Introduction
A major reason for mortality from solid tumors,
including non-small cell lung cancer (NSCLC), is cancer metastasis
(1). Epithelial-mesenchymal
transition (EMT) is a crucial early event during metastasis. In the
process of EMT, cells dissolve cadherins and tight junctions, lose
cell polarity and exhibit multiple mesenchymal cell properties such
as becoming more motile and invasive (2).
Transforming growth factor-β (TGF-β) plays various
roles in the process of malignant progression (3), and was initially identified as an
important inducer of EMT (4). In
the canonical SMAD-dependent signaling, TGF-β binds to the cell
membrane surface type II TGF-β receptor (TGFβR2) which then
recruits the type I TGF-β receptor (TGFβR1) and activates its
serine/threonine protein kinase. Thus, activated TGFβR1 leads to
phosphorylation of SMAD2 and SMAD3. Activated SMAD2 and SMAD3
interact with SMAD4 to form a transcriptional complex that
translocates into the nucleus to regulate the expression of
specific genes (5). As a hallmark
of TGF-β-induced EMT, an increased expression of E-cadherin and a
decreased expression of N-cadherin are closely associated with
TGF-β-induced phosphorylated SMAD3 (6,7). In
addition, TGF-β activated MAP kinase (8), Rho-like GTPase (9) and phosphatidylinositol-3-kinase
(PI3K)/AKT non-SMAD pathways during TGF-β-induced EMT (10).
As a member of the Ski family, the c-Ski protein was
encoded by the SKI gene in humans and first termed by
investigators from Sloan-Kettering Institute (11). Notably, Ski directly interacts with
the SMAD complex to prevent the phosphorylation of SMAD2/3 and
block the TGF-β signaling pathway (12–14).
In addition to interacting with SMAD proteins, Ski functions as a
direct antagonist of TGFβR1 (15).
Ski was also identified to interact with non-SMAD signaling,
including Akt (16) and p38
signaling (17) pathways. The
abovementioned findings suggested that Ski is involved in
SMAD-dependent and -independent TGF-β signaling pathways.
Given that TGF-β signaling mediates cytostasis
(3) and Ski is a key negative
regulator of TGF-β signaling (18),
Ski expression was found to be elevated in several types of cancer,
including melanoma, esophageal, colorectal and pancreatic cancer,
and leukemia, suggesting that Ski plays an oncogenic role in cancer
(19–23). By contrast, Shinagawa et al
reported that Ski+/− mice increased the
susceptibility of chemical-induced tumorigenesis, suggesting that
Ski is a tumor-suppressor (24).
Wang et al found that Ski suppressed tumor metastasis in
pancreatic cancer (25). Although
Ski has particular roles in the tumor development and progression,
to the best of our knowledge, few studies have focused on the
functions of Ski in lung cancer. In view of the role of TGF-β
signaling in cell EMT or invasion, Ski may act as a tumor
metastasis suppressor by inhibiting the TGF-β signaling pathway and
have a close relationship with the metastasis and recurrence of
human types of cancer, including NSCLC. However, the effect of Ski
on TGF-β-induced EMT and cell invasion in NSCLC remains to be
determiend.
In the present study, we found that the expression
of Ski was significantly lower in metastatic NSCLC than
non-metastatic cells. Moreover, Ski mRNA expression was
downregulated in NSCLC tissues from patients with lymph-node or
distance metastasis. Mechanistically, Ski inhibited TGF-β-induced
EMT and cell invasion by repressing SMAD-dependent signaling in
NSCLC.
Materials and methods
Cell culture
Human A549, LTEP-α-2, 95C and 95D NSCLC cell lines
were purchased from the Cell Bank of the Chinese Academy of
Science. The cells were cultured in Roswell Park Memorial Institute
(RPMI)-1640 medium (HyClone, Logan, UT, USA) with 50 U/ml each of
penicillin and streptomycin, and 10% heat-inactivated fetal bovine
serum (FBS) in humidified incubators at 37°C with 5%
CO2.
NSCLC tissue samples
Forty-six paired NSCLC tissues and adjacent
non-cancerous lung tissues were obtained after informed consent
from patients in the First Affiliated Hospital of Soochow
University. NSCLC patients had not received chemotherapy or
radiotherapy prior to tissue sampling. According to whether NSCLC
patients had local lymph-node or distance metastasis, NSCLC tissue
samples were classified into the non-metastatic (N0M0) and
metastatic (N1-2 and/or M1) groups. The tissues were snap-frozen
and stored in an ultra-deep freezer at −80°C. The present study was
approved by the Academic Advisory Board of Soochow University.
Reagents and antibodies
Human recombinant TGF-β1 was purchased from R&D
Systems Inc. (Minneapolis, MN, USA). TGF-β1 was diluted in sterile
4 mM HCl containing 1 mg/ml bovine serum albumin (BSA). Antibodies
used for western blot analysis were as follows: mouse
anti-E-cadherin and anti-N-cadherin (BD Biosciences, Franklin
Lakes, NJ, USA), rabbit anti-SMAD3, anti-phospho-SMAD3 (Cell
Signaling Technology, Inc., Danvers, MA, USA), rabbit anti-Ski and
mouse anti-β-actin (Abcam, San Francisco, CA, USA), and
anti-mouse/rabbit secondary antibodies (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). SIS3 (a special inhibitor of SMAD3
phosphorylation) was purchased from Santa Cruz Biotechnology.
Lentivirus production, transduction and
generation of stable cell lines
The full-length coding sequence region of human
SKI gene was amplified by PCR using the primers: forward,
5′-CCGCTCGAGGAGCCGGAGCGCACCATGGAGG-3′
and reverse, 5′-CGCGGATCCAAACACTCGCTTGGTAATAGGCAC-3′).
It was then cloned into a lentivirus expression vector
pLVX-IRES-Neo with restriction endonucleases XhoI and
BamH1 (underscored) to generate a pLVX-IRES-Neo-Ski plasmid,
which was in turn co-transfected with three packaging plasmids
(Lenti-THT packaging mix) (both from Clontech Laboratories, Inc.,
San Francisco, CA, USA) into 293T cells using Lipofectamine 2000
(Life Technologies, Carlsbad, CA, USA). The empty pLVX-IRES-Neo
vector was used as a negative control (Mock). After 48-h transient
transfection, the lentivirus-containing supernatant was collected
and applied to infect A549 cells. After 48 h, the stable cells were
selected with 400 µg/ml of G418. To obtain a stable Ski
knockdown A549 cell line, we synthesized a small hairpin RNA
(shRNA) containing a 19-bp interfering sequence (25) against SKI transcript:
5′-TGATGAAAGAGGCCAACGAGTTCAAGAGACTCGTTGGCCTCTTTCATCTTTTTTC-3′, and
cloned it into a lentiviral vector pLentiLox3.7 (pLL3.7) with
restriction endonucleases HpaI and XhoI to generate a
pLL3.7-sh-Ski vector. A scrambled sequence,
5′-TGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAACATTTTTTC-3′,
was designed as a negative control shRNA. Using Lipofectamine 2000,
the pLL3.7-sh-Ski vector or negative control vector was in turn
co-transfected with two lentiviral package plasmids (pVSVG and
pΔ8.9) into 293T cells and the packaged lentiviruses were harvested
48 h later for subsequent A549 infection. The infected A549 cells
were cultured and the monoclonal stable cells were selected by EGFP
and verified by western blotting.
Reporter gene construct and luciferase
assays
The TGF-β1-inducible luciferase reporter plasmid
(PAI-1-pGL3-Luc) containing the SMAD-binding elements of the
plasminogen activator inhibitor-1 (PAI-1) gene promoter region were
generated as previously described (7). This plasmid was transfected into cells
using Lipofectamine 2000. After 6-h transfection, TGF-β1 (5 ng/ml)
was added for 24 h, and the luciferase activity was determined by
the Dual-Luciferase Reporter Assay kit (Promega Corporation,
Madison, WI, USA).
Western blot analysis
Cells were lysed using a RIPA lysis buffer (Cell
Signaling Technology, Inc.) with protease inhibitor and phosphatase
inhibitor cocktails (Sigma, St. Louis, MO, USA). Following
centrifugation at 12,000 rpm for 15 min, the total protein products
in the collected supernatant were separated by SDS-PAGE under
reducing condition and transferred to a nitrocellulose membrane
(Millipore, Bedford, MA, USA). The membrane was blocked with 1%
BSA/TBST buffer for 1 h at room temperature and incubated with
primary antibodies overnight at 4°C. The membranes were washed
three times in TBST buffer and incubated with HRP-conjugated
secondary antibodies for 2 h at room temperature. Protein detection
was performed using the enhanced chemiluminescence system (ECL;
Pierce, Rockford, IL, USA). Experiments were performed in
triplicate and normalized by the expression of β-actin.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
When cells were grown to 80% confluence on 6-well
plates, total RNA was isolated using RNAiso Plus kit (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer’s instructions.
Synthesis of cDNA with reverse transcriptase was conducted using an
M-MLV First Strand kit (Invitrogen, Carlsbad, CA, USA). The
concentration of total RNA and cDNA were measured on a NanoDrop
2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA,
USA). RT-qPCR was performed using Platinum SYBR-Green qPCR
SuperMix-UDG kit (Invitrogen) according to the manufacturer’s
instructions on ABI Prism 7500 Sequence Detection System (Applied
Biosystems, Foster City, CA, USA). β-actin was used as an internal
control for mRNA qualification and the 2−ΔΔCt method was
applied in the analysis of the quantitative data. The primers used
for RT-qPCR were: Ski, 5′-CGACGTGAAGGAGAAATTCG-3′ (forward), and
5′-GGACTGGGAAGAGGTGTCAT-3′ (reverse) (25); MMP-2, 5′-TGATCTTGACCAGAATACCATCGA-3′
(forward), and 5′-GGCTTGCGAGGGAAGAAGTT-3′ (reverse).
Cell invasion assay
Cell invasion assay was performed using Transwell
plates (BD Biosciences) with polycarbonate filters of 8-µm
pore size. Matrigel (Discovery Labware, Bedford, MA, USA) was used
to coat the upper surface of the filter in each of the upper
chamber. Cells (5×104) with serum-free RPMI-1640 were
seeded in the upper chambers. In each lower chamber, 20% FBS medium
was placed as a chemoattractant. After 6 h, the cells were
stimulated with 5 ng/ml TGF-β1. After 24 h incubation, the cells on
the upper surface of the filter membrane were wiped and the invaded
cells on the lower surface were then fixed in 100% methanol and
stained with 1% crystal violet. Three microscopic fields
(magnification, ×100) were photographed and counted/chamber, and
results presented as the mean ± SD of results from three replicate
experiments.
Statistical analysis
For the cell lines, data were analyzed using an
unpaired t-test (two-tailed) to determine statistically significant
differences between two groups. Comparisons between
clinicopathological characteristics and expression ratios (T/N) of
Ski mRNA in tissue samples were performed with non-parametric tests
(Mann-Whitney U test for two groups, Kruskall-Wallis test for three
or more groups). Data were presented as mean ± standard deviation
(SD). P<0.05 was considered to indicate a statistically
significant result. The statistical analysis was performed using
GraphPad Prism software version 5.0 (GraphPad Software Inc., San
Diego, CA, USA).
Results
Ski expression is reduced in metastatic
NSCLC cells and tissues
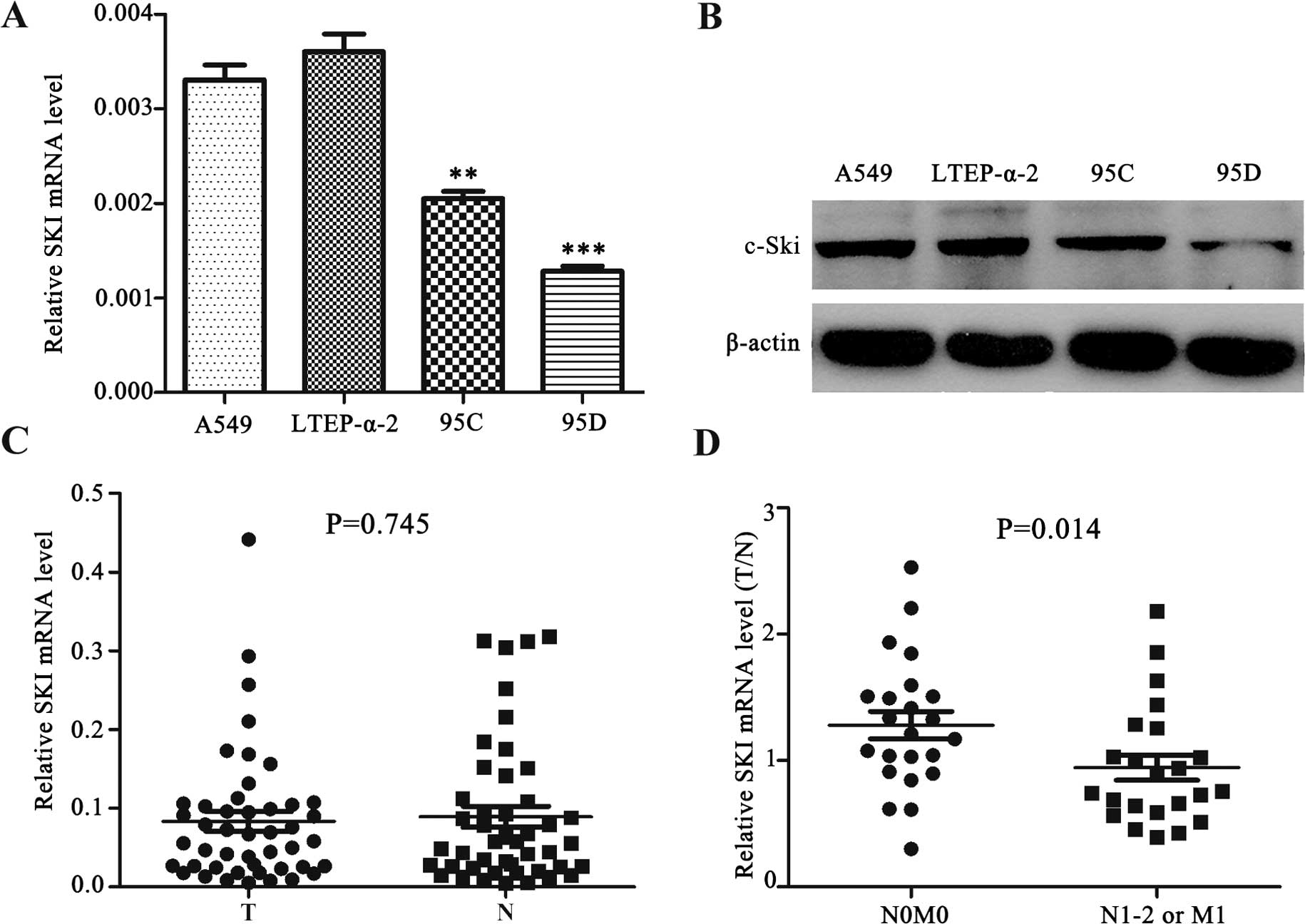
To examine whether the expression of Ski is
associated with NSCLC, we determined Ski expression in a panel of
NSCLC cell lines, including A549 and LTEP-α-2 (lung adenocarcinoma
cell lines), 95C (low metastatic giant-cell lung carcinoma cell
line) and 95D (high metastatic giant-cell lung carcinoma cell
line). We found that the mRNA and protein levels of Ski were
reduced in 95C and 95D cells, particularly in 95D cells, when
compared with A549 and LTEP-α-2 (Fig.
1A and B). We then detected Ski mRNA expression in 46 paired
NSCLC tissues (T) and adjacent cancer-free lung tissues (N). As
shown in Fig. 1C, no significant
difference in Ski mRNA level was observed between NSCLC and paired
adjacent cancer-free lung tissues (P=0.745). However, after
classifying NSCLCs by metastatic status into two groups (N0M0 vs.
N1-2 and/or M1), we found that the ratio of Ski mRNA level (T/N)
was significantly lower in the metastatic group (N1-2 and/or M1)
than the non-metastatic group (N0M0) (P=0.014; Fig. 1D). The results suggested that Ski
played a tumor metastasis-suppressing role in NSCLC.
Ski inhibits TGF-β-induced EMT and
TGF-β-mediated tran-scriptional response in NSCLC cells
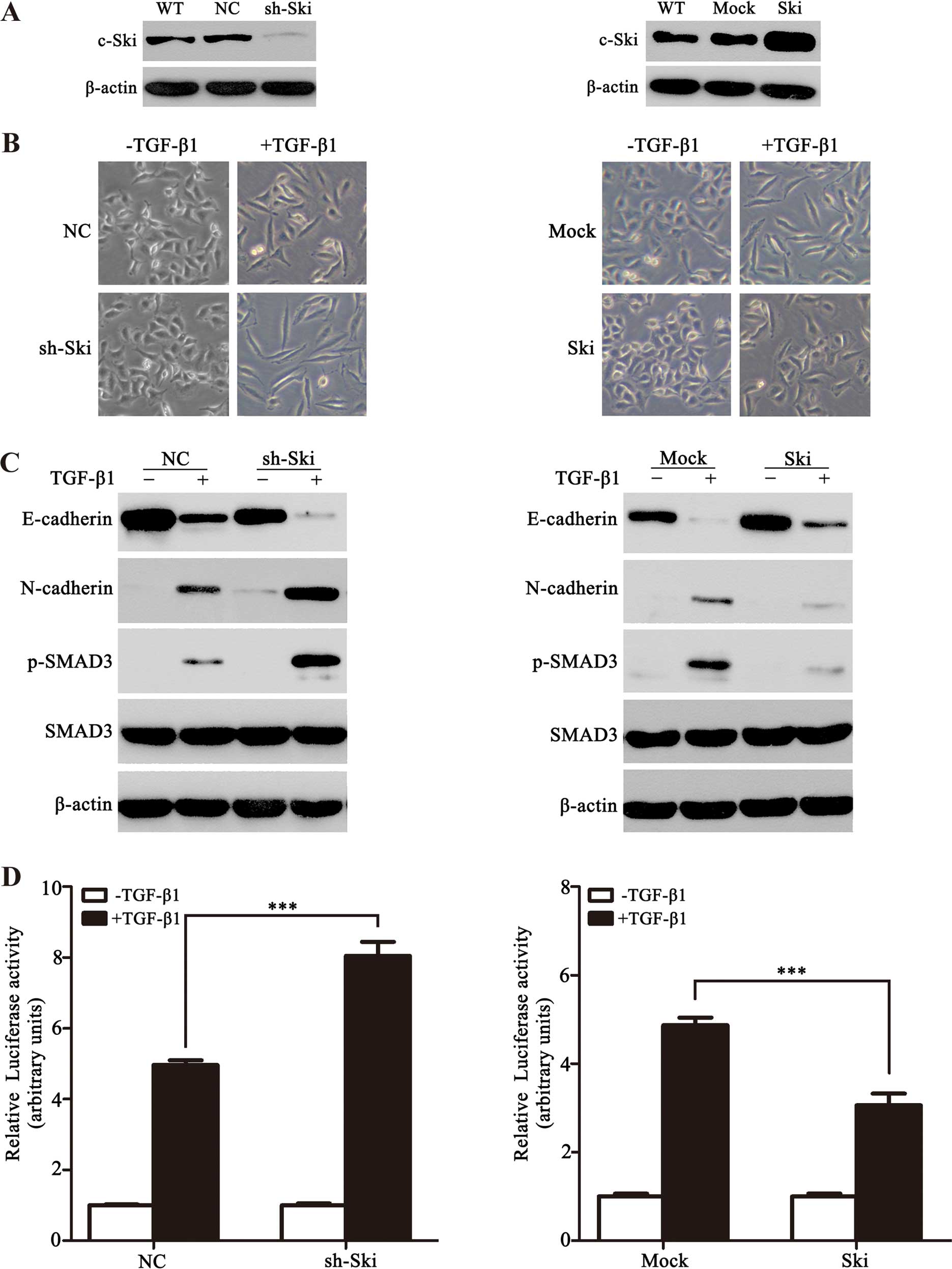
To elucidate the function of Ski involved in
TGF-β-induced EMT in vitro, we first generated A549
monoclonal cells with stable knockdown of Ski (A549-sh-Ski) and
overexpression of Ski (A549-Ski) (Fig.
2A). Following TGF-β1 stimulation, the A549 cell clones
exhibited a spindle-shaped and fibroblast-like morphology and
exhibited an EMT phenotype. Notably, Ski-silenced A549 cells had
more significant features of EMT when compared with A549 cells
overexpressing Ski (Fig. 2B).
Moreover, we examined the expression of TGF-β-induced EMT markers
(E-cadherin and N-cadherin) in the two stable clones, and found
that in the presence of TGF-β1, A549-sh-Ski cells exhibited
decreased E-cadherin and increased N-cadherin when compared with
control stable cells (Fig. 2C, left
panel), whereas an opposite result was observed in the A549-Ski
cells (Fig. 2C, right panel). To
determine whether Ski affects TGF-β-mediated activation of SMAD3 in
EMT, we examined the phosphorylated-SMAD3 (p-SMAD3) level. In
response to TGF-β1, A549-sh-Ski and A549-Ski cells showed an
increase and a reduction in the p-SMAD3 level, respectively
(Fig. 2C), indicating that Ski
represses the TGF-β-induced phosphorylation of SMAD3 in NSCLC
cells. This result was supported by PAI-1 promoter activity
obtained in A549-sh-Ski or A549-Ski cells. As shown in Fig. 2D, PAI-1 promoter activity was high
in A549-sh-Ski cells but low in A549-Ski cells. The findings
demonstrated that Ski significantly inhibited TGF-β-induced EMT and
TGF-β/SMAD-mediated transcriptional response in NSCLC cells.
Ski represses TGF-β-induced cell invasion
in NSCLC cells
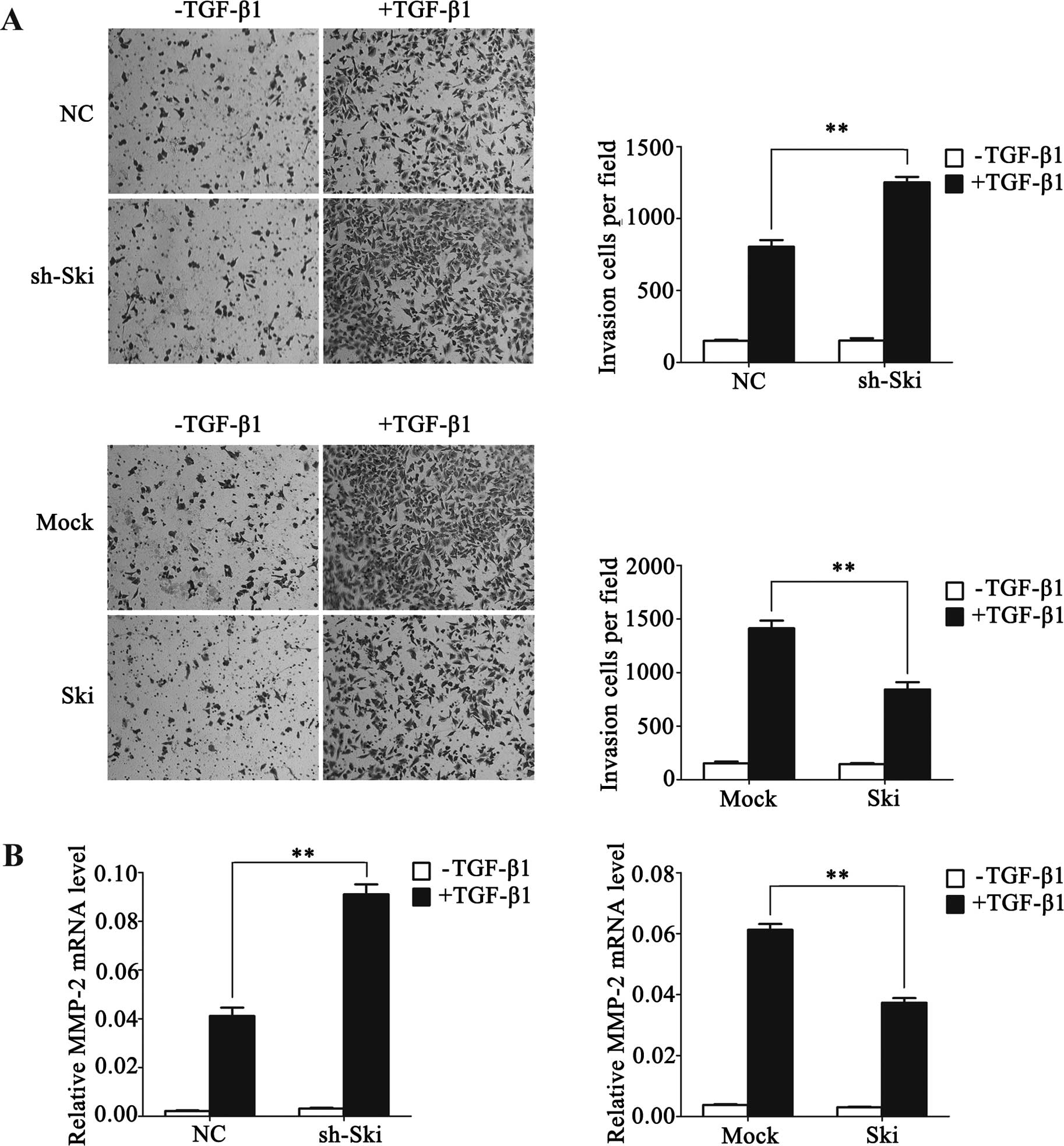
Given the fact that the acquisition of an enhanced
invasion is one of the hallmarks of TGF-β-induced EMT in
development and disease (2), and
our above findings suggested that Ski represses TGF-β-inducible
EMT, we determined whether Ski affected tumor invasiveness in A549
cells. To assess this, Matrigel-coated Transwell assays were
performed in A549-sh-Ski and A549-Ski cells in the presence or
absence of TGF-β1. As a result, following TGF-β1 stimulation,
A549-sh-Ski cells invading through Matrigel were significantly
enhanced when compared with control cells although A549-Ski cell
invasive ability was attenuated (Fig.
3A). Furthermore, we found that Ski apparently weakened the
TGF-β-induced expression of MMP-2, which was widely recognized as
an enhancer of cell invasion (Fig.
3B). Collectively, the results indicated that Ski played an
inhibitory role in TGF-β-induced cell invasion in NSCLC cells,
supporting our observation that Ski expression was downregulated in
metastatic NSCLC cells and tissues.
Ski prevents TGF-β-induced EMT and cell
invasion by inhibiting SMAD-dependent TGF-β signaling in NSCLC
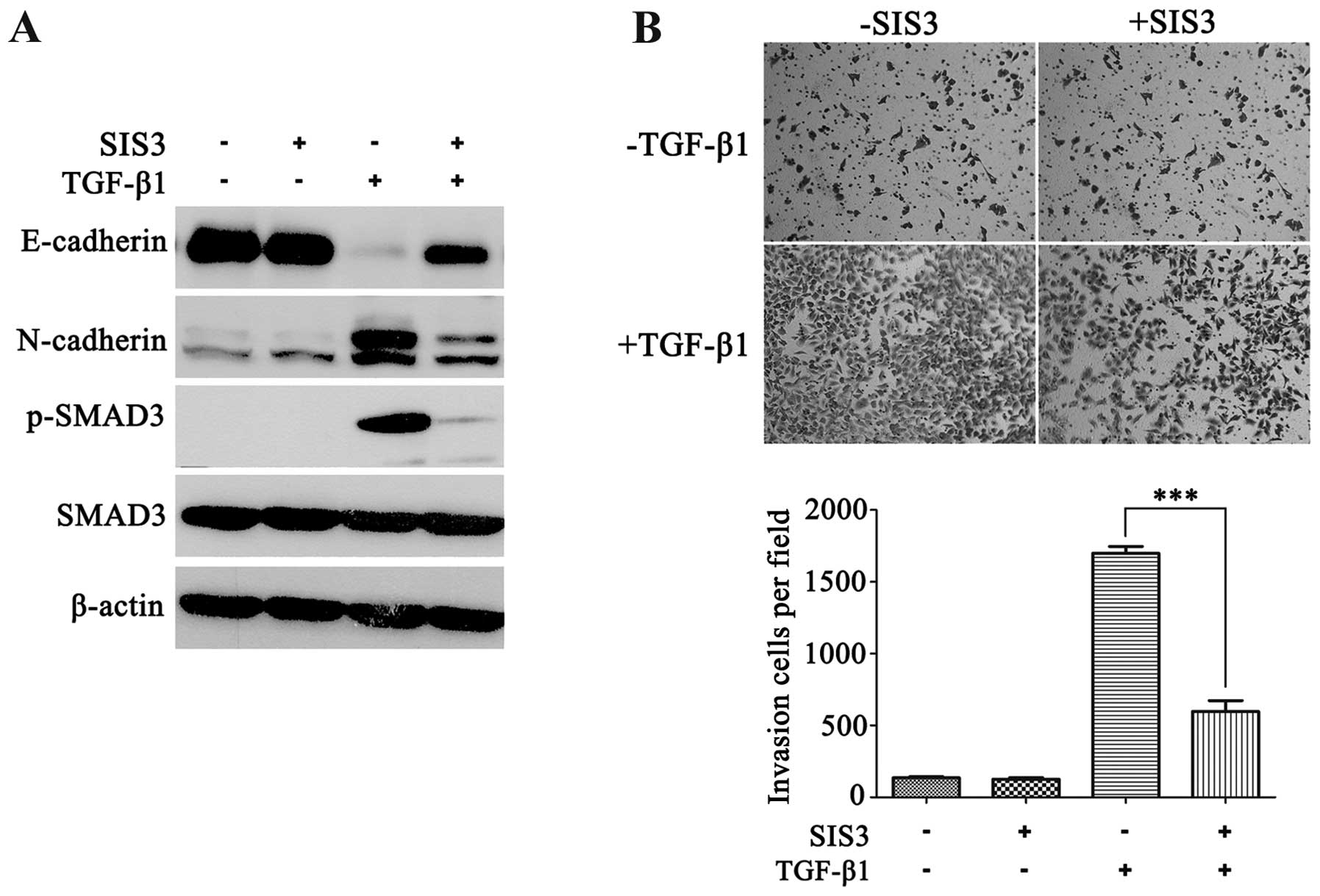
The above findings suggested the involvement of Ski
in TGF-β-induced EMT and invasion by SMAD-dependent signaling.
Other non-SMAD signaling pathways can also be activated during
TGF-β-induced EMT (26,27). To exclude the possibility that Ski
is involved in non-SMAD pathways affecting TGF-β-induced EMT and
invasion in NSCLC, we first used SIS3, a specific inhibitor of
SMAD3 phosphorylation, to block the TGF-β/SMAD canonical pathway in
A549 cells. As shown in Fig. 4A,
SIS3 markedly reduced the TGF-β-induced p-SMAD3 level, restored
E-cadherin expression and weakened N-cadherin expression. Moreover,
SIS3 significantly repressed TGF-β-induced invasion in A549 cells
(Fig. 4B). The results suggested
that p-SMAD3 was essential for TGF-β-induced EMT and cell invasion
in NSCLC.
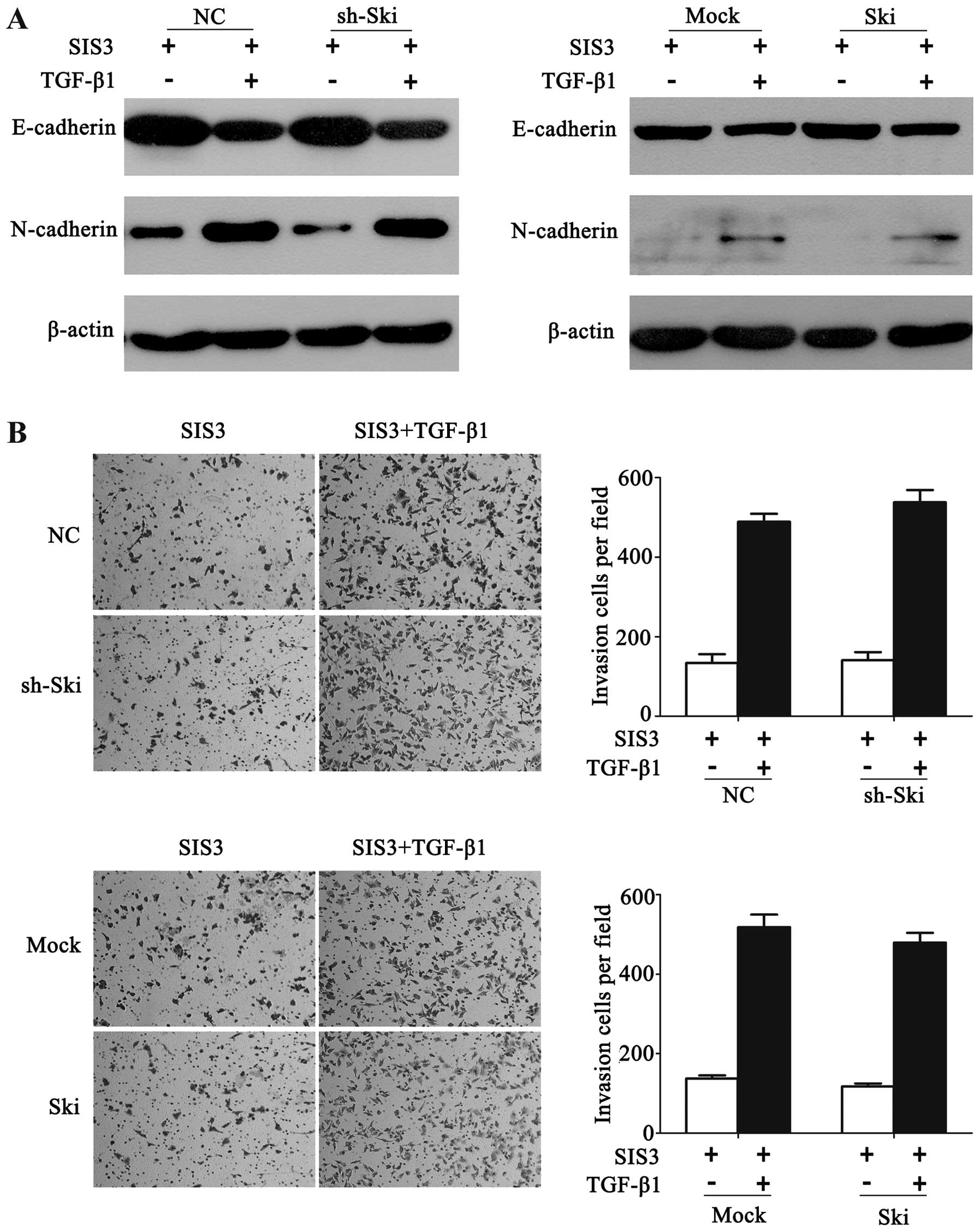
Following the pre-treatment of SIS3 followed by
TGF-β1 stimulation, A549-sh-Ski or A549-Ski cells did not
demonstrate any significant changes of E- and N-cadherin expression
compared with their corresponding control cells (Fig. 5A). Similarly, Ski knockdown or
overexpression did not exert any significant effect on the invasive
ability of A549 cells in the presence of SIS3 and TGF-β1 (Fig. 5B). The results therefore indicated
that Ski affected TGF-β-induced EMT and cell invasion by inhibiting
SMAD-dependent TGF-β signaling.
Discussion
Lung cancer has a high morbidity and mortality tumor
worldwide and nearly ~85% of all lung cancers belong to NSCLC
(28). Most cancer deaths are due
to tumor metastasis, which promotes to make a switch of early-stage
tumors into invasive malignancies (1). Therefore, understanding the molecular
basis of tumor metastasis is necessary for cancer diagnosis and
treatment. EMT is a fatal event during tumor metastasis (29). As a well-known EMT inducer, TGF-β
plays an important role in tumor metastasis (27). Ski was reported to function as a
negative regulator of TGF-β signaling (18). However, the role of Ski in
TGF-β-induced EMT and invasion was poorly understood in NSCLC. In
the present study, our findings show that Ski expression was lower
in metastatic NSCLC cells and tissues than the non-metastatic ones.
Of note, we identified that Ski prevents TGF-β-induced EMT and cell
invasion through the SMAD-dependent pathway in NSCLC cells.
Ski was primarily identified to serve as an oncogene
by promoting anchorage-independent growth of chicken and quail
embryo fibroblast cells (30).
Subsequently, Ski was reported to be a prognostic factor in
TGF-β-positive advanced gastric cancer and promote tumor growth in
diffuse-type gastric carcinoma (31,32).
In the present study, no significant difference in the Ski
expression level was found between NSCLC tissues and paired
adjacent non-cancer lung tissues. However, we detected that the
downregulated expression of Ski was significantly associated with
NSCLC metastasis. The results support those of Le Scolan et
al who reported that a reduced Ski expression in lung cancer
cells enhances tumor metastasis in vivo (33). Thus, Ski appears to function as a
metastasis suppressor in NSCLCs. Nevertheless, Javelaud et
al observed that there was no correlation between Ski and
melanoma metastasis (34). These
data suggest that Ski functions differently depending on the stages
of tumor progression and tumor types.
Although there are various roles of Ski playing in
cancer development and progression, the molecular mechanisms by
which Ski influences TGF-β-induced EMT and cell invasion in NSCLC
are not largely understood. Specifically, the knockdown of Ski
significantly altered the expression levels of EMT-related genes in
breast cancer cells (33),
suggesting that Ski is involved in EMT. In the present study, we
found that Ski significantly inhibited TGF-β-induced EMT and
invasion in NSCLC cells. This is not surprising since TGF-β
promotes EMT through a combination of SMAD-dependent and
SMAD-independent pathways (26,27),
and Ski interacts with these signaling pathways. Recently, Wang
et al reported that the knockdown of Ski significantly
enhanced TGF-β-induced EMT in SMAD4-deficient pancreatic cancer
cells (25). That finding suggests
that Ski regulates TGF-β-induced EMT through non-SMAD pathways in
pancreatic cancer. By contrast, our findings revealed that Ski was
not invovled in non-SMAD pathways affecting TGF-β-induced EMT and
invasion in NSCLC. However, Ski repressed TGF-β-induced EMT and
invasion by inhibiting SMAD-dependent signaling in NSCLC.
In conclusion, to the best of our knolwedge, this is
the first study to show that Ski is downregulated in metastatic
NSCLC cells and tissues. Moreover, cell-based and biochemical
assays indicate that Ski prevents TGF-β-induced EMT and cell
invasion by repressing the canonical SMAD signaling. Our findings
that Ski expression inhibits NSCLC cell invasion by controlling
TGF-β/Smad signaling, and provide new insights into therapeutic
strategies for NSCLC metastasis.
Acknowledgments
The present study was supported in part by §grants
from the National Natural Science Foundation of China (81372277 and
81171894 to H.-T. Zhang), the Jiangsu Province’s Key Provincial
Talents Program (RC2011106 to J. Zhao), the Jiangsu Province’s
Outstanding Medical Academic Leader Program (LJ201138), the ‘333’
Project of Jiangsu Province Government (to H.-T. Zhang), the
Graduate Innovation Project of Jiangsu Province (CXZZ13_0830 to Z.
Lei), the Soochow Scholar Project of Soochow University (to H.-T.
Zhang), the Suzhou Key Laboratory for Molecular Cancer Genetics
(SZS201209 to H.-T. Zhang), and A Project Funded by the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (PAPD).
References
|
1
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pasche B: Role of transforming growth
factor beta in cancer. J Cell Physiol. 186:153–168. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miettinen PJ, Ebner R, Lopez AR and
Derynck R: TGF-beta induced transdifferentiation of mammary
epithelial cells to mesenchymal cells: Involvement of type I
receptors. J Cell Biol. 127:2021–2036. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Massagué J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000. View Article : Google Scholar
|
|
6
|
Roberts AB, Tian F, Byfield SD, Stuelten
C, Ooshima A, Saika S and Flanders KC: Smad3 is key to
TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis,
tumor suppression and metastasis. Cytokine Growth Factor Rev.
17:19–27. 2006. View Article : Google Scholar
|
|
7
|
Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu
Z, Zhao J and Zhang HT: JAK/STAT3 signaling is required for
TGF-β-induced epithelial-mesenchymal transition in lung cancer
cells. Int J Oncol. 44:1643–1651. 2014.PubMed/NCBI
|
|
8
|
Yan Z, Winawer S and Friedman E: Two
different signal transduction pathways can be activated by
transforming growth factor beta 1 in epithelial cells. J Biol Chem.
269:13231–13237. 1994.PubMed/NCBI
|
|
9
|
Edlund S, Landström M, Heldin CH and
Aspenström P: Transforming growth factor-beta-induced mobilization
of actin cytoskeleton requires signaling by small GTPases Cdc42 and
RhoA. Mol Biol Cell. 13:902–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Turck CM, Teumer JK and Stavnezer E:
Unique sequence, ski, in Sloan-Kettering avian retroviruses with
properties of a new cell-derived oncogene. J Virol. 57:1065–1072.
1986.PubMed/NCBI
|
|
12
|
Akiyoshi S, Inoue H, Hanai J, Kusanagi K,
Nemoto N, Miyazono K and Kawabata M: c-Ski acts as a
transcriptional co-repressor in transforming growth factor-beta
signaling through interaction with smads. J Biol Chem.
274:35269–35277. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo K, Stroschein SL, Wang W, Chen D,
Martens E, Zhou S and Zhou Q: The Ski oncoprotein interacts with
the Smad proteins to repress TGFbeta signaling. Genes Dev.
13:2196–2206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu W, Angelis K, Danielpour D, Haddad MM,
Bischof O, Campisi J, Stavnezer E and Medrano EE: Ski acts as a
co-repressor with Smad2 and Smad3 to regulate the response to type
beta transforming growth factor. Proc Natl Acad Sci USA.
97:5924–5929. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrand N, Atfi A and Prunier C: The
oncoprotein c-Ski functions as a direct antagonist of the
transforming growth factor-β type I receptor. Cancer Res.
70:8457–8466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Band AM, Björklund M and Laiho M: The
phosphatidylinositol 3-kinase/Akt pathway regulates transforming
growth factor-β signaling by destabilizing ski and inducing Smad7.
J Biol Chem. 284:35441–35449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Li P, Zhang Y, Li GB, Zhou YG, Yang
K and Dai SS: c-Ski inhibits the proliferation of vascular smooth
muscle cells via suppressing Smad3 signaling but stimulating p38
pathway. Cell Signal. 25:159–167. 2013. View Article : Google Scholar
|
|
18
|
Luo K: Ski and SnoN: Negative regulators
of TGF-beta signaling. Curr Opin Genet Dev. 14:65–70. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reed JA, Bales E, Xu W, Okan NA,
Bandyopadhyay D and Medrano EE: Cytoplasmic localization of the
oncogenic protein Ski in human cutaneous melanomas in vivo:
Functional implications for transforming growth factor beta
signaling. Cancer Res. 61:8074–8078. 2001.PubMed/NCBI
|
|
20
|
Fukuchi M, Nakajima M, Fukai Y, Miyazaki
T, Masuda N, Sohda M, Manda R, Tsukada K, Kato H and Kuwano H:
Increased expression of c-Ski as a co-repressor in transforming
growth factor-beta signaling correlates with progression of
esophageal squamous cell carcinoma. Int J Cancer. 108:818–824.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buess M, Terracciano L, Reuter J,
Ballabeni P, Boulay JL, Laffer U, Metzger U, Herrmann R and
Rochlitz C: Amplification of SKI is a prognostic marker in early
colorectal cancer. Neoplasia. 6:207–212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heider TR, Lyman S, Schoonhoven R and
Behrns KE: Ski promotes tumor growth through abrogation of
transforming growth factor-beta signaling in pancreatic cancer. Ann
Surg. 246:61–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ritter M, Kattmann D, Teichler S, Hartmann
O, Samuelsson MK, Burchert A, Bach JP, Kim TD, Berwanger B, Thiede
C, et al: Inhibition of retinoic acid receptor signaling by Ski in
acute myeloid leukemia. Leukemia. 20:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shinagawa T, Nomura T, Colmenares C, Ohira
M, Nakagawara A and Ishii S: Increased susceptibility to
tumorigenesis of ski-deficient heterozygous mice. Oncogene.
20:8100–8108. 2001. View Article : Google Scholar
|
|
25
|
Wang P, Chen Z, Meng ZQ, Fan J, Luo JM,
Liang W, Lin JH, Zhou ZH, Chen H, Wang K, et al: Dual role of Ski
in pancreatic cancer cells: Tumor-promoting versus
metastasis-suppressive function. Carcinogenesis. 30:1497–1506.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Derynck R and Akhurst RJ: Differentiation
plasticity regulated by TGF-beta family proteins in development and
disease. Nat Cell Biol. 9:1000–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Massagué J: TGFbeta in Cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lei Z, Liu RY, Zhao J, Liu Z, Jiang X, You
W, Chen XF, Liu X, Zhang K, Pasche B, et al: TGFBR1 haplotypes and
risk of non-small-cell lung cancer. Cancer Res. 69:7046–7052. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Colmenares C and Stavnezer E: The ski
oncogene induces muscle differentiation in quail embryo cells.
Cell. 59:293–303. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakao T, Kurita N, Komatsu M, Yoshikawa K,
Iwata T, Utsunomiya T and Shimada M: Expression of thrombospondin-1
and Ski are prognostic factors in advanced gastric cancer. Int J
Clin Oncol. 16:145–152. 2011. View Article : Google Scholar
|
|
32
|
Kiyono K, Suzuki HI, Morishita Y, Komuro
A, Iwata C, Yashiro M, Hirakawa K, Kano MR and Miyazono K: c-Ski
overexpression promotes tumor growth and angiogenesis through
inhibition of transforming growth factor-beta signaling in
diffuse-type gastric carcinoma. Cancer Sci. 100:1809–1816. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le Scolan E, Zhu Q, Wang L, Bandyopadhyay
A, Javelaud D, Mauviel A, Sun L and Luo K: Transforming growth
factor-beta suppresses the ability of Ski to inhibit tumor
metastasis by inducing its degradation. Cancer Res. 68:3277–3285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Javelaud D, van Kempen L, Alexaki VI, Le
Scolan E, Luo K and Mauviel A: Efficient TGF-β/SMAD signaling in
human melanoma cells associated with high c-SKI/SnoN expression.
Mol Cancer. 10:22011. View Article : Google Scholar
|