Introduction
Colorectal cancer is the third most common cancer
worldwide, and its incidence in South Korea has dramatically
increased as a result of changes in the economy and lifestyle
(1). Although genetic
predisposition is an important contributing factor for the
occurrence of colorectal cancer, the main mechanisms underlying the
pathogenesis of colorectal cancer remain largely unclear. Previous
studies suggest that diet plays a significant role in colorectal
cancer development and progression (2,3).
Moreover, cellular migration and invasion are important features of
colorectal cancer progression, and therefore interception of the
migration and invasion of colorectal cancer cells could be a
potential therapeutic target. Despite improvements in conventional
therapies for colorectal cancer, the quality of life of patients
with colorectal cancer remains poor, and new therapeutic targets or
preventive tools for colorectal cancer are therefore urgently
needed.
The cancer preventive role of 3,3′-diindolylmethane
(DIM) in several types of cancer cells has been highlighted, and
several studies have proposed DIM as a potent natural dietary
compound that reduces colorectal cancer risk and performs several
antitumor activities (4–11). These functions include inhibition of
colorectal cancer cell proliferation by suppression of YAP through
an Akt-dependent process, alteration of cell cycle progression, and
activation of caspase-8 to induce apoptosis (6,10,11).
Movement of cells in the extracellular matrix (ECM) and their
adhesion to the ECM are critical processes in the invasion of
metastatic colorectal cancer cells. Despite extensive efforts to
understand the mechanism underlying adhesion to the ECM during
colorectal cancer metastasis, the effects of DIM on the adhesion
and migratory properties of colorectal cancer cells have not been
elucidated. To clarify the effect of DIM on the metastatic
functions of colorectal cancer, we investigated its effect on
migration and invasion of cultured colorectal cancer cells. In the
present study, we showed that DIM significantly suppressed the cell
migratory and invasive properties of colorectal cancer cells and
that these effects may be in part mediated through inactivation of
FOXM1 and the resultant downregulation of urokinase type
plasminogen activator (uPA) and matrix metalloprotease 9
(MMP9).
Materials and methods
Materials
Antibodies specific for E-cadherin, uPA, FOXM1 and
GAPDH were purchased from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA), and the antibody against MMP9 was purchased from
R&D Systems, Inc. (Minneapolis, MN, USA). DIM was purchased
from LKT Laboratories, Inc. (St. Paul, MN, USA).
Cell culture
The DLD-1 and HCT116 cell lines were obtained from
the University of Texas M.D. Anderson Cancer Center (Houston, TX,
USA). RPMI-1640 medium and Dulbecco’s modified Eagle’s medium
(DMEM)/F12 medium (both from Gibco, Grand Island, NY, USA) were
used for cell culture. Cells were grown in RPMI-1640 (DLD-1) or
DMEM/F12 (HCT116) supplemented with 10% fetal bovine serum (FBS;
Gibco), 100 mg/ml streptomycin and 100 IU/ml penicillin in a 5%
CO2 humidified atmosphere at 37°C. The DLD-1 and HCT116
cells were treated with various concentrations of DIM in FBS-free
medium.
Wound healing assay
To detect the effect of DIM on the migration of the
HCT116 and DLD-1 colorectal cancer cells, we performed a wound
healing assay as previously described (12). Control and DIM-treated colorectal
cancer cells were grown to confluency, and a wound was made through
the monolayer using a 200-μl pipette tip. Accurate
measurements of the wounds were taken during the time course to
calculate the migration rate according to the equation: Percentage
of wound healing = [(wound length at 0 h) − (wound length at 24 or
48 h)]/(wound length at 0 h) x 100.
Matrigel invasion assay
BD BioCoat™ Matrigel™ Invasion Chambers (BD
Biosciences, San Jose, CA, USA) were used for the in vitro
cell invasion assay according to the manufacturer’s protocol.
Briefly, the Matrigel-coated chambers were rehydrated in a
humidified tissue culture incubator at 37°C in a 5% CO2
atmosphere. Cells (2.5x104) were suspended in 500
μl medium in each Matrigel-coated Transwell insert and the
lower chamber of the Transwell was filled with 500 μl of
medium. After incubation, the cultures were washed and stained with
a Diff-Quik kit (Sysmex Corp., Kobe, Japan). Cells on the upper
side of the insert membrane were removed and cells that migrated to
the lower side of the membrane were counted on an inverted
microscope (magnification, x100). Five fields were randomly
selected, and the invasion rates were calculated as previously
described (12).
RNA isolation and real-time PCR
Total RNA was isolated from the DLD-1 and HCT116
cells before and after DIM (100 μM) treatment. Reverse
transcription was carried out with a PrimeScript™ RT reagent kit
(Takara Bio Inc., Otsu, Shiga, Japan) according to the
manufacturer’s protocol. Quantitative real-time PCR was performed
using SYBR Premix Ex Taq (Takara Bio Inc.) in an ABI Prism
7900 Sequence Detection System (Applied Biosystems, Foster City,
CA, USA). The PCR program was initiated at 95°C for 30 sec followed
by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The results
were normalized to those for GAPDH and were calculated from
threshold cycle numbers. Primer sequences were as follows:
E-cadherin sense, 5′-GGATTGCAAATTC CTGCCATTC-3′ and antisense,
5′-AACGTTGTCCCGGGTG TCA-3′; uPA sense, 5′-AGAATTCACCACCATCGAGA-3′
and antisense, 5′-ATCAGCTTCACAACAGTCAT-3′; MMP9 sense,
5′-GACCTCAAGTGGCACCACCA-3′ and antisense,
5′-GTGGTACTGCACCAGGGCAA-3; FOXM1 sense, 5′-AC GTCCCCAAGCCAGGCTC-3
and antisense, 5′-CTACTG TAGCTCAGGAATAA-3′; GAPDH sense,
5′-GTCTCCTC TGACTTCAACAGCG-3′ and antisense, 5′-ACCACCCTGTT
GCTGTAGCCAA-3′.
Western blot analysis
Colorectal cancer DLD-1 and HCT116 cells were
treated with DIM at the indicated concentrations for 48 h. Cell
lysates were prepared by suspending the cells in lysis buffer
(Intron Biotechnology, Seoul, Korea), and western blot analysis was
performed as previously described (6,13).
Briefly, cell extractions were incubated on ice for 20 min and
centrifuged at 13,000 x g for 5 min at 4°C (6). The protein concentration was
determined using a BSA protein assay kit (Pierce, Rockford, IL,
USA). Whole lysate was resolved on an SDS-PAGE gel, and transferred
to a PVDF membrane (Bio-Rad, Hercules, CA, USA) (6).
Statistical analysis
The statistical significance of differences between
groups was tested by one-way ANOVA and then later compared among
groups with an unpaired Student’s t-test. The experimental data are
presented as the means ± SEM. A p-value <0.05 was considered to
indicate a statistically significant result. All experiments were
repeated more than three times.
Results
DIM inhibits the migration of colorectal
cancer cells
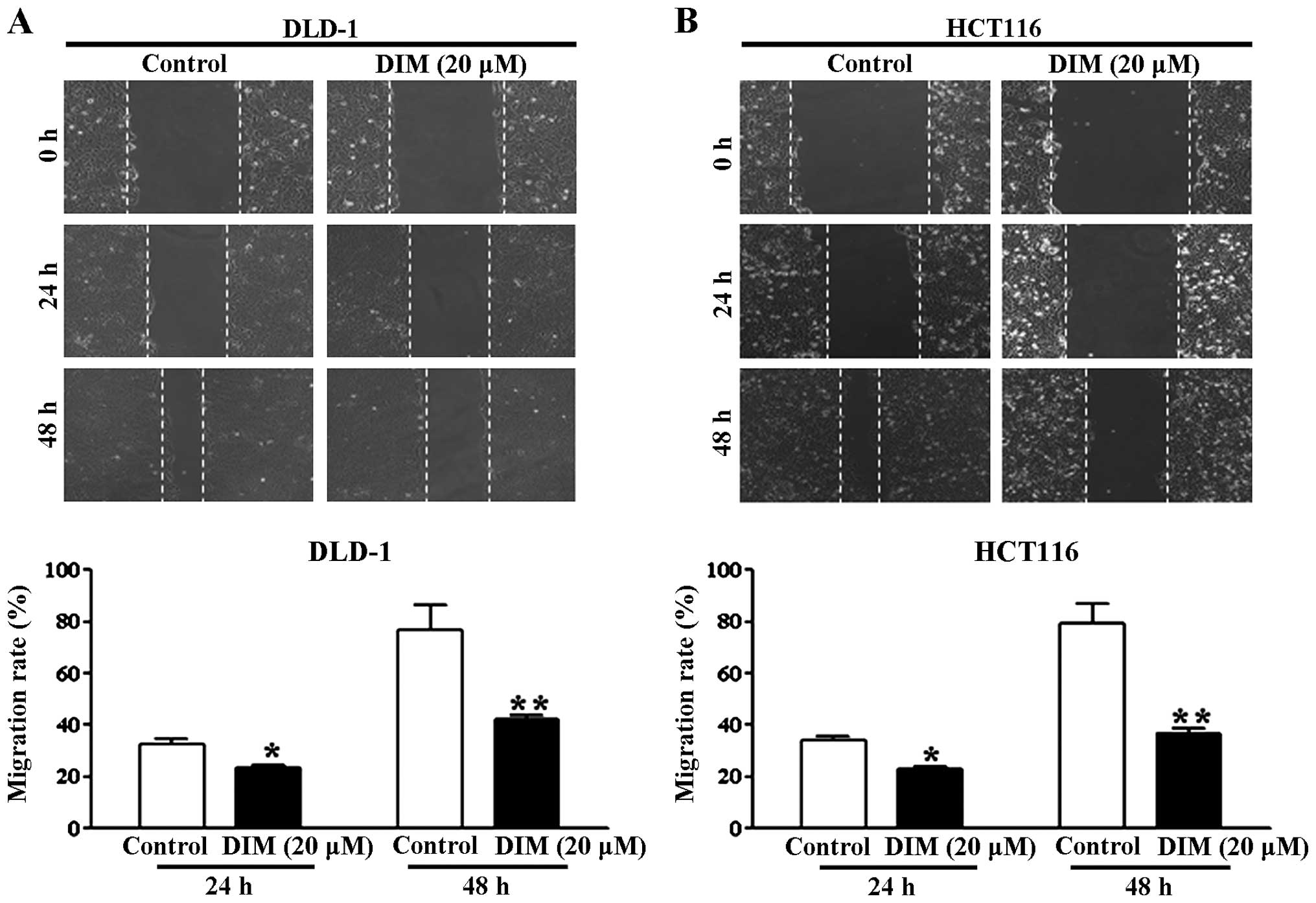
A wound healing assay was performed to investigate
the effect of DIM on colorectal cancer cell migration. This assay
tested the effect of DIM on the migration of the DLD-1 and HCT116
cells based on the ability of cells to interact with extracellular
matrix in the presence of DIM. In the presence of 20 μM DIM,
the migratory ability of the DLD-1 and HCT116 cells maintained in
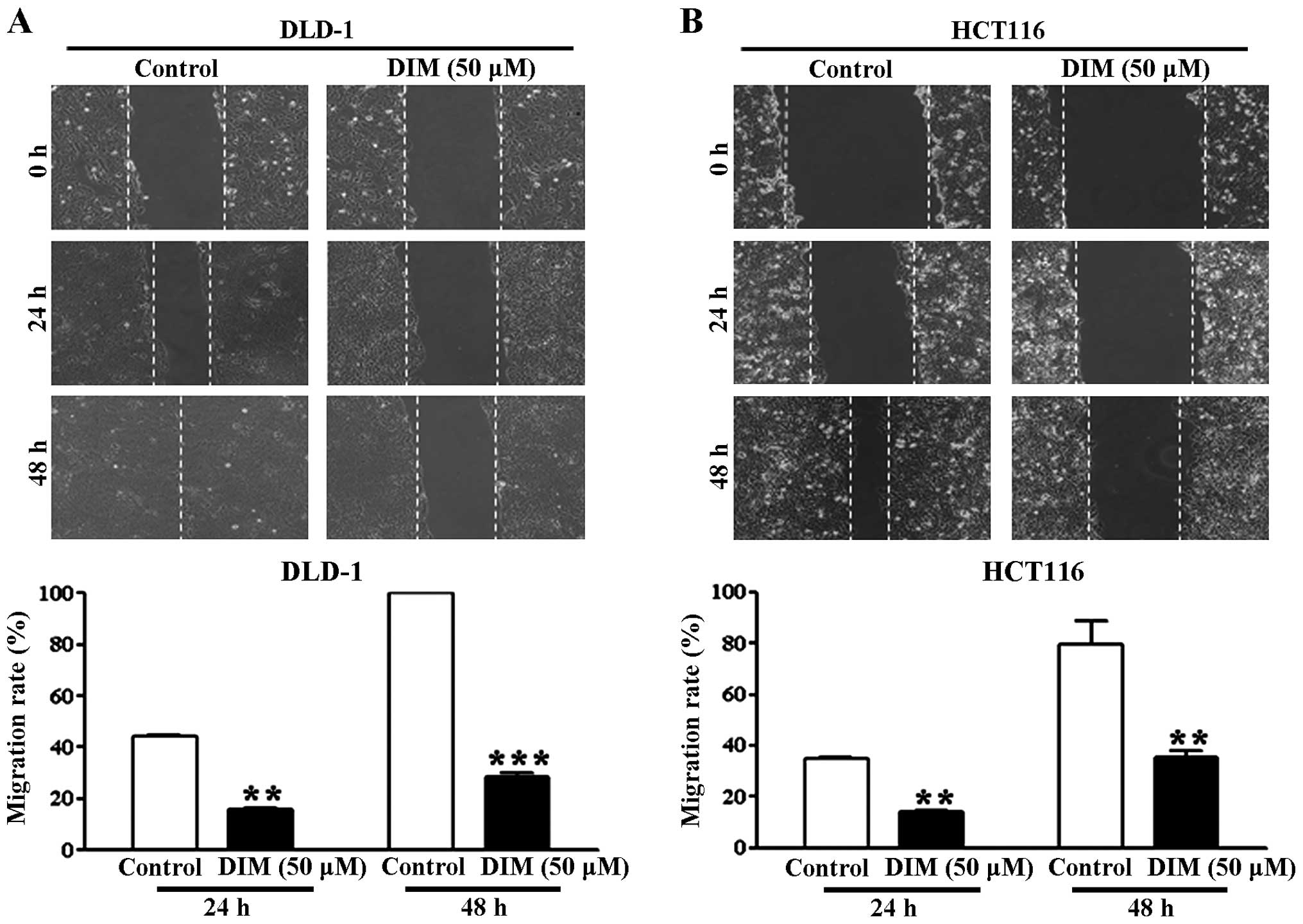
5% FBS was significantly reduced at 24 and 48 h (Fig. 1A and B). Treatment of the DLD-1 and
HCT116 cells with 50 μM DIM in the presence of 10% FBS had
an even greater antimigratory effect at 24 and 48 h (Fig. 2). These results indicate that DIM
has an antimigratory effect on colorectal cancer cells.
DIM inhibits the invasion of colorectal
cancer cells
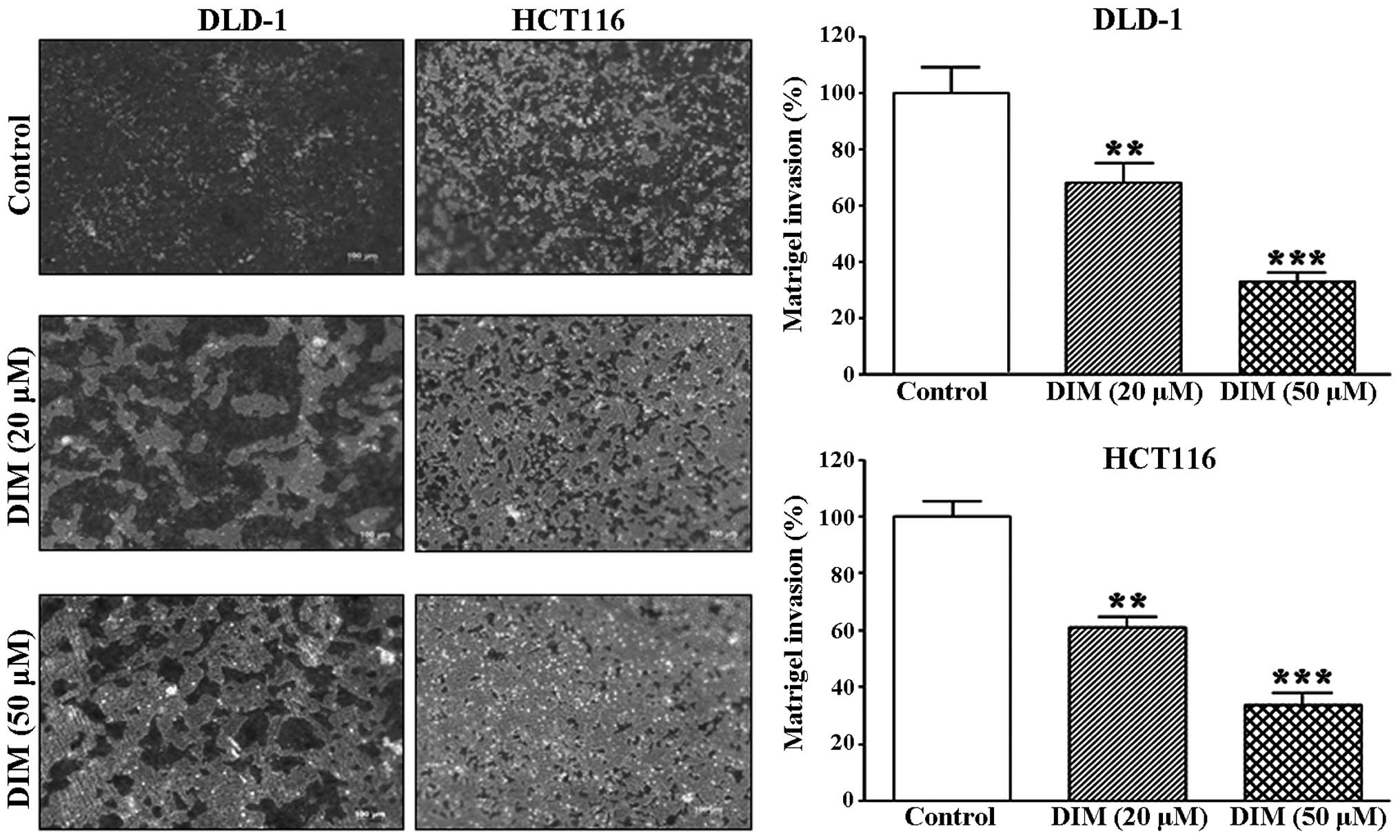
We further investigated the effect of DIM on
colorectal cancer cell invasion using a Matrigel invasion assay. We
found that DIM significantly inhibited the invasion of the DLD-1
and HCT116 cells in a dose-dependent manner; 20 μM DIM
significantly suppressed the invasion rates by 20 and 40% in the
DLD-1 and HCT116 cells, respectively, whereas 50 μM DIM
suppressed the invasion rates of both cell lines by 70% (Fig. 3). These data suggest that DIM
significantly inhibits the migration and invasion rates of
colorectal cancer cells.
Effect of DIM on the expression of
migration-related mRNAs and proteins in colorectal cancer
cells
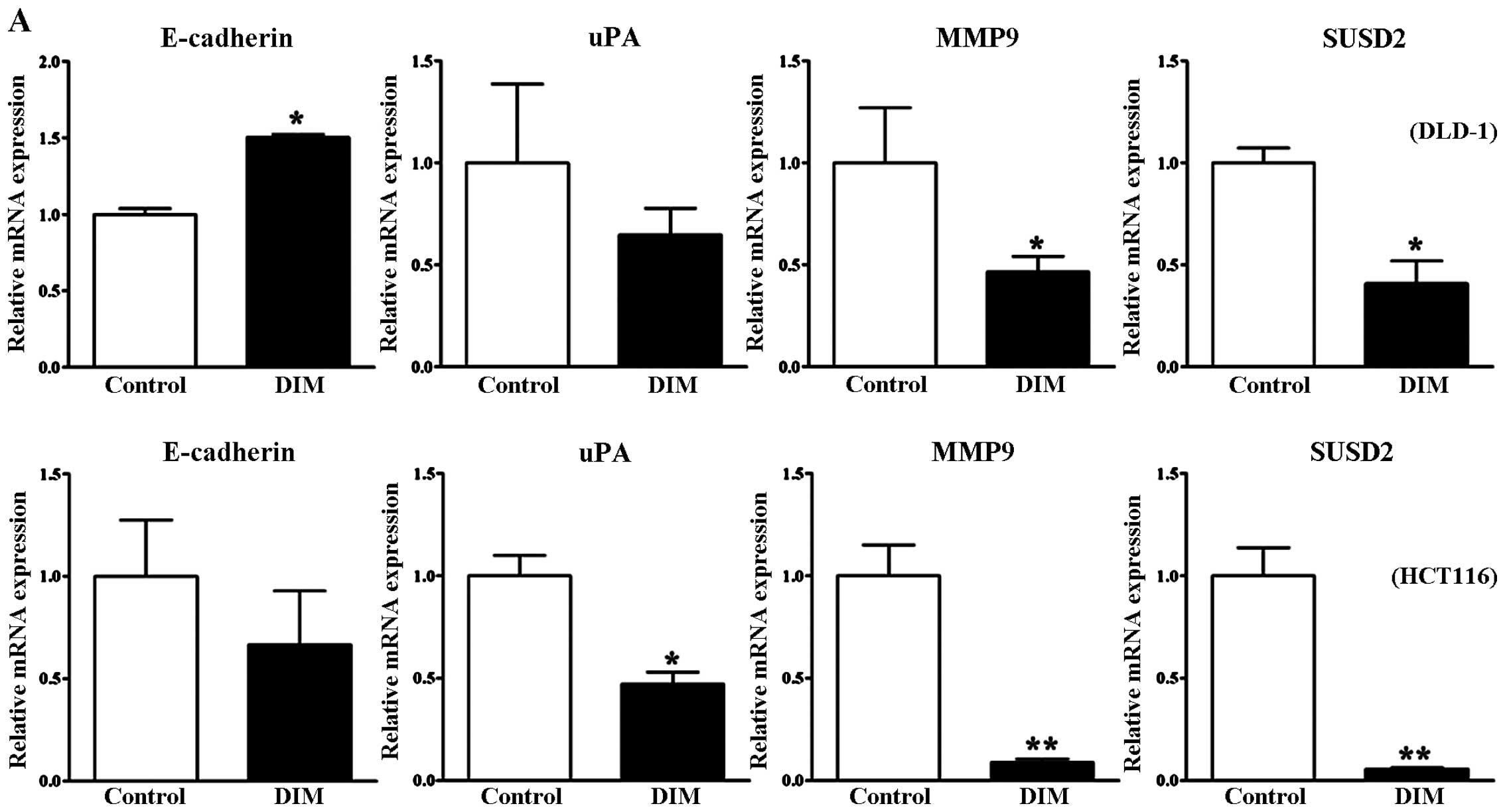
To further study the effect of DIM on the migration
and invasion of colorectal cancer cells, we performed RT-PCR and
western blot analyses to investigate the underlying molecular
events. Loss of E-cadherin expression is used as an indicator of
invasive epithelial cancers. In the present study, DIM treatment
induced a significant increase in the E-cadherin mRNA level in the
DLD-1 cells, yet there was no significant change in the HCT 116
cells (Fig. 4A). However,
E-cadherin protein levels were significantly increased following
DIM treatment in both the DLD-1 and HCT116 cells in a
dose-dependent manner (Fig. 4B).
Therefore, our data indicated that DIM increased E-cadherin
expression and thus suppressed the invasiveness and progression of
colon cancer cells. We further found that treatment with 100
μM DIM significantly decreased the mRNA levels of genes
related to invasion and cancer progression such as uPA, MMP9 and
SUSD2 in DLD-1 and HCT-116 cells (Fig.
4A). The protein levels of uPA and MMP9 were also downregulated
in a dose-dependent manner after DIM treatment (Fig. 4B). These results suggest that DIM
inhibits the migration and invasion of colorectal cancer cells by
targeting uPA and MMP9.
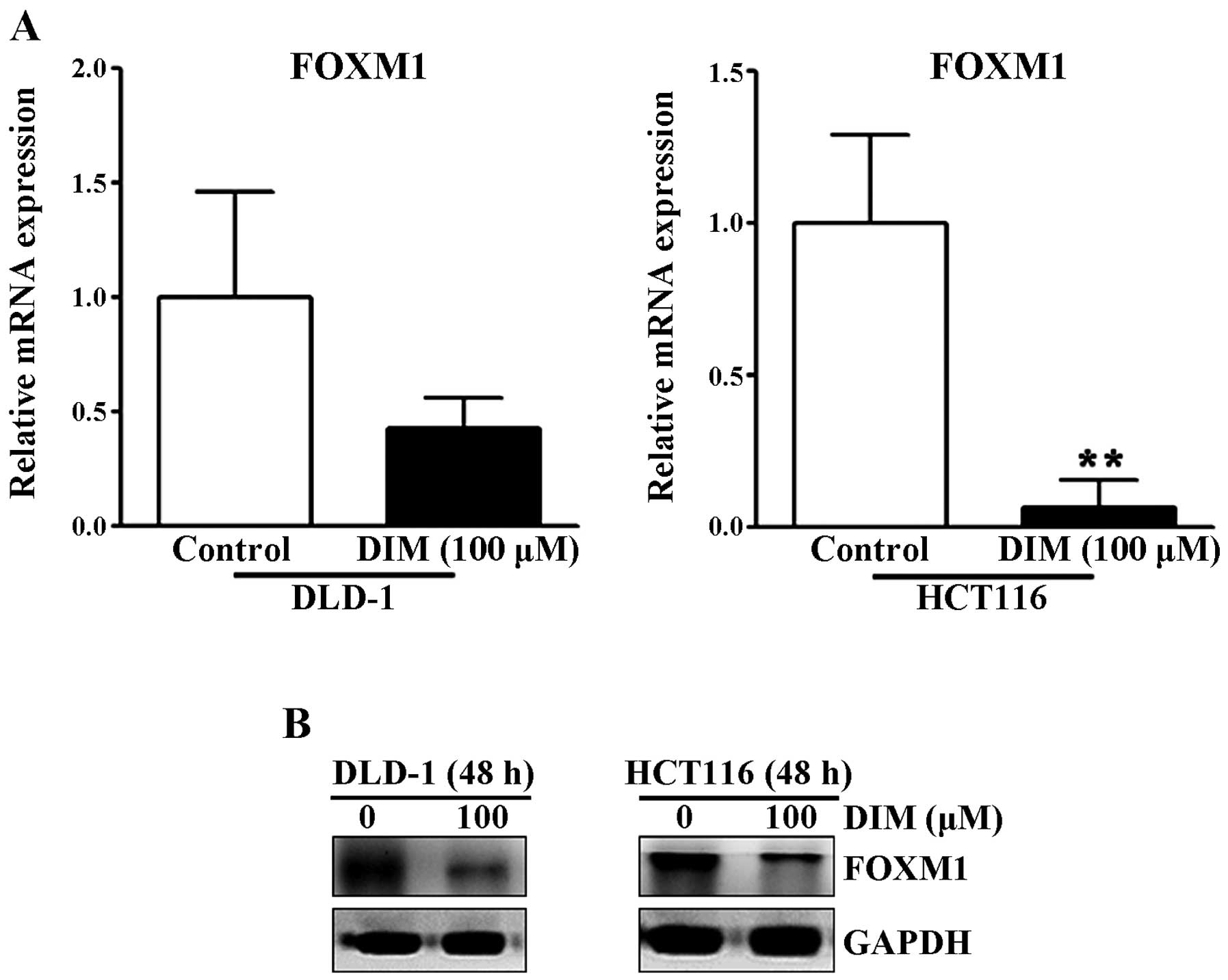
Effect of DIM on FOXM1 protein and mRNA
expression levels in colorectal cancer cells
The transcription factor forkhead box M1 (FOXM1) is
an important regulator of cell differentiation and proliferation
(14). Since FOXM1 is highly
activated in most human cancers and is overexpressed in a number of
aggressive human carcinomas (14),
we examined whether DIM alters the expression levels of FOXM1 in
colorectal cancer cells. As shown in Fig. 5, DIM significantly reduced the mRNA
and protein levels of FOXM1 in the DLD-1 and HCT116 cells at 48 h,
suggesting that DIM significantly inhibits the migration and
invasion rates of colorectal cancer cells by inactivation of
FOXM1.
Discussion
DIM is considered to be a promising natural cancer
preventive agent. Although DIM has been proven to impart antitumor
effects on numerous cancers both in vitro and in vivo
(4–9,11,13,15–19),
its antimetastatic effects in colorectal cancer and the underlying
mechanism have not been fully elucidated. In the present study, we
demonstrated that DIM inhibits the migration and invasion of
colorectal cancer cells by inactivation of FOXM1 and subsequent
downregulation of uPA and MMP9.
In the present study, we found that there was a
significant reduction in cellular migration of colorectal cancer
cells (DLD-1 and HCT116) following treatment with 20 and 50
μM DIM. Treatment with 50 μM DIM with 10% FBS of the
colorectal cancer cells resulted in a more pronounced inhibition of
migration than treatment with 20 μM DIM with 5% FBS. DIM at
concentrations of 20 and 50 μM also significantly inhibited
the invasion rates of the DLD-1 and HCT116 cells in a Matrigel
invasion assay. The inhibition of cancer cell metastatic function
by DIM has been reported by many researchers in various types of
cancer including prostate, breast, ovarian, thyroid and
nasopharyngeal cancers (15–23). A
previous study on the antitumor effects and chemopreventive role of
DIM in colorectal cancer proposed that DIM inhibited colorectal
cancer cells by inactivation of YAP and induction of G1 and G2 cell
cycle arrest (6,8). However, the effects of DIM on invasion
and metastasis in colorectal cancer cells have not previously been
described, and this is the first study to show attenuation of the
adhesion and migratory properties of colorectal cancer cells by
DIM.
As our data showed that DIM regulated colorectal
cancer cell invasion and metastasis, we further investigated
whether DIM altered the expression levels of metastasis marker
proteins in colorectal cancer cells. Indeed, DIM significantly
induced expression of E-cadherin, an indicator protein of invasive
function in epithelial cancer cells, in both the DLD-1 and HCT116
cells. Our findings suggest that induction of E-cadherin expression
by DIM treatment represents loss of epithelial invasive function in
colorectal cancer cells. We also found that DIM decreased the
protein levels of uPA and MMP9 and significantly downregulated mRNA
levels of uPA, MMP9, and SUSD2 in the DLD-1 and HCT116 cells. Our
results are consistent with those of previous studies in other
cancer cells. For example, inhibition of invasion in prostate
cancer by DIM was previously shown to be mediated by MMP9 and uPA,
which regulated the bioavailability of vascular endothelial growth
factor (18). Downregulation of uPA
by DIM has also been reported to contribute to the inhibition of
cell growth and migration of breast cancer cells (15). Therefore, our results strongly
indicated that inhibition of uPA and MMP9 by DIM attenuates the
invasion and metastasis of colorectal cancer cells.
Forkhead box M1 (FOXM1) is a member of the FOX
family of transcriptional factors and a cell cycle regulator that
is essential for cell cycle progression (24). FOXM1 has been reported to play an
important role in the promotion of cancer cell proliferation and to
be involved in malignant tumor development in a variety of cancers,
including lung, liver, ovarian and breast cancer (24–30).
Moreover, overexpression of FOXM1 is associated with tumor invasion
and metastasis and predictive of poor prognosis in many cancer
types, including ovarian, esophageal, lung, liver and bladder
cancer (31–35). Chu et al reported that
overexpression of FOXM1 stimulated migration/invasion and is
correlated with a poor prognosis of colorectal cancer (36). Activation of FOXM1 increases
colorectal cancer progression and metastasis by activation of uPA
receptor expression (37).
Therefore, it is obvious that abnormal regulation of FOXM1 plays a
critical role in tumor development and metastasis of colorectal
cancer.
Since elevated expression of FOXM1 has been observed
in human colorectal cancer (36,37),
we investigated whether DIM altered the expression levels of FOXM1
in colorectal cancer cells. We found that DIM significantly
suppressed the mRNA and protein levels of FOXM1 in two different
colorectal cancer cells (DLD-1 and HCT116). These observations are
in agreement with previous studies demonstrating that DIM
effectively downregulated FOXM1 in various breast cancer cell lines
(38–40). Downregulation of FOXM1 has been
found to inhibit the expression of metastasis factors that are
involved in the degradation of the ECM, such as uPA and MMP9, in
breast cancer cells (41). In the
present study, we found that the inhibition of migration and
metastasis by DIM was accompanied by downregulation of FOXM1, uPA
and MMP9. Therefore, the antimetastatic effects of DIM in
colorectal cancer cells may be associated with inhibition of FOXM1,
which in turn may induce downregulation of uPA and MMP9 expression.
However, further studies are needed to elucidate how DIM
specifically controls the expression of FOXM1 and its target
genes.
In conclusion, DIM inhibited the cell migratory and
invasive properties of colorectal cancer cells, most likely through
downregulation of uPA and MMP9 mediated by suppression of the FOXM1
transcription factor. Our results suggest the potential application
of FOXM1 downregulation by DIM as a novel approach for the
treatment of aggressive colorectal cancer.
Acknowledgments
This study was supported by a National Research
Foundation of Korea (NRF) grant funded by the Korea Government
(MISP) (no. 2008-0062279).
References
|
1
|
Byun JY, Yoon SJ, Oh IH, Kim YA, Seo HY
and Lee YH: Economic burden of colorectal cancer in Korea. J Prev
Med Pub Health. 47:84–93. 2014. View Article : Google Scholar
|
|
2
|
Irrazábal T, Belcheva A, Girardin SE,
Martin A and Philpott DJ: The multifaceted role of the intestinal
microbiota in colon cancer. Mol Cell. 54:309–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis P, Hold GL and Flint HJ: The gut
microbiota, bacterial metabolites and colorectal cancer. Nat Rev
Microbiol. 12:661–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kandekar S, Preet R, Kashyap M, Renu
Prasad MU, Mohapatra P, Das D, Satapathy SR, Siddharth S, Jain V,
Choudhuri M, et al: Structural elaboration of a natural product:
Identification of 3,3′-diindolylmethane aminophosphonate and urea
derivatives as potent anticancer agents. ChemMedChem. 8:1873–1884.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Li X and Guo B: Chemopreventive
agent 3,3′-diindolylmethane selectively induces proteasomal
degradation of class I histone deacetylases. Cancer Res.
70:646–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li XJ, Leem SH, Park MH and Kim SM:
Regulation of YAP through an Akt-dependent process by 3,
3′-diindolylmethane in human colon cancer cells. Int J Oncol.
43:1992–1998. 2013.PubMed/NCBI
|
|
7
|
Park C, Choi YW, Hyun SK, Kwon HJ, Hwang
HJ, Kim GY, Choi BT, Kim BW, Choi IW, Moon SK, et al: Induction of
G1 arrest and apoptosis by schisandrin C isolated from Schizandra
chinensis Baill in human leukemia U937 cells. Int J Mol Med.
24:495–502. 2009.PubMed/NCBI
|
|
8
|
Choi HJ, Lim Y and Park JH: Induction of
G1 and G2/M cell cycle arrests by the dietary compound
3,3′-diindolylmethane in HT-29 human colon cancer cells. BMC
Gastroenterol. 9:392009. View Article : Google Scholar
|
|
9
|
Bhatnagar N, Li X, Chen Y, Zhou X, Garrett
SH and Guo B: 3,3′-Diindolylmethane enhances the efficacy of
butyrate in colon cancer prevention through down-regulation of
survivin. Cancer Prev Res. 2:581–589. 2009. View Article : Google Scholar
|
|
10
|
Pappa G, Strathmann J, Löwinger M, Bartsch
H and Gerhäuser C: Quantitative combination effects between
sulforaphane and 3,3′-diindolylmethane on proliferation of human
colon cancer cells in vitro. Carcinogenesis. 28:1471–1477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim EJ, Park SY, Shin HK, Kwon DY, Surh YJ
and Park JH: Activation of caspase-8 contributes to
3,3′-diindolylmethane-induced apoptosis in colon cancer cells. J
Nutr. 137:31–36. 2007.
|
|
12
|
Lee KB, Ye S, Park MH, Park BH, Lee JS and
Kim SM: p63-Mediated activation of the β-catenin/c-Myc signaling
pathway stimulates esophageal squamous carcinoma cell invasion and
metastasis. Cancer Lett. 353:124–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li XJ, Park ES, Park MH and Kim SM:
3,3′-Diindolylmethane suppresses the growth of gastric cancer cells
via activation of the Hippo signaling pathway. Oncol Rep.
30:2419–2426. 2013.PubMed/NCBI
|
|
14
|
Halasi M and Gartel AL: Targeting FOXM1 in
cancer. Biochem Pharmacol. 85:644–652. 2013. View Article : Google Scholar
|
|
15
|
Ahmad A, Kong D, Wang Z, Sarkar SH,
Banerjee S and Sarkar FH: Down-regulation of uPA and uPAR by
3,3′-diindolylmethane contributes to the inhibition of cell growth
and migration of breast cancer cells. J Cell Biochem. 108:916–925.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang X, Tou JC, Hong C, Kim HA, Riby JE,
Firestone GL and Bjeldanes LF: 3,3′-Diindolylmethane inhibits
angiogenesis and the growth of transplantable human breast
carcinoma in athymic mice. Carcinogenesis. 26:771–778. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kandala PK and Srivastava SK:
Diindolylmethane suppresses ovarian cancer growth and potentiates
the effect of cisplatin in tumor mouse model by targeting signal
transducer and activator of transcription 3 (STAT3). BMC Med.
10:92012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong D, Li Y, Wang Z, Banerjee S and
Sarkar FH: Inhibition of angiogenesis and invasion by
3,3′-diindolylmethane is mediated by the nuclear factor-kappaB
downstream target genes MMP-9 and uPA that regulated
bioavailability of vascular endothelial growth factor in prostate
cancer. Cancer Res. 67:3310–3319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahimi M, Huang KL and Tang CK:
3,3′-Diindolylmethane (DIM) inhibits the growth and invasion of
drug-resistant human cancer cells expressing EGFR mutants. Cancer
Lett. 295:59–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu T, Chen C, Li F, Chen Z, Xu Y, Xiao B
and Tao Z: 3,3′-Diindolylmethane inhibits the invasion and
metastasis of nasopharyngeal carcinoma cells in vitro and in vivo
by regulation of epithelial mesenchymal transition. Exp Ther Med.
7:1635–1638. 2014.PubMed/NCBI
|
|
21
|
Ribaux P, Irion O and Cohen M: An active
product of cruciferous vegetables, 3,3′-diindolylmethane, inhibits
invasive properties of extravillous cytotrophoblastic cells. Neuro
Endocrinol Lett. 33:133–137. 2012.
|
|
22
|
Rajoria S, Suriano R, George A, Shanmugam
A, Schantz SP, Geliebter J and Tiwari RK: Estrogen induced
metastatic modulators MMP-2 and MMP-9 are targets of
3,3′-diindolylmethane in thyroid cancer. PLoS One. 6:e158792011.
View Article : Google Scholar
|
|
23
|
Rajoria S, Suriano R, Wilson YL, Schantz
SP, Moscatello A, Geliebter J and Tiwari RK: 3,3′-Diindolylmethane
inhibits migration and invasion of human cancer cells through
combined suppression of ERK and AKT pathways. Oncol Rep.
25:491–497. 2011.
|
|
24
|
Xu N, Jia D, Chen W, Wang H, Liu F, Ge H,
Zhu X, Song Y, Zhang X, Zhang D, et al: FoxM1 is associated with
poor prognosis of non-small cell lung cancer patients through
promoting tumor metastasis. PLoS One. 8:e594122013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalin TV, Wang IC, Ackerson TJ, Major ML,
Detrisac CJ, Kalinichenko VV, Lyubimov A and Costa RH: Increased
levels of the FoxM1 transcription factor accelerate development and
progression of prostate carcinomas in both TRAMP and LADY
transgenic mice. Cancer Res. 66:1712–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim IM, Ackerson T, Ramakrishna S,
Tretiakova M, Wang IC, Kalin TV, Major ML, Gusarova GA, Yoder HM,
Costa RH, et al: The Forkhead Box m1 transcription factor
stimulates the proliferation of tumor cells during development of
lung cancer. Cancer Res. 66:2153–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balli D, Zhang Y, Snyder J, Kalinichenko
VV and Kalin TV: Endothelial cell-specific deletion of
transcription factor FoxM1 increases urethane-induced lung
carcinogenesis. Cancer Res. 71:40–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calvisi DF, Pinna F, Ladu S, Pellegrino R,
Simile MM, Frau M, De Miglio MR, Tomasi ML, Sanna V, Muroni MR, et
al: Forkhead box M1B is a determinant of rat susceptibility to
hepatocarcinogenesis and sustains ERK activity in human HCC. Gut.
58:679–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chandran UR, Ma C, Dhir R, Bisceglia M,
Lyons-Weiler M, Liang W, Michalopoulos G, Becich M and Monzon FA:
Gene expression profiles of prostate cancer reveal involvement of
multiple molecular pathways in the metastatic process. BMC Cancer.
7:642007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Millour J, Constantinidou D, Stavropoulou
AV, Wilson MS, Myatt SS, Kwok JM, Sivanandan K, Coombes RC, Medema
RH, Hartman J, et al: FOXM1 is a transcriptional target of ERalpha
and has a critical role in breast cancer endocrine sensitivity and
resistance. Oncogene. 29:2983–2995. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wen N, Wang Y, Wen L, Zhao SH, Ai ZH, Wang
Y, Wu B, Lu HX, Yang H, Liu WC, et al: Overexpression of FOXM1
predicts poor prognosis and promotes cancer cell proliferation,
migration and invasion in epithelial ovarian cancer. J Transl Med.
12:1342014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takata A, Takiguchi S, Okada K, Takahashi
T, Kurokawa Y, Yamasaki M, Miyata H, Nakajima K, Mori M and Doki Y:
Clinicopathological and prognostic significance of FOXM1 expression
in esophageal squamous cell carcinoma. Anticancer Res.
34:2427–2432. 2014.PubMed/NCBI
|
|
33
|
Kong FF, Qu ZQ, Yuan HH, Wang JY, Zhao M,
Guo YH, Shi J, Gong XD, Zhu YL, Liu F, et al: Overexpression of
FOXM1 is associated with EMT and is a predictor of poor prognosis
in non-small cell lung cancer. Oncol Rep. 31:2660–2668.
2014.PubMed/NCBI
|
|
34
|
Xia L, Huang W, Tian D, Chen Z, Zhang L,
Li Y, Hu H, Liu J, Chen Z, Tang G, et al: ACP5, a direct
transcriptional target of FoxM1, promotes tumor metastasis and
indicates poor prognosis in hepatocellular carcinoma. Oncogene.
33:1395–1406. 2014. View Article : Google Scholar
|
|
35
|
Liu D, Zhang Z and Kong CZ: High FOXM1
expression was associated with bladder carcinogenesis. Tumour Biol.
34:1131–1138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chu XY, Zhu ZM, Chen LB, Wang JH, Su QS,
Yang JR, Lin Y, Xue LJ, Liu XB and Mo XB: FOXM1 expression
correlates with tumor invasion and a poor prognosis of colorectal
cancer. Acta Histochem. 114:755–762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li D, Wei P, Peng Z, Huang C, Tang H, Jia
Z, Cui J, Le X, Huang S and Xie K: The critical role of
dysregulated FOXM1-PLAUR signaling in human colon cancer
progression and metastasis. Clin Cancer Res. 19:62–72. 2013.
View Article : Google Scholar :
|
|
38
|
Ahmad A, Ali S, Wang Z, Ali AS, Sethi S,
Sakr WA, Raz A and Rahman KM: 3,3′-Diindolylmethane enhances
taxotere-induced growth inhibition of breast cancer cells through
downregulation of FoxM1. Int J Cancer. 129:1781–1791. 2011.
View Article : Google Scholar
|
|
39
|
Ahmad A, Sakr WA and Rahman KM: Novel
targets for detection of cancer and their modulation by
chemopreventive natural compounds. Front Biosci. 4:410–425. 2012.
View Article : Google Scholar
|
|
40
|
Ahmad A, Ali S, Ahmed A, Ali AS, Raz A,
Sakr WA and Rahman KM: 3, 3′-Diindolylmethane enhances the
effectiveness of herceptin against HER-2/neu-expressing breast
cancer cells. PLoS One. 8:e546572013. View Article : Google Scholar
|
|
41
|
Ahmad A, Wang Z, Kong D, Ali S, Li Y,
Banerjee S, Ali R and Sarkar FH: FoxM1 down-regulation leads to
inhibition of proliferation, migration and invasion of breast
cancer cells through the modulation of extra-cellular matrix
degrading factors. Breast Cancer Res Treat. 122:337–346. 2010.
View Article : Google Scholar
|