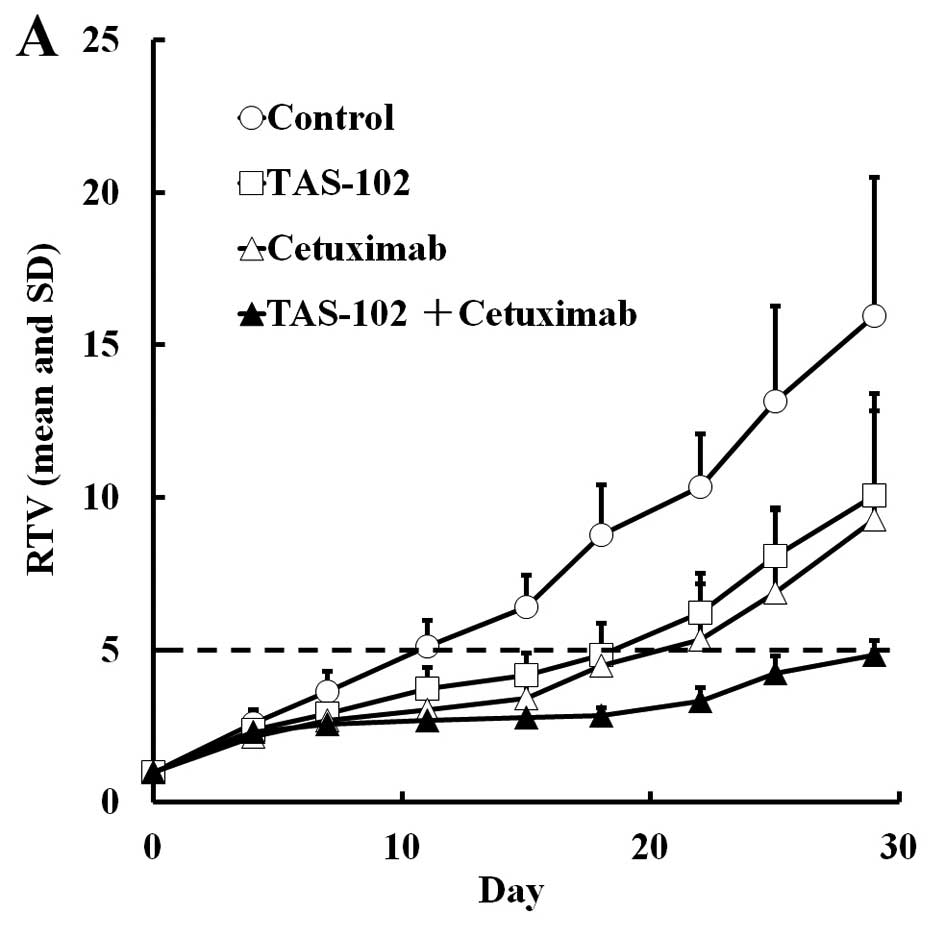
We evaluated the efficacy of cetuximab and
panitumumab combined with TAS-102 in the SW48 xenograft model.
TAS-102 and cetuximab both suppressed tumor growth compared to the
vehicle alone (P<0.05 and 0.01, respectively, Table III), and combined cetuximab and
TAS-102 significantly suppressed tumor growth compared to each
monotherapy on day 29. Similarly, combined TAS-102 and cetuximab
significantly extended the RTV5 compared to either drug alone.
TAS-102 caused a significant reduction in the body weight compared
to untreated mice (P<0.01) at the nadir on day 15 and 18, but
the mice recovered and the weight loss was <10% on day 29. Thus,
the toxicity of TAS-102 seemed to be tolerable (Fig. 4). Interestingly, combined cetuximab
and TAS-102 did not result in significant body weight loss, despite
having superior antitumor efficacy (Fig. 4).
 | Table IIIAntitumor activity and body weight
changes in mice implanted with human colorectal tumor SW48 after
treatment with TAS-102 and cetuximab. |
Table III
Antitumor activity and body weight
changes in mice implanted with human colorectal tumor SW48 after
treatment with TAS-102 and cetuximab.
| Group | Dose (mg/kg) | Schedule | RTVa (mean ± SD) | TGIb (%) | RTV5c (days) | BWCd
|
|---|
| (Mean ± SD, g) | (%) |
|---|
| Control | – | – | 15.95±4.54 | 0 | 11.65±2.10 | 2.2±1.4- | 8.6 |
| TAS-102 | 150 | Day 1–14
(b.i.d.) | 10.05±3.22f | 37.0 | 19.65±5.25h | −1.3±0.4e | −5.3 |
| Cetuximab | 4.4 | Day 1, 5, 8,
12 | 9.29±2.79e | 41.7 | 21.15±3.92i | 3.0±1.2 NS | 11.6 |
| Combination | 150+4.4 | | 4.85±0.46e,g | 69.6 | >28.34i,j | 0.9±0.8 NS | 3.6 |
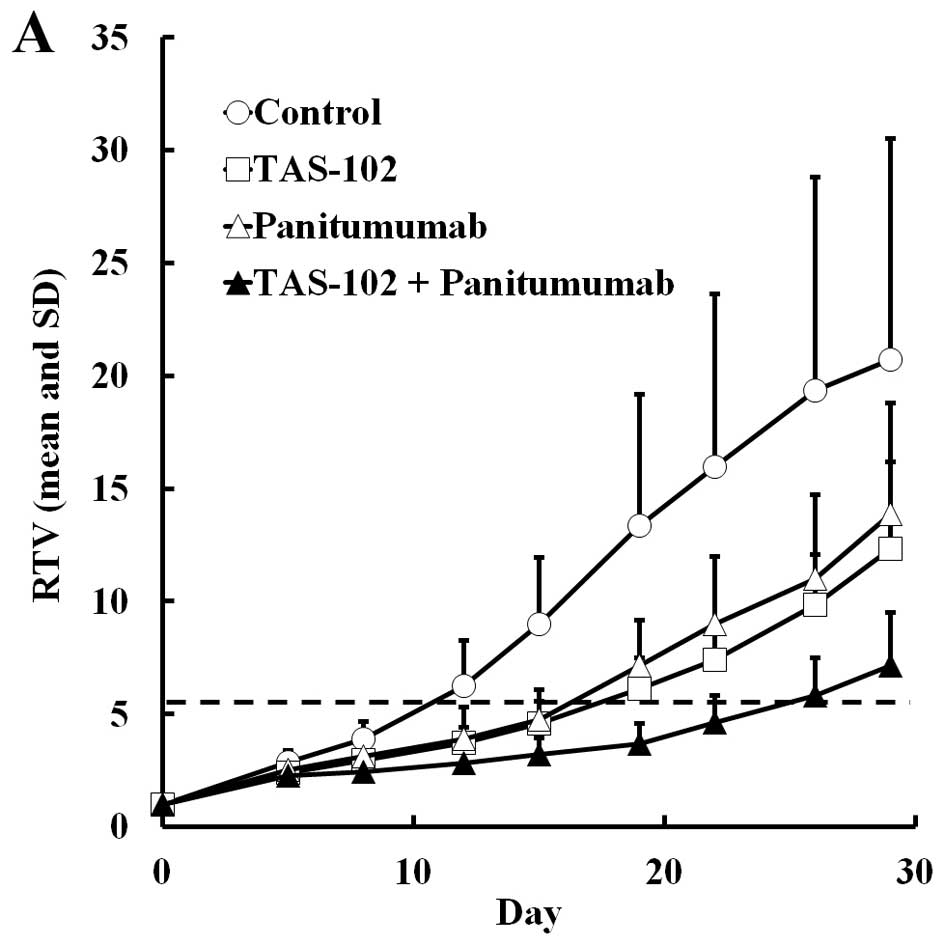
TAS-102 or panitumumab monotherapy tended to inhibit
tumor growth but these reductions were not significant, since the
standard deviation of RTV in the control group varied only in this
experiment. Combined TAS-102 and panitumumab significantly reduced
tumor volume and extended RTV5 (P<0.05 and 0.01, respectively,
Table IV), while the combined
therapy also resulted in less weight loss than TAS-102 alone,
despite showing a superior antitumor effect (Fig. 5).
 | Table IVAntitumor activity and body weight
changes in mice implanted with human colorectal tumor SW48 after
treatment with TAS-102 and panitumumab. |
Table IV
Antitumor activity and body weight
changes in mice implanted with human colorectal tumor SW48 after
treatment with TAS-102 and panitumumab.
| Group | Dose (mg/kg) | Schedule | RTVa (mean ± SD) | TGIb (%) | RTV5c (days) | BWCd
|
|---|
| (Mean ± SD, g) | (%) |
|---|
| Control | – | – | 20.70±9.81 | 0 | 11.51±4.84 | 0.6±1.5- | 2.3 |
| TAS-102 | 150 | Day 1–14
(b.i.d.) | 12.33±3.86 NS | 40.5 | 16.40±2.37 NS | −1.8±1.6e | −7.1 |
| Panitumumab | 3 | Day 1, 5, 8,
12 | 13.86±4.94 NS | 33.1 | 15.59±4.33 NS | 0.3±1.1 NS | 1.0 |
| Combination | 150+3 | | 7.15±2.34e | 65.5 | >23.85f,g | 0.7±1.0 NS | 2.8 |
Discussion
In the present study, we found that combined
bevacizumab and TAS-102 suppresses tumor growth to a significantly
greater degree than either drug alone in nude mice with colorectal
cancer, but had no significant effect on the body weight. Thus,
bevacizumab appears to enhance the antitumor effect of TAS-102
without increasing its toxicity.
We used two colorectal cancer cell lines: SW48,
which is KRAS wild-type, and HCT116, which carries a
KRAS mutation. TAS-102 was effective regardless of the
KRAS status, at least in the present study. In a randomized
phase-II trial for metastatic colorectal cancer patients who were
refractory or intolerant to standard chemotherapies, TAS-102 also
improved overall survival regardless of the KRAS tumor
status (16). It has also been
reported that the effect of bevacizumab is not influenced by the
KRAS status (27,28). Furthermore, combined TAS-102 and
bevacizumab showed superior antitumor efficacy to TAS-102 alone,
and therefore, this combination therapy may be beneficial to
patients with both mutated and wild-type KRAS tumors.
In order to evaluate the mechanism underlying the
enhanced antitumor effect of combined TAS-102 and bevacizumab, we
measured FTD and its phosphorylated forms in tumors, as these are
the active components and metabolites of TAS-102. Phosphorylated
FTD levels were increased by combining TAS-102 and bevacizumab in
both SW48 and HCT116 tumors. Tumor blood vessels are generally
poorly organized and hyperpermeable, with an impaired gradient
between vascular and interstitial pressure and, consequently, a
diminished blood supply (29). This
may also limit the accumulation of FTD in tumors. Bevacizumab
inhibits angiogenesis through antagonizing vascular endothelial
growth factor and may therefore normalize tumor vasculature,
improving tumor blood supply and increasing FTD accumulation and
its subsequent phosphorylation in the tumor.
We also evaluated the combination of TAS-102 and the
anti-epidermal growth factor receptor antibodies, cetuximab and
panitumumab, in SW48 and KRAS wild-type tumors. Both
enhanced the antitumor effect of TAS-102. Interestingly, combining
TAS-102 with cetuximab or panitumumab reduced the weight loss that
occurred after TAS-102 monotherapy. We observed no severe toxicity
after combination treatment, as reflected by the absence of weight
loss or drug-related deaths. However, other toxicities were not
evaluated. In some clinical studies, most frequently observed
toxicities were gastrointestinal and hematologic in phase II and
III of TAS-102 (16,17). Careful monitoring of the overall
side effects, including hematological toxicities, will be needed to
evaluate the efficacy of these combination therapies in clinical
studies.
In conclusion, we have demonstrated that
bevacizumab, cetuximab and panitumumab enhance the antitumor effect
of TAS-102 in colorectal cancer. These combination therapies may be
proven to be promising options for patients suffering from cancer
that is refractory to the existing drugs. A clinical study of
combined TAS-102 and bevacizumab therapy is ongoing (no.
UMIN000012883), and we expect that its outcome will be highly
informative.
Acknowledgments
We would like to thank Editage (www.editage.jp) for the English language editing.
Abbreviations:
|
BWC
|
body weight change
|
|
FTD
|
trifluridine
|
|
F3dTTP
|
α,α,α-trifluorothymidine
triphosphate
|
|
HPMC
|
hydroxypropyl methylcellulose
|
|
LLOQ
|
lower limit of quantification
|
|
RTV
|
relative tumor volume
|
|
RTV5
|
time taken for the relative tumor
volume to increase five-fold
|
|
TGI
|
tumor growth inhibition
|
|
LC-MS/MS
|
liquid chromatography-tandem mass
spectrometry
|
|
TPI
|
tipiracil hydrochloride
|
|
F3dTMP
|
α,α,α-trifluorothymidine
monophosphate
|
|
F3dTDP
|
α,α,α-trifluorothymidine
diphosphate
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Armand JP, Ducreux M, Mahjoubi M,
Abigerges D, Bugat R, Chabot G, Herait P, de Forni M and Rougier P:
CPT-11 (irinotecan) in the treatment of colorectal cancer. Eur J
Cancer. 31A:1283–1287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saltz LB, Clarke S, Diaz-Rubio E, et al:
Bevacizumab in combination with oxaliplatin-based chemotherapy as
first-line therapy in metastatic colorectal cancer: A randomized
phase III study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sobrero AF, Maurel J, Fehrenbacher L, et
al: EPIC: Phase III trial of cetuximab plus irinotecan after
fluoropyrimidine and oxaliplatin failure in patients with
metastatic colorectal cancer. J Clin Oncol. 26:2311–2319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Peeters M, Siena S, et al:
Open-label phase III trial of panitumumab plus best supportive care
compared with best supportive care alone in patients with
chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol.
25:1658–1664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grothey A, Van Cutsem E, Sobrero A, et al:
CORRECT Study Group: Regorafenib monotherapy for previously treated
metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar
|
|
7
|
Emura T, Nakagawa F, Fujioka A, Ohshimo H,
Yokogawa T, Okabe H and Kitazato K: An optimal dosing schedule for
a novel combination antimetabolite, TAS-102, based on its
intracellular metabolism and its incorporation into DNA. Int J Mol
Med. 13:249–255. 2004.PubMed/NCBI
|
|
8
|
Dexter DL, Wolberg WH, Ansfield FJ, Helson
L and Heidelberger C: The clinical pharmacology of
5-trifluoromethyl-2′-deoxyuridine. Cancer Res. 32:247–253.
1972.PubMed/NCBI
|
|
9
|
Tanaka N, Sakamoto K, Okabe H, et al:
Repeated oral dosing of TAS-102 confers high trifluridine
incorporation into DNA and sustained antitumor activity in mouse
models. Oncol Rep. 32:2319–2326. 2014.PubMed/NCBI
|
|
10
|
Fukushima M, Suzuki N, Emura T, Yano S,
Kazuno H, Tada Y, Yamada Y and Asao T: Structure and activity of
specific inhibitors of thymidine phosphorylase to potentiate the
function of antitumor 2′-deoxyribonucleosides. Biochem Pharmacol.
59:1227–1236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emura T, Suzuki N, Fujioka A, Ohshimo H
and Fukushima M: Potentiation of the antitumor activity of α, α,
α-trifluorothymidine by the co-administration of an inhibitor of
thymidine phosphorylase at a suitable molar ratio in vivo. Int J
Oncol. 27:449–455. 2005.PubMed/NCBI
|
|
12
|
Emura T, Suzuki N, Yamaguchi M, Ohshimo H
and Fukushima M: A novel combination antimetabolite, TAS-102,
exhibits antitumor activity in FU-resistant human cancer cells
through a mechanism involving FTD incorporation in DNA. Int J
Oncol. 25:571–578. 2004.PubMed/NCBI
|
|
13
|
Emura T, Murakami Y, Nakagawa F, Fukushima
M and Kitazato K: A novel antimetabolite, TAS-102 retains its
effect on FU-related resistant cancer cells. Int J Mol Med.
13:545–549. 2004.PubMed/NCBI
|
|
14
|
Murakami Y, Kazuno H, Emura T, Tsujimoto
H, Suzuki N and Fukushima M: Different mechanisms of acquired
resistance to fluorinated pyrimidines in human colorectal cancer
cells. Int J Oncol. 17:277–283. 2000.PubMed/NCBI
|
|
15
|
Utsugi T: New challenges and inspired
answers for anticancer drug discovery and development. Jpn J Clin
Oncol. 43:945–953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshino T, Mizunuma N, Yamazaki K, et al:
TAS-102 monotherapy for pretreated metastatic colorectal cancer: A
double-blind, randomised, placebo-controlled phase 2 trial. Lancet
Oncol. 13:993–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshino T, Mayer R, Falcon A, et al:
RECOURSE study group: Results of a multicenter, randomized,
double-blind, phase III study of TAS-102 vs. placebo, with best
supportive care (BSC), in patients (pts) with metastatic colorectal
cancer (MCRC) refractory to standard therapies (RECOURSE). Ann
Oncol. 25(Suppl 2): 1–117. 2014. View Article : Google Scholar
|
|
18
|
Welch S, Spithoff K, Rumble RB and Maroun
J; Gastrointestinal Cancer Disease Site Group: Bevacizumab combined
with chemotherapy for patients with advanced colorectal cancer: A
systematic review. Ann Oncol. 21:1152–1162. 2010. View Article : Google Scholar
|
|
19
|
Van Cutsem E, Köhne CH, Hitre E, et al:
Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bokemeyer C, Bondarenko I, Makhson A, et
al: Fluorouracil, leucovorin, and oxaliplatin with and without
cetuximab in the first-line treatment of metastatic colorectal
cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar
|
|
21
|
Douillard JY, Siena S, Cassidy J, et al:
Randomized, phase III trial of panitumumab with infusional
fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4
alone as first-line treatment in patients with previously untreated
metastatic colorectal cancer: The PRIME study. J Clin Oncol.
28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dunn EF, Iida M, Myers RA, Campbell DA,
Hintz KA, Armstrong EA, Li C and Wheeler DL: Dasatinib sensitizes
KRAS mutant colorectal tumors to cetuximab. Oncogene. 30:561–574.
2011. View Article : Google Scholar :
|
|
23
|
Nukatsuka M, Saito H, Nakagawa F,
Tsujimoto H, Sakamoto K, Tsukioka S, Uchida J, Kiniwa M, Kobunai T
and Takechi T: Combination therapy using oral S-1 and targeted
agents against human tumor xenografts in nude mice. Exp Ther Med.
3:755–762. 2012.PubMed/NCBI
|
|
24
|
Balin-Gauthier D, Delord JP, Rochaix P,
Mallard V, Thomas F, Hennebelle I, Bugat R, Canal P and Allal C: In
vivo and in vitro antitumor activity of oxaliplatin in combination
with cetuximab in human colorectal tumor cell lines expressing
different level of EGFR. Cancer Chemother Pharmacol. 57:709–718.
2006. View Article : Google Scholar
|
|
25
|
Bauer P, Röhmel J, Maurer W and Hothorn L:
Testing strategies in multi-dose experiments including active
control. Stat Med. 17:2133–2146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shelton JW, Waxweiler TV, Landry J, Gao H,
Xu Y, Wang L, El-Rayes B and Shu HK: In vitro and in vivo
enhancement of chemoradiation using the oral PARP inhibitor ABT-888
in colorectal cancer cells. Int J Radiat Oncol Biol Phys.
86:469–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Price TJ, Hardingham JE, Lee Ck, et al:
Impact of KRAS and BRAF gene mutation status on outcomes from the
phase III AGITG MAX trial of capecitabine alone or in combination
with bevacizumab and mitomycin in advanced colorectal cancer. J
Clin Oncol. 29:2675–2682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim ST, Park KH, Shin SW and Kim YH: Dose
KRAS mutation status affect on the effect of VEGF therapy in
metastatic colon cancer patients? Cancer Res Treat. 46:48–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: a new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|