Introduction
Mushrooms are a valuable part of a balanced
nutrition. The use of mushrooms for food as well as for medicine is
deep-rooted in most cultures. Many mushrooms contain healthy
micronutrients, vitamins and mineral nutrients. Fungal
polysaccharide is a type of active organic compound that is found
in medicinal fungi, fruiting bodies and mycelium (1). Polysaccharides are polymeric
carbohydrate molecules composed of long chains of monosaccharide
units bound together by glycosidic linkages and upon hydrolysis
yield the constituent monosaccharides or oligosaccharides. They
range in structure from linear to highly branched. Polysaccharides
are often quite heterogeneous, containing slight modifications of
the repeating unit. Depending on the structure, these
macromolecules can have properties that are distinct from their
monosaccharide building blocks. They may be amorphous or even
insoluble in water (2). Recently,
an increasing number of fungal polysaccharides have been reported
to exhibit a variety of biological activities, including
immunostimulatory, antitumor and antioxidant properties (3–8).
Gomphus clavatus Gray grows in Xiaojin
Country of Sichuan Province in China at an elevation of 3,770
meters. A novel heteropolysaccharide was isolated from the fruiting
bodies of Gomphus clavatus Gray through Sephadex G-200 and
DEAE-cellulose columns. Its chemical structure and antioxidant and
anticancer activities were characterized for the first time.
Overall, Gomphus clavatus Gray may be an ideal source of
antioxidant and anticancer agents.
Materials and methods
Chemicals
The fresh fruiting bodies of Gomphus clavatus
Gray were collected in Xiaojing Country of Sichuan Province, China,
and were authenticated by Professor Zhirong Yang (College of Life
Sciences, Sichuan University, Chengdu, China). The fruiting bodies
of Gomphus clavatus Gray were crushed and stored at 4°C
before being used in the Key Laboratory of Southwest China Wild
Resources Conservation, School of Life Sciences, China West Normal
University. Sephadex G-200 and DEAE-cellulose 52 were purchased
from Sigma-Aldrich (Shanghai, China). Trifluoroacetic acid (TFA),
standard monosaccharides and dextrans of different molecular
weights (MWs) were purchased from Beijing Biodee Biotechnology Co.,
Ltd. (Beijing, China). All other reagents used were of analytical
grade.
Extraction, purity and fractionation of
the polysaccharides from Gomphus clavatus Gray
Dried and powdered Gomphus clavatus Gray (280
g) was precisely weighed and then extraction was carried out with
2,000 ml distilled water at 90°C for 6 h. After extraction, the
extractive was filtrated and then centrifuged at 10,000 rpm for 25
min in a high-speed centrifuge and subsequently concentrated in a
vacuum. Then the supernatant was added with 3 volumes of 95% EtOH
to precipitate crude polysaccharides (GCG; 26.0 g, recovery 9.3%).
The Sevag method was used for the deproteination (9), and the crude polysaccharides (10 g)
were redissolved in 50 ml of distilled water, and purified with a
DEAE-cellose column (Tris-HCl, pH 7.0, 4.5×50 cm, Cl−)
equilibrated with distilled water. The polysaccharides were
fractionated and eluted stepwise with NaCl solutions at different
concentrations (0, 0.1, 0.2, 0.3, 0.4, 0.5 and 1.0 mol/l NaCl). The
eluate was monitored by the phenol-sulfuric acid method (10). The pure water elution was
concentrated and purified on a Sephadex G-200 column (2.6x60 cm).
The resulting product was concentrated and passed through a 7-kDa
membrane for 48 h to eliminate small-molecular compounds. The
Gomphus clavatus Gray polysaccharide, named GCG-1, was
obtained by the above processes and then lyophilized. The yield
rate of GCG-1 was 0.11% (0.300 g) form the starting material.
Measurement of the molecular weight (MW)
of GCG-1
The MW of the polysaccharide fraction was identified
by high-performance gel permeation chromatography (HPGPC) (11). An aliquot (5 mg) of the dry sample
was dissolved in 10 ml of double-distilled water and filtered
through a membrane filter (0.22-μm). The calibration curve
was prepared from the standard T-series Dextran (T-500, T-110,
T-70, T-40 and T-10). The data were analyzed using GPC software
(Millennium 32 software).
Monosaccharide composition analysis of
GCG-1
The polysaccharide GCG-1 (6.0 mg) was hydrolyzed
with 2 M trifluoroacetic acid (TFA) at 110°C for 8 h (12). After removing the excess acid with
methyl alcohol (MeOH) when the hydrolysis was completed, the
samples were dissolved with distilled water for analyzing the
monosaccharide composition. One part of the hydrolysate (2.0 mg)
was used for thin layer chromatography analysis (TLC) with
developing solvent [acetoacetate-pyridine-ethanol-water solution
(8:5:1.5:1)] and developer system (85% phosphoric acid solution 140
ml containing 8 ml diphenylamine, 8 g aniline) (13). The other (2.0 mg) was dissolved in
pyridine (0.2 ml). The derivatization reaction was initiated by
addition of hexamethyldisilazane (0.2 ml) and trimethylchlorosilane
(0.2 ml) (14). The resulting
supernatant was examined by chromatographymass spectrometry (GC-MS)
on a column of Rtx-5sil MS (5% phenylmethylsiloxane 30 mm × 0.25 mm
× 0.25 μm) at a temperature program of 50–230°C with a rate
of 2°C/min (15,16).
Methylation analysis
The polysaccharide was methylated using methyliodide
(MeI) according to the method of Hakomori (16). The completeness of methylation was
confirmed by the disappearance of the hydroxyl absorption in
infrared (IR) spectrum at 3,400 cm−1. The permethylated
product was depolymerized with 90% formic acid at 100°C for 4 h and
further hydrolyzed with 2 M TFA at 100°C for 8 h. The resulting
products were derivatized using the derivatization reagent and
analyzed by GC-MS.
UV and IR spectral analysis
GCG-1 was tested in UV light from 200 to 400 nm.
FT-IR spectra of the sample were measured by grinding a mixture of
polysaccharide with dry KBr and then pressing in a mold. Fourier
transform IR spectra of the GCG-1 film were collected using a
Thermo Nicolet 6700 spectrometer operating in the range of
400–4,000 cm−1 at a resolution of 4 cm−1.
Nuclear magnetic resonance (NMR)
experiment
The polysaccharide was dissolved in deuteroxide
accompanied by ultrasonic wave processing for 30 min. Then the
Varian Unity INOVA 400/45 was used to perform the 1H NMR
spectra (GCG-1, 80 mg) and 13C NMR spectra (GCG-1, 40
mg) analysis with tetramethylsilane as internal standard.
Determination of
1,1-diphenyl-2-picrylhydrazyl-free (DPPH−) radical
scavenging activity of GCG-1
The DPPH− radical scavenging activity of
the polysaccharide sample was measured according to the method
described by Braca et al (17,18).
Antiradical activity was measured by a decrease in absorbance at
517 nm of a solution of purple-colored DPPH in methanol brought
about by the sample. Absorbance at 517 nm was determined after 30
min using a UV-visible spectrometer; a lower absorbance of the
reaction mixture indicated higher free radical scavenging activity.
The capability to scavenge the DPPH radical was calculated using
the following equation: Scavenging effect (%) = (1 − A sample/A
control) x 100, where A control is the absorbance of the control
(DPPH− solution without sample), A sample is the test
sample (DPPH− solution plus test sample or positive
control). Vitamin C (Vc) and butylated hydroxytoluene (BHT) were
used as positive control.
Scavenging activity of the
2,2′-azino-bis(3-ethylbenzthi-azoline-6-suphonic acid) diammonium
(ABTS) radical of GCG-1
ABTS radical scavenging activity of the
polysaccharide extracts and fractions was measured by the ABTS
cation decolorization assay as described by Auddy et al
(19,20). The ABTS radical cation
(ABTS+) was produced by reaction of 7 mM stock solution
of ABTS with 2.45 mM ammonium persulphate (APS) and allowing the
mixture at room temperature in the dark for 16 h. Then 2 ml of
various concentrations of the sample and 2 ml of ABTS+
radical solution (0.7 mM) were added. The absorbance was measured
immediately at 734 nm. A control reaction was carried out without
the extract. The percentage of scavenging of hydrogen radicals was
calculated as follows: Scavenging effect (%) = [1 − (A sample - A
sample blank)/A control] x 100, where A control is the absorbance
of the control group in the ABTS+ radical generation
system, A sample is the absorbance of the test group and A sample
blank is the absorbance of the samples only. Vc was used as a
positive control.
Cell lines and culture
PC12 cells [American Type Culture Collection (ATCC)
USA] were maintained in Dulbecco’s modified Eagle’s medium (DMEM),
which contained 10% fetal bovine serum (FBS) and antibiotics (100
U/ml penicillin, 100 mg/ml streptomycin) at 37°C in a humidified
atmosphere containing 5% CO2. Human hepatoma G-2 cells
were purchased from the North Sichuan Medical College, Institute of
Biochemistry and Molecular Immunology and maintained in MEM (Gibco
Co., Carlsbad, CA, USA) supplemented with 10% FBS (Evergreen
Biological Products Co., China), 100 U/ml penicillin, 100
μg/ml streptomycin and pH 7.4 RPMI-1640 (all from Gibco Co.)
at 37°C in a humidified atmosphere containing 5%
CO2.
Antioxidant activity assay
In the present study, PC12 cells were seeded into
96-well plates at a concentration of 5x104 cells/ml
using DMEM. After 24 h, the PC12 cells were pretreated with GCG-1
for 2 h before H2O2 (300 mM solution)
exposure for 1 h. After the H2O2 was
withdrawn, cells were then further incubated in the fresh medium
for another 6 h at 37°C. Then, Cell Counting Kit-8 (CCK-8) solution
(10 μl) was added to each well. After incubating for 4 h,
the absorbance measurement was determined at 450 nm using a
Universal microplate reader (Bio-Rad, USA). The damage inhibitory
effect was expressed as: Damage inhibitory effect (%) =
[(As − A)]/[(A0 − A)] x 100%, where
As is the absorbance in the presence of the sample and
H2O2, A0 is the absorbance of the
control in the absence of the sample and
H2O2, and A is the absorbance only in the
presence of H2O2.
Quantitative RT-PCR detection of related
gene expression
The HepG-2 cells were harvested after stimulation by
various concentrations of GCG-1 for 4 h. The total cellular RNA was
extracted using TRIzol reagent and reverse-transcribed into cDNA
using oligo(dT)18 primers (both from Invitrogen, USA).
Amplification of each target cDNA was performed in a cycler system
(Bio-Rad). PCR products were quantified using SYBR-Green I and
β-actin was used as an endogenous control to normalize expression
levels. The relative expression abundance was calculated by the
following formula: Relative expression abundance = moles of
detected mRNA/moles of β-actin mRNA.
Statistical analysis
All data are presented as means ± standard deviation
(SD) of three replications. Statistical analyses were performed
using the Student’s t-test and one-way analysis of variance. Values
of P<0.05 were considered to indicate statistically significant
findings.
Statement of the use of humans and
experimental animals
The present study was carried out on humans
following the international and national regulations. The study
also followed internationally recognized guidelines on animal
welfare, as well as local and national regulations.
Results and Discussion
Extraction, purity and composition of
polysaccharides
The crude polysaccharide, named GCGP, was obtained
from the fruiting bodies of Gomphus clavatus Gray with a
yield of 9.3%. After fractionation with Sephadex G-200 and
DEAE-cellulose 52 column chromatography, 200 mg of GCG-1 was
obtained from the 0.1 M NaCl eluate. GCG-1 was eluted from
gel-filtration chromatography on Sephadex G-200 column and was
detected by the phenol-sulfuric acid assay as a single peak and it
had the same optical rotation: [α]20D − 11.4°
(c 0.5, water) with different low concentration of ethanol using
HK7-SGW-1 automatic optical polarimeter. HPGPC of the
polysaccharide fraction showed that each fraction was represented
by a broad and symmetrical peak on the chromatograms. The dextran
standards were used to create a calibration curve for elucidating
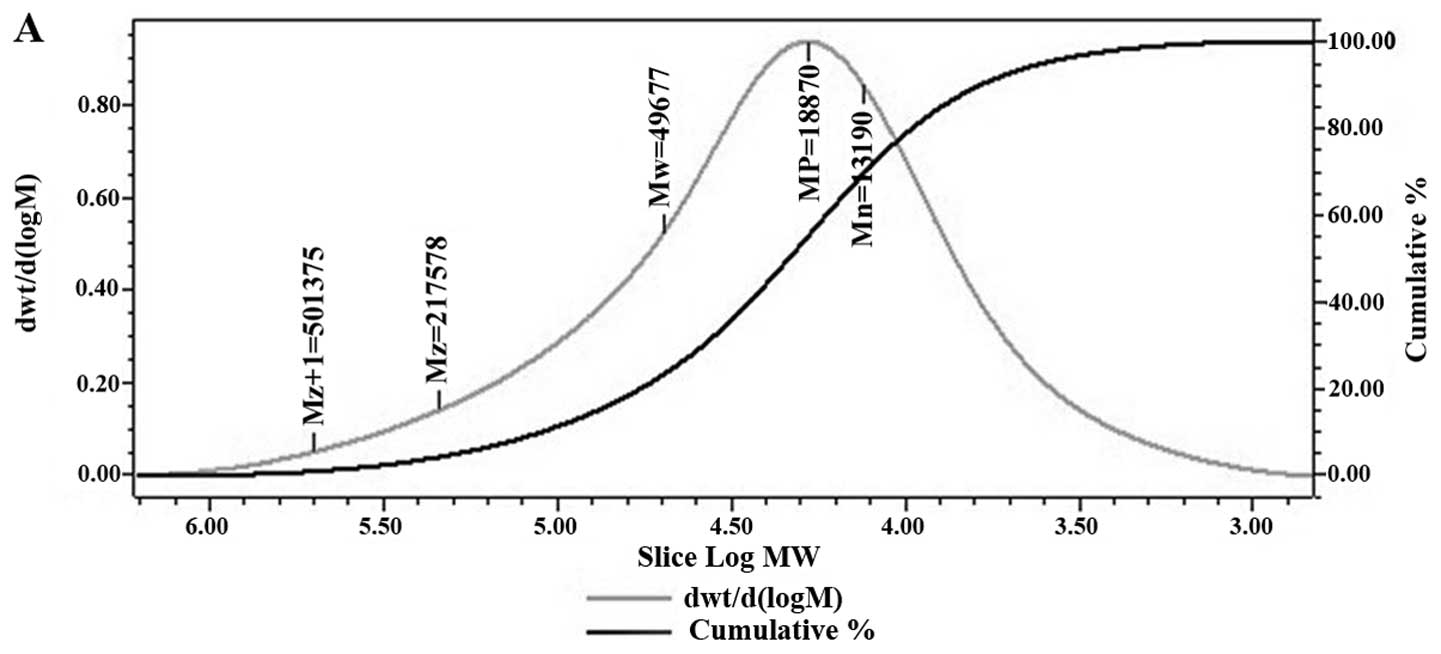
the molecular weight of GCG-1. The average molecular weight of
GCG-1 was ~50,000 Da and the polydispersity was 3.77 (Fig. 1A). The composition analysis of
polysaccharides is an important step to control the quality and
obtain basic information about polysaccharides. In the present
study, the GCG-1 polysaccharide sample was hydrolyzed with TFA and
then the component monosaccharides were analyzed by TLC. It was
shown that the GCG-1 polysaccharides had a composition of D-glucose
and D-galactose. GCG-1 was in good agreement with the
D-configuration monosaccharides according to the GC-MS
analysis.
Structure elucidation of GCG-1
The IR spectrum of the sample showed that the
absorption was very obvious at >3,000 cm−1, which was
caused by the stretching vibration and angular vibration of O-H
linkage. The intensity of bands ~3,416 cm−1 in the IR
spectrum (Fig. 1B) was due to the
hydroxyl stretching vibration of the polysaccharide and as expected
they were broad. The absorption peak at 2,932 cm−1 was
C–H stretching vibration absorption peak of GCG-1, and the bands in
the region of 1,653 cm−1 were due to associated water
(21). The strong absorption bands
at 1,404 cm−1 were due to C-H bending vibration and the
bands in the region of 400–702 cm−1 were due to C-H
rocking vibration. The strong absorption bands at 1,046 and 1,077
cm−1 in the range of 1,200–1,000 cm−1 in the
IR spectrum suggested that the monosaccharides in GCG-1 had a
pyranose-ring (22). Moreover, the
characteristic absorption at 917 cm−1 indicated
α-configurations (23), which was
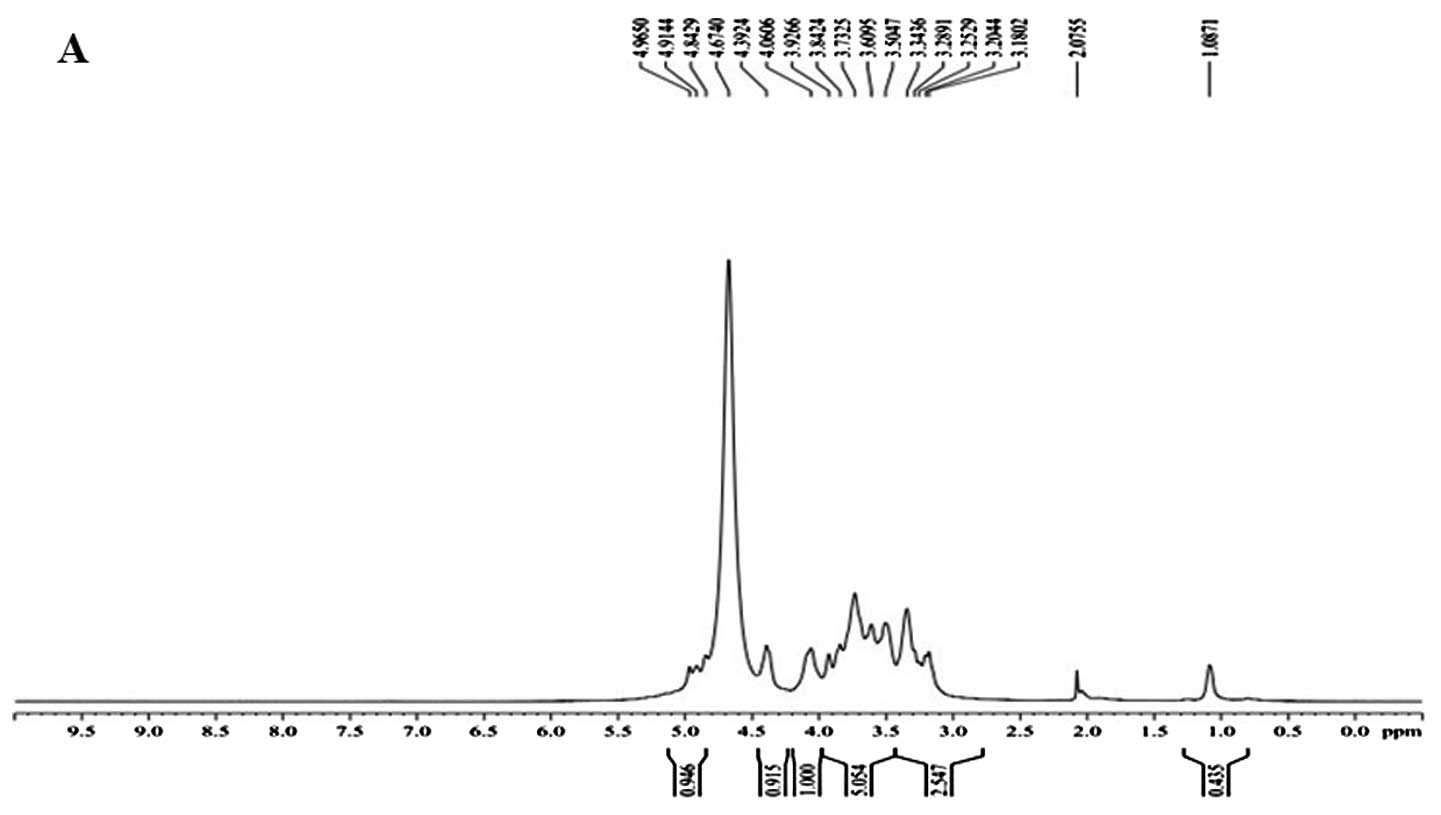
in good agreement with the anomeric proton signals at δ 4.965, δ
4.914 and δ 4.842 in the 1H NMR (400 MHz) spectrum
(Fig. 2A). δ 4.674 was the hydrogen
signal of water. The signals at δ 3.180–4.392 are the signal peaks
of remaining proton which were mostly formed by a number of signal
peaks which overlapped.
The resonances in the region of 101–103 ppm in the
13C NMR (400 MHz) spectrum of GCG-1 were due to the
anomeric carbon atoms of β-D-glucosepyranose (β-D-Glup) and
α-D-galactopyranose (α-D-Galp) (24). Signals at δ 103.657 could be
attributed to C-1 of →3)-α-D-Gal-(1→; δ 103.026 to C-1 of
α-D-Gal-(1→; δ 102.925 to C-1 of →4)-β-D-Glu-(1→ (Fig. 2B) (Table
I).
 | Table I13C NMR chemical shift
data (δ, ppm) for polysaccharide GCG-1. |
Table I
13C NMR chemical shift
data (δ, ppm) for polysaccharide GCG-1.
| Sugar residues | Chemical shifts, δ
(ppm)
|
|---|
| C1 | C2 | C3 | C4 | C5 | C6 |
|---|
|
→4)-β-D-Glu-(1→ | 102.925 | 65.234 | 66.840 | 69.585 | 73.406 | 75.650 |
|
→4,6)-β-D-Glu-(1→ | 101.411 | 61.107 | 66.590 | 68.899 | 73.104 | 74.956 |
|
→3)-α-D-Gal-(1→ | 103.657 | 60.110 | 67.543 | 70.424 | 74.184 | 76.034 |
| α-D-Gal-(1→ | 103.026 | 60.806 | 67.221 | 70.093 | 73.519 | 75.952 |
The methylated products of GCG-1 were hydrolyzed
with acid, converted into alditol acetate and analyzed by GC-MS.
Experiment data were collected and are listed in Table II. The information in MS showed
that fragment ion peaks were consistent with data of
D-configuration monosaccharide fragment ion peaks which can be
concluded that galactose and glucose residues had D configuration,
respectively. Methylation analysis for GCG-1 proved that the
α-D-galactopyranose residues were 2,4,6-tri-substituted and
2,3,4,6-tetra-substituted, the β-D-Glup) residues were
2,3-bis-substituted and 2,3,6-trisubstituted (Table II and Fig. 3). Results of the methylated linkage
analysis of GCG-1 indicated that the branched residue was (1→4,
6)-linked-β-D-glucosepyranose and also revealed that
(1→4)-linked-β-D-glucosepyranose possibly formed the backbone
structure. Residues of branch structures were terminated with
α-D-galactopyranose residues. The relative amounts of (1→4,
6)-linked-β-D-glucosepyranose indicated that approximate branch
ratios could theoretically be 60%, corresponding to an average two
branching points at each three backbone residues. It was concluded
that GCG-1 had a backbone of (1→4)-β-D-glucopyranose residues which
branch at O-6 based on the experimental results. The branches were
mainly composed of two with (1→3)-α-D-galactopyranose residue. The
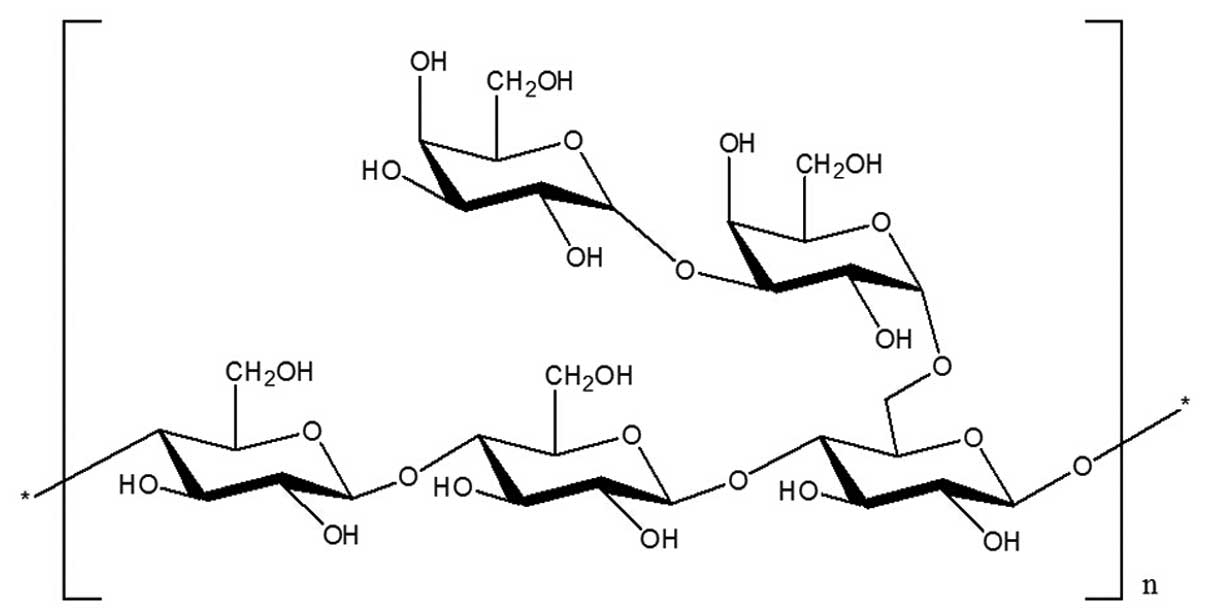
predicted structure of the novel polysaccharide GCG-1 is shown in
Fig 4.
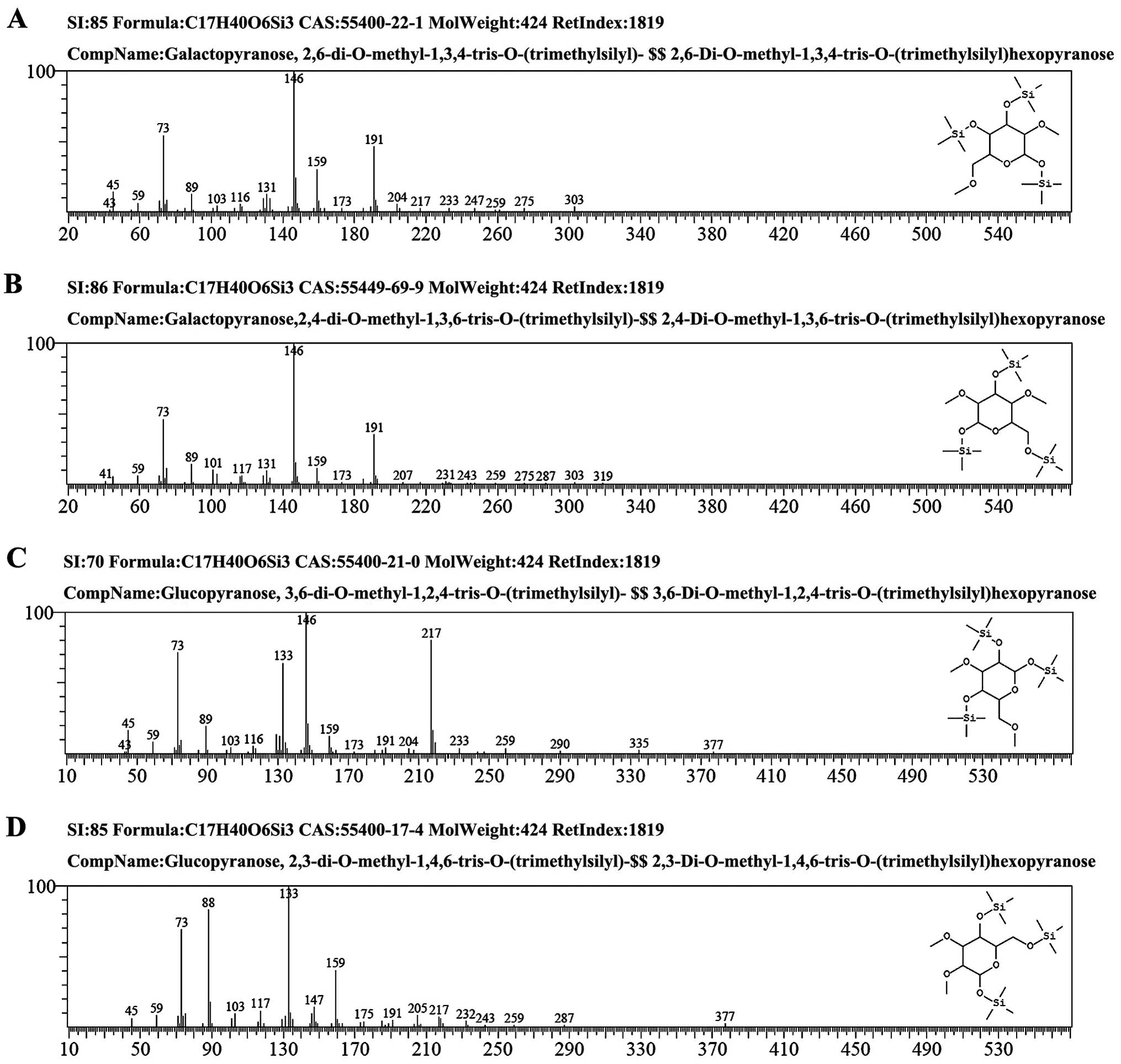 | Figure 3The GC-MS spectra of GCG-1. (A) The
fragment ion peaks of
2,6-di-O-methyl-1,3,4-tris-O-(trimethylsilyl)-galactopyranose. (B)
The fragment ion peaks of
2,4-di-O-methyl-1,3,6-tris-O-(trimethylsilyl)-galactopyranose. (C)
The fragment ion peaks of
3,6-di-O-methyl-1,2,4-tris-O-(trimethylsilyl)-glucopyranose. (D)
The fragment ion peaks of
2,3-di-O-methyl-1,4,6-tris-O-(trimethylsilyl)-glucopyranose. GC-MS,
gas chromatography-mass spectrometry; GCG-1, Gomphus
clavatus Gray polysaccharide. |
 | Table IIGC-MS results of the methylation
analysis of GCG-1. |
Table II
GC-MS results of the methylation
analysis of GCG-1.
| Methylated
sugar | Linkage | m/z |
|---|
|
2,3,6-Me3-Glu | 1,4- | 45, 59, 73, 89,
101, 133, 146, 159, 204, 217, 233 |
|
2,3-Me2-Glu | 1,4, 6- | 45, 59, 73, 88,
103, 117, 133, 147, 159, 175, 205, 217, 232 |
|
2,4,6-Me3-Gal | 1, 3- | 45, 59, 73, 89,
101, 117, 131, 146, 159, 173, 204, 217, 233 |
|
2,3,4,6-Me4-Gal | T- | 45, 59, 73, 89,
103, 117, 146, 159, 173, 191, 207, 231 |
Determination of DPPH− radical
scavenging activity of GCG-1
The decrease in absorbance of the DPPH−
radical caused by antioxidants is due to the reaction between
antioxidant molecules and radical progress which results in the
scavenging of the radical by hydrogen donation. It is visually
noticeable as a change in color from purple to yellow. GCG-1
exhibited a comparable antioxidant activity with that of standard
ascorbic acid at varying concentrations tested. There was a
dose-dependent increase in the percentage of antioxidant activity
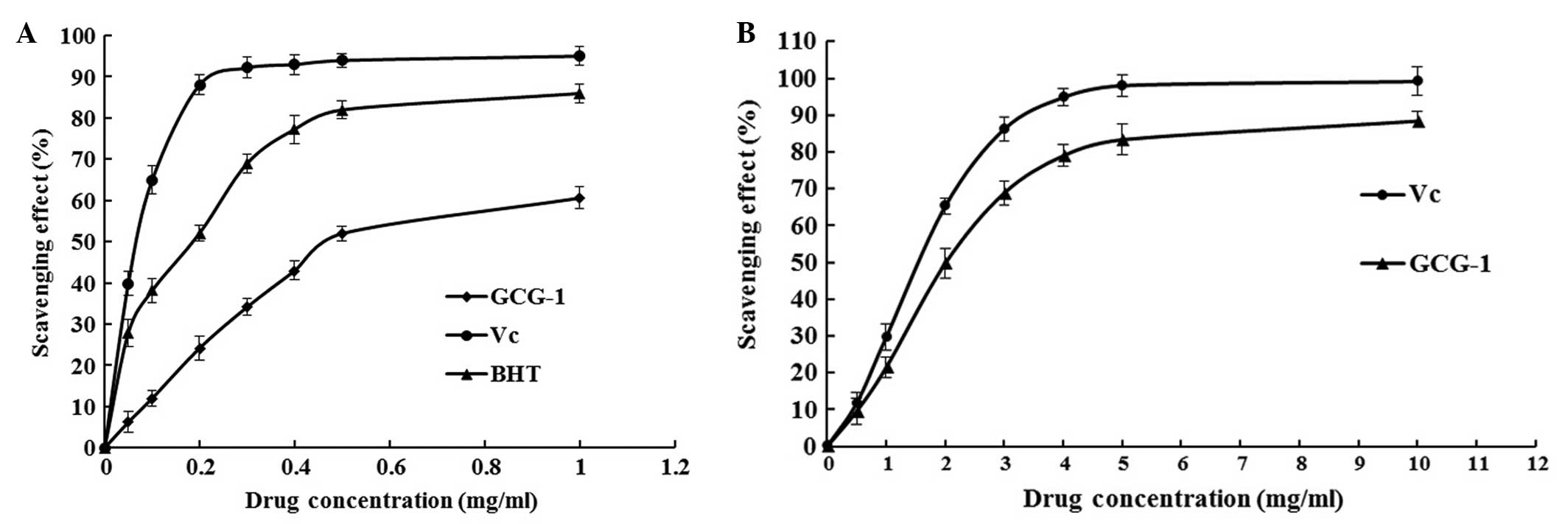
for all concentrations tested (Fig.
5). These results showed that the IC50 value of
GCG-1 for eliminating DPPH− radicals was ~0.467
mg•ml−1, which indicated that GCG-1 had a noticeable
effect on scavenging the DPPH− radical, particularly at
high addition quantity. However, the inhibitory ability was lower
than that of BHT and Vc.
Determination of ABTS+ radical
cation scavenging activity of GCG-1
The ABTS radical scavenging activity of GCG-1 was
measured spectrophotometrically at 734 nm. The results of
antioxidant activity of GCG-1 are expressed as shown in Fig. 5B. Absorbance of the ABTS+
radical cation was decreased dose-dependently, and the
IC50 value of GCG-1 was 2.520 mg•ml−1.
However, the scavenging activity was lower than that of Vc.
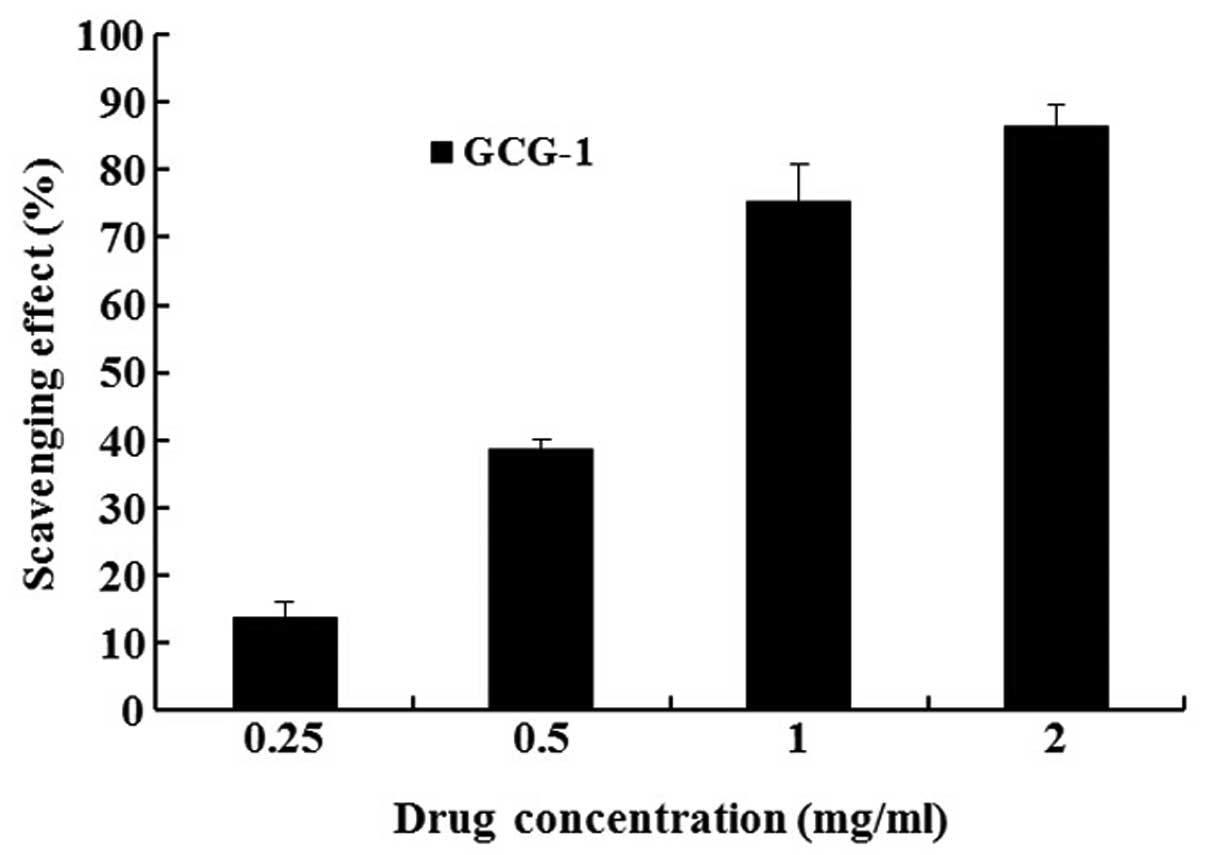
Antioxidant activity analysis of GCG-1 by
CCK-8
In the CCK-8 experiments, we determined the
protective effect of GCG-1 on PC12 cells from hydrogen peroxide
(H2O2)-induced injury. After pretreatment
with 0.25, 0.5, 1, 2 mg•ml−1 of GCG-1, the PC12 cells
were protected from H2O2 (300 mM) injury in a
dose-dependent manner, with cell viability rates of 13.8, 38.5,
75.2 and 86.3%, respectively (Fig.
6). Thus, we confirmed that GCG-1 attenuates the injury on PC12
cells induced by H2O2.
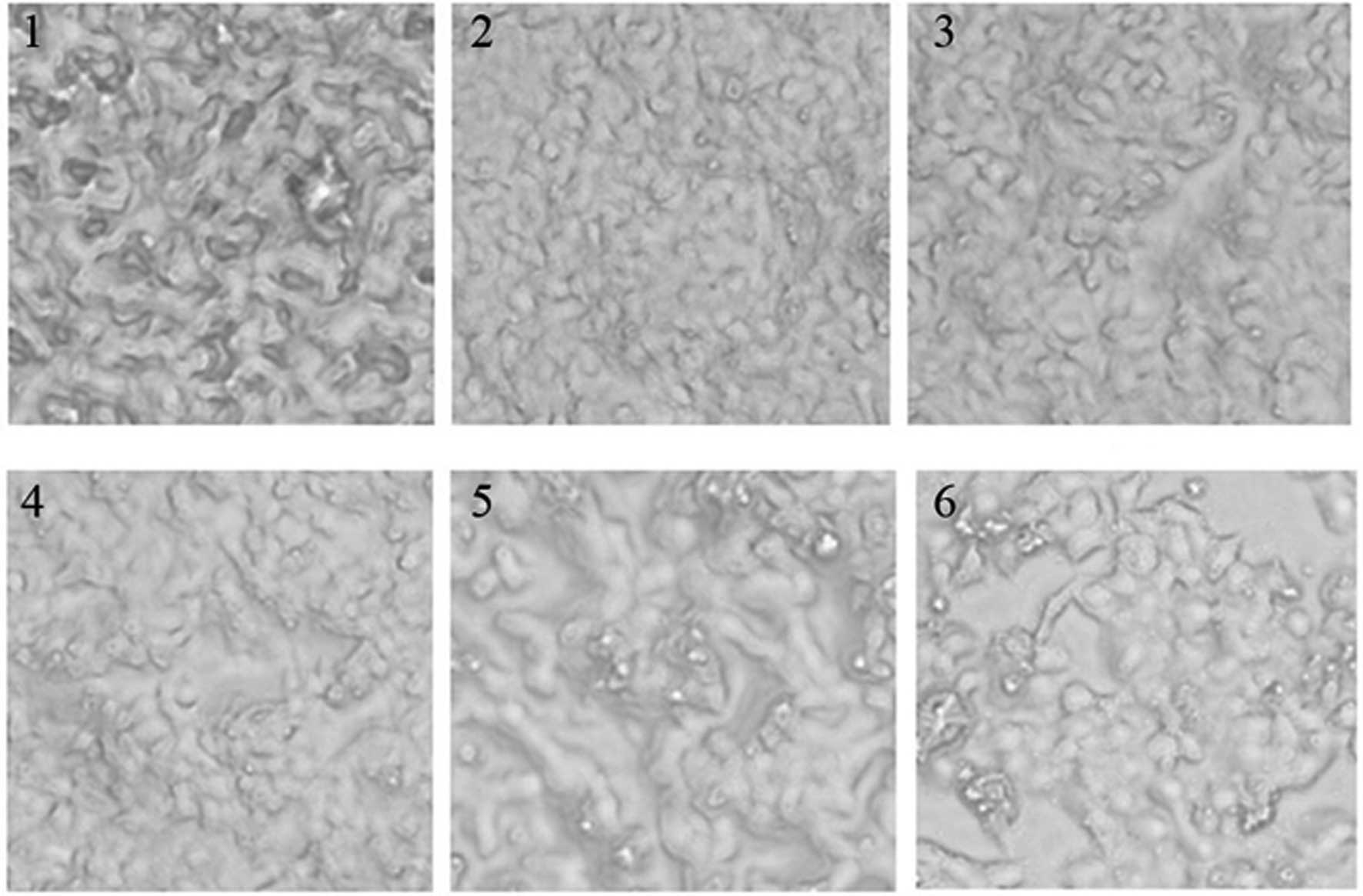
Effect of GCG-1 on the morphology of
human hepatoma HepG-2 cells
The 96-well plates were placed under an inverted
microscope, and images recorded the changes in cell morphology for
different concentrations of GCG-1 in order to measure the effect of
GCG-1. GCG-1 exhibited high anticancer activity as observed from
the cell morphology, examples are shown in Fig. 7.
GCG-1 affects the expression of mRNA
expression of housekeeping genes in HepG-2 cells
Housekeeping genes are typically constitutive genes
that are required for the maintenance of basic cellular function,
and are expressed in all cells of an organism under normal and
pathophysiological conditions. These genes tend to produce proteins
at steady rates, and errors in their expression can lead to cell
death. Quantitative RT-PCR results showed that GCG-1 affected the
mRNA expression of various housekeeping genes in HepG-2 cells
compared to the untreated cells (Table III).
 | Table IIImRNA expression of housekeeping genes
in HepG-2 cells treated with GCG-1. |
Table III
mRNA expression of housekeeping genes
in HepG-2 cells treated with GCG-1.
| Gene symbol | a | b | c | d | e | f | g |
|---|
| APC | 30.55 | 12.39 | 28.02 | 6.91 | 5.49 | 0.02 | 44.79 ↑ |
| RUNX3 | 37.00 | 18.84 | 34.48 | 13.37 | 5.48 | 0.02 | 44.48 ↑ |
| CDKN2B | 33.30 | 15.14 | 32.46 | 11.35 | 3.80 | 0.07 | 13.88 ↑ |
| IL2 | 38.27 | 20.11 | 37.69 | 16.58 | 3.54 | 0.09 | 11.59 ↑ |
| WWOX | 29.25 | 11.09 | 36.91 | 15.80 | −4.71 | 26.08 | 26.08 ↓ |
| MDM2 | 23.93 | 5.77 | 30.28 | 9.17 | −3.40 | 10.52 | 10.52 ↓ |
Cyclin-dependent kinase inhibitor 2B (Cdkn2b) gene
lies adjacent to the tumor-suppressor gene CDKN2A in a region that
is frequently mutated and deleted in a wide variety of tumors
(25). Adenomatous polyposis coli
(APC) is classified as a tumor-suppressor gene which prevents the
uncontrolled growth of cells resulting in cancerous tumors
(26). The APC protein produced by
the APC gene controls how often a cell divides, how it attaches to
other cells within a tissue, or whether a cell moves within or away
from a tissue thus determining whether a cell may develop into a
tumor. Runt-related transcription factor 3 (Runx3) gene encodes a
member of the runt domain-containing family of transcription
factors. A heterodimer of this protein and a β subunit forms a
complex that binds to the core DNA sequence 5′-PYGPYGGT-3′ found in
a number of enhancers and promoters, and can either activate or
suppress transcription. IL-2 is a lymphokine that induces the
proliferation of responsive T cells. In addition, it acts on
various B cells, via receptor-specific binding (27), as a growth factor and antibody
production stimulant (28). The
protein is secreted as a single glycosylated polypeptide, and
cleavage of a signal sequence is required for its activity
(29). Quantitative RT-PCR results
showed a significant upregulation in the levels of Apc, Cdkn2b,
IL-2 and Runx3 mRNA in the GCG-1-treated HepG-2 cells (Table III). Particularly the expression
levels of APC and Runx3 mRNA in the HepG-2 cells increased 44.79
and 44.48, respectively. Yet, the expression levels of Cdkn2b and
IL-2 mRNA only increased 13.88 and 11.59, respectively.
The WW domain-containing oxidoreductase (WWOX) gene
encodes a member of the short-chain dehydrogenase/reductase (SDR)
protein family. Expression of the encoded protein is able to induce
apoptosis, while defects in this gene are associated with multiple
types of cancer. Mdm2 is an important negative regulator of the p53
tumor suppressor. Mdm2 protein functions both as an E3 ubiquitin
ligase that recognizes the N-terminal transactivation domain (TAD)
of the p53 tumor suppressor and an inhibitor of p53 transcriptional
activation. Quantitative RT-PCR results showed a significant
reduction in the levels of MDM2 and WWOX mRNA in the GCG-1-treated
HepG-2 cells (Table III), which
were 26.08 and 10.52, respectively (Table III). Further research is ongoing
to determine the bioactive principle(s) of GCG-1 responsible for
its anticancer activity.
In conclusion, according to the above results, it
was concluded that the novel polysaccharide obtained from
Gomphus clavatus Gray is a heteropolysaccharide, namely
GCG-1. The purified polysaccharide prepared (GCG-1) was confirmed
to be of high purity. The present study also showed that GCG-1
consisted of two monosaccharides, namely D-Glu and D-Gal in a ratio
3:2 by GC-MS. Structural study demonstrated that GCG-1 had a
backbone of (1→4)-β-D-glucopyranose residues which branch at O-6
based on the experimental results. The branches were mainly
composed of two with a (1→3)-α-D-galactopyranose residue. The
purified polysaccharide prepared in the present study was confirmed
to be of high purity. Antioxidation test in vitro showed
that it possessed strong free radical scavenging activity, which
may be comparable to Vc and BHT. In PC12 cells as determined by the
antioxidant effect assay, we found that GCG-1 significantly
attenuated PC12 cell damage caused by hydrogen peroxide.
Antioxidation test in vitro showed that it possessed strong
free radical scavenging activity, which may be comparable to Vc and
BHT. Moreover, GCG-1 induced the apoptosis of HepG-2 cells and
affected the mRNA expression of various housekeeping genes in the
HepG-2 cells. Overall, Gomphus clavatus Gray may be one
ideal sources for antioxidant and anticancer agents.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (31400016 and 31200012), the
Application Foundation Project of Sichuan Province (2013JY0094),
the Science and Technology Support Project of Sichuan Province
(2014SZ0020 and 2014FZ0024), the Cultivate Major Projects of
Sichuan Province (14CZ0016), the Open Foundation of Microbial
Resources and Drug Development of Key Laboratory and of Guizhou
Province (GZMRD-2014-002), and the Doctor Startup Foundation
Project of China West Normal University (11B019 and 11B020).
References
|
1
|
Hibbett DS, Binder M, Bischoff JF,
Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM,
Lücking R, et al: A higher-level phylogenetic classification of the
Fungi. Mycol Res. 111:509–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertozzi CR and Kiessling LL: Chemical
glycobiology. Science. 291:2357–2364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borchers AT, Stern JS, Hackman RM, Keen CL
and Gershwin ME: Mushrooms, tumors, and immunity. Proc Soc Exp Biol
Med. 221:281–293. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rudd PM, Elliott T, Cresswell P, Wilson IA
and Dwek RA: Glycosylation and the immune system. Science.
291:2370–2376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Angeli JP, Ribeiro LR, Gonzaga ML, Soares
SA, Ricardo MP, Tsuboy MS, Stidl R, Knasmueller S, Linhares RE and
Mantovani MS: Protective effects of β-glucan extracted from
Agaricus brasiliensis against chemically induced DNA damage in
human lymphocytes. Cell Biol Toxicol. 22:285–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li SP, Zhao KJ, Ji ZN, Song ZH, Dong TT,
Lo CK, Cheung JK, Zhu SQ and Tsim KW: A polysaccharide isolated
from Cordyceps sinensis, a traditional Chinese medicine, protects
PC12 cells against hydrogen peroxide-induced injury. Life Sci.
73:2503–2513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang F, Hou Y, Ding X, Hou W, Song B, Wang
T, Li J and Zeng Y: Structure elucidation and antioxidant effect of
a polysaccharide from Lactarius camphoratum (Bull) Fr. Int J Biol
Macromol. 62:131–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Staub AM: Removal of protein - Sevag
method. Methods Carb Chem. 5:5–6. 1965.
|
|
10
|
Dubois M, Gilles KA, Hamilton JK, Rebers
PA and Smith F: Colorimetric method for determination of sugars and
related substances. Anal Chem. 28:350–356. 1956. View Article : Google Scholar
|
|
11
|
Yamamoto Y, Nunome T, Yamauchi R, Kato K
and Sone Y: Structure of an exocellular polysaccharide of
Lactobacillus helveticus TN-4, a spontaneous mutant strain of
Lactobacillus helveticus TY1-2. Carbohydr Res. 275:319–332. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu RM, Yin Y, Yang W, Ma W, Yang L, Chen
X, Zhang Z, Ye B and Song L: Structural elucidation and biological
activity of a novel polysaccharide by alkaline extraction from
cultured Cordyceps militaris. Car Poly. 75:166–171. 2009.
View Article : Google Scholar
|
|
13
|
Partridge SM: Aniline hydrogen phthalate
as a spraying reagent for chromatography of sugars. Nature.
164:443–446. 1949. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Q, Zhang ZY, Lin Y and Fang J:
Studies on two polysaccharides from Stephania tetrandra. Acta
Biochim Biophys Sin. 27:261–265. 1995.
|
|
15
|
Chen Y, Xie MY, Nie SP, Li C and Wang YX:
Purification, composition analysis and antioxidant activity of a
polysaccharide from the fruiting bodies of Ganoderma atrum. Food
Chem. 107:231–241. 2008. View Article : Google Scholar
|
|
16
|
Hakomori S: A rapid permethylation of
glycolipid, and polysaccharide catalyzed by methylsulfinyl
carbanion in dimethyl sulfoxide. J Biochem. 55:205–208.
1964.PubMed/NCBI
|
|
17
|
Braca A, De Tommasi N, Di Bari L, Pizza C,
Politi M and Morelli I: Antioxidant principles from Bauhinia
terapotensis. J Nat Prod. 64:892–895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye H, Wang KQ, Zhou CH, Liu J and Zeng X:
Purification, antitumor and antioxidant activities in vitro of
polysaccharides from the brown seaweed Sargassum pallidum. Food
Chem. 111:428–432. 2008. View Article : Google Scholar
|
|
19
|
Auddy B, Ferreira M, Blasina F, Lafon L,
Arredondo F, Dajas F, Tripathi PC, Seal T and Mukherjee B:
Screening of antioxidant activity of three Indian medicinal plants,
traditionally used for the management of neurodegenerative
diseases. J Ethnopharmacol. 84:131–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao W, Li XQ, Liu L, Wang M, Fan HT, Li C,
Lv Z, Wang X and Mei Q: Structural analysis of water-soluble
glucans from the root of Angelica sinensis (Oliv) Diels. Carbohydr
Res. 341:1870–1877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barker SA, Bourne EJ, Stacey M and Whiffen
DH: Infra-red spectra of carbohydrates. Part I. Some derivatives of
D-glucopyranose. J Chem Soc Chem Commu. 171–176. 1954.
|
|
22
|
Kim YT, Kim EH, Cheong C, Williams DL, Kim
CW and Lim ST: Structural characterization of β-D-(1→3, 1→6)-linked
glucans using NMR spectroscopy. Carbohydr Res. 328:331–341. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu XM and Tu PF: Isolation and
characterization of α-(1→6)-glucans from Cistanche deserticola. J
Asian Nat Prod Res. 7:823–828. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang ZJ, Luo DH and Liang ZY: Structure of
polysaccharides from the fruiting body of Hericium erinaceus Pers.
Carbohydr Polym. 57:241–247. 2004. View Article : Google Scholar
|
|
25
|
Basu S, Liu Q, Qiu Y and Dong F: Gfi-1
represses CDKN2B encoding p15INK4B through interaction
with Miz-1. Proc Natl Acad Sci USA. 106:1433–1438. 2009. View Article : Google Scholar
|
|
26
|
Nishisho I, Nakamura Y, Miyoshi Y, Miki Y,
Ando H, Horii A, Koyama K, Utsunomiya J, Baba S and Hedge P:
Mutations of chromosome 5q21 genes in FAP and colorectal cancer
patients. Science. 253:665–669. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yokota T, Arai N, Lee F, Rennick D,
Mosmann T and Arai K: Use of a cDNA expression vector for isolation
of mouse interleukin 2 cDNA clones: Expression of T-cell
growth-factor activity after transfection of monkey cells. Proc
Natl Acad Sci USA. 82:68–72. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cerretti DP, McKereghan K, Larsen A,
Cantrell MA, Anderson D, Gillis S, Cosman D and Baker PE: Cloning,
sequence, and expression of bovine interleukin 2. Proc Natl Acad
Sci USA. 83:3223–3227. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mott HR, Driscoll PC, Boyd J, Cooke RM,
Weir MP and Campbell ID: Secondary structure of human interleukin 2
from 3D heteronuclear NMR experiments. Biochemistry. 31:7741–7744.
1992. View Article : Google Scholar : PubMed/NCBI
|