Introduction
The incidence of oral cancer accounts for ~5% of all
the malignant tumors of body, and the 5-year survival rate is ~64%
(1). The incidence of oral cancer
in China is 5–6/100.000 individuals. Squamous epithelial cancer is
most common in oral and maxillofacial malignancies, accounting for
~80% of oral and maxillofacial tumors (2). Tongue squamous cell carcinoma (TSCC)
has the highest incidence of oral cancers with cervical lymph-node
metastasis occurring in early stage, rendering it an early event
for the development of TSCC. Thus, it is particularly important to
identify clinical treatment as well as prediction of recurrence and
prognosis (3).
Recent findings have shown that there is an
abnormality of microRNA (miR) expression in a variety of tumors.
This influences the biological behavior, such as proliferation,
apoptosis, differentiation, movement, invasion, metastasis and
angiogenesis, of tumor cells through the regulation of downstream
target genes and plays a role in oncogenes or tumor-suppressor
genes, which are involved in tumorigenesis and the development of
tumors, a multi-step process (4,5).
Recent studies have found miR-214 is associated with a variety of
malignancies, which plays a tumor suppressor or tumor promotion
effect through multiple signal transduction pathways. The knockdown
of miR-214 which promotes apoptosis and inhibits proliferation in
TSCC Tca8113 cells (6), and in
nasopharyngeal (7) and
hepatocellular carcinoma (8), has
been previously reported.
The p53 gene is considered to be associated
with cancer in humans. Proliferating cell nuclear antigen, also
known as cyclin, is an auxiliary protein of DNA polymerase δ,
involved in the regulation of DNA synthesis and closely associated
with cell cycle proliferation (9).
Qi and Zhang showed that alisertib induced apoptosis of human TSCC
by activation of the p53 pathway (10). Yasumoto et al reported that
apoptosis-related gene expression after hyperthermia harbors wild-
or mutated-type p53 in human TSCC (11). Nagler et al identified that
the possible biological significance of markers is an independent
role for p53 in TSCC (12). Tumor
occurrence is associated with hindered apoptosis. The
Bcl-2/Bax gene is important for the inhibition of apoptosis,
and upregulation of the Bcl-2/Bax signal may play an important role
in the occurrence and development of TSCC Tca8113 (13,14).
Xie et al reported that the Bcl-2/Bax expression ratio has a
prognostic value in TSCC (15).
Cantharidin is an active pharmaceutical ingredient
for cantharide, which has a good effect on malignant tumors such as
liver (16), esophageal (17), lung (18) and gastric (19) cancer, and has been used in clinical
treatment of cancer over a long period of time. As reported,
cantharidin induces tumor cell apoptosis. However, the estimated
anticancer effect of cantharidin on cell proliferation and
apoptosis of human TSCC remains to be determined. Thus, we
investigated the anticancer effect of cantharidin on the behavior
of human TSCC TCA8113 cells and investigated the molecular
mechanisms related involved in this activity.
Materials and methods
Reagents and instruments
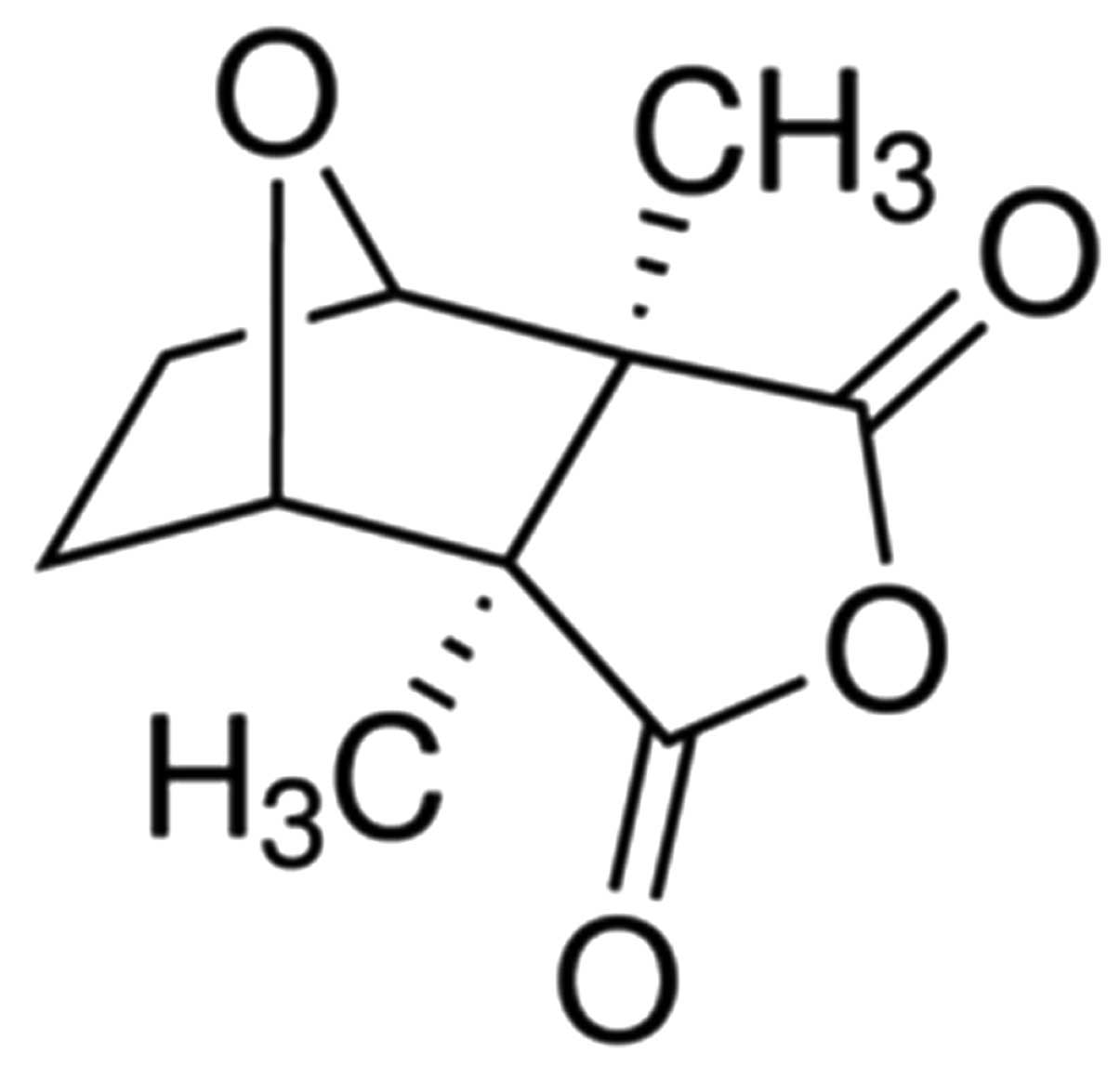
The cantharidin chemical structure (Sigma-Aldrich,
St. Louis, MO, USA), with a purity of ≥95% is shown in Fig. 1. Dulbecco’s modified Eagle’s medium
(DMEM) was purchased from Invitrogen Life Technologies (Carlsbad,
CA, USA). Fetal bovine serum (FBS) was purchased from HyClone,
Invitrogen Co. (Australia).
3.3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) was purchased from Sigma-Aldrich. Annexin V-fluorescein
isothiocyanate (V-FITC)/propidium iodide (PI) double staining kits
were purchased from Sigma-Aldrich.
Cell line and culture conditions
Human TSCC TCA8113 cells were obtained from the
Shanghai Usea Biotech Company (Shanghai, China). TCA8113 cells were
cultured in DMEM containing 10% FBS, 1% penicillin/streptomycin
(Invitrogen Life Technologies), and maintained at 37°C in a
humidified atmosphere of 5% CO2.
Detection of cell proliferation by MTT
assay
Proliferation of TCA8113 cells was screened by MTT
colorimetric assay. Briefly, TCA8113 cells (2×103
cells/well) were seeded in 96-well plates and treated with
cantharidin (0, 2.5, 5, 10, 20, 40 and 80 μM) for 24, 48 or 72 h
following treatment, respectively. TCA8113 cells were added with 20
μl MTT (5 mg/ml) and the cells were cultured for an additional 4 h.
DMSO was added to each well and agitated for 20 min. The absorbance
was measured with Multiskan Spectrum microplate reader (Thermo
Fisher Scientific, Waltham, MA, USA) at 490 nm.
Detection of cell cytotoxicity by lactate
dehydrogenase (LDH) assay
Proliferation of TCA8113 cells was screened by LDH
assay. Briefly, TCA8113 cells (2×103 cells/well) were
seeded in 96-well plates and treated with cantharidin (10, 20 and
40 μM) for 48 h following treatment. Each well was added with 100
μl LDH solution and incubated at room temperature for 30 min. The
absorbance was read at 490 nm using a multiwell spectrophotometer
(XL-818; Bio-Tek, Winooski, VT, USA).
Detection of cell apoptosis by flow
cytometry
Apoptosis of TCA8113 cells was analyzed by flow
cytometry. Briefly, TCA8113 cells (2×106 cells/well)
were seeded in 6-well plates and treated with cantharidin (10, 20
and 40 μM) for 48 h following treatment. TCA8113 cells were
collected and washed twice with ice-cold phosphate-buffered saline
(PBS). TCA8113 cells (1×106 cells/ml) were resuspended
with binding buffer according to the manufacturer’s instructions
(Sigma-Aldrich). Annexin V-FITC (10 μl) was added into TCA8113
cells and stained for 30 min in the dark. The samples were
immediately analyzed by flow cytometry.
Detection of caspase-9 and -3 activities
by colorimetric protease assay
Apoptosis of TCA8113 cells was analyzed using flow
cytometry. Briefly, TCA8113 cells (2×106 cells/well)
were seeded in 6-well plates and treated with cantharidin (10, 20
and 40 μM) for 48 h following treatment. TCA8113 cells were washed
with twice PBS. The cells were lysed on ice in a buffer and
cultured for 30 min. The total proteins were determined using a BSA
kit (Pierce, Rockford, IL, USA). Equal protein was mixed with
reaction buffer (Ac-DEVD-pNA for caspase-3, Ac-LEHD-pNA for37°C for
2 h in the dark. Caspase-3/9 activity was measured at an absorbance
of 405 nm.
Detection of miR-214 expression by
quantitative polymerase chain reaction (qPCR)
miR-214 expression levels were measured with qPCR.
Briefly, TCA8113 cells (2×106 cells/well) were seeded in
6-well plates and treated with cantharidin (10, 20 and 40 μM) for
48 h following treatment. Total RNA was isolated from renal tissues
using TRIzol, according to the manufacturer’s instructions
(Invitrogen, Chicago, IL, USA). cDNAs were produced and detected
using the TaqMan 7900 (ABI) Real-Time PCR system, according to the
manufacturer’s protovarian (Qiagen, Valencia, CA, USA). The
expression of miR-214 was measured using a SYBR-Green kit,
according to the manufacturer’s instructions (Qiagen). The primers
were compound and purchased from BeastBio Co., Ltd. (Shanghai,
China). miR-214-F, 5′-AGCATAATACAGCAGGCACAGAC-3′ and miR-214-R
5′-AAAGGTTGTTCTCCACTCTCTCAC-3′; U6-F, 5′-CGCTTCGGCAGCACATATACTA-3′
and U6-R, 5′-CGCTTCACGAATTTGCGTGTCA.
Detection of p53, Bcl-2 and Bax protein
expression by western blotting
p53 and Bax protein expression levels were detected
with western blotting. Briefly, TCA8113 cells (2×106
cells/well) were seeded in 6-well plates and treated with
cantharidin (10, 20 and 40 μM) for 48 h following treatment.
TCA8113 cells were washed with twice PBS. The cells were lysed on
ice in a buffer and cultured for 30 min. The lysed solution was
centrifuged at 12,000 x g for 10 min at 4°C. The total proteins
were determined using a BSA kit. Equivalent amounts of protein were
separated by 10% SDS-PAGE and then transferred to polyvinylidene
difluoride membranes (Millipore, Billerica, MA, USA). The membranes
were blocked with Tris-buffered saline (TBS) containing 5% w/v
non-fat milk to block non-specific binding sites. The membranes
were blocked and incubated overnight with anti-p53 (1:1,000),
anti-Bcl-2 (1:1,000), anti-Bax (1:1,500) (all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-β-actin (1:500;
Sangon Biotech, Shanghai, China) overnight at 4°C. The membranes
were then incubated with IgG-conjugated goat anti-rabbit secondary
antibodies at room temperature for 2 h using enhanced
chemiluminescent reagents (Tiangen, Beijing, China) and exposed to
X-ray film.
Transfection of miR-214 and
anti-miR-214
The TCA8113 cells (2×106 cells/well) were
seeded in 6-well plates 24 h prior to transfection. miR-214
inhibitors and mimics were established by BeastBio Co., Ltd.
miR-214 inhibitors (10 nM duplex/well) and miR-214 mimics (10 nM
duplex/well) were performed using Lipofectamine 2000 (Invitrogen
Life Technologies).
Statistical analysis
Analysis of variance (ANOVA) or Student’s t-test was
performed with SPSS software. Data were presented as mean ± SD.
P<0.05 was considered to indicate a statistically significant
result.
Results
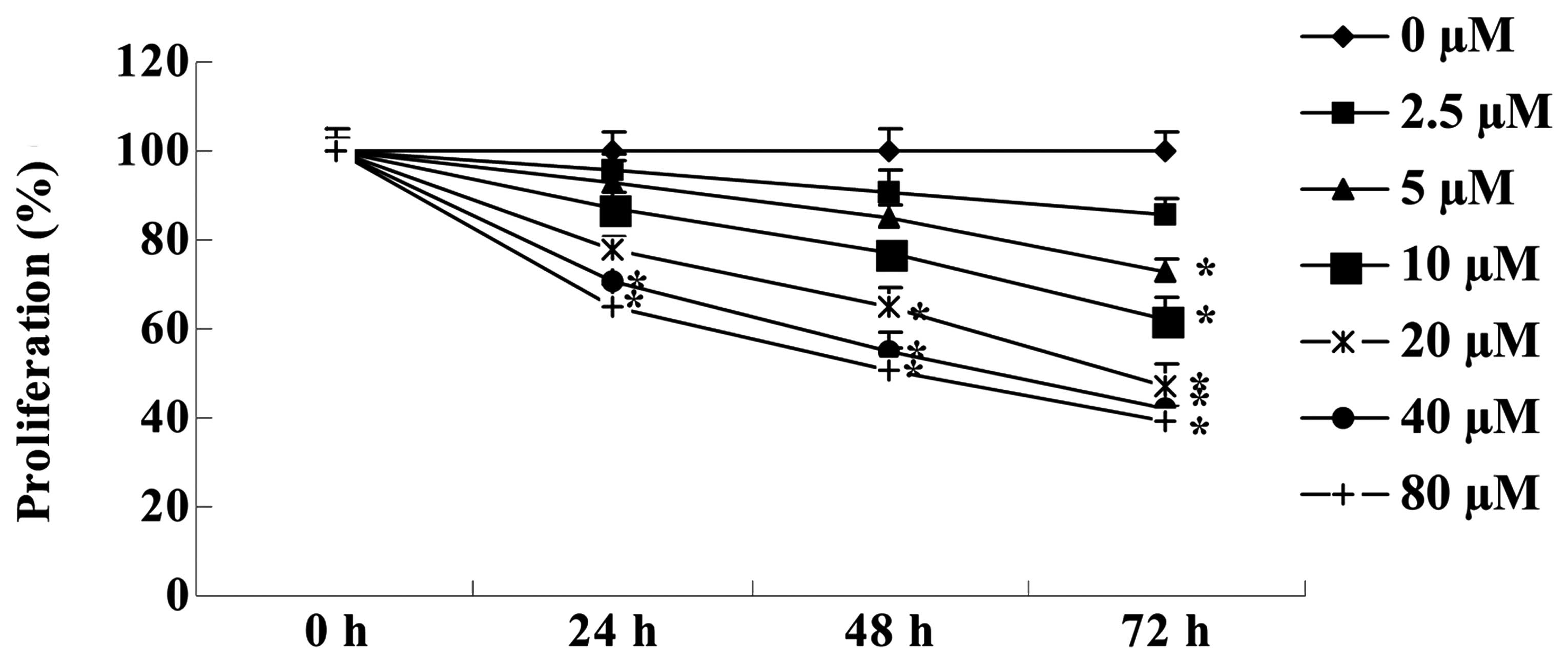
Cantharidin inhibits the proliferation of
TSCC Tca8113 cells
To confirm the effect of cantharidin on the
proliferation of TSCC Tca8113 cells, an MTT assay was used to
measure the growth of Tca8113 cella. The results showed that
cantharidin inhibited the proliferation of Tca8113 cells in a dose-
and time-dependent manner (Fig. 2).
After 72 h, the anticancer effect of cantharidin (5, 10, 20, 40 and
80 μM) markedly inhibited the proliferation of Tca8113 cells. After
48 h, treatment with cantharidin (20, 40 and 80 μM) significantly
inhibited the proliferation of Tca8113 cells. Treatment with
cantharidin (40 and 80 μM) significantly inhibited the
proliferation of Tca8113 cells for 24 h.
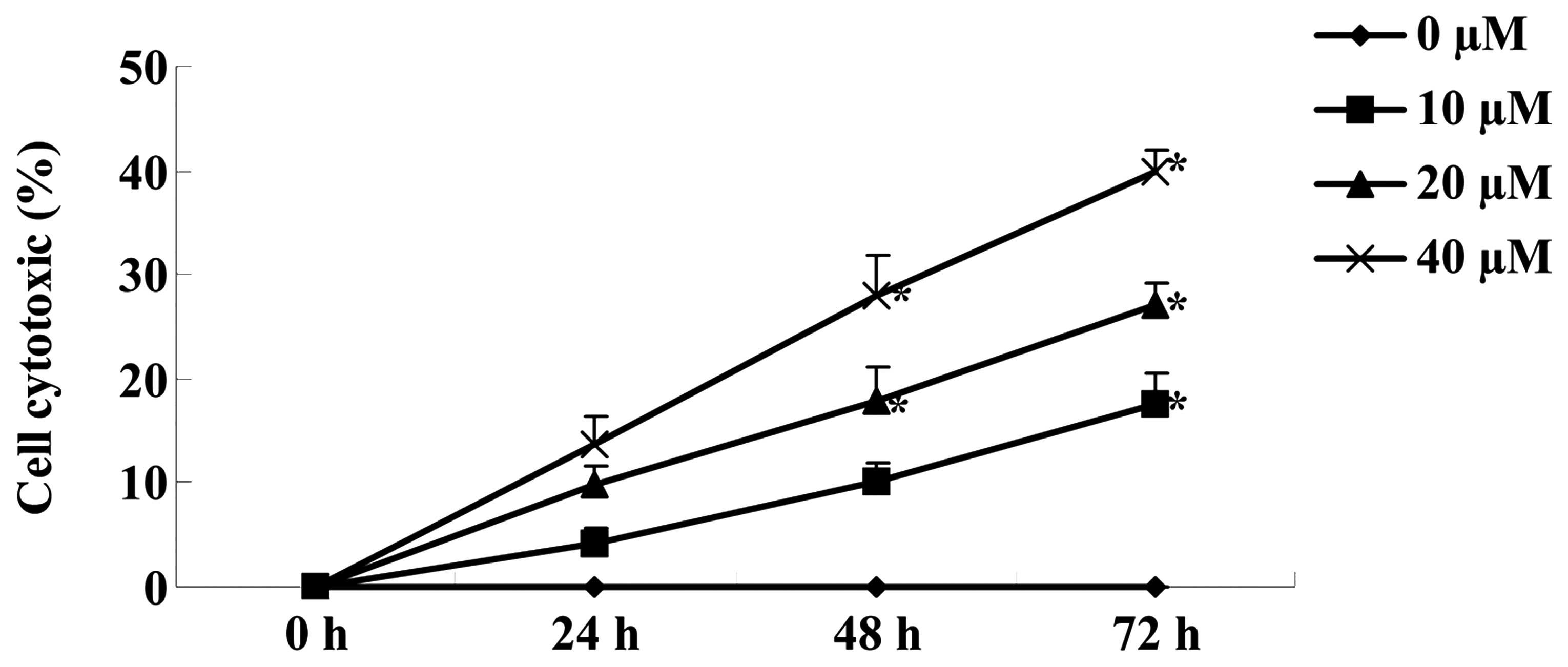
Cantharidin promotes cytotoxicity of TSCC
Tca8113 cells
An LDH assay was used to investigate the effect of
cantharidin on the cytotoxicity of TSCC Tca8113 cells treated with
0, 10, 20 and 40 μM cantharidin for 48 h. In the Tca8113 cells
treated with 0, 10, 20 and 40 μM cantharidin, cantharidin increased
the cytotoxicity of Tca8113 cells in a dose- and time-dependent
manner (Fig. 3). After 48 and 72 h,
cantharidin (20 and 40 μM) notably increased the cytotoxicity of
Tca8113 cells (Fig. 3).
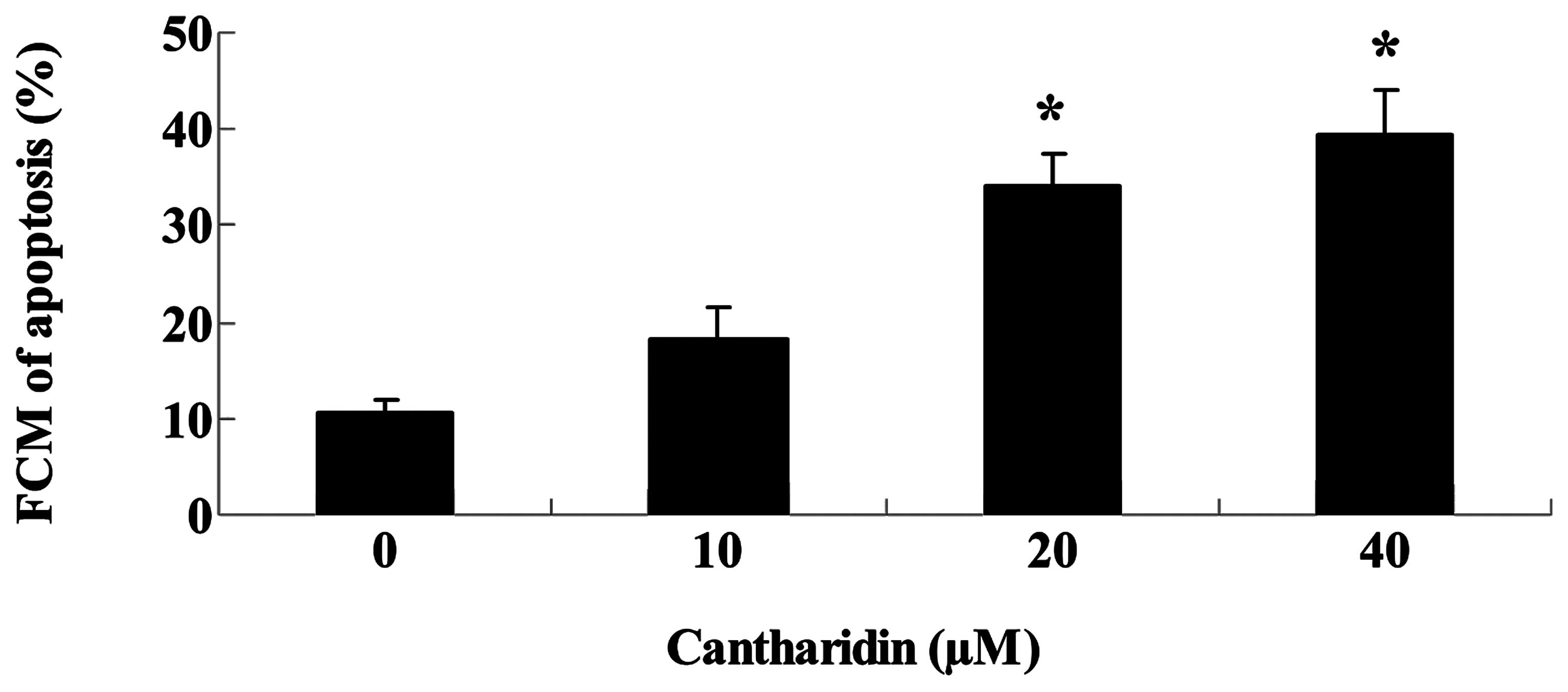
Cantharidin promotes apoptosis of TSCC
Tca8113 cells
To investigate whether cantharidin induced
inhibition of TSCC Tca8113 cells through cell apoptotic pathways,
apoptosis of Tca8113 cells was measured by flow cytometry. After 48
h, treatment with cantharidin (20 and 40 μM) significantly promoted
the apoptosis of Tca8113 cells (Fig.
4).
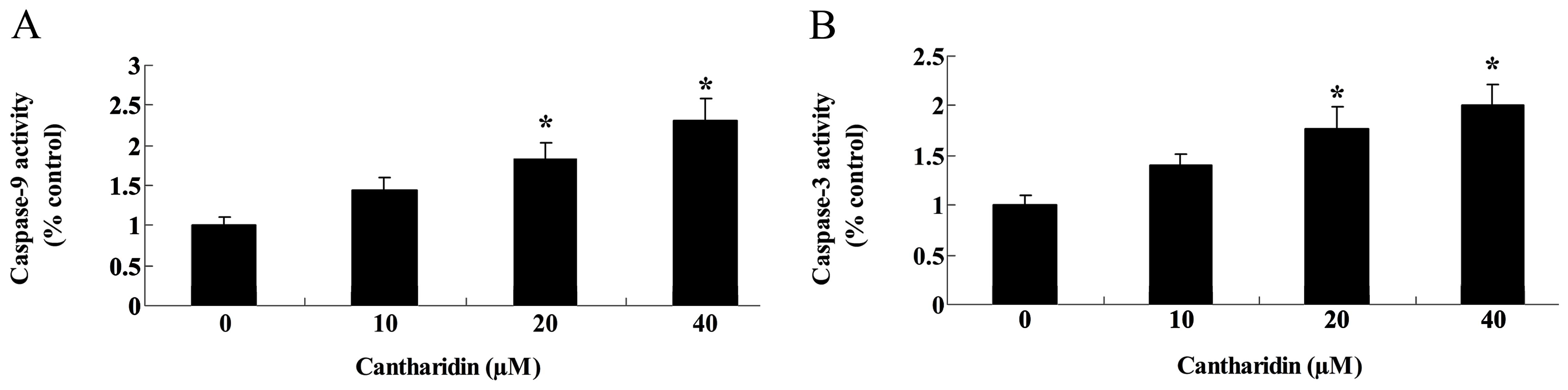
Cantharidin induces caspase-9 and -3
activities of TSCC Tca8113 cells
The possible signaling pathways through which
cantharidin induced apoptosis in Tca8113 cells were investigated to
detect caspase-9 and -3 activities of Tca8113 cells. Fig. 5 shows that caspase-9 and -3
activities of Tca8113 cells were markedly increased by treatment
with cantharidin (20 and 40 μM).
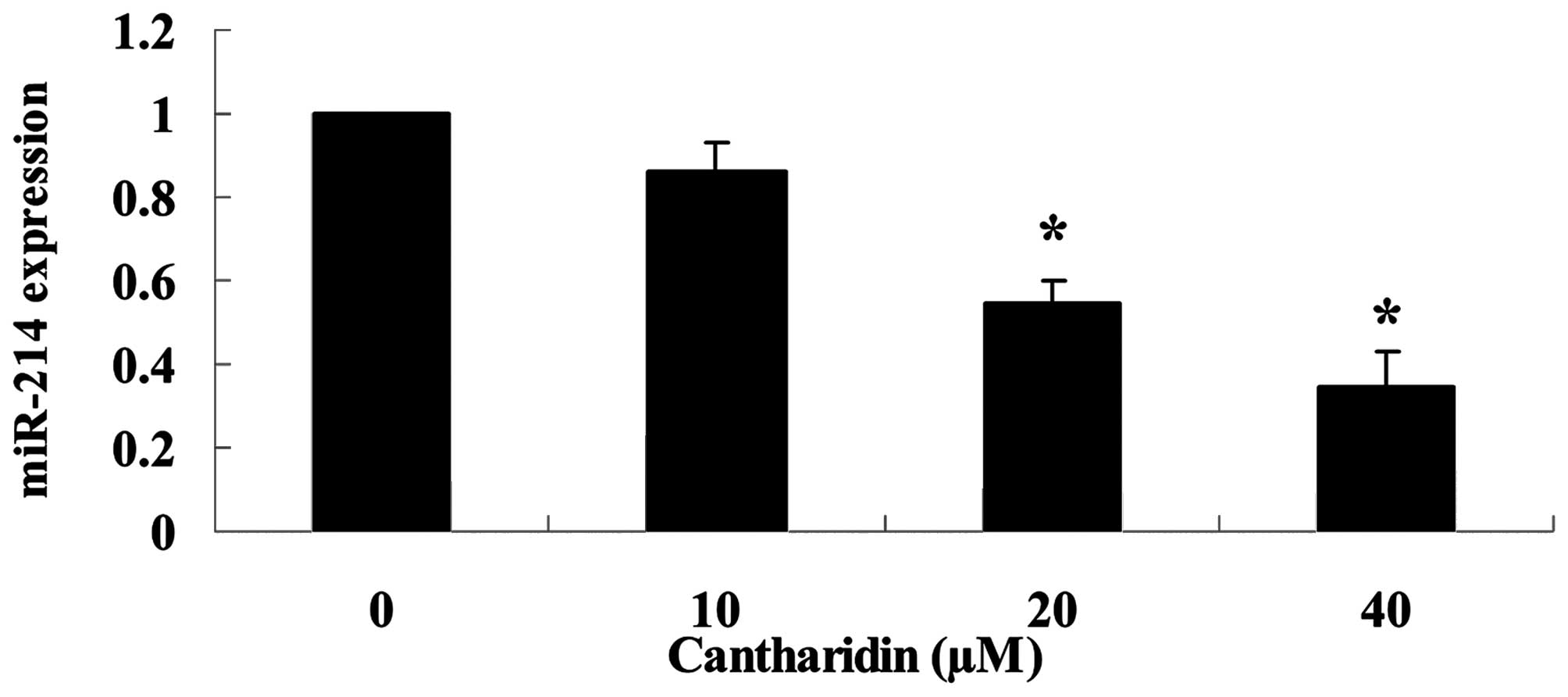
Cantharidin suppresses miR-214 expression
of TSCC Tca8113 cell
To investigate the functional role of miR-214
expression in TSCC Tca8113 cell, we carried out miR-214 expression
of Tca8113 cells using qPCR. miR-214 expression level was markedly
downregulated by treatment with cantharidin (20 and 40 μM) in
Tca8113 cells (Fig. 6).
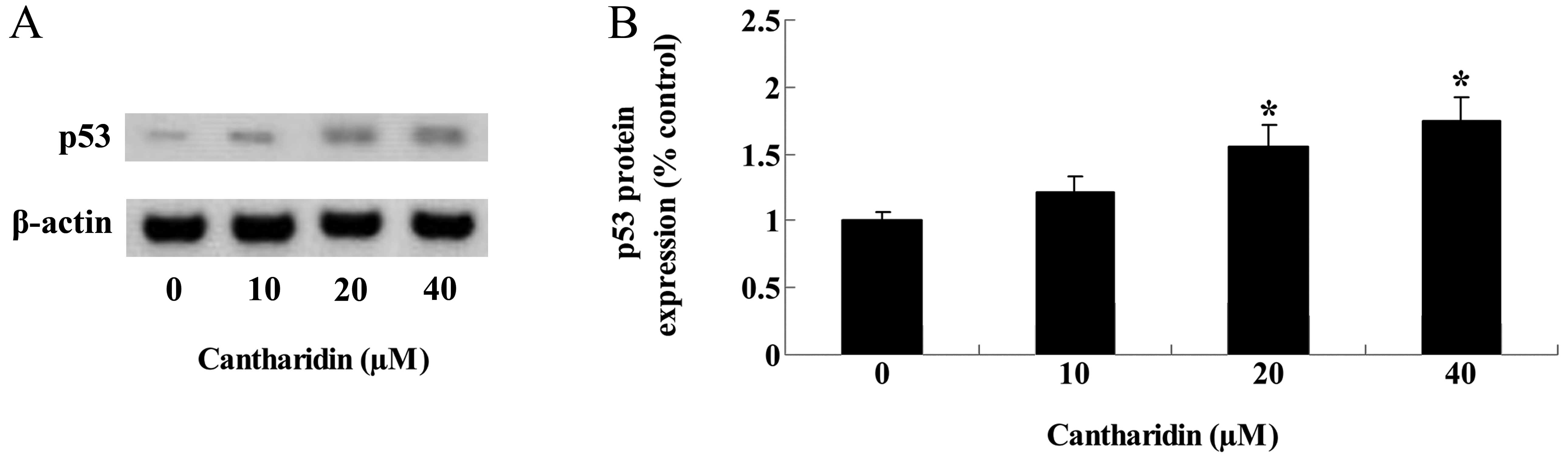
Cantharidin increases p53 protein
expression of TSCC Tca8113 cells
The underlying signaling pathways through which
cantharidin regulated the proliferation and apoptosis of TSCC
Tca8113 cells were examined, and p53 protein expression of Tca8113
cells was examined using western blotting. After 48 h, treatment
with cantharidin (20 and 40 μM) repaired and markedly increased p53
protein expression of Tca8113 cells (Fig. 7A and B).
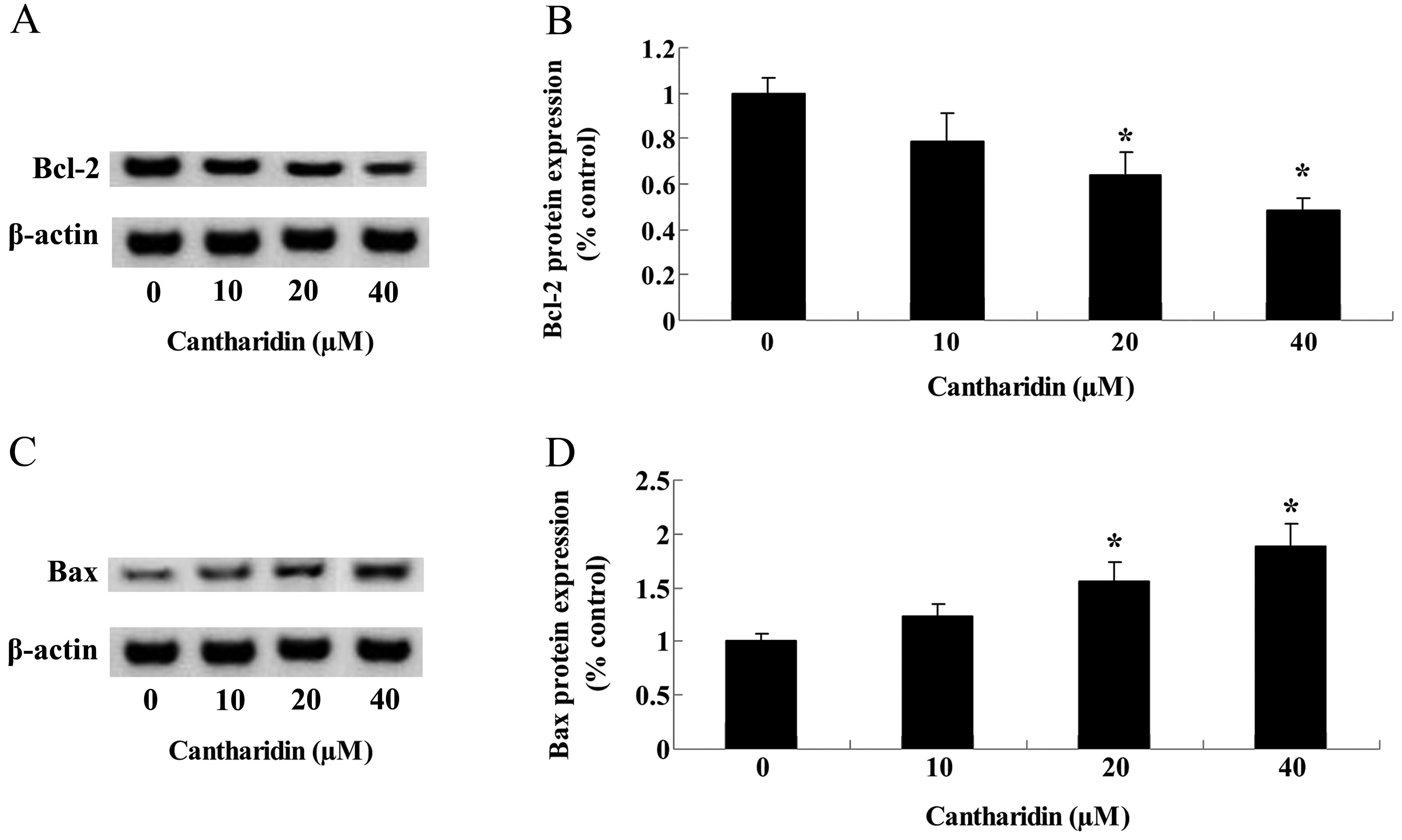
Cantharidin adjusts Bcl-2/Bax protein
expression of TSCC Tca8113 cells
To investigate the potential connection between
Bcl-2/Bax protein expression and the potential anticancer effect of
cantharidin on TSCC Tca8113 cells, western blotting was used to
analyze Bcl-2/Bax protein expression in Tca8113 cells. After 48 h,
Bcl-2 protein expression was suppressed by treatment with
cantharidin (20 and 40 μM) in Tca8113 cells (Fig. 8A and B). By contrast, the Bax
protein expression of Tca8113 cells was activated by treatment with
cantharidin (20 and 40 μM) for 48 h (Fig. 8C and D).
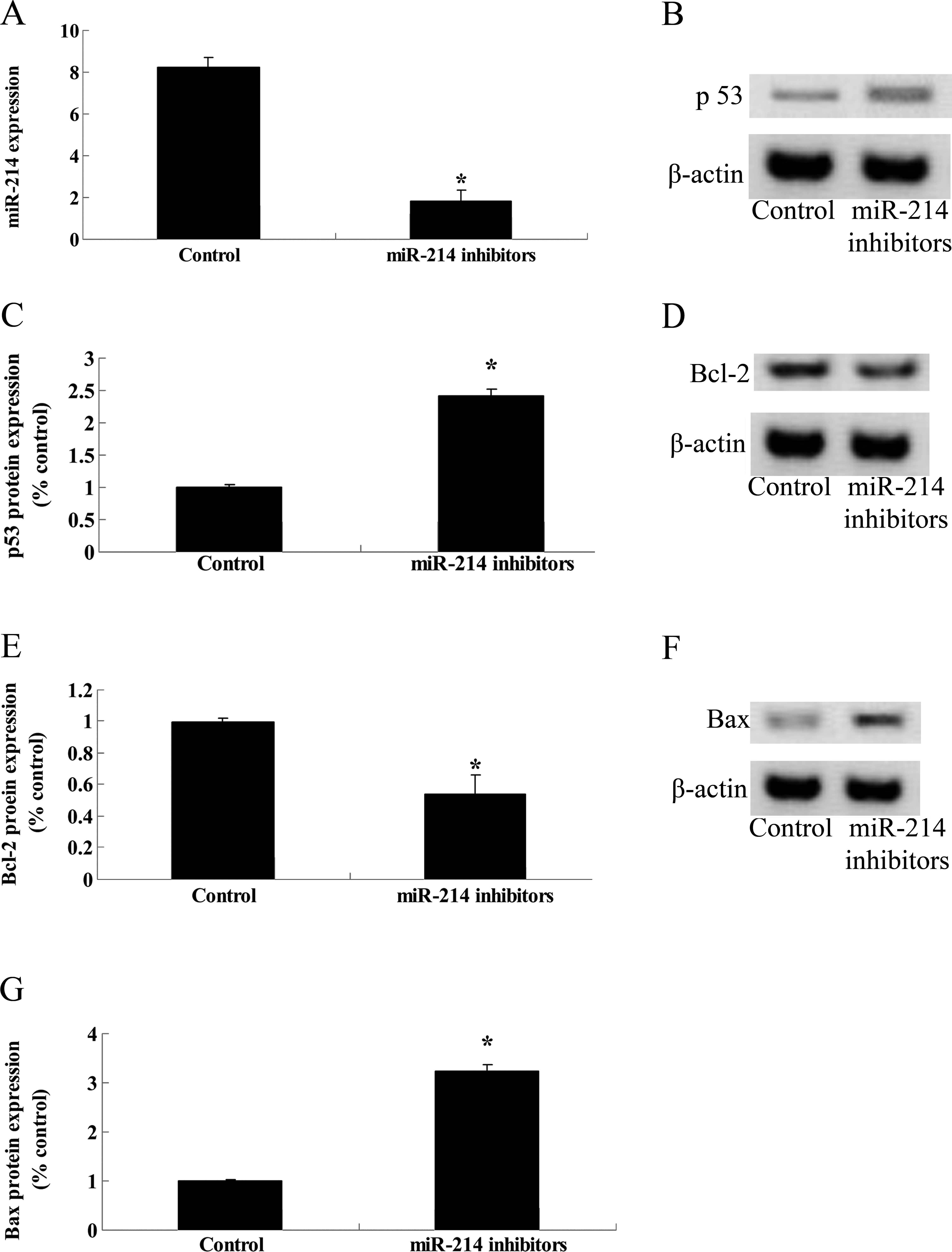
Anti-miR-214 and expression of p53 and
Bcl-2/Bax proteins in TSCC Tca8113 cells
To investigate the potential connection between
miR-214 silencing and the potential anticancer effect of
cantharidin on TSCC Tca8113 cells, miR-21 inhibitors were
transfected into Tca8113 cells to detect the p53 and Bcl-2/Bax
protein expression in Tca8113 cells. Firstly, miR-21 expression of
Tca8113 cells were significantly inhibited by miR-21 inhibitors
(Fig. 9A). p53 protein expression
of Tca8113 cells was subsequently increased by miR-21 inhibitors
(Fig. 9B and C). Bcl-2 protein
expression was attenuated and Bax protein expression was enhanced
following miR-21 transfection (Fig. 9D
and G). These results suggest that miR-214 inhibition regulated
p53 and Bcl-2/Bax expression in Tca8113 cells.
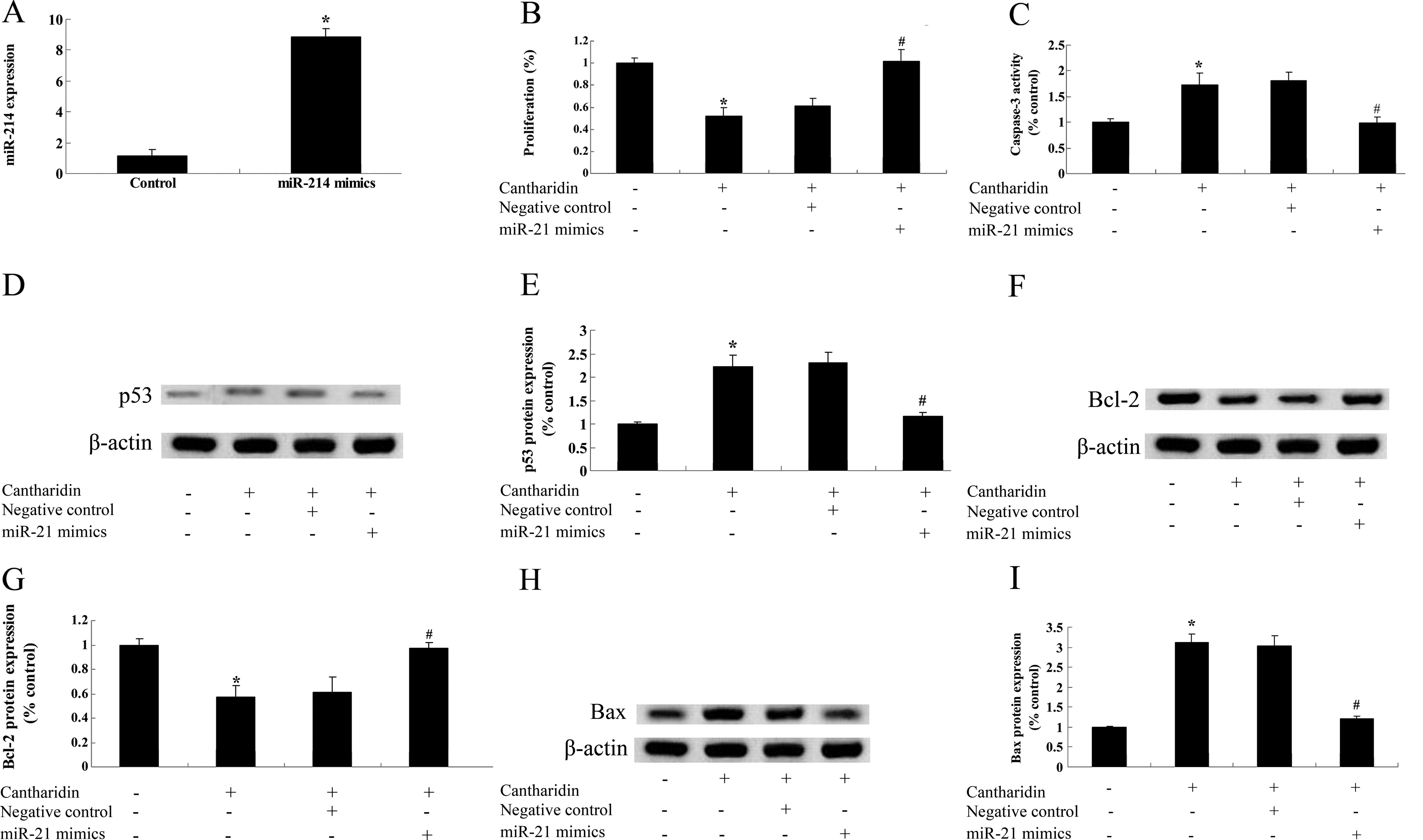
Overexpression of miR-214 and the
anticancer effect of cantharidin on TSCC Tca8113 cells
To assess the potential connection between miR-214
overexpression and the anticancer effect of cantharidin on TSCC
Tca8113 cells, miR-214 mimics were transfected into Tca8113 cells.
The result in Fig. 10A shows that
miR-214 mimics significantly increased the expression levels of
miR-214 in Tca8113 cells. However, miR-214 mimics reversed the
potential anticancer effect of cantharidin on the proliferation,
thereby reducing caspase-3 activation in Tca8113 cells (Fig. 10B and C). Notably, p53 and Bax
protein expression of Tca8113 cells were weakened by miR-214
overexpression (Fig. 10D and E, and H
and I). miR-214 overexpression increased Bcl-2 protein
expression of Tca8113 cells (Fig. 10F
and G). The results suggested that overexpression of miR-214
reduces the anticancer effect of cantharidin on TSCC Tca8113 cells
regulated by p53 and Bcl-2/Bax expression in Tca8113 cells.
Discussion
Oral cancer refers to oral and maxillofacial
malignancies, of which the majority are squamous epithelial cancer,
including tongue, buccal, gum, palate, lip and jaw bone cancer,
oral floor carcinoma and oropharyngeal cancer (3). TSCC is a common oral cancer. The
occurrence and development of cancer is a multi-step process with
multi-gene mutations, while tumor growth and metabolism are
associated with growth scores and self-programmed death (20). Autophagy, as a form of programmed
death, is a highly conserved cellular behavior, closely associated
with cell growth and proliferation, as well as the tumor
development process (21). In the
present study, treatment with cantharidin significantly inhibited
the proliferation and increased cytotoxicity of TSCC Tca8113 cells
in a dose- and time-dependent manner. Cantharidin also stimulated
cell apoptosis and enhanced caspase-9/3 activities of TSCC Tca8113
cells. The results were in concordance with those of other studies.
For example, Zhang et al suggested that cantharidin induced
apoptosis by activating caspase-3, -7, -8 and -9 in human gastric
cancer (19). Hsia et al
reported that cantharidin induces apoptosis by activating caspase-3
and -8 in H460 human lung cancer cells (22). Therefore, cantharidin is an optimal
potential therapeutic agent for TSCC.
Results of miRNAs studies have identified that a
large number of miRNAs with abnormal expressions regulate the
development of various types of cancer including pancreatic cancer
by regulating the expression of target genes. Thus, large-scale
screening of the abnormal expression of miRNAs in the tumor
development process may provide a comprehensive understanding of
the biological characteristics of tumor in human (23). Findings of a recent study showed
that miR-214 is abnormally expressed in a variety of malignancies.
However, the expression in different tumors are not the same, with
certain specificity (24). We found
that cantharidin decreased the miR-214 expression levels of Tca8113
cells. This result was consistent with that of the study by Lu
et al whereby cantharidin exerted an anticancer effect by
miR-214 modulating in hepatocellular carcinoma (8). However, the detailed mechanisms on how
cantharidin regulates miR-214 expression remain to be determined in
future studies.
The p53 tumor-suppressor gene has been extensively
studied. The p53 gene is a tumor-suppressor gene that is
associated with human cancer and plays an important role in the
regulation of the cell cycle (25).
Its product, p53 protein, inhibits tumorigenesis, although
following gene mutation the p53 protein loses its tumor-suppressor
effect, with the promotion of the activity of malignancy (26). In the present study, we found that
treatment with cantharidin effectively activated p53 protein
expression of Tca8113 cells. Hsia et al showed that
cantharidin inhibited DNA repair-associated protein levels (p53 and
H2A.X) and induced DNA damage in NCI-H460 human lung cancer cells
(18). Kuo et al reported
that cantharidin induced the apoptosis of human bladder cancer by
promoting the p53 level (27).
Apoptosis is a type of cell death with specific
morphological change, which is a physiological process caused by a
series of gene actions. Bcl-2 oncogene is a cancer gene found at
t(14;18) chromosomal translocation breakpoint, of which the
overexpression protects tumor cells from apoptosis (28). Cantharidin reduced the Bcl-2 protein
expression and activated the Bax protein expression of Tca8113
cells in the present study. Li et al suggested that
cantharidin induced oxidative stress-independent growth inhibition
of pancreatic cancer cells through suppression of the expression of
anti-apoptotic Bcl-2 (29). These
findings suggested cantharidin induced apoptosis via Bcl-2/Bax in
human bladder carcinoma (30),
pulmonary carcinoma (31), and
pancreatic cancer (29) cells.
The present study has limitations. To clarify the
mechanism involved in the suppression of Tca8113 cells, the effect
of cantharidin on miR-214, p53 and Bcl-2/Bax expression was
examined. We found that the miR-214 silence expression stimulated
the p53 protein expression and inhibited the Bcl-2/Bax signaling
pathway in Tca8113 cells. Additionally, miR-451 overexpression
reversed the anticancer effect of cantharidin on TSCC Tca8113
cella, attenuated p53 protein and stiumlated the Bcl-2/Bax
signaling pathway in TSCC Tca8113 cells. With respect to the
anticancer effect of cantharidin on TSCC and its mechanism, further
studies on knockdown of miR-214 may provide more information for an
improved understanding of the effect of cantharidin affected by p53
and Bcl-2/Bax signal on the proliferation and apoptosis in
TSCC.
In conclusion, the results show the anticancer
effect of cantharidin inhibits cell proliferation and promotes
apoptosis of TSCC. Knockdown of miR-214 activates the effect of
cantharidin on cell proliferation and apoptosis in vivo. Our
findings suggest that cantharidin is an important potential
therapeutic agent in TSCC and a potential therapeutic target for
TSCC patients.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 31101042).
References
|
1
|
Shao Y, Sha XY, Bai YX, Quan F and Wu SL:
Effect of A disintegrin and metalloproteinase 10 gene silencing on
the proliferation, invasion and migration of the human tongue
squamous cell carcinoma cell line TCA8113. Mol Med Rep. 11:212–218.
2015.
|
|
2
|
Li H, Huang D, Gao Z, Chen Y, Zhang L and
Zheng J: Scutellarin inhibits the growth and invasion of human
tongue squamous carcinoma through the inhibition of matrix
metalloproteinase-2 and -9 and αvβ6 integrin. Int J Oncol.
42:1674–1681. 2013.PubMed/NCBI
|
|
3
|
Addeo R, Napolitano A, Montella L and
Ricciardiello F: Squamous cell carcinoma of the tongue in a female
with advanced breast cancer: A case report of an elderly patient
presenting with two types of cancer. Oncol Lett. 8:235–237.
2014.PubMed/NCBI
|
|
4
|
Chiyomaru T, Seki N, Inoguchi S, Ishihara
T, Mataki H, Matsushita R, Goto Y, Nishikawa R, Tatarano S, Itesako
T, et al: Dual regulation of receptor tyrosine kinase genes EGFR
and c-Met by the tumor-suppressive microRNA-23b/27b cluster in
bladder cancer. Int J Oncol. 46:487–496. 2015.
|
|
5
|
Jin D, Fang Y, Li Z, Chen Z and Xiang J:
Epithelial-mesenchymal transition-associated microRNAs in
colorectal cancer and drug-targeted therapies (Review). Oncol Rep.
33:515–525. 2015.
|
|
6
|
Yu ZW, Zhong LP, Ji T, Zhang P, Chen WT
and Zhang CP: MicroRNAs contribute to the chemoresistance of
cisplatin in tongue squamous cell carcinoma lines. Oral Oncol.
46:317–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang ZC, Li YY, Wang HY, Fu S, Wang XP,
Zeng MS, Zeng YX and Shao JY: Knockdown of miR-214 promotes
apoptosis and inhibits cell proliferation in nasopharyngeal
carcinoma. PLoS One. 9:e861492014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu S, Gao Y, Huang X and Wang X:
Cantharidin exerts anti-hepatocellular carcinoma by miR-214
modulating macrophage polarization. Int J Biol Sci. 10:415–425.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozaki T and Nakagawara A: p53: The
attractive tumor suppressor in the cancer research field. J Biomed
Biotechnol. 2011:6039252011. View Article : Google Scholar
|
|
10
|
Qi L and Zhang Y: Alisertib (MLN8237), a
selective Aurora-A kinase inhibitor, induces apoptosis in human
tongue squamous cell carcinoma cell both in vitro and in vivo.
Tumour Biol. Nov 4–2014.Epub ahead of print. View Article : Google Scholar
|
|
11
|
Yasumoto J, Imai Y, Takahashi A, Ohnishi
K, Yuki K, Kirita T and Ohnishi T: Analysis of apoptosis-related
gene expression after X-ray irradiation in human tongue squamous
cell carcinoma cells harboring wild-type or mutated p53 gene. J
Radiat Res. 44:41–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagler RM, Kerner H, Laufer D, Ben-Eliezer
S, Minkov I and Ben-Itzhak O: Squamous cell carcinoma of the
tongue: The prevalence and prognostic roles of p53, Bcl-2, c-erbB-2
and apoptotic rate as related to clinical and pathological
characteristics in a retrospective study. Cancer Lett. 186:137–150.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niu XW, Feng J, Peng ZH, et al:
Receptor-related mechanism of proliferation inhibilion and
apoptosis induetion of human tongue squamous cell line Tca8113 by
retinoids. Di Yi Jun Yi Da Xue Xue Bao. 25:935–941. 2005.PubMed/NCBI
|
|
14
|
Saleh HA, Jackson H, Khatib G and Banerjee
M: Correlation of bcl-2 oncoprotein immunohistochemical expression
with proliferation index and histopathologic parameters in
colorectal neoplasia. Pathol Oncol Res. 5:273–279. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie X, Clausen OP, De Angelis P and Boysen
M: The prognostic value of spontaneous apoptosis, Bax, Bcl-2, and
p53 in oral squamous cell carcinoma of the tongue. Cancer.
86:913–920. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeh CB, Su CJ, Hwang JM and Chou MC:
Therapeutic effects of cantharidin analogues without bridging ether
oxygen on human hepatocellular carcinoma cells. Eur J Med Chem.
45:3981–3985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barr AC, Wigle WL, Flory W, Alldredge BE
and Reagor JC: Cantharidin poisoning of emu chicks by ingestion of
Pyrota insulata. J Vet Diagn Invest. 10:77–79. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsia TC, Lin JH, Hsu SC, Tang NY, Lu HF,
Wu SH, Lin JG and Chung JG: Cantharidin induces DNA damage and
inhibits DNA repair-associated protein levels in NCI-H460 human
lung cancer cells. Environ Toxicol. Mar 17–2014.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Chen Z, Zhou X, Xu W, Wang G,
Tang X, Luo L, Tu J, Zhu Y, Hu W, et al: Cantharidin induces
G2/M phase arrest and apoptosis in human gastric cancer
SGC-7901 and BGC-823 cells. Oncol Lett. 8:2721–2726.
2014.PubMed/NCBI
|
|
20
|
Wang K, Lin C, Wang C, Shao Q, Gao W, Song
B, Wang L, Song X, Qu X and Wei F: Silencing Kif2a induces
apoptosis in squamous cell carcinoma of the oral tongue through
inhibition of the PI3K/Akt signaling pathway. Mol Med Rep.
9:273–278. 2014.
|
|
21
|
Fang QG, Shi S, Liu FY and Sun CF: Tongue
squamous cell carcinoma as a possible distinct entity in patients
under 40 years old. Oncol Lett. 7:2099–2102. 2014.PubMed/NCBI
|
|
22
|
Hsia TC, Yu CC, Hsu SC, Tang NY, Lu HF,
Huang YP, Wu SH, Lin JG and Chung JG: Cantharidin induces apoptosis
of H460 human lung cancer cells through mitochondria-dependent
pathways. Int J Oncol. 45:245–254. 2014.PubMed/NCBI
|
|
23
|
Su Y, Ni Z, Wang G, Cui J, Wei C, Wang J,
Yang Q, Xu Y and Li F: Aberrant expression of microRNAs in gastric
cancer and biological significance of miR-574-3p. Int
Immunopharmacol. 13:468–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu M, Liu J, Wang L, Wu H, Zhou C, Zhu H,
Xu N and Xie Y: Association of serum microRNA expression in
hepatocellular carcinomas treated with transarterial
chemoembolization and patient survival. PLoS One. 9:e1093472014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HP, Li TM, Tsao JY, Fong YC and Tang
CH: Curcumin induces cell apoptosis in human chondrosarcoma through
extrinsic death receptor pathway. Int Immunopharmacol. 13:163–169.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marx J: New tumor suppressor may rival
p53. Science. 264:344–345. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuo JH, Chu YL, Yang JS, Lin JP, Lai KC,
Kuo HM, Hsia TC and Chung JG: Cantharidin induces apoptosis in
human bladder cancer TSGH 8301 cells through mitochondria-dependent
signal pathways. Int J Oncol. 37:1243–1250. 2010.PubMed/NCBI
|
|
28
|
Saigusa S, Inoue Y, Tanaka K, Okugawa Y,
Toiyama Y, Uchida K, Mohri Y and Kusunoki M: Lack of M30 expression
correlates with factors reflecting tumor progression in rectal
cancer with preoperative chemoradiotherapy. Mol Clin Oncol.
2:99–104. 2014.PubMed/NCBI
|
|
29
|
Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y,
Xu Z and Han X: Cantharidin, a potent and selective PP2A inhibitor,
induces an oxidative stress-independent growth inhibition of
pancreatic cancer cells through G2/M cell-cycle arrest and
apoptosis. Cancer Sci. 101:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huan SK, Lee HH, Liu DZ, Wu CC and Wang
CC: Cantharidin-induced cytotoxicity and cyclooxygenase 2
expression in human bladder carcinoma cell line. Toxicology.
223:136–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang WD, Zhao HR, Yan Y, Wang XH, Zong ZH
and Liu Y: Apoptosis induced by cantharidin in human pulmonary
carcinoma cells A549 and its molecular mechanisms. Zhonghua Zhong
Liu Za Zhi. 27:330–334. 2005.PubMed/NCBI
|