Introduction
Malignant tumors of the vulva account for 5% of
cancers of the female genital tract in the USA (1). Squamous cell carcinomas (SCC) make up
70% of all vulvar cancers. The incidence rate is much higher in
older (20:100,000) than in younger (1:100,000) women (1). Little is known about the acquired
genomic changes of this type of cancer as only few cases have been
cytogenetically and/or molecularly analyzed (2–4).
Chromosome and array-based comparative genomic hybridization (CGH)
analysis (4) has shown, among other
imbalances, loss of chromosomal band 3p14 leading to deregulation
of the FHIT gene. Expression array analyses also identified
a number of other genes whose expression profile was altered.
Notable among the downregulated genes were MAL (in 2q11),
KRT4 (in 12q13) and OLFM4 (in 13q14), whereas
upregulated genes included SPRR2G (in 1q21.3) and
S100A7A (in 1q21.3). No mutation analyses have so far been
performed in this tumor type. Vulva malignant melanoma (MM), the
second most common malignancy in this organ or site, is an
aggressive cancer carrying poor overall prognosis (5). Only five vulvar MM cases have been
cytogenetically characterized; they showed complex karyotypes with
no recurrent aberration. No molecular genetic data on vulvar MM
cases have been published.
To gain more information on the genetics of vulvar
tumors, we analyzed 22 SCC, two MM, and one atypical squamous cell
hyperplasia (AH) case for expression of the high-mobility group
AT-hook genes HMGA2 and HMGA1. We then searched them
for mutations in the isocitrate dehydrogenase 1 (IDH1) and 2
(IDH2) and telomerase reverse transcriptase (TERT)
genes, and checked them for the methylation status of the promoter
of O6-methylguanine-DNA methyltransferase (MGMT).
Since these genes have all been found mutated, deregulated and/or
methylated in various types of cancer (6–9), they
seemed to be a reasonable starting point for the characterization
of the genetic profile also of vulvar cancers.
Materials and methods
Tumor material
The material consisted of fresh samples from 22 SCC,
two MM and one AH, all arising in the vulva and surgically removed
at The Norwegian Radium Hospital between 1998 and 2009 (Table I). The tumors have been previously
characterized for chromosomal aberrations and genomic imbalances
(3); a subset of them was also
investigated for their expression profile (4).
 | Table IOverview of the results for the vulva
tumors. |
Table I
Overview of the results for the vulva
tumors.
| Case/lab no. | Diagnosis | IDH1 | IDH2 | TERT | MGMT
methylated | HMGA1 | HMGA2 ex1–3 | HMGA2 ex1–5 |
Immunohisto-chemistry score |
|---|
| 1/02-167 | SCC | − | − | − | − | + | + | − | >50 |
| 2/02-848 | SCC | − | − | − | − | + | + | − | 1–10 |
| 3/02-869 | SCC | − | − | − | − | + | + | + | >50 |
| 4/02-1060 | SCC | IDH1G105 | − | − | − | + | + | + | 11–50 |
| 5/02-1171 | SCC | − | − | C254T | − | + | + | + | >50 |
| 6/03-48 | AH | − | − | − | − | NA | NA | NA | NA |
| 7/03-830 | SCC | − | − | C228T | + | NA | NA | NA | >50 |
| 8/03-1011 | SCC | − | − | C228T | − | + | + | + | − |
| 9/03-1088 | SCC | − | − | − | − | + | − | − | NA |
| 10/04-1190 | SCC | − | − | C228T | − | NA | NA | NA | >50 |
| 11/06-19 | SCC | − | − | C228T | − | + | + | + | 1–10 |
| 12/06-125 | SCC | − | − | C228T | − | + | + | + | 11–50 |
| 13/06-709 | SCC | − | − | − | − | + | + | + | 1–10 |
| 14/09-733 | SCC | − | − | − | − | + | + | + | 11–50 |
| 15/09-818 | SCC | NA | NA | NA | NA | + | + | + | 1–10 |
| 16/68-98 | SCCIS | − | − | − | − | NA | NA | NA | >50 |
| 17/02-99 | MM | − | − | − | − | NA | NA | NA | 1–10 |
| 18/00-647 | SCC | − | − | − | − | NA | NA | NA | − |
| 19/00-651 | SCC | − | − | − | − | NA | NA | NA | >50 |
| 20/00-1127 | MM | − | − | − | − | NA | NA | NA | >50 |
| 21/01-61 | SCC | − | − | − | − | NA | NA | NA | >50 |
| 22/01-99 | SCC | − | − | − | − | NA | NA | NA | − |
| 23/01-134 | SCC | − | − | − | − | NA | NA | NA | − |
| 24/01-777 | SCC | − | − | − | − | NA | NA | NA | 11–50 |
| 25/01-981 | SCC | − | − | − | − | NA | NA | NA | 1–10 |
DNA and RNA extraction and cDNA
synthesis
DNA extraction was performed on 24 samples (no
frozen material was available for case 15). The DNA was extracted
using the Maxwell 16 extractor (Promega, Madison, WI, USA) and the
Maxwell 16 Tissue DNA Purification kit (Promega) according to the
manufacturer’s recommendations. RNA was extracted from the 12
samples from which we had sufficient material to extract both DNA
and RNA. The RNA was extracted using the miRNeasy kit (Qiagen,
Hilden, Germany) and QIAcube (Qiagen). The concentration and purity
of both DNA and RNA were measured with the NanoVue
spectrophotometer (GE Healthcare, Pittsburgh, PA, USA). One
microgram of extracted RNA was reverse-transcribed in a 20
μl reaction volume using the iScript Advanced cDNA Synthesis
kit according to the manifacturer’s instructions (Bio-Rad
Laboratories, Oslo, Nor way).
Molecular analyses
All primers used in the PCR reactions are listed in
Table II. All PCR reactions were
run on a Bio-Rad C100 Thermal Cycler (Bio-Rad Laboratories). Three
microliters of the PCR products were stained with GelRed (Biotium,
Hayward, CA, USA) and analyzed by electrophoresis through 1.0%
agarose gel. The gel was scanned with G-Box (Syngene, Los Altos,
CA, USA) and the images were acquired using GeneSnap (Syngene). The
remaining 22 μl of the amplified fragments were purified
using the QIAquick PCR Purification kit (Qiagen). Direct sequencing
was performed using the light run sequencing service of GATC
Biotech (http://www.gatc-biotech.com/en/sanger-services/lightrun-sequencing.html).
The BLAST (http://blast.ncbi.nlm.nih.gov/blast.cgi) and BLAT
(http://genome.ucsc.edu/cgi-bin/hgblat) programs were
used for computer analysis of sequence data.
 | Table IIPrimers used in the PCR
reactions. |
Table II
Primers used in the PCR
reactions.
| Primer name | Primer
sequence |
|---|
| IDH1-rs1-86F |
5′-CTCCTGATGAGAAGAGGGTTGAG-3′ |
| IDH1-rs1-321R |
5′-ACACATACAAGTTGGAAATTTCTGG-3′ |
| IDH2-rs12-42F |
5′-CTTGGGGTTCAAATTCTGGTTGA-3′ |
| IDH2-rs12-315R |
5′-GCTAGGCGAGGAGCTCCAGTC-3′ |
| TERT-PromF2 |
5′-GCCGGGCTCCCAGTGGATTCG-3′ |
| TERT-PromR2 |
5′-GGCTTCCCACGTGCGCAGCAG-3′ |
| HMGA2-846F1 |
5′-CCACTTCAGCCCAGGGACAACCT-3′ |
| HMGA2-982-F1 |
5′-CAAGAGTCCCTCTAAAGCAGCTCA-3′ |
| HMGA2-1021R1 |
5′-CCTCTTGGCCGTTTTTCTCCAGTG-3′ |
| HMGA2-1112R1 |
5′-CCTCTTCGGCAGACTCTTGTGAGGA-3′ |
| HMGA1-284F1 |
5′-CAGCCATCACTCTTCCACCTGC-3′ |
| HMGA1-648R1 |
5′-CTGTCCAGTCCCAGAAGGAAGCT-3′ |
| ABL1-91F1 |
5′-CAGCGGCCAGTAGCATCTTGACTTTG-3′ |
| ABL1-404R1 |
5′-CTCAGCAGATACTCAGCGGCATTGC-3′ |
| A3RNV-RACE |
5′-ATCGTTGAGACTCGTACCAGCAGAGTCACGAGAGAGACTACACGGTACTGGTTTTTTTTTTTTTTT-3′ |
| A3R1New |
5′-TCGTTGAGACTCGTACCAGCAGAGTCAC-3′ |
| A3R3 |
5′-CGAGAGAGACTACACGGTACTGGT-3′ |
| MSP-MGMT-MetF |
5′-TTTCGACGTTCGTAGGTTTTCGC-3′ |
| MSP-MGMT-MetR |
5′-GCACTCTTCCGAAAACGAAACG-3′ |
|
MSP-MGMT-UnmetF |
5′-TTTGTGTTTTGATGTTTGTAGGTTTTTGT-3′ |
|
MSP-MGMT-UnmetR |
5′AACTCCACACTCTTCCAAAAACAAAACA3′ |
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
cDNA equivalent to 10 ng RNA was amplified using the
Takara Premix Ex Taq (Takara-Bio, Europe/SAS,
Saint-Germain-en-Laye, France). The primer combination HMGA2-846F1
and HMGA2-1021R1 was used to amplify the region between exons 1 and
3, whereas the primer combination HMGA2-846F1 and HMGA2-1112R1 was
used for exons 1 to 5. Expression of the housekeeping gene
ABL1 was monitored as the internal control. The PCR cycling
program for both HMGA2 and ABL1 was as follows: 30
sec at 94°C, followed by 35 cycles of 7 sec at 98°C and 2 min at
68°C and a final step at 68°C for 5 min. The RT-PCR products were
analyzed by electrophoresis. The primers HMGA1-284F1 and
HMGA1-648R1 were used to amplify the HMGA1 transcript. The
PCR cycling program for HMGA1 was as follows: 30 sec at 94°C
followed by 35 cycles of 7 sec at 98°C, 30 sec at 55°C, 60 sec at
72°C and a final extension for 2 min at 72°C.
3′ Rapid amplification of cDNA ends - PCR
(3′RACE-PCR)
For 3′-RACE-PCR, 100 ng of total RNA were
reverse-transcribed in a 20 μl reaction volume with
A3RNV-RACE as a primer and using the iScript Select cDNA Synthesis
kit according to the manufacturer’s instructions (Bio-Rad
Laboratories). One microliter was used as a template and amplified
using the outer primer combination HMGA2-846F1/A3R-1New. One
microliter of the amplified products was used as template in nested
PCR with the primers HMGA2-982F1 and A3R3. For both PCRs the 25
μl reaction volume contained 12.5 μl of Premix Ex Taq
(Takara-Bio), template and 0.4 μM of each of the forward and
reverse primers. PCR cycling consisted of an initial step of
denaturation at 94°C for 30 sec followed by 35 cycles of 7 sec at
98°C, 30 sec at 55°C, 90 sec at 72°C and a final extension for 5
min at 72°C. The PCR products were analyzed by electrophoresis,
purified and sequenced.
Polymerase chain reaction (PCR)
IDH1 and IDH2
DNA was first amplified in a 25 μl reaction
volume using Takara Premix Ex Taq and 1 μl of the primer
combination IDH1-rs1-86F and IDH1-rs1-321R for IDH1, and
IDH2-rs12-42F and IDH2-rs12-315R for IDH2. The thermal
cycling for IDH1 included an initial step at 94°C for 30
sec, followed by 35 cycles at 98°C for 7 sec, 55°C for 30 sec, 1
min at 77°C, followed by a final step at 68°C for 5 min. The
thermal cycling for IDH2 was set to 94°C for 30 sec,
followed by 35 cycles of 7 sec at 98°C, 30 sec at 58°C, 1 min at
77°C and a final step at 68°C for 5 min. The PCR products were
analyzed by electrophoresis, purified, and sequenced. Mutated and
wild-type plasmids for IDH1 and IDH2 were used to
check the accuracy of our analyses. The mutated plasmids harbored
the mutations IDH1R132 and IDH2R172 for IDH1
and IDH2, respectively. Serial dilutions up to 10% of
mutated/wild-type plasmids were analyzed using the same protocol,
giving informative results for all dilutions. IDH1 mutation
was spotted even at the lowest concentration of the mutated plasmid
(10%), whereas the IDH2 analysis showed that the mutation
could be identified only at concentration >20%.
TERT
We amplified the TERT promoter region with
PCR in order to detect the possible mutations −C228T and −C250T
which correspond to positions 124 and 146 nt upstream of the
TERT ATG start site (7),
respectively. DNA was amplified in 25 μl PCR volume
containing 1X PrimeSTAR GXL buffer (Takara bio), 200 μM of
each dNTP, 0.4 μM of each of the primers, TERTpromF2 and the
reverse primer TRETpromR2, 1.25 units of PrimeSTAR GXL DNA
polymerase and 20 ng of genomic DNA. The PCR program started with
an initial denaturation at 94°C for 30 sec, followed by 35 cycles
of 7 sec at 98°C, 30 sec and 90 sec at 68°C and a final extension
for 5 min at 68°C. The PCR products were analyzed by
electrophoresis, purified and sequenced.
Methylation-specific PCR (MSP)
Methylation analysis of the MGMT promoter was
performed using the primers and PCR conditions described by
Esteller et al (8) (Table II).
Immunohistochemistry
Formalin-fixed paraffin-embedded sections from 23
samples were analyzed for protein expression of HMGA2 using the
FLEX+ system (DakoA/S, Glostrup, Denmark). The procedures and the
scoring approach were as reported by Hetland et al (10).
Results
Results of the gene analyses
A complete overview of the results for all the gene
analyses is given in Table I.
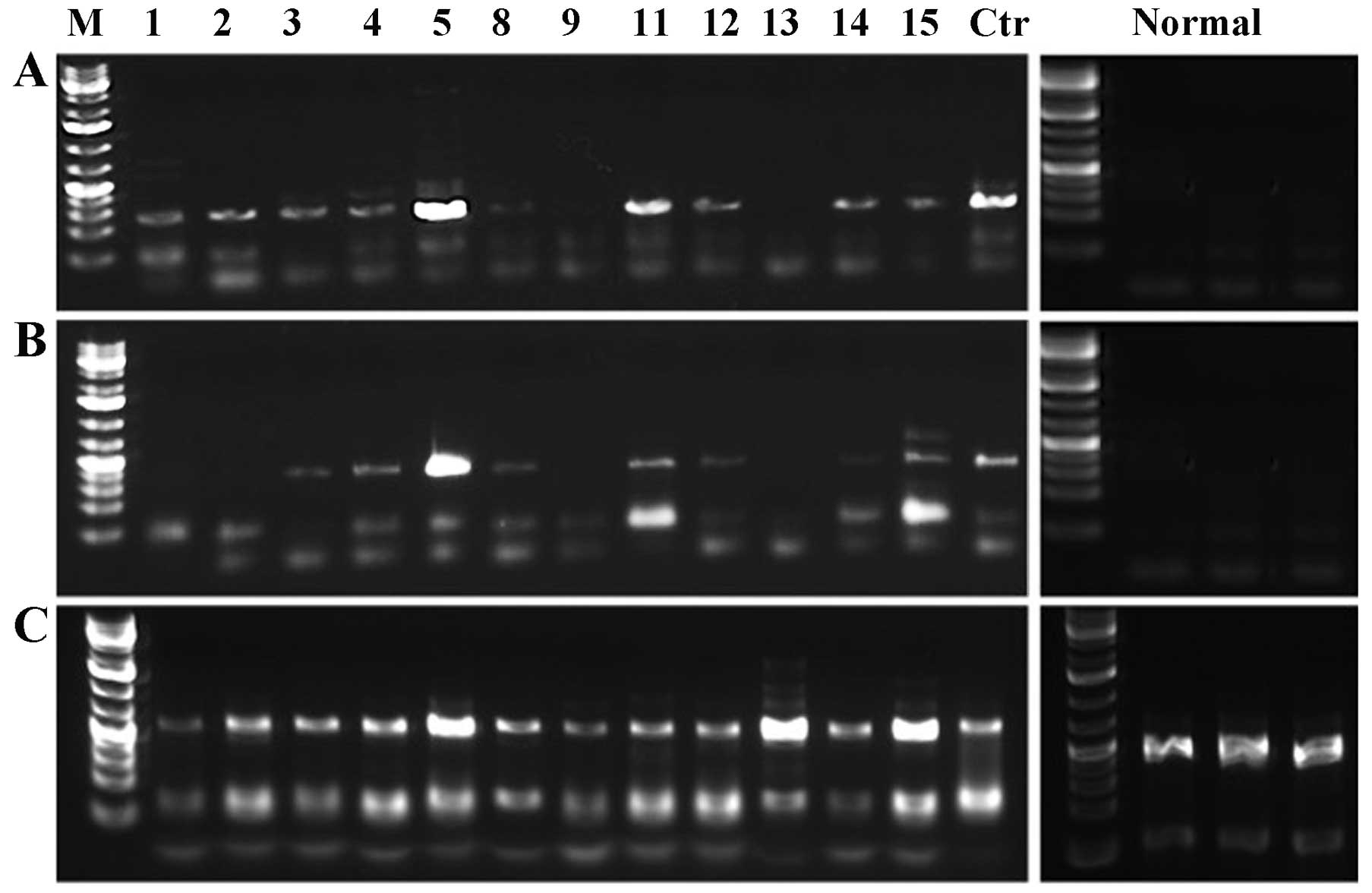
HMGA1 and HMGA2 expression
The 12 tumors from which RNA was available were
tested for expression of HMGA1 and HMGA2 giving
informative results for all samples (Table I). The 3 samples from the normal
vulva tissue used as controls showed no expression of HMGA2
but expression of HMGA1 (Fig.
1). The HMGA1 gene was expressed both in the 12 tumors
and in the controls. For the HMGA2 gene, the samples were
run for two parallel PCR reactions which amplified exons 1–3 and
exons 1–5, respectively. Eight cases showed expression of
HMGA2. Cases 1 and 2 showed expression of a truncated
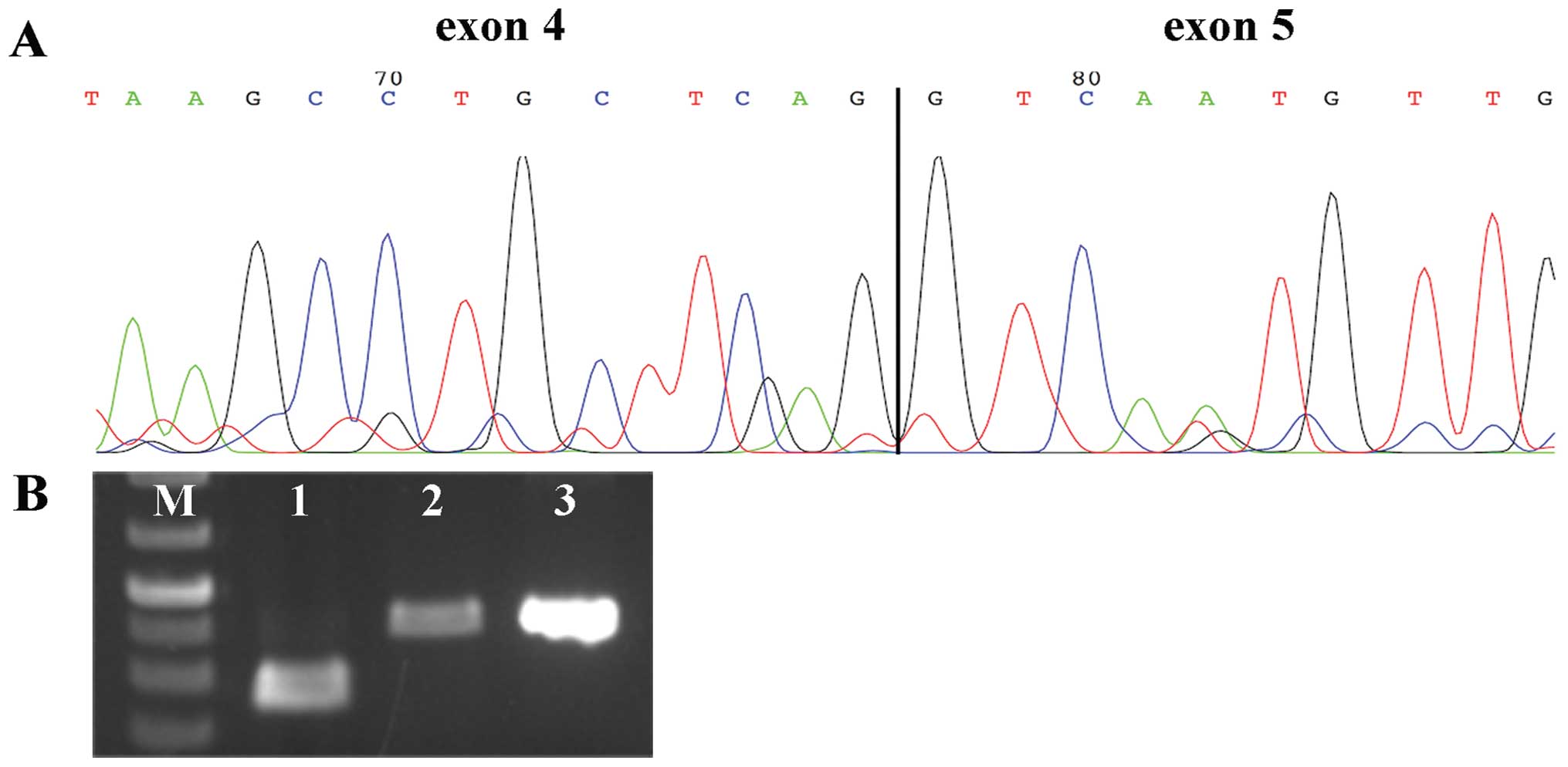
HMGA2, i.e., exons 1–3. 3′-RACE PCR was performed in search
of possible fusion transcripts in these two cases, and analysis of
the sequences revealed the presence in both of them of transcript
variant 3 of HMGA2 (accession no. NM_001300918.1) (Fig. 2A). The HMGA2 variant 3
contains alternative 3′ coding region and 3′ UTR compared to the
normal transcript; this explains why the transcripts found in case
1 and 2 could not be amplified by our primers. The HMGA2 protein
expression status was further investigated by immunohistochemistry
in the 23 tumors from which material was available (Table I). Four tumors (cases 8, 18, 22 and
23) were negative for HMGA2 immunostaining, but in all other cases
HMGA2 expression was noted. Cases 8 and 13 showed
contrasting/opposite results with the two methods used, RT-PCR
analysis and immunohistochemistry. More specifically, case 8 was
found negative for expression of HMGA2 by immunohistochemistry but
positive by RT-PCR, whereas case 13 was found positive by
immunohistochemistry but negative by RT-PCR. An additional RT-PCR
analysis for the latter case was performed following the same
protocol, but using a higher concentration of cDNA (30 ng instead
of 10 ng) since the immunohistochemistry was positive but with a
low score (1–10). We then observed that HMGA2
was also expressed in case 13 in its entire length (Fig. 2B). The opposite results obtained in
case 8 may be due to different part of the biopsy being used for
molecular analyses and/or immunohistochemistry.
TERT
All of the 24 cases from which DNA was extracted
were analyzed for mutations in the promoter region of TERT.
Five tumors (cases 7, 8 and 10–12) showed the C228T. Case 5 showed
a C254T unclassified variant.
IDH1 and IDH2 mutation
Twenty-four samples were analyzed for mutations in
IDH1 and IDH2. More precisely, the following mutation
sites were investigated: IDH1R100, IDH1R109 and IDH1R132 of
IDH1 and IDH2R140, IDH2R149 and IDH2R172 of IDH2. All
gave informative results whereas only one sample, case 4 was
positive for the SNPI DH1G105.
MGMT
We assessed MGMT promoter methylation using
MSP of the 24 samples from which DNA was extracted. All of the
tumors gave informative results; however, only one tumor, case 7,
was found to have MGMT promoter methylation.
Discussion
The high-mobilty group AT-hook proteins are
non-histone proteins involved in a wide variety of nuclear
processes from chromatin dynamics to gene regulation; there are two
proteins belonging to this group, HMGA1 and HMGA2. The HMGA family
genes are expressed during embryonic development (11) but are largely unexpressed in adult
normal tissues (12). However, high
expression levels of HMGA2 have been noted in different
benign tumors such as lipomas (9),
pleiomorphic adenomas of the salivary gland (13), uterine leiomyomas (14) and lung hamartomas (15). In these tumors, HMGA2 was
found disrupted due to rearrangement of chromosome arm 12q. The
alterations involve exon 3 and cause deletion of downstream regions
leading to a truncated transcript that can evade gene silencing.
Alternatively, chromosomal rearrangement of 12q13–15 may lead to
formation of a fusion gene. In order to detect a truncated
transcript of HMGA2, if present, we used two sets of primers
for parallel amplification of our samples, one for exons 1–3 and
one for exons 1–5. We found a truncated gene in cases 1 and 2
leading to the expression of exons 1–3. We further characterized
these transcripts by 3′RACE-PCR searching for possible fusion
partners. The karyotypic data on both tumors were normal, so
possibly a cryptic rearrangement involving chromosome 12 may be
present; alternatively, the cells carrying the chromosomal
aberration of interest did not divide in vitro. Sequence
analysis of the transcripts showed the HMGA2 splicing
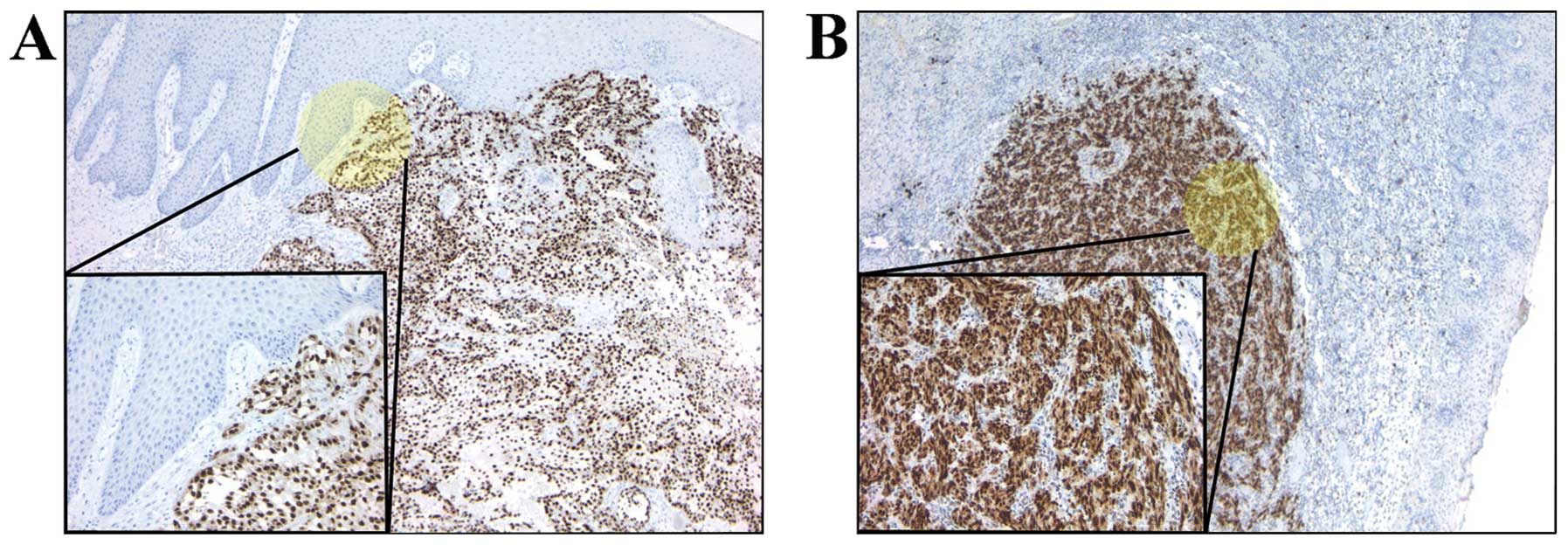
variant 3 in both cases. Moreover, immunohistochemistry analysis
revealed that HMGA2 was expressed in the majority of tumors, SCC as
well as MM (Fig. 3).
This is the first time that expression of the
HMGA2 gene has been assessed in tumors of the vulva. The
finding that the entire transcript is expressed in 83% of the
samples (including the two cases with the variant form of the gene)
give a hint that the gene may be involved in tumorigenesis or tumor
progression. Expression of HMGA2 has hitherto mostly been
noted in benign tumors with only sparse or anecdotal information on
expression in malignant ones (16).
The finding of 20 out of 24 malignant vulvar tumors, both SCC and
MM, showing HMGA2 expression therefore was unexpected.
Unfortunately, we did not have sufficient material to investigate
for HMGA2 expression in the only premalignant lesion, the
AH, of the present series.
TERT is the gene for the telomerase reverse
transcriptase. Its involvement in cancer is well known and many
studies have shown that mutations in the promoter region can
increase telomerase expression (7).
We focused on the most frequent mutations, i.e., C228T and C250T,
which have been noted in a large number of tumors of different
types (17). These mutations
introduce a new binding site (TTCCGG) for members of the
E-twenty-six/ternary complex factor (Ets/TCF) transcription factor
family (17). The C228T mutation
was found in 5 of the 6 cases with mutation (out of 24 tumors
analyzed). Despite the low frequency of the TERT mutation in
tumors of the vulva, 25%, it may be important in a subset of SCC.
Notably, a single nucleotide variant, C254T, was identified in case
5. This variant/mutation has not been previously reported. Its
location is close to the C228T mutation; however, it is not
involved in the formation of a new binding site for Ets/TCF.
Unfortunately, we did not have material from either normal tissue
or blood from this patient and so we could not establish whether
the variant was a germ line polymorphism, a rare normal trait or a
new cancer-associated mutation.
The IDH1 and IDH2 genes express two
forms of isocitrate dehydrogenase. Mutations in one of these genes
can lead to an enzyme that produces 2-hydroxyglutarate. This
metabolite is an inhibitor of α-ketoglutarate-dependent oxygenases,
whose impaired activity can cause genome-wide methylations that
have an impact on the expression of various genes. Mutations in
IDH1 and/or IDH2 have been identified in gliomas
(6) as well as in hematological
malignancies (18). Our analyses
showed no mutations of these genes in vulva tumors. SNP IDH1G105,
an adverse prognostic factor in CN-AML (19), was present in only a single tumor
(case 4). IDH1 and IDH2 are probably not involved in
vulvar tumorigenesis.
MGMT encodes O6-methylguanine DNA
methyltranferase, a DNA repair enzyme that removes alkyl adducts
from the O6-position of guanine. Expression of this gene
can lead to resistance against alkylating cytostatics. Methylation
of the MGMT promoter makes the cells more sensitive to
alkylating drugs as was demonstrated in different type of cancer,
not least gliomas (20). Due to its
efficacy as a prognostic and predictive tumor marker, the
assessment of MGMT promoter methylation status has become
one of the most requested analyses for gliomas (21). Our analysis found this gene altered
in only a single sample, suggesting that methylation of the
MGMT promoter of is not a frequent event in vulvar
tumorigenesis.
Acknowledgments
The present study was supported by grants from the
Norwegian Radium Hospital Foundation, the Norwegian Cancer Society,
the South-East Norway Regional Health Authority and the Research
Council of Norway through its Centres of Excellence funding scheme,
project no. 179571.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Teixeira MR, Kristensen GB, Abeler VM and
Heim S: Karyotypic findings in tumors of the vulva and vagina.
Cancer Genet Cytogenet. 111:87–91. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Micci F, Teixeira MR, Scheistrøen M,
Abeler VM and Heim S: Cytogenetic characterization of tumors of the
vulva and vagina. Genes Chromosomes Cancer. 38:137–148. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Micci F, Panagopoulos I, Haugom L,
Dahlback HS, Pretorius ME, Davidson B, Abeler VM, Tropé CG,
Danielsen HE and Heim S: Genomic aberration patterns and expression
profiles of squamous cell carcinomas of the vulva. Genes
Chromosomes Cancer. 52:551–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tcheung WJ, Selim MA, Herndon JE,
Abernethy AP and Nelson KC: Clinicopathologic study of 85 cases of
melanoma of the female genitalia. J Am Acad Dermatol. 67:598–605.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Håvik AB, Lind GE, Honne H, Meling TR,
Scheie D, Hall KS, van den Berg E, Mertens F, Picci P, Lothe RA,
Heim S and Brandal P: Sequencing IDH1/2 glioma mutation hotspots in
gliomas and malignant peripheral nerve sheath tumors. Neuro Oncol.
16:320–322. 2014. View Article : Google Scholar :
|
|
7
|
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda
C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL,
Giovanella BC, et al: TERT promoter mutations occur frequently in
gliomas and a subset of tumors derived from cells with low rates of
self-renewal. Proc Natl Acad Sci USA. 110:6021–6026. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteller M, Hamilton SR, Burger PC, Baylin
SB and Herman JG: Inactivation of the DNA repair gene
O6-methylguanine-DNA methyltransferase by promoter
hypermethylation is a common event in primary human neoplasia.
Cancer Res. 59:793–797. 1999.PubMed/NCBI
|
|
9
|
Schoenmakers EF, Wanschura S, Mols R,
Bullerdiek J, Van den Berghe H and Van de Ven WJ: Recurrent
rearrangements in the high mobility group protein gene, HMGI-C, in
benign mesenchymal tumours. Nat Genet. 10:436–444. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hetland TE, Holth A, Kaern J, Flørenes VA,
Tropé CG and Davidson B: HMGA2 protein expression in ovarian serous
carcinoma effusions, primary tumors, and solid metastases. Virchows
Arch. 460:505–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiappetta G, Avantaggiato V, Visconti R,
Fedele M, Battista S, Trapasso F, Merciai BM, Fidanza V, Giancotti
V, Santoro M, et al: High level expression of the HMGI (Y) gene
during embryonic development. Oncogene. 13:2439–2446.
1996.PubMed/NCBI
|
|
12
|
Rogalla P, Drechsler K, Frey G, Hennig Y,
Helmke B, Bonk U and Bullerdiek J: HMGI-C expression patterns in
human tissues. Implications for the genesis of frequent mesenchymal
tumors. Am J Pathol. 149:775–779. 1996.PubMed/NCBI
|
|
13
|
Geurts JM, Schoenmakers EF and Van de Ven
WJ: Molecular characterization of a complex chromosomal
rearrangement in a pleomorphic salivary gland adenoma involving the
3′-UTR of HMGIC. Cancer Genet Cytogenet. 95:198–205. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mine N, Kurose K, Nagai H, Doi D, Ota Y,
Yoneyama K, Konishi H, Araki T and Emi M: Gene fusion involving
HMGIC is a frequent aberration in uterine leiomyomas. J Hum Genet.
46:408–412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kazmierczak B, Meyer-Bolte K, Tran KH,
Wöckel W, Breightman I, Rosigkeit J, Bartnitzke S and Bullerdiek J:
A high frequency of tumors with rearrangements of genes of the
HMGI(Y) family in a series of 191 pulmonary chondroid hamartomas.
Genes Chromosomes Cancer. 26:125–133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nyquist Kb, Panagopoulos I, Thorsen J,
Roberto R, Wik HS, Tierens A, Heim S and Micci F: t(12;13)(q14;q31)
leading to HMGA2 upregulation in acute myeloid leukaemia. Br J
Haematol. 157:769–771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heidenreich B, Rachakonda PS, Hemminki K
and Kumar R: TERT promoter mutations in cancer development. Curr
Opin Genet Dev. 24:30–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patel KP, Barkoh BA, Chen Z, Ma D, Reddy
N, Medeiros LJ and Luthra R: Diagnostic testing for IDH1 and IDH2
variants in acute myeloid leukemia an algorithmic approach using
highresolution melting curve analysis. J Mol Diagn. 13:678–686.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wagner K, Damm F, Gohring G, Gorlich K,
Heuser M, Schafer I, Ottmann O, Lubbert M, Heit W, Kanz L, et al:
Impact of IDH1 R132 mutations and an IDH1 single nucleotide
polymorphism in cytogenetically normal acute myeloid leukemia: SNP
rs11554137 is an adverse prognostic factor. J Clin Oncol.
28:2356–2364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Margison GP and Santibáñez-Koref MF:
O6-alkylguanine-DNA alkyltransferase: role in
carcinogenesis and chemotherapy. Bioessays. 24:255–266. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Håvik AB, Brandal P, Honne H, Dahlback HS,
Scheie D, Hektoen M, Meling TR, Helseth E, Heim S, Lothe RA and
Lind GE: MGMT promoter methylation in gliomas-assessment by
pyrosequencing and quantitative methylation-specific PCR. J Transl
Med. 10:362012. View Article : Google Scholar : PubMed/NCBI
|