Introduction
Acute myeloid leukemia (AML) is a highly
heterogeneous hematologic malignancy that displays diverse
responses to chemotherapy. Although a number of clinical factors
affect treatment outcomes, the cytogenetic features of AML are
generally accepted as strong predictors of therapeutic response
(1). Pediatric and adult patients
carrying the t(8;21) chromosomal translocation, which is one of the
most frequent AML subtypes, are part of a favorable risk group
(2). Although these patients are
initially highly responsive to treatment, a number of patients
relapse and fail to achieve long-term disease-free survival (DFS)
(3–5). However, little is currently known
concerning the functional mechanism by which t(8;21) is associated
with both a higher remission rate in some patients, and
occasionally with poor outcomes in others, except in cases that are
characterized by other factors such as distinctive
immunophenotypical changes and multidrug resistance genes (6–8).
Cytarabine is a nucleoside analog that is
intensively used for the treatment of AML (9–11).
Because of its hydrophilic nature, cytarabine can only enter cells
via nucleoside transporters such as concentrative nucleoside
transporters (CNT1/2/3) and equilibrative nucleoside transporters
(ENT1/2) (12,13). Cytarabine must also be converted to
its active form by specific enzymes such as deoxycytidine kinase
(dCK) (14). Several groups have
reported that low gene expression and protein activity of these
factors confers resistance to nucleoside drugs of leukemia cells in
in vivo and in vitro studies (15–18).
Alternatively, activation of certain components of the metabolic
machinery such as cytidine deaminase (CDA), ecto-5′-nucleotidase
(CD73) and 5′,3′-nucleotidase (NT5C), may cause resistance to
cytarabine therapy (19–22). Microarray analysis of an in
vitro gemcitabine-resistant model revealed upregulation of
ribonucleotide reductase M subunits (RRM1/2) as part of the
intracellular detoxification process (23). Nevertheless, the prognostic
implications of changes in the expression of these genes have not
been elucidated in specific subpopulations, such as in AML patients
with t(8;21).
In the present study, we hypothesized that specific
genes that are closely involved in the antitumor action of
cytarabine may be associated with the clinical response to
treatment of AML patients carrying t(8;21). Therefore, we attempted
to identify a novel marker that was predictive of outcomes of
patients carrying the t(8;21) abnormality and evaluated the
prognostic impact of the candidate genes.
Materials and methods
Patient samples
Bone marrow (BM) mononuclear cells (MNCs) from
adults newly diagnosed with AML and 4 healthy donors were collected
at Chonnam National University Hwasun Hospital and Catholic
University Seoul St. Mary’s Hospital. Written informed consent for
the cryopreservation and use of the samples for further research
were obtained from the patients. The Institutional Review Board
approved all research on the human subjects participating in the
present study. The 54 individuals in the test study and the 44
individuals in the validation study had M2 subtype disease
according to the French-American-British (FAB) classification
criteria; their clinical information is summarized in Table I. In the test cohort, 16 patients
had normal karyotypes, 25 patients had the cytogenetically t(8;21)
abnormality, and 13 patients had diverse chromosomal abnormalities.
In the validation cohort, 15 patients had normal karyotypes, 19
patients had t(8;21), and 10 patients had other abnormalities.
Intensive remission induction therapy was executed by
administration of the combination of 12 mg/m2/day
idarubicin for 3 days with 100 mg/m2/day cytarabine for
7 days or N4-behenoyl-1-D-arabinofuranosyl
cytosine (BH-AC) at either 300 mg/m2/day for patients
younger than 40 years or 200 mg/m2/day for patients
older than 40 years. Four patients received cytarabine alone at a
dose of 100 mg/m2/day; one patient, daunorubicin plus
cytarabine; one patient, cytarabine plus etoposide. Patients who
failed to achieve complete remission (CR) after the first round of
induction chemotherapy received re-induction chemotherapy with the
same regimen. Patients achieving a CR received 3 courses of
high-dose Ara-C (3 g/m2 every 12 h/day on days 1, 3 and
5) or the combination of idarubicin and BH-AC for consolidation
therapy. Thirty-three patients in the test cohort and 13 patients
in the validation cohort received hematopoietic stem cell
transplantation (HSCT).
 | Table IPatient samples. |
Table I
Patient samples.
| Category | Test | Validation |
|---|
| Patients, n | 54 | 44 |
| Age, median years
(range) Gender, n (%) | 41 (20–72) | 49 (17–73) |
| Male | 28 (51.9) | 26 (59.1) |
| Female | 26 (48.1) | 18 (40.9) |
| Blasts, mean %
(range) | 50 (2–92) | 60 (20–91) |
| M2 (FAB), n | 54 | 44 |
| Cytogenetics,
n | | |
| t(8;21) | 25 | 19 |
| Normal | 16 | 15 |
| Others | 13 | 10 |
RNA preparation and real-time polymerase
chain reaction (real-time PCR)
Total RNA was extracted from the BM-MNCs of patients
using the Qiagen RNA isolation kit (Qiagen, Venlo, The Netherlands)
and converted to cDNA with a reverse transcription kit (Invitrogen,
Carlsbad, CA, USA). The cDNA was mixed with SYBR-Green PCR Master
Mix (PE Applied Biosystems, Foster City, CA, USA) and specific
primers for 8 genes including CD73, CDA, NT5C,
dCK, CNT3, ENT1, RRM1 and RRM2.
Amplification reactions were performed in triplicate using the ABI
Prism 7900 Sequence Detection system (PE Applied Biosystems).
GAPDH served as an experimental control. Primer sequences,
PCR conditions, and data processing were previously described
(24). In the validation study, the
expression levels of the membrane transporters, CNT1,
CNT2, CNT3, ENT1 and ENT2, were
evaluated by the method described above. However, we excluded the
results for CNT1 and CNT2 from further analysis
because they had low or undetectable signals that were not as
reliable.
Survival estimate and correlation
analyses
The study population was separated based on the
cytogenetic abnormalities of the patients. For the purpose of
statistical analysis of the target gene, the patients were also
subdivided into 3 groups (low, intermediate and high) according to
candidate gene expression tertiles. DFS was defined as the time
from first remission to relapse or death. Overall survival (OS) was
defined as the time from diagnosis to the date of death or last
followup. Kaplan-Meier estimates were used to construct survival
curves. Statistical differences in the treatment outcomes were
determined by the univariate analyses with log-rank test and Cox
proportional hazards univariate model. In multivariate analysis, a
backward stepwise method of Cox proportional hazards model was used
to investigate the association of clinical variables and gene
expression pattern with DFS and OS and to assess hazard ratio (HR)
with 95% confidence interval (CI). Correlations between clinical
and molecular features were obtained by Pearson’s correlation
statistics and represented as a correlation coefficient (r). All
statistical analyses were performed using SPSS version 12.0 (SPSS,
Inc., Chicago, IL, USA).
Results
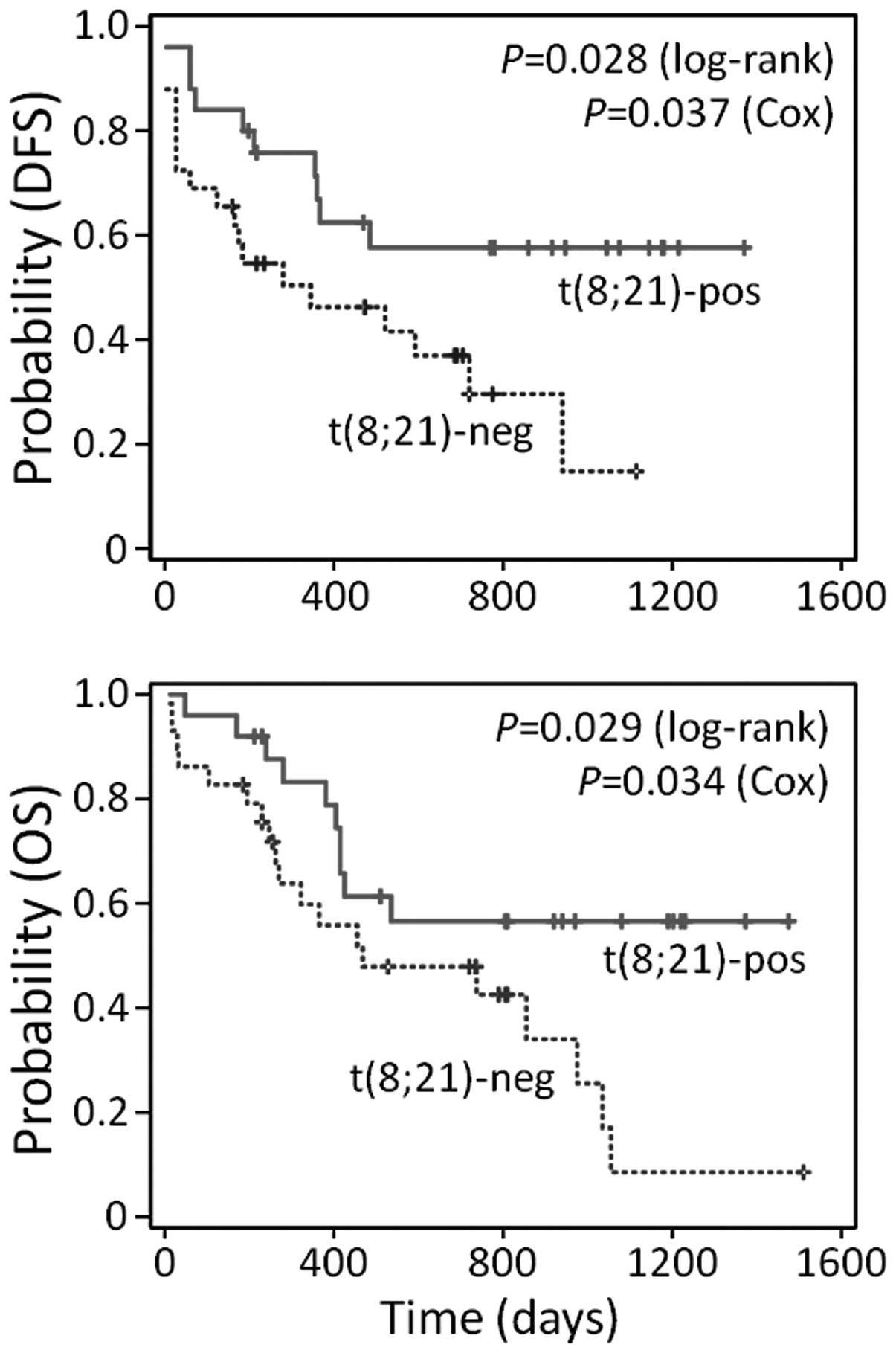
Prolonged survival in patients carrying
t(8;21)
We collected BM-MNCs from 54 newly diagnosed
patients with M2 subtype AML according to the FAB classification
who received cytarabine-based chemotherapy. Although it is well
established that AML patients with the t(8;21) abnormality respond
better to chemotherapy than patients with normal cytogenetics or
other cytogenetic abnormalities (1,2,25), we
confirmed this in our study population. In a univariate analysis,
Kaplan-Meier estimates showed longer survival rates in terms of DFS
(P=0.028 in log-rank; P=0.037 in Cox model) and OS (P=0.029 in
log-rank; P=0.034 in Cox model) in the t(8;21)-positive AML cohort
compared to the t(8;21)-negative group (Fig. 1).
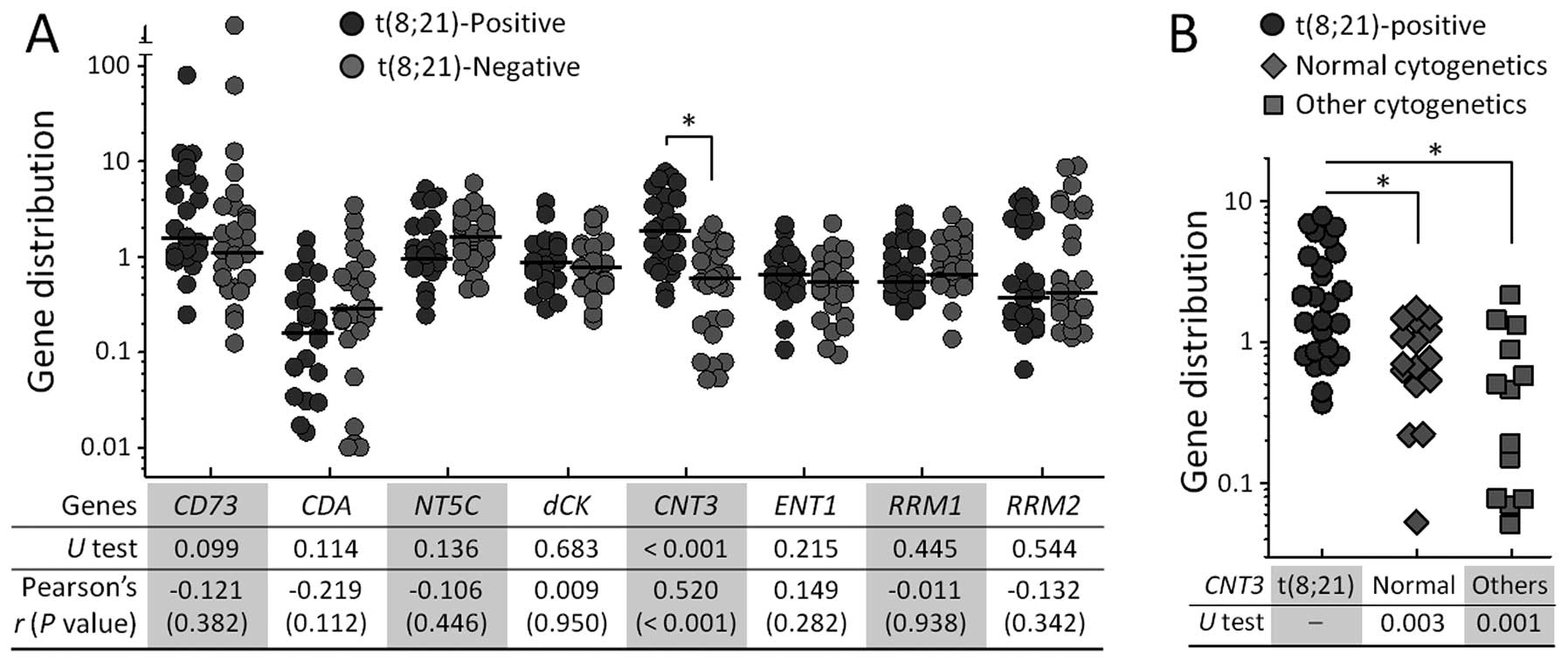
Elevated CNT3 expression in AML patients
with t(8;21)
To explore possible pathological reasons for the
better response to cytarabine-based therapy in t(8;21)-positive
patients, we examined the mRNA expression levels of 8 candidate
genes in a total of 54 AML patients. These genes included
CD73, CDA, NT5C, dCK, CNT3,
ENT1, RRM1 and RRM2, which are genes that are
closely involved in metabolic processing of nucleoside analogs. Of
the 8 genes, only CNT3 mRNA levels were significantly
different between the t(8;21)-positive and -negative patients in
non-parametric statistical analysis with Mann-Whitney U test
(P<0.001); no significant differences were observed for the
remaining genes (Fig. 2A). Using
the level of CNT3 in the healthy donors as a reference, the
median expression values were 1.89 (range, 0.36–7.67) for
t(8;21)-positive patients and 0.63 (range, 0.05–2.16) for
t(8;21)-negative patients. For the study of correlation between
expression patterns of the candidate genes and cytogenetic
characteristics of the patients, we conducted Pearson’s correlation
analysis. This analysis revealed that only the CNT3 level
had a definite correlation with the presence of cytogenetic
abnormalities (r=0.520; P<0.001) (at bottom in Fig. 2A).
To further clarify the distinctive expression
patterns of CNT3 in patients, we divided the patients into 3
groups: t(8;21) (n=25), normal cytogenetics (n=16), and other
cytogenetic abnormalities (n=13). The patients carrying t(8;21)
still had higher CNT3 expression compared to either of the
other patient groups, even though the t(8;21)-negative population
was divided into 2 subpopulations (Fig.
2B).
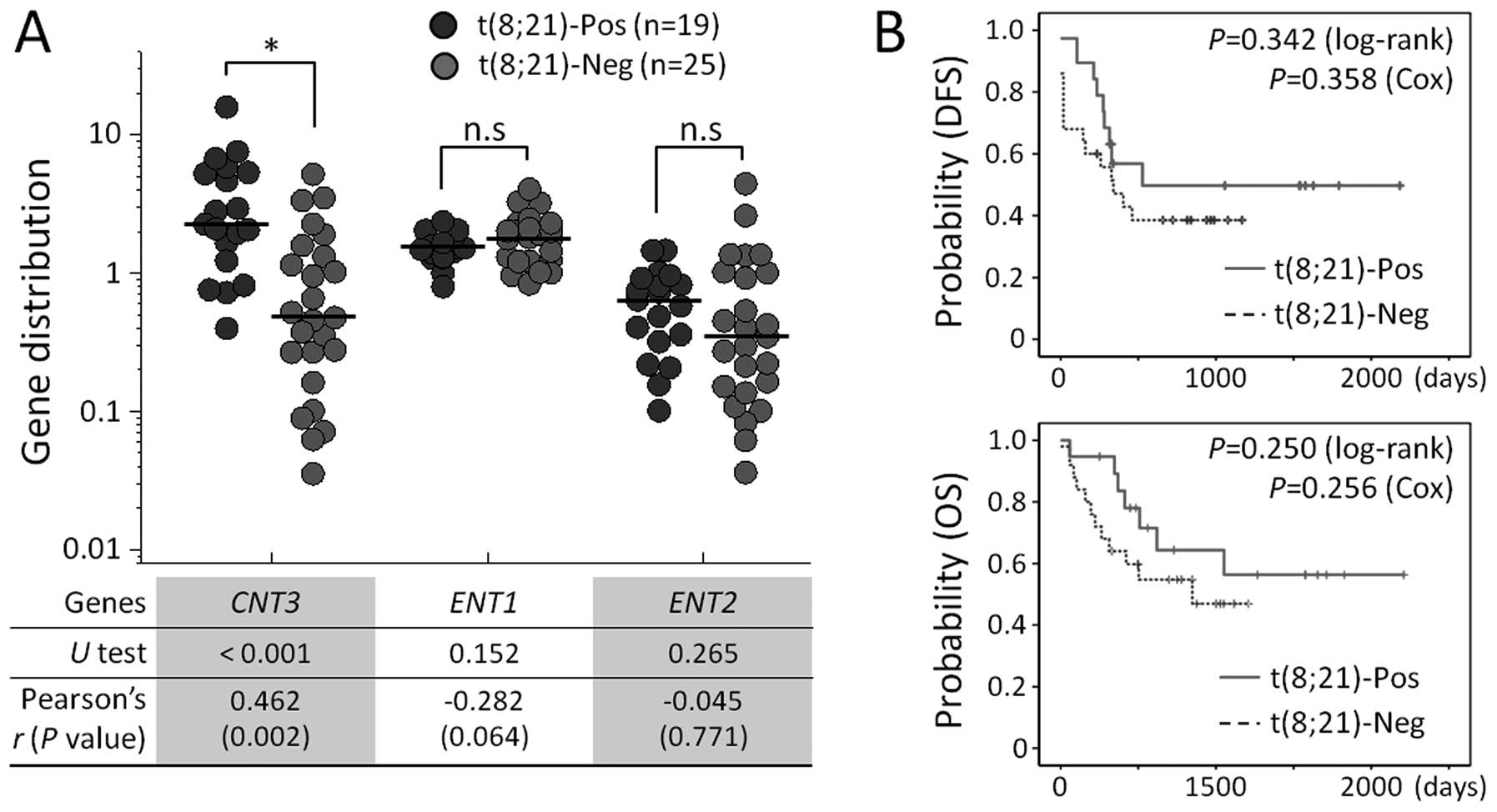
The t(8;21) abnormality-specific increase in
CNT3 expression was confirmed in a validation cohort of 44
patients diagnosed with M2 FAB subtype AML. To further examine the
association between nucleoside transporter genes and cytogenetic
abnormalities, we analyzed expression levels of solute carrier
(SLC) family genes, including SLC28 (CNT1, CNT2 and
CNT3) and SLC29 (ENT1 and ENT2). The levels of
CNT1 and CNT2 mRNA were low or undetectable in most
of the validation cohort and, therefore, results for these genes
were excluded from the following analysis. As shown in Fig. 3A, we observed that CNT3
expression was significantly elevated in patient samples that were
t(8;21)-positive, compared to those that were t(8;21)-negative
(P<0.001 in U test and P=0.002 in Pearson’s correlation
statistics). The median expression values were 2.23 (range,
0.39–15.76) for t(8;21)-positive patients and 0.47 (0.04–5.12) for
t(8;21)-negative patients. Levels of ENT1 and ENT2
were not statistically different between the two groups. We also
compared DFS and OS between t(8;21)-positive and -negative patient
groups. Prolonged survival was observed in patients harboring
t(8;21), although the results did not reach statistical
significance for survival indices (P=0.342 for DFS and P=0.250 for
OS in log-rank tests; Fig. 3B). The
t(8;21)-specific higher expression of CNT3 was consistent
with the observation in Fig. 2 and
implies a particular contribution of CNT3 to favorable
outcomes of these patients.
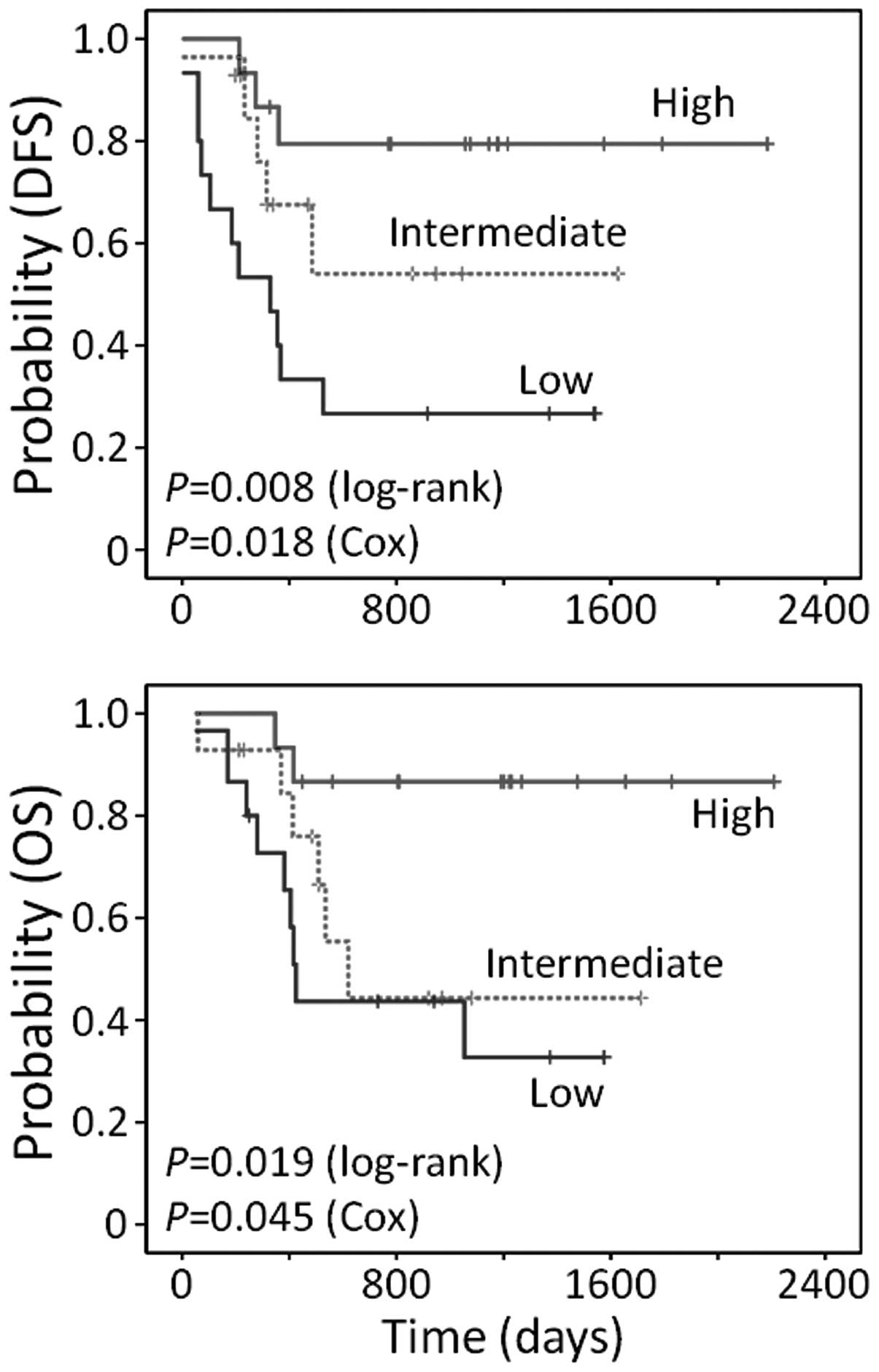
Prognostic impact of CNT3 expression on
treatment outcomes
We hypothesized that expression of CNT3 in
patients with t(8;21) could enable more precise prediction of
treatment outcomes. To examine the potential role of CNT3 in
predicting outcomes, 45 t(8;21)-positive patients from the test and
validation cohorts were clustered into 3 groups (tertiles) based on
their levels of CNT3 expression (high, intermediate or low).
First, the prognostic impact of CNT3 was evaluated by
univariate analysis. As shown in Fig.
4, patients with high CNT3 expression had significantly
longer survival compared to the other two patient groups (DFS,
P=0.008; OS, P=0.019 in log-rank tests). In a Cox proportional
hazards univariate model, HRs for the patients in the high
CNT3 tertile compared to the patients in the low tertile
were 0.18 (95% CI, 0.05–0.63; P=0.008) for DFS and 0.14 (95% CI,
0.03–0.66; P=0.013) for OS. However, when the prognostic value of
CNT3 was investigated in a separate, restricted group of
patients lacking the t(8;21), there were no significant differences
in survival among the subpopulations with different CNT3
expression levels (data not shown).
We conducted a multivariate analysis using the Cox
proportional hazards model, which adjusted for the influence of the
remission induction (RI) regimen, hematopoietic stem cell
transplantation (HSCT), blast percentage, age, gender and
CNT3 mRNA; this model confirmed that high CNT3
expression was an independent risk factor associated with DFS (HR,
0.12; P=0.007) and OS (HR, 0.06; P=0.010) (Table II). Alternatively, clinical
outcomes of patients with t(8;21)-negative AML were affected by the
RI regimen and HSCT, but not by the CNT3 expression status
(data not shown). These findings suggest that CNT3
expression levels can be used to stratify overall clinical outcomes
after cytarabine-based standard chemotherapy in AML patients with
t(8;21).
 | Table IIMultivariate analysis of clinical
outcomes in the t(8;21)-positive patients. |
Table II
Multivariate analysis of clinical
outcomes in the t(8;21)-positive patients.
| Variable | Disease-free
survival
| Overall survival
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| CNT3
expression |
| CNT3
<1.34 (n=15) | 1 (reference) | | 1 (reference) | |
| CNT3 ≥1.34
and <3.37 (n=14) | 0.44
(0.14–1.38) | 0.158 | 0.80
(0.24–2.64) | 0.717 |
| CNT3 ≥3.37
(n=15) | 0.12
(0.03–0.56) | 0.007 | 0.06
(0.01–0.52) | 0.010 |
| RI regimen |
| Ida/AraC
(n=24) | 1 (reference) | | 1 (reference) | |
| Ida/BHAC
(n=20) | 1.11
(0.36–3.45) | 0.860 | 2.20
(0.67–7.23) | 0.196 |
| HSCT |
| Yes (n=24) | 1 (reference) | | 1 (reference) | |
| No (n=20) | 1.27
(0.45–3.59) | 0.658 | 1.66
(0.51–5.39) | 0.401 |
| Blast (%) |
| <50 (n=16) | 1 (reference) | | 1 (reference) | |
| ≥50 (n=27) | 0.47
(0.14–1.56) | 0.214 | 0.46
(0.12–1.72) | 0.251 |
| Age (years) |
| ≤60 (n=39) | 1 (reference) | | 1 (reference) | |
| >60 (n=5) | 0.54
(0.11–2.53) | 0.431 | 0.69
(0.14–3.51) | 0.654 |
| Gender |
| Male (n=26) | 1 (reference) | | 1 (reference) | |
| Female (n=18) | 1.37
(0.46–4.14) | 0.573 | 1.80
(0.52–6.27) | 0.355 |
Discussion
We showed that cytogenetic abnormality-specific
expression of CNT3 in leukemic blasts from AML patients
correlated with favorable responses to cytarabine-based
chemotherapy and prolonged survival of patients with t(8;21). We
also found that CNT3 expression level was an independent
predictor of the response to cytarabine-based chemotherapy in the
t(8;21)-positive AML patients.
The t(8;21) translocation is one of the most
frequent cytogenetic abnormalities, occurring in a large proportion
(up to 15%) of AML patients. This type of AML has a high remission
rate to chemotherapy, particularly when high-dose cytarabine is
administered (2,9,26).
Nevertheless, some patients with t(8;21) experience relapse and
fail to maintain long-term survival (3–5). For
this reason, most studies concerning the treatment response in AML
with t(8;21) have been devoted to identification of useful markers
that can be measured at diagnosis to predict poor outcomes.
Representative clinically significant examples include the
association of natural killer cell antigen CD56 and P-glycoprotein
with inferior outcomes in t(8;21)-positive AML patients (6–8,27).
However, neither of these factors is linked with favorable outcomes
in patients carrying this aberration. Although a limited number of
patients were examined in the present study, we were able to
provide the first example of a gene related to cytarabine
metabolism that can explain the varied responses of these patients
to cytarabine therapy.
Nucleoside transporters including both influx and
efflux pumps and drug metabolism-associated enzymes have been
steadily identified as predictive markers of clinical outcomes and
as potential therapeutic targets (24,28–31).
In this study, we examined two types of specialized influx pumps of
nucleoside analogs, ENTs and CNTs, as potential candidates for
predicting patient outcomes. We chose these transporters because of
previous reports indicating that they have a broad spectrum of
substrate flux and are predominantly expressed in BM cells
(32–34). CNT3 expression significantly
correlated with the presence of the t(8;21) aberration in both the
test and validation data sets. Previous reports indicated that
ENT1 was clinically significant for cytarabine-mediated
therapy; however, the present study found a slight but
insignificant correlation between ENT1 and the t(8;21)
translocation in correlation analysis and survival analysis in AML
patients with t(8;21) (33,35,36).
These results indicate that CNT3, but not ENT1 or
other functional genes, is a specific marker of cytogenetics in AML
and is also a critical factor in the responsiveness of
t(8;21)-positive patients to cytarabine.
Nucleoside transporters are required not only for
nucleotide synthesis, but also for import of a variety of
nucleoside-derived anticancer drugs. Specifically, CNT3 plays a
role in the uptake of a broad range of nucleosides and their
analogs, while CNT1 and CNT2 are responsible for specialized
substrates (pyrimidine and purine nucleosides for CNT1 and CNT2,
respectively) (34). Most studies
that have only analyzed CNT3 have used in vitro assays to
identify a critical role of CNT3 in the antitumor effect of
nucleoside drugs and the resistance to these drugs (34,37,38).
Alternatively, a study by Mackey et al (30) showed a negative correlation between
CNT3 and clinical outcomes after fludarabine therapy in patients
with chronic lymphocytic leukemia. Furthermore, distinct expression
of CNT3 has not been reported in AML with specific
cytogenetics, even though there were several genome-wide approaches
to dissect gene profiles in large numbers of patients (39–41).
The present study is the first to demonstrate that CNT3
levels can be used to stratify patients based on survival outcomes
in, at least in part, t(8;21)-positive but not t(8;21)-negative AML
patients. We questioned why the prognostic potential of CNT3
preferentially applied to t(8;21)-positive AML patients. For the
survival analysis, we divided t(8;21)-positive or -negative
patients into subpopulations based on their CNT3 expression
level. The median values for CNT3 expression were 2.07
(range, 0.36–15.76) for the t(8;21)-positive cohort and 0.56
(range, 0.04–5.12) for the t(8;21)-negative cohort. One potential
explanation for this observation is that, at least in the
t(8;21)-negative population, the small range of CNT3
expression might be insufficient to allow stratification for
outcome prediction. Although we did not determine whether
CNT3 transcript levels were consistent with protein
expression levels in individual patients, and we did not ensure
that the protein encoded by the CNT3 gene was functional, it
is believed that low mRNA levels are indicative of low CNT3 protein
levels, which are insufficient for CNT3 to carry out its novel
function.
In the CNT nucleoside transporter family, expression
levels of CNT1 and CNT2 are known to be upregulated
by extracellular stimuli including cell proliferation and
activation (42–44). However, the mechanism of
transcriptional regulation of CNT3 remains largely unknown.
Based on our novel finding that CNT3 was upregulated in AML
patients with t(8;21) but not in patients with other cytogenetic
changes, we hypothesize that there may be a physiological
interaction between the AML1 or AML1-ETO chimera proteins, which
are derived from the t(8;21) translocation, and the promoter region
of CNT3. Indeed, a virtual analysis for promoter prediction
performed using transcription factor binding site search software
identified 3 putative binding sites for AML1 at −3438 to −3433,
−888 to −883, and +526 to +531 relative to the transcription start
site of CNT3. Therefore, additional study is necessary to
identify a role of these putative binding sites.
In summary, our results imply that higher
CNT3 expression in AML patients with t(8;21) contributes to
the prolonged survival observed in this population. Our results
also suggest that CNT3 can be used to predict clinical responses to
cytarabine-based therapies in AML patients with t(8;21).
Acknowledgments
The present study was supported by grant
A111218-GM06 of the National Project for Personalized Genomic
Medicine, Ministry for Health and Welfare, the National Research
Foundation of Korea (NRF) grant 2011-0001388 funded by the Korean
government and a Korea University grant.
References
|
1
|
Grimwade D, Walker H, Oliver F, Wheatley
K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, et
al The Medical Research Council Adult and Children’s Leukaemia
Working Parties: The importance of diagnostic cytogenetics on
outcome in AML: Analysis of 1,612 patients entered into the MRC AML
10 trial. Blood. 92:2322–2333. 1998.PubMed/NCBI
|
|
2
|
Estey EH: Acute myeloid leukemia: 2012
update on diagnosis, risk stratification, and management. Am J
Hematol. 87:89–99. 2012. View Article : Google Scholar
|
|
3
|
Bloomfield CD, Herzig GP, Peterson BA and
Wolff SN: Long-term survival of patients with acute myeloid
leukemia: Updated results from two trials evaluating postinduction
chemotherapy. Cancer. 80(Suppl): 2186–2190. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O’Brien S, Kantarjian HM, Keating M,
Gagnon G, Cork A, Trujillo J and McCredie KB: Association of
granulocytosis with poor prognosis in patients with acute
myelogenous leukemia and translocation of chromosomes 8 and 21. J
Clin Oncol. 7:1081–1086. 1989.
|
|
5
|
Prébet T, Boissel N, Reutenauer S, Thomas
X, Delaunay J, Cahn JY, Pigneux A, Quesnel B, Witz F, Thépot S, et
al Acute Leukemia French Association; Groupe Ouest-Est des
leucémies et autres maladies du sang (GOELAMS); Core Binding Factor
Acute Myeloid Leukemia (CBF AML) intergroup: Acute myeloid leukemia
with translocation (8;21) or inversion (16) in elderly patients
treated with conventional chemotherapy: A collaborative study of
the French CBF-AML intergroup. J Clin Oncol. 27:4747–4753. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baer MR, Stewart CC, Lawrence D, Arthur
DC, Byrd JC, Davey FR, Schiffer CA and Bloomfield CD: Expression of
the neural cell adhesion molecule CD56 is associated with short
remission duration and survival in acute myeloid leukemia with
t(8;21)(q22;q22). Blood. 90:1643–1648. 1997.PubMed/NCBI
|
|
7
|
Schaich M, Koch R, Soucek S, Repp R,
Ehninger G and Illmer T: A sensitive model for prediction of
relapse in adult acute myeloid leukaemia with t(8;21) using white
blood cell count, CD56 and MDR1 gene expression at diagnosis. Br J
Haematol. 125:477–479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang DH, Lee JJ, Mun YC, Shin HJ, Kim YK,
Cho SH, Chung IJ, Seong CM and Kim HJ: Predictable prognostic
factor of CD56 expression in patients with acute myeloid leukemia
with t(8:21) after high dose cytarabine or allogeneic hematopoietic
stem cell transplantation. Am J Hematol. 82:1–5. 2007. View Article : Google Scholar
|
|
9
|
Bloomfield CD, Lawrence D, Byrd JC,
Carroll A, Pettenati MJ, Tantravahi R, Patil SR, Davey FR, Berg DT,
Schiffer CA, et al: Frequency of prolonged remission duration after
high-dose cytarabine intensification in acute myeloid leukemia
varies by cytogenetic subtype. Cancer Res. 58:4173–4179.
1998.PubMed/NCBI
|
|
10
|
Weick JK, Kopecky KJ, Appelbaum FR, Head
DR, Kingsbury LL, Balcerzak SP, Bickers JN, Hynes HE, Welborn JL,
Simon SR, et al: A randomized investigation of high-dose versus
standard-dose cytosine arabinoside with daunorubicin in patients
with previously untreated acute myeloid leukemia: A Southwest
Oncology Group study. Blood. 88:2841–2851. 1996.PubMed/NCBI
|
|
11
|
Schnetzke U, Fix P, Spies-Weisshart B,
Schrenk K, Glaser A, Fricke HJ, La Rosée P, Hochhaus A and Scholl
S: Efficacy and feasibility of cyclophosphamide combined with
intermediate- dose or high-dose cytarabine for relapsed and
refractory acute myeloid leukemia (AML). J Cancer Res Clin Oncol.
140:1391–1397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Damaraju VL, Damaraju S, Young JD, Baldwin
SA, Mackey J, Sawyer MB and Cass CE: Nucleoside anticancer drugs:
The role of nucleoside transporters in resistance to cancer
chemotherapy. Oncogene. 22:7524–7536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Visser F, King KM, Baldwin SA,
Young JD and Cass CE: The role of nucleoside transporters in cancer
chemotherapy with nucleoside drugs. Cancer Metastasis Rev.
26:85–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Momparler RL and Fischer GA: Mammalian
deoxynucleoside kinase. I. Deoxycytidine kinase: Purification,
properties, and kinetic studies with cytosine arabinoside. J Biol
Chem. 243:4298–4304. 1968.PubMed/NCBI
|
|
15
|
Galmarini CM, Thomas X, Graham K, El
Jafaari A, Cros E, Jordheim L, Mackey JR and Dumontet C:
Deoxycytidine kinase and cN-II nucleotidase expression in blast
cells predict survival in acute myeloid leukaemia patients treated
with cytarabine. Br J Haematol. 122:53–60. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Molina-Arcas M, Bellosillo B, Casado FJ,
Montserrat E, Gil J, Colomer D and Pastor-Anglada M: Fludarabine
uptake mechanisms in B-cell chronic lymphocytic leukemia. Blood.
101:2328–2334. 2003. View Article : Google Scholar
|
|
17
|
Song JH, Kim SH, Kweon SH, Lee TH, Kim HJ,
Kim HJ and Kim TS: Defective expression of deoxycytidine kinase in
cytarabine-resistant acute myeloid leukemia cells. Int J Oncol.
34:1165–1171. 2009.PubMed/NCBI
|
|
18
|
Takagaki K, Katsuma S, Kaminishi Y, Horio
T, Nakagawa S, Tanaka T, Ohgi T and Yano J: Gene-expression
profiling reveals down-regulation of equilibrative nucleoside
transporter 1 (ENT1) in Ara-C-resistant CCRF-CEM-derived cells. J
Biochem. 136:733–740. 2004. View Article : Google Scholar
|
|
19
|
Abraham A, Varatharajan S, Abbas S, Zhang
W, Shaji RV, Ahmed R, Abraham A, George B, Srivastava A, Chandy M,
et al: Cytidine deaminase genetic variants influence RNA expression
and cytarabine cytotoxicity in acute myeloid leukemia.
Pharmacogenomics. 13:269–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galmarini CM, Cros E, Graham K, Thomas X,
Mackey JR and Dumontet C: 5′-(3′)-nucleotidase mRNA levels in blast
cells are a prognostic factor in acute myeloid leukemia patients
treated with cytarabine. Haematologica. 89:617–619. 2004.PubMed/NCBI
|
|
21
|
Momparler RL, Eliopoulos N, Bovenzi V,
Létourneau S, Greenbaum M and Cournoyer D: Resistance to cytosine
arabi-noside by retrovirally mediated gene transfer of human
cytidine deaminase into murine fibroblast and hematopoietic cells.
Cancer Gene Ther. 3:331–338. 1996.PubMed/NCBI
|
|
22
|
Ujházy P, Berleth ES, Pietkiewicz JM,
Kitano H, Skaar JR, Ehrke MJ and Mihich E: Evidence for the
involvement of ecto-5′-nucleotidase (CD73) in drug resistance. Int
J Cancer. 68:493–500. 1996. View Article : Google Scholar
|
|
23
|
Smid K, Bergman AM, Eijk PP, Veerman G,
van Haperen VW, van den Ijssel P, Ylstra B and Peters GJ:
Micro-array analysis of resistance for gemcitabine results in
increased expression of ribonucleotide reductase subunits.
Nucleosides Nucleotides Nucleic Acids. 25:1001–1007. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song JH, Kweon SH, Kim HJ, Lee TH, Min WS,
Kim HJ, Kim YK, Hwang SY and Kim TS: High TOP2B/TOP2A expression
ratio at diagnosis correlates with favourable outcome for standard
chemotherapy in acute myeloid leukaemia. Br J Cancer. 107:108–115.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho EK, Bang SM, Ahn JY, Yoo SM, Park PW,
Seo YH, Shin DB and Lee JH: Prognostic value of AML 1/ETO fusion
transcripts in patients with acute myelogenous leukemia. Korean J
Intern Med. 18:13–20. 2003.PubMed/NCBI
|
|
26
|
Downing JR: The AML1-ETO chimaeric
transcription factor in acute myeloid leukaemia: Biology and
clinical significance. Br J Haematol. 106:296–308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schaich M, Harbich-Brutscher E, Pascheberg
U, Mohr B, Soucek S, Ehninger G and Illmer T: Association of
specific cytogenetic aberrations with mdr1 gene expression in adult
myeloid leukemia and its implication in treatment outcome.
Haematologica. 87:455–464. 2002.PubMed/NCBI
|
|
28
|
Jin G, Matsushita H, Asai S, Tsukamoto H,
Ono R, Nosaka T, Yahata T, Takahashi S and Miyachi H: FLT3-ITD
induces ara-C resistance in myeloid leukemic cells through the
repression of the ENT1 expression. Biochem Biophys Res Commun.
390:1001–1006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jordheim LP and Dumontet C: Review of
recent studies on resistance to cytotoxic deoxynucleoside
analogues. Biochim Biophys Acta. 1776:138–159. 2007.PubMed/NCBI
|
|
30
|
Mackey JR, Galmarini CM, Graham KA, Joy
AA, Delmer A, Dabbagh L, Glubrecht D, Jewell LD, Lai R, Lang T, et
al: Quantitative analysis of nucleoside transporter and metabolism
gene expression in chronic lymphocytic leukemia (CLL):
Identification of fludarabine-sensitive and -insensitive
populations. Blood. 105:767–774. 2005. View Article : Google Scholar
|
|
31
|
Guo Y, Köck K, Ritter CA, Chen ZS, Grube
M, Jedlitschky G, Illmer T, Ayres M, Beck JF, Siegmund W, et al:
Expression of ABCC-type nucleotide exporters in blasts of adult
acute myeloid leukemia: Relation to long-term survival. Clin Cancer
Res. 15:1762–1769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Griffiths M, Beaumont N, Yao SY, Sundaram
M, Boumah CE, Davies A, Kwong FY, Coe I, Cass CE, Young JD, et al:
Cloning of a human nucleoside transporter implicated in the
cellular uptake of adenosine and chemotherapeutic drugs. Nat Med.
3:89–93. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hubeek I, Stam RW, Peters GJ, Broekhuizen
R, Meijerink JP, van Wering ER, Gibson BE, Creutzig U, Zwaan CM,
Cloos J, et al: The human equilibrative nucleoside transporter 1
mediates in vitro cytarabine sensitivity in childhood acute myeloid
leukaemia. Br J Cancer. 93:1388–1394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ritzel MW, Ng AM, Yao SY, Graham K, Loewen
SK, Smith KM, Ritzel RG, Mowles DA, Carpenter P, Chen XZ, et al:
Molecular identification and characterization of novel human and
mouse concentrative Na+-nucleoside cotransporter
proteins (hCNT3 and mCNT3) broadly selective for purine and
pyrimidine nucleosides (system cib). J Biol Chem. 276:2914–2927.
2001. View Article : Google Scholar
|
|
35
|
Galmarini CM, Thomas X, Calvo F, Rousselot
P, El Jafaari A, Cros E and Dumontet C: Potential mechanisms of
resistance to cytarabine in AML patients. Leuk Res. 26:621–629.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stam RW, den Boer ML, Meijerink JP, Ebus
ME, Peters GJ, Noordhuis P, Janka-Schaub GE, Armstrong SA,
Korsmeyer SJ and Pieters R: Differential mRNA expression of
Ara-C-metabolizing enzymes explains Ara-C sensitivity in MLL
gene-rearranged infant acute lymphoblastic leukemia. Blood.
101:1270–1276. 2003. View Article : Google Scholar
|
|
37
|
Fotoohi AK, Lindqvist M, Peterson C and
Albertioni F: Involvement of the concentrative nucleoside
transporter 3 and equilibrative nucleoside transporter 2 in the
resistance of T-lymphoblastic cell lines to thiopurines. Biochem
Biophys Res Commun. 343:208–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karim H, Hashemi J, Larsson C, Moshfegh A,
Fotoohi AK and Albertioni F: The pattern of gene expression and
gene dose profiles of 6-Mercaptopurine- and 6-Thioguanine-resistant
human leukemia cells. Biochem Biophys Res Commun. 411:156–161.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Verhaak RG, Wouters BJ, Erpelinck CA,
Abbas S, Beverloo HB, Lugthart S, Löwenberg B, Delwel R and Valk
PJ: Prediction of molecular subtypes in acute myeloid leukemia
based on gene expression profiling. Haematologica. 94:131–134.
2009. View Article : Google Scholar :
|
|
40
|
Balgobind BV, Van den Heuvel-Eibrink MM,
De Menezes RX, Reinhardt D, Hollink IH, Arentsen-Peters ST, van
Wering ER, Kaspers GJ, Cloos J, de Bont ES, et al: Evaluation of
gene expression signatures predictive of cytogenetic and molecular
subtypes of pediatric acute myeloid leukemia. Haematologica.
96:221–230. 2011. View Article : Google Scholar :
|
|
41
|
Ichikawa H, Tanabe K, Mizushima H, Hayashi
Y, Mizutani S, Ishii E, Hongo T, Kikuchi A and Satake M: Common
gene expression signatures in t(8;21)- and inv(16)-acute myeloid
leukaemia. Br J Haematol. 135:336–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Duflot S, Riera B, Fernández-Veledo S,
Casadó V, Norman RI, Casado FJ, Lluís C, Franco R and
Pastor-Anglada M: ATP-sensitive K+ channels regulate the
concentrative adenosine transporter CNT2 following activation by
A1 adenosine receptors. Mol Cell Biol. 24:2710–2719.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fernández-Veledo S, Valdés R, Wallenius V,
Casado FJ and Pastor-Anglada M: Up-regulation of the high-affinity
pyrimidine-preferring nucleoside transporter concentrative
nucleoside transporter 1 by tumor necrosis factor-alpha and
interleukin-6 in liver parenchymal cells. J Hepatol. 41:538–544.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Soler C, Felipe A, García-Manteiga J,
Serra M, Guillén-Gómez E, Casado FJ, MacLeod C, Modolell M,
Pastor-Anglada M and Celada A: Interferon-gamma regulates
nucleoside transport systems in macrophages through signal
transduction and activator of transduction factor 1
(STAT1)-dependent and -independent signalling pathways. Biochem J.
375:777–783. 2003. View Article : Google Scholar : PubMed/NCBI
|