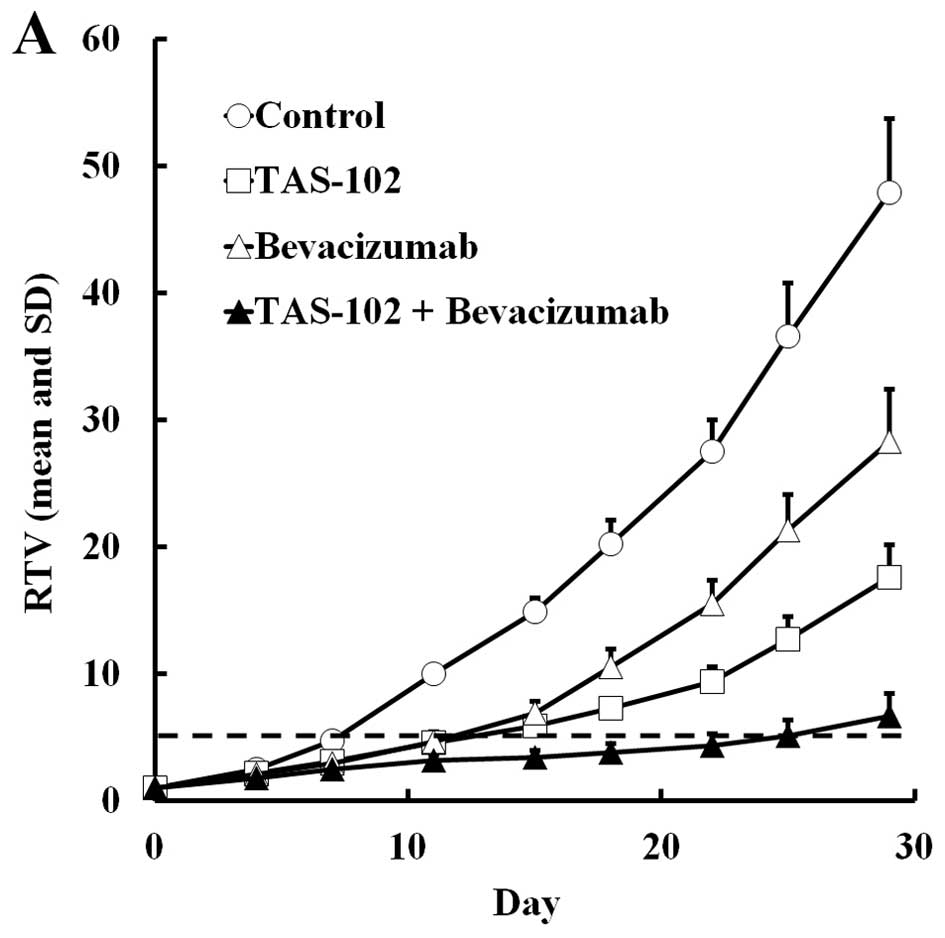
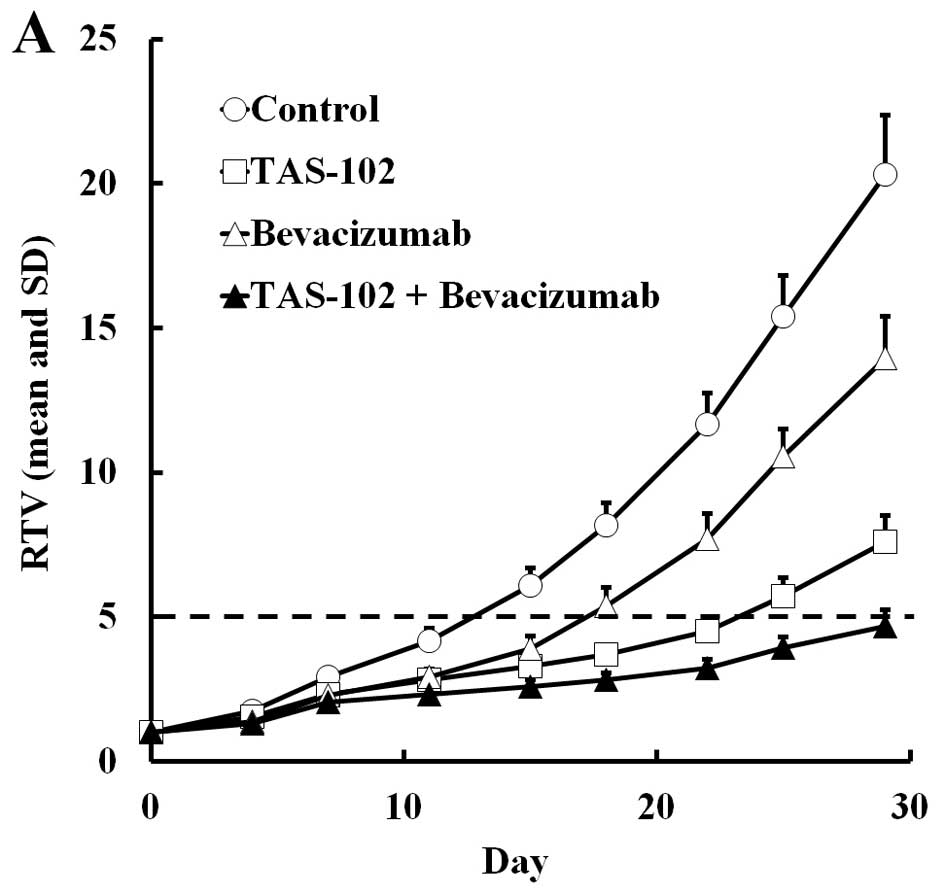
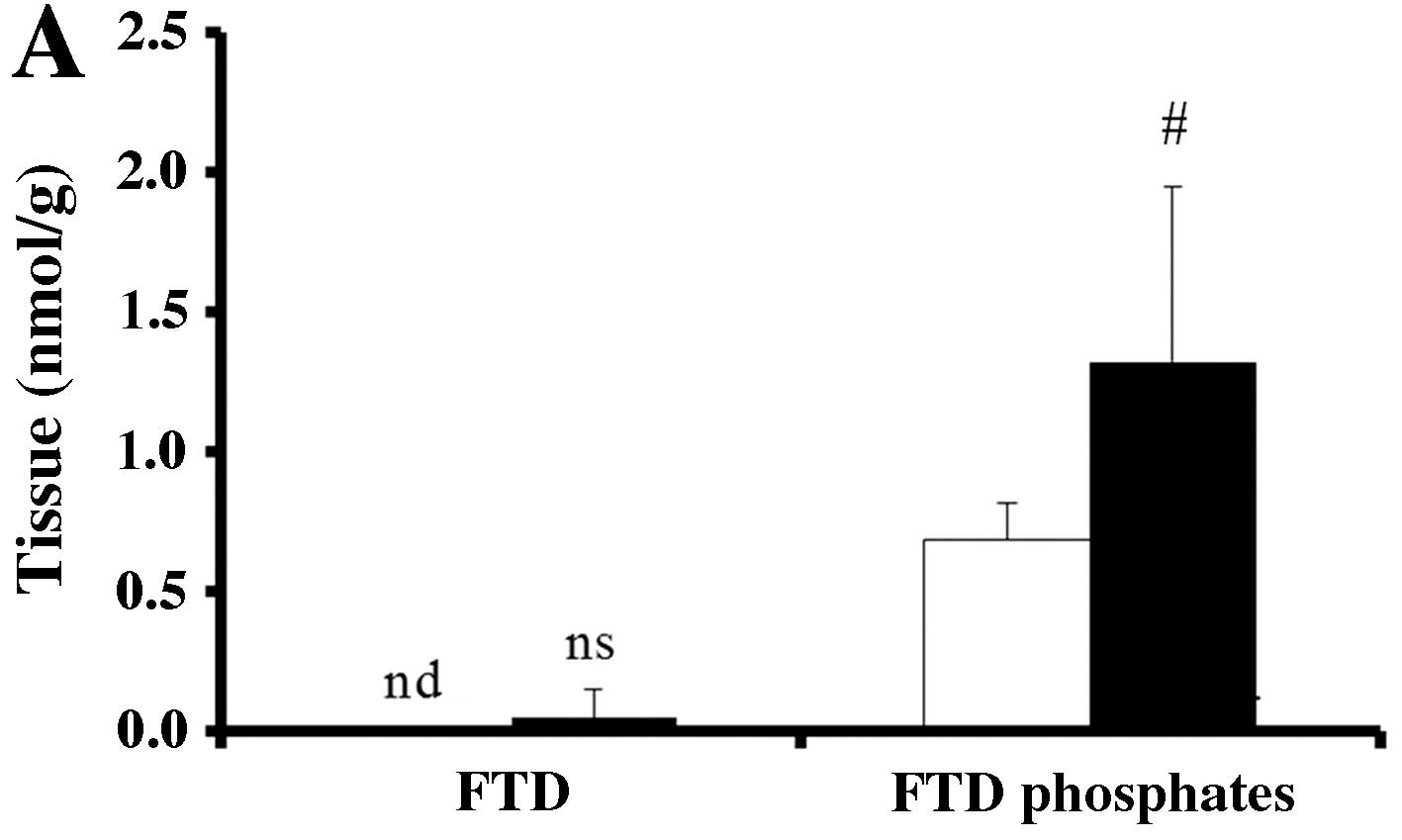
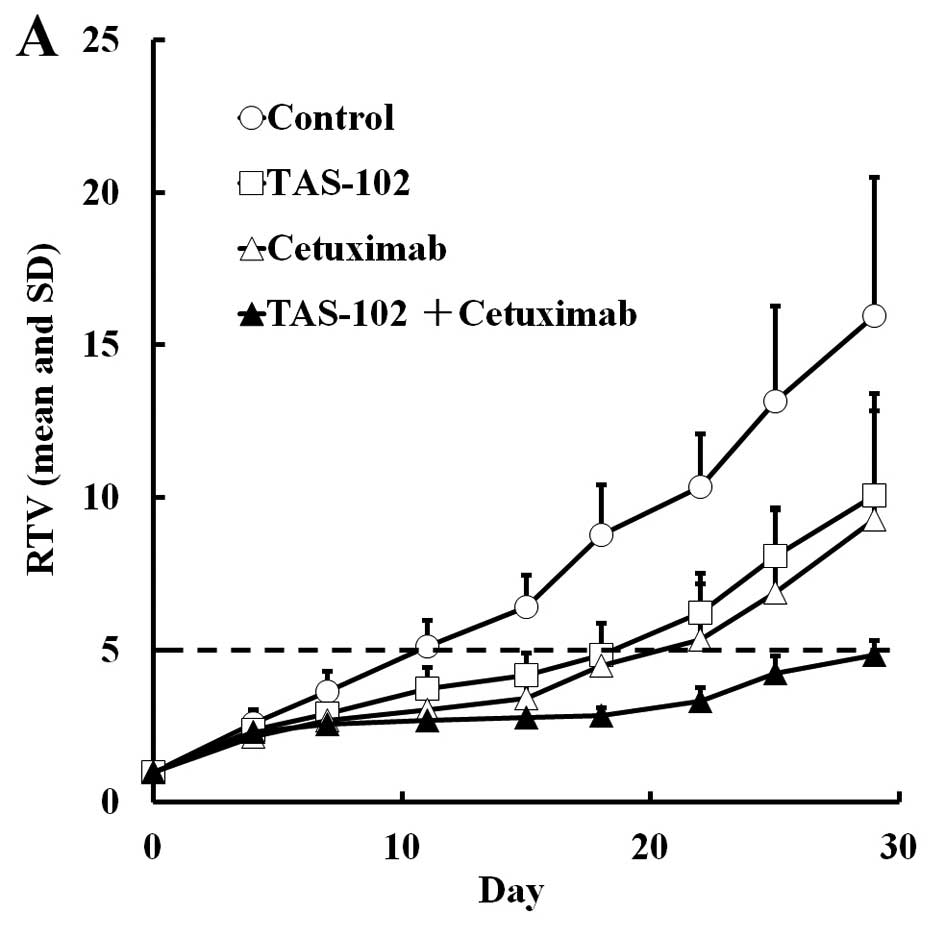
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Armand JP, Ducreux M, Mahjoubi M,
Abigerges D, Bugat R, Chabot G, Herait P, de Forni M and Rougier P:
CPT-11 (irinotecan) in the treatment of colorectal cancer. Eur J
Cancer. 31A:1283–1287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saltz LB, Clarke S, Diaz-Rubio E, et al:
Bevacizumab in combination with oxaliplatin-based chemotherapy as
first-line therapy in metastatic colorectal cancer: A randomized
phase III study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sobrero AF, Maurel J, Fehrenbacher L, et
al: EPIC: Phase III trial of cetuximab plus irinotecan after
fluoropyrimidine and oxaliplatin failure in patients with
metastatic colorectal cancer. J Clin Oncol. 26:2311–2319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Peeters M, Siena S, et al:
Open-label phase III trial of panitumumab plus best supportive care
compared with best supportive care alone in patients with
chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol.
25:1658–1664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grothey A, Van Cutsem E, Sobrero A, et al:
CORRECT Study Group: Regorafenib monotherapy for previously treated
metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar
|
|
7
|
Emura T, Nakagawa F, Fujioka A, Ohshimo H,
Yokogawa T, Okabe H and Kitazato K: An optimal dosing schedule for
a novel combination antimetabolite, TAS-102, based on its
intracellular metabolism and its incorporation into DNA. Int J Mol
Med. 13:249–255. 2004.PubMed/NCBI
|
|
8
|
Dexter DL, Wolberg WH, Ansfield FJ, Helson
L and Heidelberger C: The clinical pharmacology of
5-trifluoromethyl-2′-deoxyuridine. Cancer Res. 32:247–253.
1972.PubMed/NCBI
|
|
9
|
Tanaka N, Sakamoto K, Okabe H, et al:
Repeated oral dosing of TAS-102 confers high trifluridine
incorporation into DNA and sustained antitumor activity in mouse
models. Oncol Rep. 32:2319–2326. 2014.PubMed/NCBI
|
|
10
|
Fukushima M, Suzuki N, Emura T, Yano S,
Kazuno H, Tada Y, Yamada Y and Asao T: Structure and activity of
specific inhibitors of thymidine phosphorylase to potentiate the
function of antitumor 2′-deoxyribonucleosides. Biochem Pharmacol.
59:1227–1236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emura T, Suzuki N, Fujioka A, Ohshimo H
and Fukushima M: Potentiation of the antitumor activity of α, α,
α-trifluorothymidine by the co-administration of an inhibitor of
thymidine phosphorylase at a suitable molar ratio in vivo. Int J
Oncol. 27:449–455. 2005.PubMed/NCBI
|
|
12
|
Emura T, Suzuki N, Yamaguchi M, Ohshimo H
and Fukushima M: A novel combination antimetabolite, TAS-102,
exhibits antitumor activity in FU-resistant human cancer cells
through a mechanism involving FTD incorporation in DNA. Int J
Oncol. 25:571–578. 2004.PubMed/NCBI
|
|
13
|
Emura T, Murakami Y, Nakagawa F, Fukushima
M and Kitazato K: A novel antimetabolite, TAS-102 retains its
effect on FU-related resistant cancer cells. Int J Mol Med.
13:545–549. 2004.PubMed/NCBI
|
|
14
|
Murakami Y, Kazuno H, Emura T, Tsujimoto
H, Suzuki N and Fukushima M: Different mechanisms of acquired
resistance to fluorinated pyrimidines in human colorectal cancer
cells. Int J Oncol. 17:277–283. 2000.PubMed/NCBI
|
|
15
|
Utsugi T: New challenges and inspired
answers for anticancer drug discovery and development. Jpn J Clin
Oncol. 43:945–953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshino T, Mizunuma N, Yamazaki K, et al:
TAS-102 monotherapy for pretreated metastatic colorectal cancer: A
double-blind, randomised, placebo-controlled phase 2 trial. Lancet
Oncol. 13:993–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshino T, Mayer R, Falcon A, et al:
RECOURSE study group: Results of a multicenter, randomized,
double-blind, phase III study of TAS-102 vs. placebo, with best
supportive care (BSC), in patients (pts) with metastatic colorectal
cancer (MCRC) refractory to standard therapies (RECOURSE). Ann
Oncol. 25(Suppl 2): 1–117. 2014. View Article : Google Scholar
|
|
18
|
Welch S, Spithoff K, Rumble RB and Maroun
J; Gastrointestinal Cancer Disease Site Group: Bevacizumab combined
with chemotherapy for patients with advanced colorectal cancer: A
systematic review. Ann Oncol. 21:1152–1162. 2010. View Article : Google Scholar
|
|
19
|
Van Cutsem E, Köhne CH, Hitre E, et al:
Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bokemeyer C, Bondarenko I, Makhson A, et
al: Fluorouracil, leucovorin, and oxaliplatin with and without
cetuximab in the first-line treatment of metastatic colorectal
cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar
|
|
21
|
Douillard JY, Siena S, Cassidy J, et al:
Randomized, phase III trial of panitumumab with infusional
fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4
alone as first-line treatment in patients with previously untreated
metastatic colorectal cancer: The PRIME study. J Clin Oncol.
28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dunn EF, Iida M, Myers RA, Campbell DA,
Hintz KA, Armstrong EA, Li C and Wheeler DL: Dasatinib sensitizes
KRAS mutant colorectal tumors to cetuximab. Oncogene. 30:561–574.
2011. View Article : Google Scholar :
|
|
23
|
Nukatsuka M, Saito H, Nakagawa F,
Tsujimoto H, Sakamoto K, Tsukioka S, Uchida J, Kiniwa M, Kobunai T
and Takechi T: Combination therapy using oral S-1 and targeted
agents against human tumor xenografts in nude mice. Exp Ther Med.
3:755–762. 2012.PubMed/NCBI
|
|
24
|
Balin-Gauthier D, Delord JP, Rochaix P,
Mallard V, Thomas F, Hennebelle I, Bugat R, Canal P and Allal C: In
vivo and in vitro antitumor activity of oxaliplatin in combination
with cetuximab in human colorectal tumor cell lines expressing
different level of EGFR. Cancer Chemother Pharmacol. 57:709–718.
2006. View Article : Google Scholar
|
|
25
|
Bauer P, Röhmel J, Maurer W and Hothorn L:
Testing strategies in multi-dose experiments including active
control. Stat Med. 17:2133–2146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shelton JW, Waxweiler TV, Landry J, Gao H,
Xu Y, Wang L, El-Rayes B and Shu HK: In vitro and in vivo
enhancement of chemoradiation using the oral PARP inhibitor ABT-888
in colorectal cancer cells. Int J Radiat Oncol Biol Phys.
86:469–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Price TJ, Hardingham JE, Lee Ck, et al:
Impact of KRAS and BRAF gene mutation status on outcomes from the
phase III AGITG MAX trial of capecitabine alone or in combination
with bevacizumab and mitomycin in advanced colorectal cancer. J
Clin Oncol. 29:2675–2682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim ST, Park KH, Shin SW and Kim YH: Dose
KRAS mutation status affect on the effect of VEGF therapy in
metastatic colon cancer patients? Cancer Res Treat. 46:48–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: a new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|