Introduction
Gastric cancer is one of the most common types of
cancers. Over 1.6 million individuals succumb to gastric cancer
each year in China. The Chinese incidence of gastric cancer
accounts for more than 40% of the worldwide occurrences. Moreover,
the progression-free survival and overall survival rates of gastric
cancer patients in China are much lower than those in Europe and
the US. Therefore, research concerning the characteristics and
pathogenesis of gastric cancer in Chinese patients is urgently
needed.
Gastric cancer can be influenced by a wide range of
genetic and environmental factors. A clear association has been
reported between gastric cancer and chronic inflammation (1–3).
Pro-inflammatory factors, including interleukin-1 (IL-1), IL-6 and
tumor necrosis factor (TNF), may not only play roles in
inflammation-associated carcinogenesis (4), but may also influence the
chemotherapeutic sensitivity during gastric cancer treatment
(5,6). IL-33 (previously known as NF-HEV), an
18-kDa protein, is a new member of the IL-1 family (7). Traditionally, the IL-1 family is well
known for their effects on host defense, immune regulation and
inflammation (7). However, recent
research suggests that the IL-1 family is also involved in cancer
development. For example IL-18, another member of the IL-1 family,
acts as a pleiotropic cytokine in many types of cancer cells, and
influences the invasion of gastric cancer cells under hypoxia
(8). A high level of IL-18 in serum
has been intensively associated with a wide variety of tumors, such
as hepatocellular (9) and
esophageal carcinoma (10) and
gastric cancer (11).
The structures of IL-33 and IL-18 are very similar.
Therefore, it is believed that IL-33 and IL-18 may have similar
biological functions related to cancer development (12). Actually, IL-33 is abundantly
expressed in the nuclei of endothelial cells in human tumor
tissues, such as in kidney, stomach, liver and pancreas tissues
(13). The serum level of IL-33
(14) was found to be significantly
increased (~6-fold) in gastric cancer patients, and a higher serum
level of IL-33 in gastric cancer patients was found to correlate
with poor prognosis.
However, the relationship between IL-33 and the
development of gastric cancer is largely unknown. In the present
study, the effects and the underlying mechanisms of IL-33 in
regards to the proliferation and drug sensitivity of gastric cancer
were explored.
Materials and methods
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The proliferation level of cells was detected by MTT
(Invitrogen, USA) assay as described below. GES-1 cells and a
gastric cancer cell line (SIBCB, Shanghai, China) were grown in
RPMI-1640 (Gibco, USA) supplemented with 10% fetal bovine serum
(FBS) (Biological Industries, Israel). The cells were trypsinized
and grown in a 96-well plate (Corning Incorporated, USA) at a
density of 1×105/well and incubated for 24 h at 37°C, in
5% CO2. IL-33 (Sigma, USA) at a final concentration of
7.8, 15.6, 31.25, 62.5, 125, 250, 500 and 1,000 pg/ml was incubated
with MGC803 and GES-1 cells, respectively for 24 h. Twenty
microliters MTT (5 mg/ml) was added into the cells and incubated
for 4 h. After removing the supernatant, 200 μl DMSO was
added to dissolve the formazan. The absorbance value (OD) was
detected at a 570-nm wavelength with a microplate reader (Thermo,
USA), with 630 nm as reference. The cell viability (%) = treatment
group OD/control group OD. The experiment was repeated three times.
For the cell protection assay, MGC803 cells were incubated with
5-FU, adriamycin, cis-platinum (DDP) and etoposide (Sigma)
coupled with 0, 20, 40, 60, 80 pg/ml IL-33. All of these
chemotherapy drugs were at the concentration of 10 μM. After
incubation with the MGC803 cells for 24 h, MTT assay was carried
out and the inhibition rate was calculated. The inhibition rate (%)
= (1 − treatment group OD/control group OD) × 100%. For evaluation
of the protective effects against DDP-induced cell inhibition,
GES-1 and gastric cancer cell lines MGC803, SGC7901 and GC9811-P
were incubated with 0, 5, 10, 20 μM DDP coupled with 60
pg/ml IL-33 for 24 h. Then MTT assays were carried out as described
above.
Flow cytometric analysis
MGC803 cells were cultured in RPMI-1640 supplemented
with 10% FBS. The cells were incubated with DDP (10 μM) and
a triple-drug combination (TDC) [5-FU, oxaliplatin (Sigma);
docetaxel; 750:75:75 μg/ml (15)] alone or with 60 pg/ml IL-33 for 24
h, and the apoptosis assay was performed with an Annexin V/PI
staining kit (Bender MedSystems, USA). The experiment was carried
out according to the instruction manual provided in the kit. After
staining, the cells were analyzed by flow cytometry (Calibur; BD,
USA), and data were collected and analyzed using FlowJo software
(version 10.0). The experiment was repeated three times.
Western blotting
Levels of activated-PARP, ST2, and MAPK
pathway-related proteins including p-ERK1/2, P38, p-JNK were
determined as described below. Confluent cultured MGC803 cells were
treated with different concentrations of the compounds including 10
μM DDP or TDC alone or with 60 pg/ml IL-33 for 24 h. The
cell lysates containing 25 μg protein were electrophoresed
on polyacrylamide gel in the presence of sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electroblotted to a polyvinylidene fluoride (PVDF) membrane
(Millipore Corporation, USA). After blocking with non-fat milk, the
PVDF membrane was incubated with the primary antibodies (Cell
Signaling Technology, USA) overnight at 4°C. Then the secondary
antibody (Cell Signaling Technology) was added and after washing
with TBS-T buffer, the PVDF membrane was visualized using ECL
chemiluminescence (Invitrogen) and exposed to X-ray film (Kodak,
USA). The bands were quantified by ImageJ (version 1.47) with
β-tubulin (Cell Signaling Technology) as the internal reference.
The experiment was repeated three times.
JNK inhibition assay
MGC803 cells were trypsinized and grown in 96-well
plates at the density of 105 cells/well. After
pretreatment with 10 μM of SP600125 (Calbiochem, USA), a
highly selective JNK inhibitor for 1 h, the MGC803 cells was
incubated with 10 μM DDP and TDC alone or with 60 pg/ml
IL-33 for 24 h. Then the cell inhibition rate was detected by MTT
assay. The experiment was repeated three times.
Matrigel invasion assay
Twenty-four-well plates with an inner chamber was
chosen for the invasion assay, and membranes with an 8-μm
pore size were precoated with 10 μg/ml Matrigel (Corning
Incorporated). The MGC803 cells were trypsinized and grown on the
inner chamber at the density of 1×106 cells/well in
RPMI-1640, with RPMI-1640 medium containing 0, 30, 60 and 120 pg/ml
IL-33 in the lower chamber separately. In addition, the positive
control was performed with RPMI-1640 containing 10% FBS in one
lower chamber and MGC803 cells in RPMI-1640 medium in the upper
chamber. After incubation at 37°C for 6 h, cells on the upper
surface of the filter were removed and cells adhering to the
undersurface of the filter membrane were dyed with 0.5% crystal
violet for 30 min. The crystal violet was washed with PBS for three
times. Cells on the lower chamber were counted under a microscope
in four fields randomly. The mean cell numbers were recorded and
analyzed. The experiment was repeated three times.
Statistical analysis
The differences were analyzed by the two-tailed
Student’s t-test using SPSS 13.0. Statistical significance was
verified at p<0.05. The graphs were constructed using GraphPad
Prism for Windows (version 5.0).
Results
Proliferative effect of IL-33 alone on
gastric cancer and normal epithelial cells
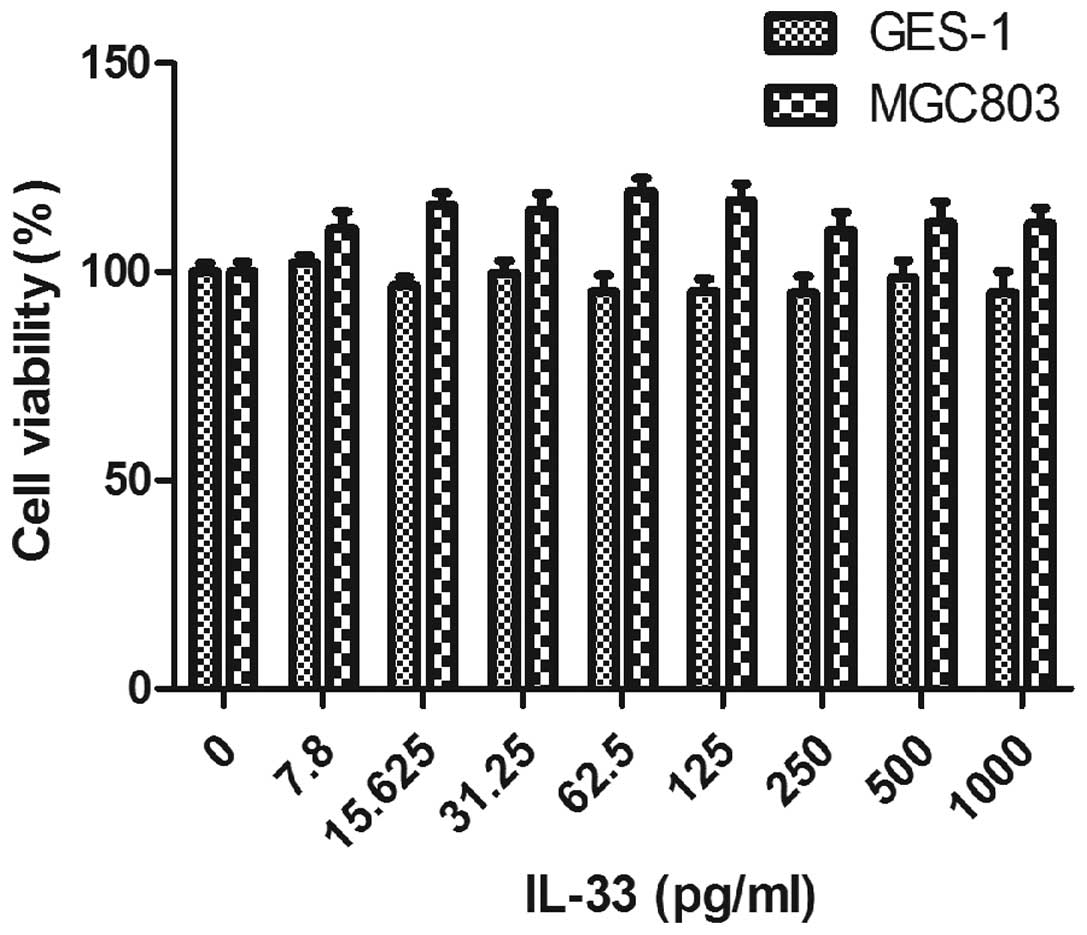
To explore the direct influence of IL-33 on the
proliferation of GES-1 and gastric cancer cells, GES-1 and MGC803
cells were incubated with different concentrations of IL-33 for 24
h. As shown in Fig. 1, IL-33
inhibited the proliferation of GES-1 cells slightly and stimulated
the proliferation of MGC803 mildly. This implied that IL-33 nearly
had no obvious effects on the proliferation of both cell lines at a
low concentration (<100 pg/ml). Thus, IL-33 at a lower
concentration was chosen for the subsequent experiments.
IL-33 protects gastric cancer MGC803
cells from apoptosis induced by a platinum drug
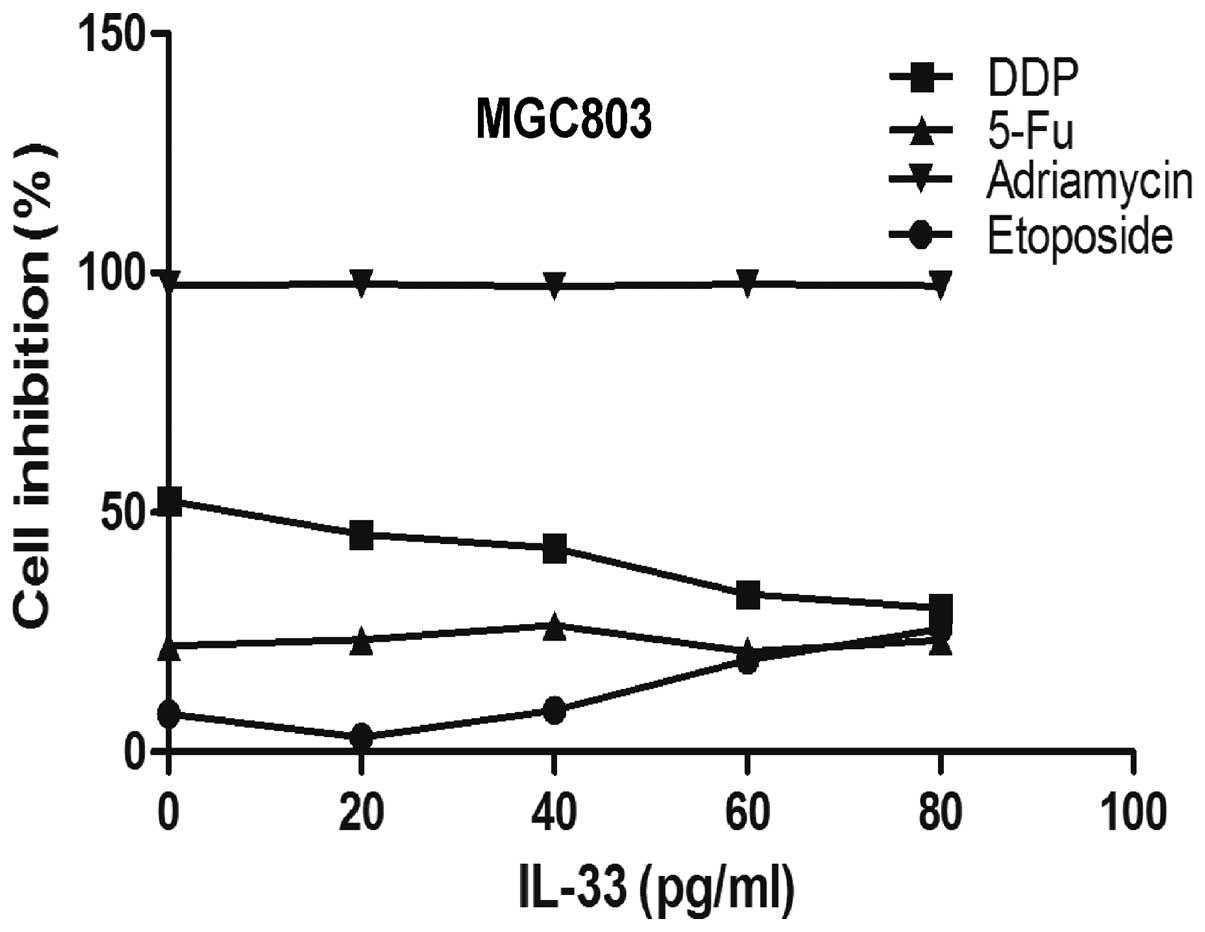
In the present study, the effect on proliferation of
different chemical compounds on MGC803 cells were assessed when
co-cultured with IL-33. MGC803 cells were incubated with 5-FU,
adriamycin, DDP and etoposide at the concentration of 10 μM
coupled with 0, 20, 40, 60 and 80 pg/ml IL-33. As shown in Fig. 2, IL-33 at 60 pg/ml significantly
reduced the apoptosis caused by DDP but not with the other chemical
compounds (p<0.05).
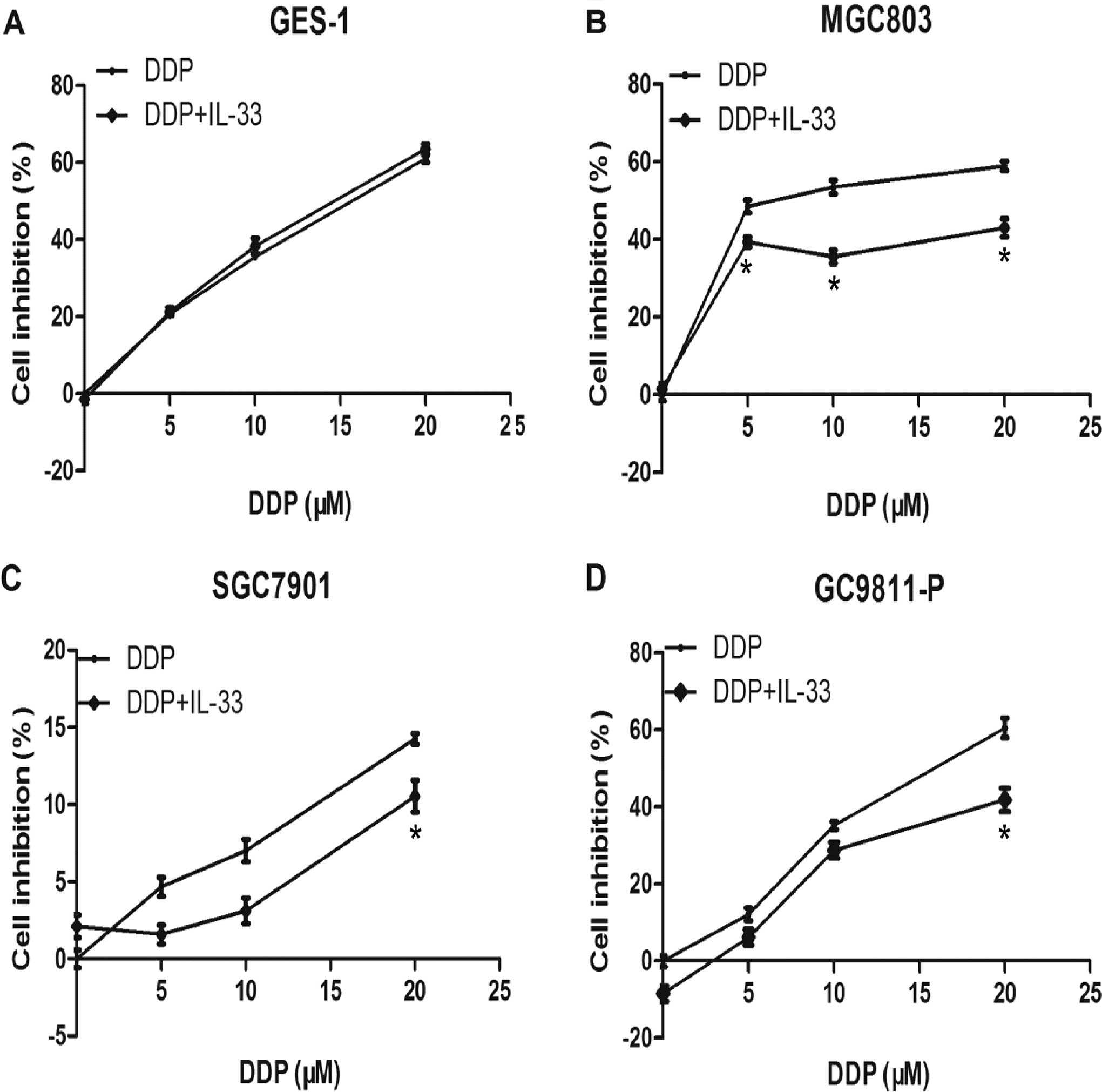
After the above experiment, GES-1 and gastric cancer
cell lines MGC803, SGC7901 and GC9811-P were cultured with 0, 5, 10
and 20 μM DDP coupled with 60 pg/ml IL-33 for 24 h. As shown
in Fig. 3, the MTT assay data
revealed that IL-33 decreased the inhibitory rate of DDP in the
gastric cancer cells, particularly in the MGC803 cells. This
implies that IL-33 protects MGC803 and other gastric cancer cells
from apoptosis induced by DDP. Yet, it had no apparent
proliferative effect on GES-1 cells. The protective effect on cell
proliferation was more obvious in the MGC803 cells at 5, 10 and 20
μM DDP with statistical significance (p<0.05), and MGC803
cells were chosen for use in the following experiments.
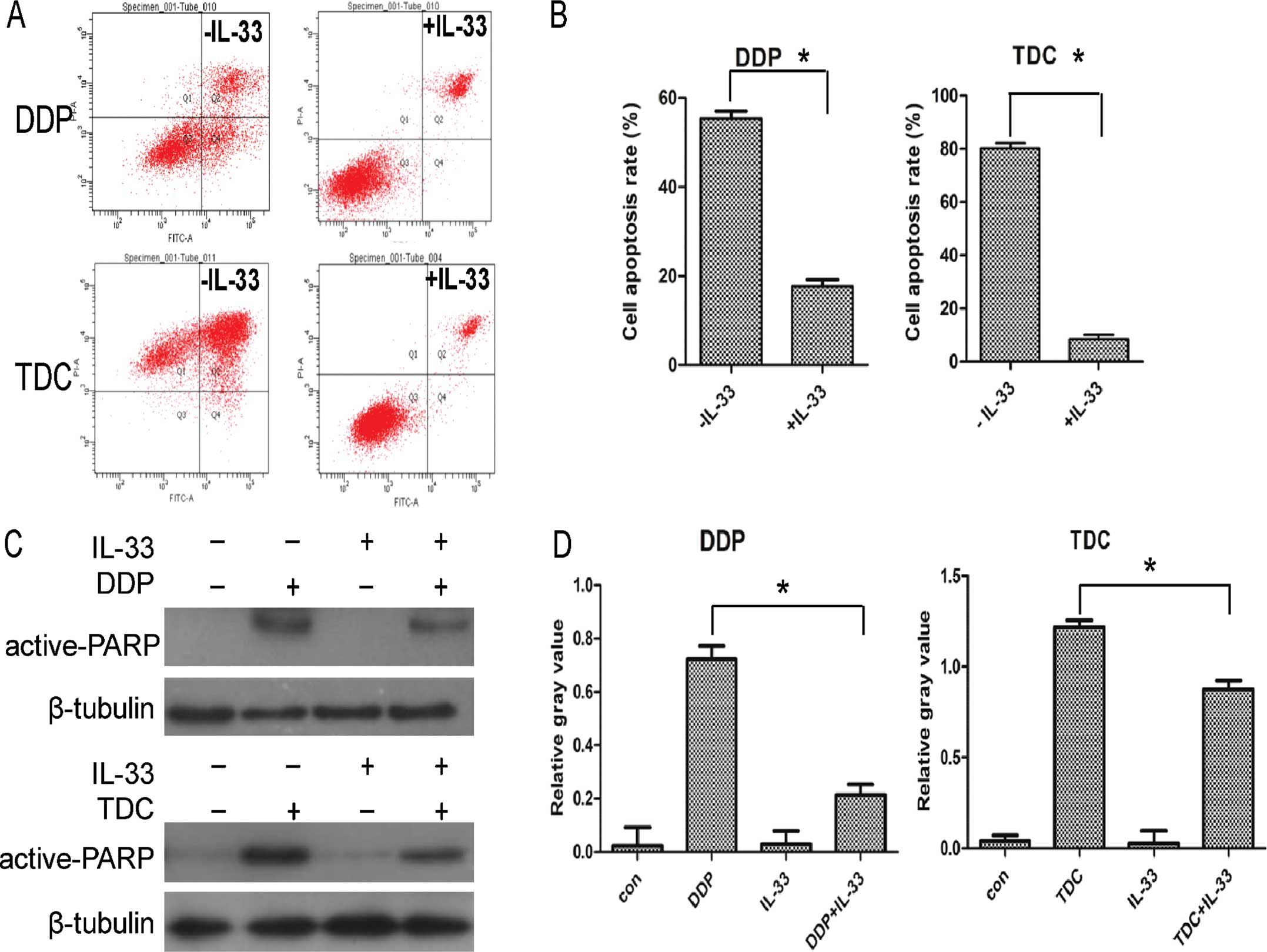
Furthermore, a flow cytometric assay was carried out
to confirm the above result. Considering the significance of
combination drug therapy, apart from DDP, MGC803 cells were
incubated with TDC containing oxaliplatin alone or with 60 pg/ml
IL-33. As shown in Fig. 4A and B,
when coupled with IL-33, the apoptosis rate of the MGC803 cells
declined markedly, and the phenomenon was more obvious in the TDC
treatment group. In addition, as shown in Fig. 4C and D, expression of apoptosis
protein active-PARP was decreased compared to the level in the
control group when cultured with IL-33; all of the above data
confirmed that IL-33 protects MGC803 cells from apoptosis induced
by DDP or platinum-based cytotoxic drugs.
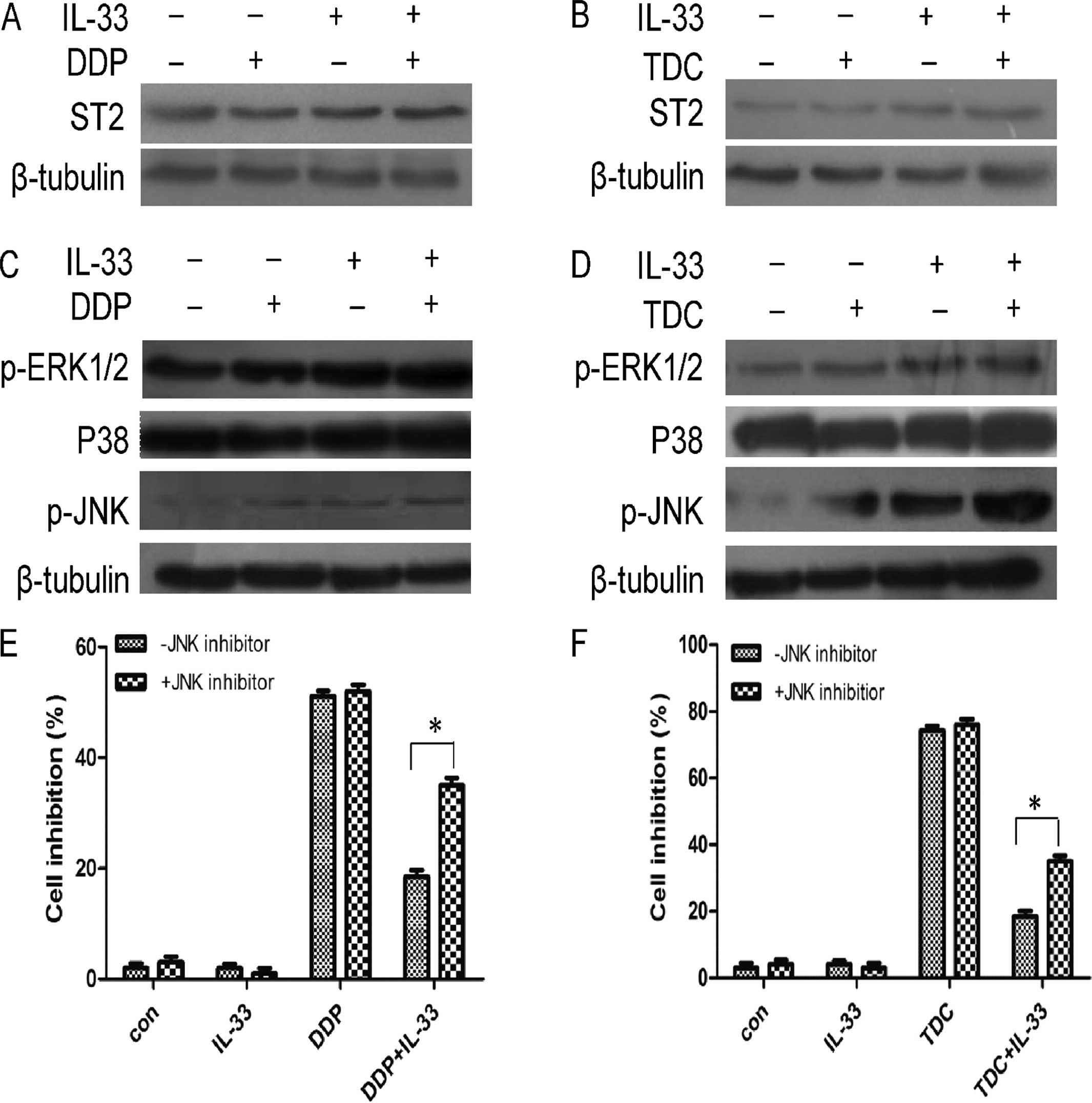
Expression of ST2 in the MGC803
cells
MGC803 cells were treated with DDP or TDC and IL-33
for 24 h, and the protein level of ST2 was detected by western
blotting. As shown in Fig. 5A and
B, compared with the untreated group, the protein expression of
ST2 was slightly increased following treatment with DDP, TDC or
IL-33 alone. When MGC803 cells were treated with both DDP or TDC
and IL-33, expression of ST2 was obviously increased compared to
the DDP or TDC group.
IL-33 may have protective a function via
the JNK pathway
The mechanism of IL-33 was explored by western
blotting. MGC803 cells were treated with DDP or TDC alone and with
IL-33 for 24 h. Phospho-MAPK (ERK1/2, p38, JNK) protein expression
was detected. As shown in Fig. 5C and
D, the p38 protein level had almost no change, yet
phospho-ERK1/2 increased slightly and expression of phospho-JNK
(p-JNK) obviously increased when the cells were incubated with
IL-33. The result showed that when the cells were treated with both
DDP or TDC and 60 pg/ml IL-33, phospho-ERK1/2 was increased
slightly, and p-JNK was increased obviously. IL-33 may play a
protective function through JNK pathway activation, and partially
through treatment with ERK1/2.
Following treatment with the JNK inhibitor, MTT
assay was carried to assess cell growth. MGC803 cells were
incubated with DDP, TDC and IL-33 alone and/or with JNK inhibitor
pretreatment for 1 h. As shown in Fig.
5E and F, compared with the inhibitor non-treatment group, the
cell viability was obviously decreased when cultured with the JNK
inhibitor. This phenomenon was more obvious in the TDC group and
the data reached statistical significance compared to the control
group (p<0.05). These data indicate that JNK plays an important
role in the IL-33 protective function.
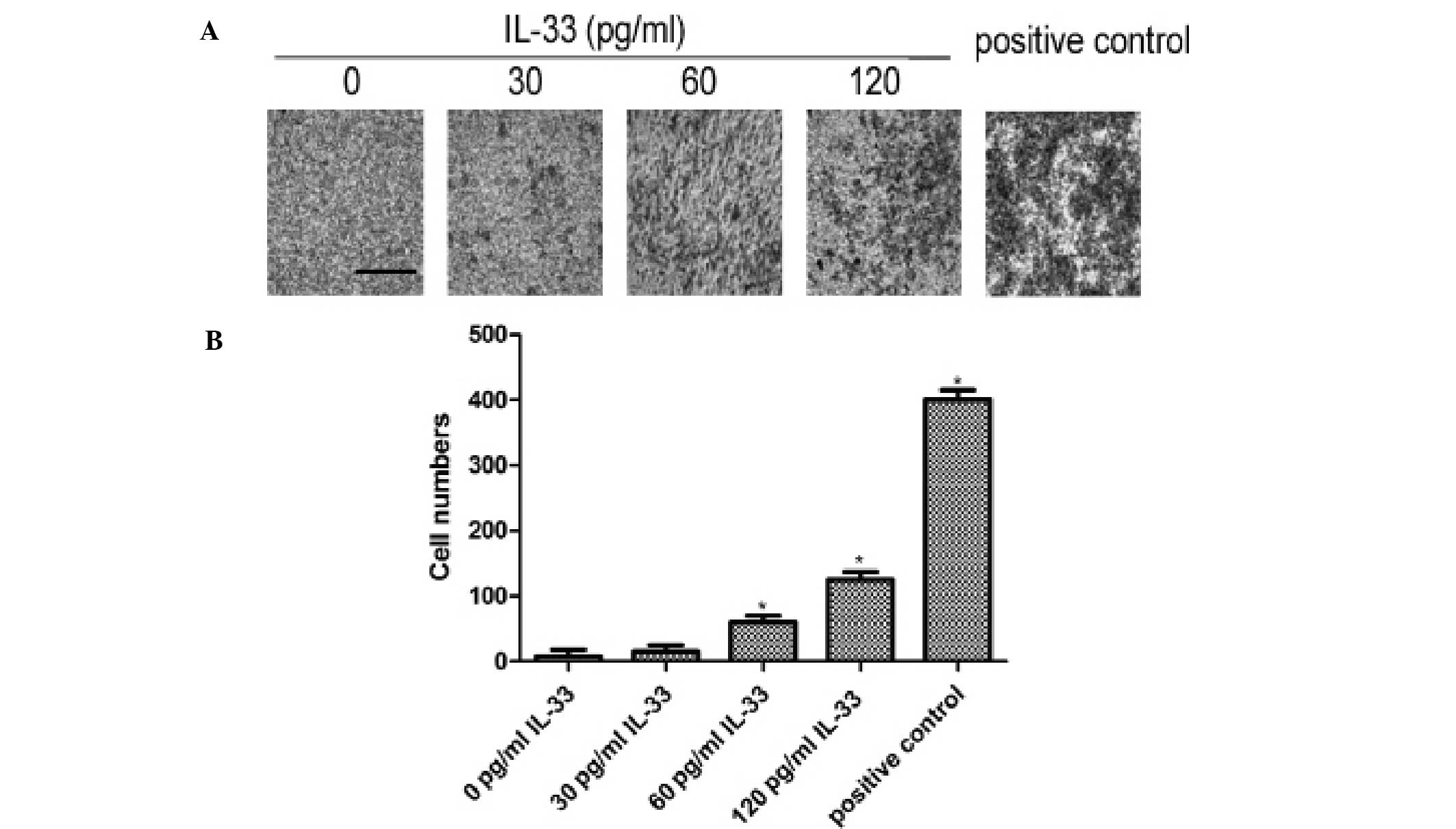
IL-33 stimulates the invasive ability of
the gastric cancer MGC803 cells
In addition to the proliferation function of IL-33,
the influence of IL-33 on cell invasion was explored via a Matrigel
invasion assay. As shown in Fig. 6,
following treatment with a higher concentration of IL-33, the
number of invasive cells was increased. The invasion data indicated
that IL-33 promoted MGC803 cell invasion, and the data reached
statistical significance compared to the control group (p<0.05).
Thus IL-33 may be an inducible factor of gastric cell invasion, and
even may promote the metastasis of gastric cancer.
Discussion
As a new member of the IL-1 family, research on
IL-33 has been mainly focused on inflammation. For example, IL-33
at 10–100 ng/ml significantly and dose-dependently enhanced the
survival of eosinophils (16), and
IL-33 promoted the proliferation of circulating fibrocytes in
patients with allergen-exacerbated asthma (17). In the present study, the
proliferative effect of IL-33 on gastric cancer cells was firstly
reported. Gastric cancer MGC803 cells and normal gastric epithelial
GES-1 cells were incubated with IL-33 at 7.8–1,000 pg/ml. Notably,
IL-33 at a low concentration (<100 pg/ml) had no obvious
proliferative effect on the GES-1 and MGC803 cells. This avoided an
individual effect of IL-33 on cell proliferation in the following
experiments.
In the present study, IL-33 decreased the apoptosis
rate of chemotherapeutic compounds in gastric cancer cell lines,
yet not in normal epithelial GES-1 cells. At present,
fluoropyrimidine, taxanes and platinum-based regimens are most
frequently used and offer a response rate of 30–50% with a median
overall survival of ≤1 year (18).
Considering the combined usage in the clinic, apart from DDP, TDC
containing oxaliplatin was applied. PARP-1 activation has been
shown to cause cell death by triggering a signaling cascade
involving the MAP kinase JNK (19).
In the present study, the apoptosis of cells was confirmed by MTT
assay, flow cytometry and the protein active-PARP level. MTT data
showed that among 5-FU, adriamycin, DDP and etoposide, IL-33
reduced the apoptosis caused by DDP in three gastric cancer cell
lines yet not in GES-1 cells. In addition, IL-33 was found to
weaken the apoptotic effect induced by TDC, consistent with the DDP
group. The present study clearly showed that IL-33 reduced the
cytotoxic effect of the chemotherapeutics on the gastric cancer
cell lines, particularly for the platinum-based therapy. However,
IL-33 had no apparent effect on the apoptosis of GES-1 cells
induced by DDP, which imply that IL-33 may selectively protect
gastric cancer cells from apoptosis rather than normal cells.
Similarly, there is another interleukin that has a similar
protective function as IL-33. IL-22 protects colorectal cancer
cells from chemotherapy by activating the STAT3 pathway (20). The results further imply that IL-33
may weaken the therapeutic effect of platinum-based
chemotherapeutics in gastric cancer.
Among the IL-1 family, ST2 was identified as an
IL-33 receptor (7). In the present
study, the ST2 protein level was increased compared to the control
group after chemotherapy, and increased further when incubated with
both chemotherapeutic agents and IL-33 in MGC803 cells. This
implied that expression of ST2 was increased after cell apoptosis
induced by DDP and TDC. It has been reported that sST2 is
associated with liver injury and cell death as well as systemic
inflammatory response (21).
Following co-culture with IL-33, ST2 was induced compared to the
DDP or TDC group, indicating that IL-33 induced the expression of
ST2. Research has shown that activation or recruitment of IL-33 in
target cells co-expressing the ST2 receptor led to increased ST2
levels in serum (13). However,
extracellular IL-33 was found to promote the expression of ST2 in
gastric cancer. The present study implied that apart from
inflammation, the IL-33/ST2 pathway affects the cell proliferation
and drug sensitivity in gastric cancer.
As found in a previous study, IL-33 was able to bind
a heterodimeric receptor complex consisting of ST2 and IL-1R
accessory protein (IL-1 RAP) (22)
and to activate NK-κB and mitogen-activated protein kinases (MAPKs)
(23). MAPK pathways involve the
extracellular signal-regulated kinases: ERK1/2, c-Jun
amino-terminal kinase JNK and p38 kinase (24). Recent data suggest that MAPKs may
mediate apoptotic signaling induced by anticancer drugs (25). For example, sustained activation of
JNK/p38 MAPK in response to cisplatin was found to lead to cell
death in ovarian carcinoma cells (26). JNK was found to be activated in
response to chemical and environmental stress and to inflammatory
cytokines (27). Research also
suggests that regulation of the ERK1/2 and P38 MAPK signaling
pathways is crucial in the context of DNA-damaging drug-induced
apoptosis and may be generally involved in the apoptosis induced by
anticancer DNA-damaging drugs, including doxorubicin and etoposide
(28). In the present study, to
reveal the protective mechanism of IL-33 in gastric cancer, the
MAPK pathway including ERK1/2, P38, JNK was detected via western
blotting. The data revealed that ERK1/2 and JNK were activated
through phosphorylation under the condition of IL-33, while JNK
activation was more obvious, which indicated that JNK has a
significant correlation with IL-33. Thus, we conclude that JNK
plays an important role in the IL-33 protective function, as well
as ERK1/2. Considering that IL-33 could promote the expression of
ST2, we conclude that IL-33 may function via the IL-33/ST2 and MAPK
pathways including JNK and ERK1/2.
To confirm these findings, MTT assay following
treatment with the JNK inhibitor was carried out. Consistent with
the present study, the cell inhibition rate was higher following
treatment with the JNK inhibitor, which implied that the JNK
inhibitor blocked the protective effects of IL-33 in gastric cancer
cells. Thus, JNK may be a key factor in the IL-33 function for
protecting gastric cancer cells from apoptosis. Other studies have
shown the significance of JNK in gastric cancer. For example,
IL-18-enhanced thrombospondin expression was blocked by the
JNK-specific inhibitor (SP600125) and it enhanced the expression of
phosphorylated JNK in human gastric cancer cell lines (29). Diversin increased the proliferative
and invasive ability of non-small cell lung cancer cells via the
JNK pathway (30).
Many inflammatory cytokines have influence on cell
proliferation, as well as on cell migration. According to a
previous study, IL-1β, besides its central role in inflammation,
has also been recognized as a factor affecting tumor progression,
angiogenesis and invasiveness (31). In the Matrigel invasion assay, we
explored the function of IL-33 in MGC803 cell invasion. The results
showed that IL-33 could promote MGC803 cell invasion. The data
revealed that IL-33 may accelerate gastric cell invasion and may be
an alarm for gastric cancer. Considering the results above, IL-33
may indicate the poor prognosis of gastric cancer patients
particularly those with a high IL-33 level in the serum (14). In addition, a high level of IL-33
was found to be a poor prognostic factor in other tumors. For
example, overexpression in head and neck squamous cell carcinoma
was found to promote migration and invasion (32). IL-33 accelerated breast cancer
growth and metastasis through increased intratumoral accumulation
of immunosuppressive cells and diminished innate antitumor
immunity. The above function was attenuated in the absence of ST2
(33). In short, a high IL-33 level
may be detrimental to the tumor and should be treated as an alarm
during therapy.
The present study demonstrated that IL-33 protected
gastric cancer cell lines rather than normal gastric epithelial
GES-1 cells from apoptosis induced by chemical compounds containing
platinum. In addition, the protective function of IL-33 in gastric
cancer cell apoptosis was mainly through the JNK pathway. IL-33
stimulated gastric cancer cell invasion. The results imply that
IL-33 may combine with ST2 to affect platinum therapy in gastric
cancer and even worsen the disease. In the clinical therapy of
gastric cancer, evaluation of the serum level of IL-33 before drug
administration is recommended. If patients have a high IL-33 serum
level, then chemotherapeutics containing platinum should be avoided
or applied cautiously. In addition, various specific molecular
inhibitors can be combined with platinum therapy, such as a JNK
inhibitor. Further in vivo and clinical research of IL-33,
particularly in regards to gastric cancer patients is
warranted.
Acknowledgments
This study was funded by Ningbo Natural Science
Foundation (no. 2011A610048)
References
|
1
|
Oguma K, Oshima H and Oshima M:
Inflammation, tumor necrosis factor and Wnt promotion in gastric
cancer development. Future Oncol. 6:515–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ernst P: Review article: the role of
inflammation in the pathogenesis of gastric cancer. Aliment
Pharmacol Ther. 13(Suppl 1): S13–S18. 1999. View Article : Google Scholar
|
|
3
|
Fox JG and Wang TC: Inflammation, atrophy,
and gastric cancer. J Clin Invest. 117:60–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Multhoff G, Molls M and Radons J: Chronic
inflammation in cancer development. Front Immunol. 2:982012.
View Article : Google Scholar :
|
|
5
|
Mosaffa F, Kalalinia F, Lage H, Afshari JT
and Behravan J: Pro-inflammatory cytokines interleukin-1 beta,
interleukin 6, and tumor necrosis factor-alpha alter the expression
and function of ABCG2 in cervix and gastric cancer cells. Mol Cell
Biochem. 363:385–393. 2012. View Article : Google Scholar
|
|
6
|
Chen CC, Chu CB, Liu KJ, Huang CY, Chang
JY, Pan WY, Chen HH, Cheng YH, Lee KD, Chen MF, et al: Gene
expression profiling for analysis acquired oxaliplatin resistant
factors in human gastric carcinoma TSGH-S3 cells: the role of IL-6
signaling and Nrf2/AKR1C axis identification. Biochem Pharmacol.
86:872–887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen Z, Seppänen H, Vainionpää S, Ye Y,
Wang S, Mustonen H and Puolakkainen P: IL10, IL11, IL18 are
differently expressed in CD14+ TAMs and play different
role in regulating the invasion of gastric cancer cells under
hypoxia. Cytokine. 59:352–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohran ZY, Ali-Eldin FA and Abdel Aal HA:
Serum interleukin-18: does it have a role in the diagnosis of
hepatitis C virus related hepatocellular carcinoma? Arab J
Gastroenterol. 12:29–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuboi K, Miyazaki T, Nakajima M, Fukai Y,
Masuda N, Manda R, Fukuchi M, Kato H and Kuwano H: Serum
interleukin-12 and interleukin-18 levels as a tumor marker in
patients with esophageal carcinoma. Cancer Lett. 205:207–214. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thong-Ngam D, Tangkijvanich P, Lerknimitr
R, Mahachai V, Theamboonlers A and Poovorawan Y: Diagnostic role of
serum interleukin-18 in gastric cancer patients. World J
Gastroenterol. 12:4473–4477. 2006.PubMed/NCBI
|
|
12
|
Smith DE: The biological paths of IL-1
family members IL-18 and IL-33. J Leukoc Biol. 89:383–392. 2011.
View Article : Google Scholar
|
|
13
|
Moussion C, Ortega N and Girard JP: The
IL-1-like cytokine IL-33 is constitutively expressed in the nucleus
of endothelial cells and epithelial cells in vivo: a novel
‘alarmin’? PLoS One. 3:e33312008. View Article : Google Scholar
|
|
14
|
Sun P, Ben Q, Tu S, Dong W, Qi X and Wu Y:
Serum interleukin-33 levels in patients with gastric cancer. Dig
Dis Sci. 56:3596–3601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Phase III study of docetaxel and cisplatin plus
fluorouracil compared with cisplatin and fluorouracil as first-line
therapy for advanced gastric cancer: a report of the V325 Study
Group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzukawa M, Koketsu R, Iikura M, Nakae S,
Matsumoto K, Nagase H, Saito H, Matsushima K, Ohta K, Yamamoto K
and Yamaguchi M: Interleukin-33 enhances adhesion, CD11b expression
and survival in human eosinophils. Lab Invest. 88:1245–1253. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bianchetti L, Marini MA, Isgrò M, Bellini
A, Schmidt M and Mattoli S: IL-33 promotes the migration and
proliferation of circulating fibrocytes from patients with
allergen-exacerbated asthma. Biochem Biophys Res Commun.
426:116–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Vita F, Giuliani F, Silvestris N,
Catalano G, Ciardiello F and Orditura M: Human epidermal growth
factor receptor 2 (HER2) in gastric cancer: a new therapeutic
target. Cancer Treat Rev. 36(Suppl 3): S11–S15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Huang S, Liu ZG and Han J:
Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic
cell death requires RIP1/TRAF2-mediated JNK1 activation. J Biol
Chem. 281:8788–8795. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu T, Wang Z, Liu Y, Mei Z, Wang G, Liang
Z, Cui A, Hu X, Cui L, Yang Y and Liu CY: Interleukin 22 protects
colorectal cancer cells from chemotherapy by activating the STAT3
pathway and inducing autocrine expression of interleukin 8. Clin
Immunol. 154:116–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bergis D, Kassis V, Ranglack A, Koeberle
V, Piiper A, Kronenberger B, Zeuzem S, Waidmann O and Radeke HH:
High serum levels of the interleukin-33 receptor soluble ST2 as a
negative prognostic factor in hepatocellular carcinoma. Transl
Oncol. 6:311–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chackerian AA, Oldham ER, Murphy EE,
Schmitz J, Pflanz S and Kastelein RA: IL-1 receptor accessory
protein and ST2 comprise the IL-33 receptor complex. J Immunol.
179:2551–2555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar
|
|
24
|
Yuan L, Wang J, Xiao H, Wu W, Wang Y and
Liu X: MAPK signaling pathways regulate mitochondrial-mediated
apoptosis induced by isoorientin in human hepatoblastoma cancer
cells. Food Chem Toxicol. 53:62–68. 2013. View Article : Google Scholar
|
|
25
|
Fan M and Chambers TC: Role of
mitogen-activated protein kinases in the response of tumor cells to
chemotherapy. Drug Resist Updat. 4:253–267. 2001. View Article : Google Scholar
|
|
26
|
Mansouri A, Ridgway LD, Korapati AL, Zhang
Q, Tian L, Wang Y, Siddik ZH, Mills GB and Claret FX: Sustained
activation of JNK/p38 MAPK pathways in response to cisplatin leads
to Fas ligand induction and cell death in ovarian carcinoma cells.
J Biol Chem. 278:19245–19256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH,
Kim BW, Choi HY, Jeong MY and Cho SG: Interplay between PI3K/Akt
and MAPK signaling pathways in DNA-damaging drug-induced apoptosis.
Biochim Biophys Acta. 1763:958–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim J, Kim C, Kim TS, Bang SI, Yang Y,
Park H and Cho D: IL-18 enhances thrombospondin-1 production in
human gastric cancer via JNK pathway. Biochem Biophys Res Commun.
344:1284–1289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luan L, Zhao Y, Xu Z, Jiang G, Zhang X,
Fan C, Liu D, Zhao H, Xu K, Wang M, et al: Diversin increases the
proliferation and invasion ability of non-small-cell lung cancer
cells via JNK pathway. Cancer Lett. 344:232–238. 2014. View Article : Google Scholar
|
|
31
|
Fontana VA, Sanchez M, Cebral E and Calvo
JC: Interleukin-1 beta regulates metalloproteinase activity and
leptin secretion in a cytotrophoblast model. Biocell. 34:37–43.
2010.PubMed/NCBI
|
|
32
|
Chen SF, Nieh S, Jao SW, Wu MZ, Liu CL,
Chang YC and Lin YS: The paracrine effect of cancer-associated
fibroblast-induced interleukin-33 regulates the invasiveness of
head and neck squamous cell carcinoma. J Pathol. 231:180–189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jovanovic IP, Pejnovic NN, Radosavljevic
GD, Pantic JM, Milovanovic MZ, Arsenijevic NN and Lukic ML:
Interleukin-33/ST2 axis promotes breast cancer growth and
metastases by facilitating intratumoral accumulation of
immunosuppressive and innate lymphoid cells. Int J Cancer.
134:1669–1682. 2014. View Article : Google Scholar
|