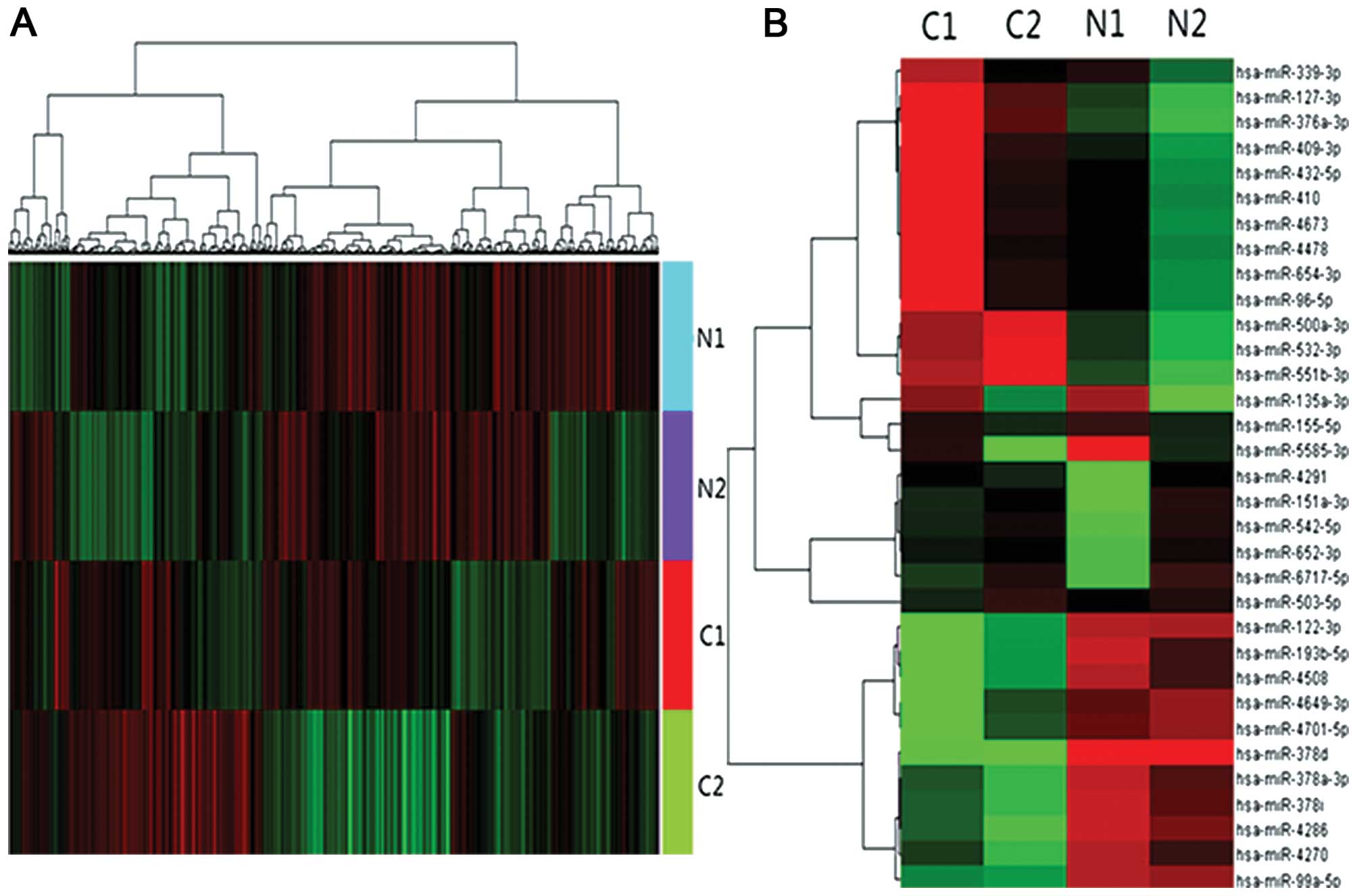
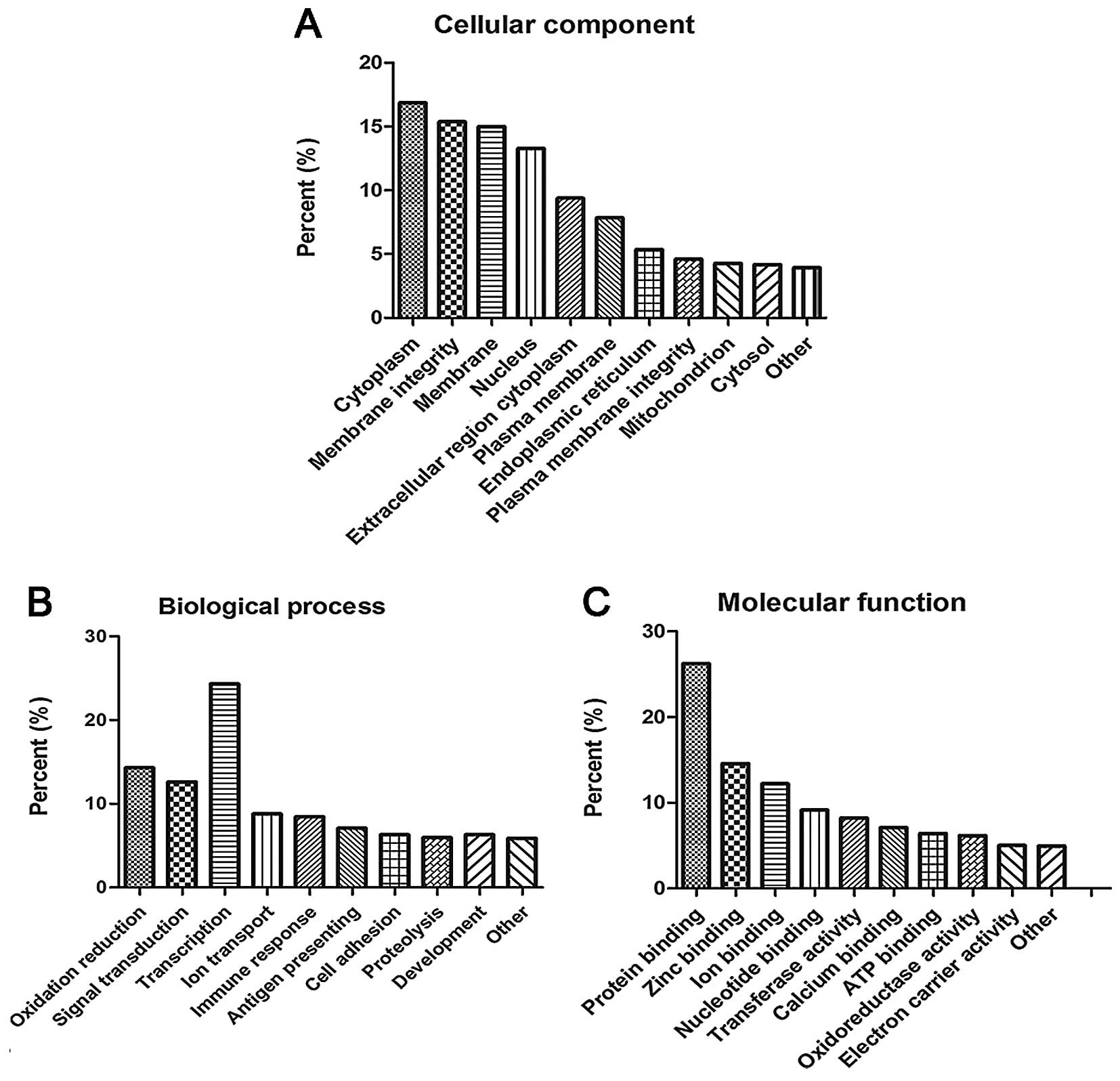
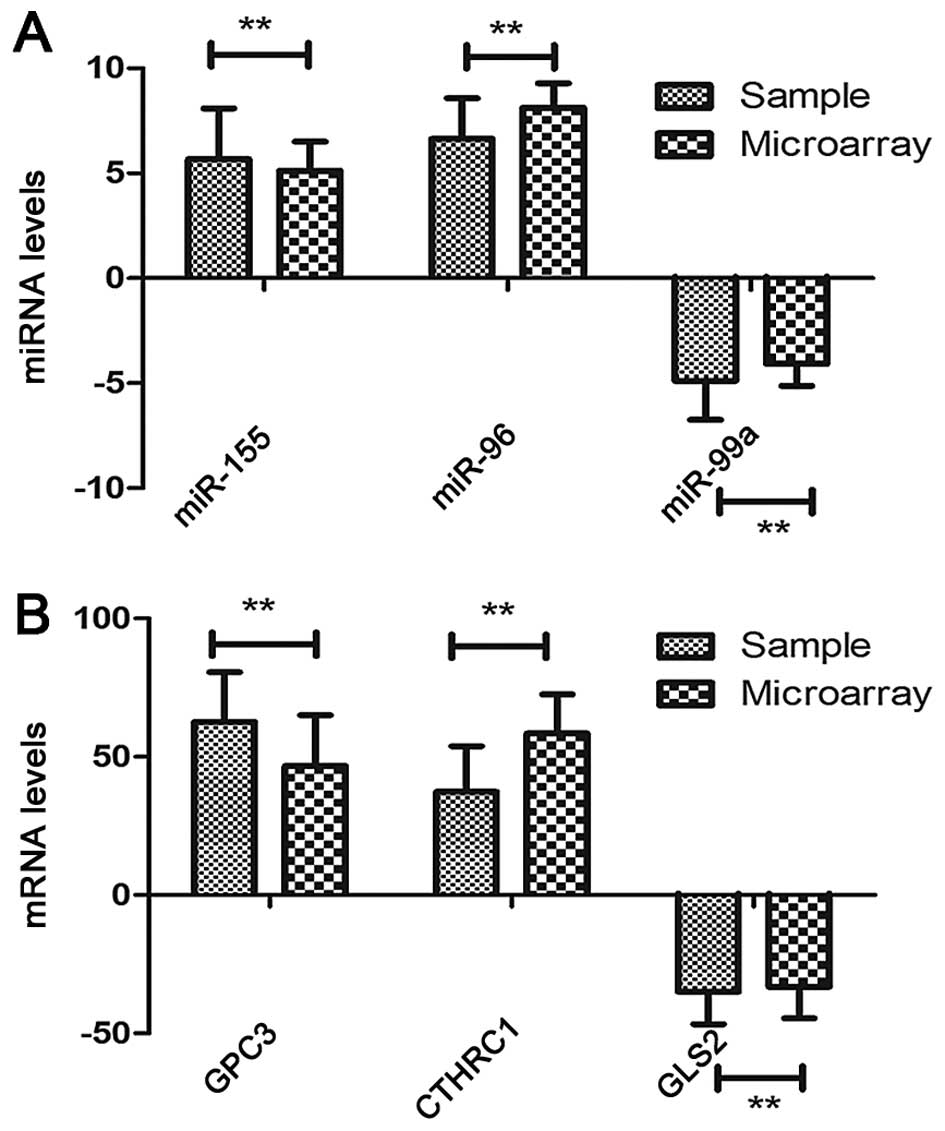
| hsa-miR-127 | Upregulation | C7orf10, GADD45G,
LRRC27, SMPD3, SOX8, SYT7, TNFRSF10D, USP47, ZBTB7A | Downregulation |
| hsa-miR-96 | Upregulation | ITPR2, FOXO1,
PROSC, SDC2, TSKU, KLHDC9, ZNF577, SLC6A13, ACO1, FAM134B, OLFM1,
PGM1, SNAI2, SLC1A2, GHR, KLF12, CBFA2T3, NCAM1, EPB41L4B, NRXN1,
TMEM50B, TP53INP2, ACADSB, AKR1D1, AQP9, AVPI1, C1orf116, CAPN5,
CASP10, CBLN3, CES3, CXorf38, CYP3A43, DBT, EFHA2, GIPR, HAPLN4,
IGSF3, ITGB8, IYD, LPA, LZTS1, MASP1, MBP, MCART6, MMAB, NF1,
PADI2, PIK3R1, PTCD3, RASSF6, RCAN1, RNF43, SEC14L2, SH3BGRL2,
SHMT1, SLC16A2, SLC23A2, SLC45A3, SLC47A1, SLC7A2, SMOC1, SPTB,
USH2A, USP15, VAPA | Downregulation |
| hsa-miR-155 | Upregulation | KALRN, VAPA, PCDH9,
ZNF236, CYP2C9, EPB41L4B, GALC, HAL, KBTBD11, MCART6, SCAMP1,
USH2A, MUT, CYR61, ACVR1C, SNX29, ETNK2, KPNA1, ACADSB, ACSM5,
ARG1, ATF3, ATXN10, BCL2L15, BHMT2, CA13, CARD11, CASP10, EGFR,
EML5, FOS, GABRP, GLS, GPD1, GRIA4, HCN3, IL28RA, MBP, MEIG1,
NBPF3, PEBP1, PIK3R1, PLN, PTCD3, PTPRD, RCAN1, SH3RF2, SLC16A2,
SLC1A2, SLC20A2, SLC7A2, SYT15, WNK3, ZNF577 | Downregulation |
| hsa-miR-654 | Upregulation | KLF12, MYO6,
PPARGC1A, RNF128, USP15, ACADSB, AKR1D1, AQP9, ATXN10, BCKDHB,
BCL2L15, BHMT, C1orf116, CAPN6, CASP10, CDC14B, CUX2, CXorf38, DBT,
EFHA2, FOXO1, GLS, GRAMD1C, HEY2, HFE2, HLF, HNRNPA2B1, IL12RB2,
ITGB8, KBTBD11, KPNA1, LPIN3, LRRC1, LRRK2, MBP, MCART6, MMAB,
MSRA, MSRB2, NRXN1, OLFM1, PLN, PRRG4, PTPRD, RASSF6, RPL28,
SCAMP1, SDC2, SEC14L3, SERPIND1, SH2D4A, SH3BGRL2, SLC1A2, SLC23A2,
SLC2A2, SNX29, SPTB, SRD5A2, SSTR1, SULT1E1, TMCO7, TUB, ZNF236,
ZNF395 | Downregulation |
| hsa-miR-339 | Upregulation | BDH2, C10orf114,
CROCC, GIPR, GREM2, KPNA1, KRBA2, PIK3R1, PSD3, RPL28, SALL3, SOX8,
SSTR1, TRPV6 | Downregulation |
| hsa-miR-432 | Upregulation | MASP1, PTCD3,
SLCO4C1, ADI1, ARMC5, PDE4DIP, SAMD4A, ABTB2, FAM134B, ITPR2,
MACROD2, SPTB, ACVR1C, ANKS1A, CXCL2, GLYAT, KBTBD11, PPM1K,
RPH3AL, SS18L1, TCF7, AQP9, BHMT2, BZRAP1, CDC14B, CES3, CUX2,
CXCL12, CXCL14, CYP2B6, DBT, EFHA2, EGFR, EPB41L4B, FAM123B, FMO4,
FOS, FOXO1, GABRP, GCHFR, GHR, GIPR, IGSF3, ITGB8, KMO, LNP1,
LRRC1, LZTS1, NCAM1, NF1, PTPRD, RCL1, SH2D4A, SLC30A4, SLC7A2,
SMPD3, TSKU, TSPAN12, TUB, VWCE, XPNPEP2, ZBTB7C | Downregulation |
| hsa-miR-410 | Upregulation | ACADSB, AIM1,
AMDHD1, EFHA2, EPB41L4B, HSD11B1, ITGB8, TPR2, LRRK2, PLN, RGS16,
SCAMP1, SLC1A2, SLC7A2, SRD5A1, TMEM50B, TNFRSF10D, ZNF577, ME1,
ABCG2, ADI1, AQP9, ATM, BCL2L15, C1orf116, C7orf58, CA13, CMBL,
CXCL12, CXCL14, DBT, FAM123B, FOSB, GALC, GFRA1, GIPC2, HS3ST3B1,
IYD, KPNA1, MYO9A, NF1, OAT, PIK3R1, PSD3, PTPRD, PUS10, RASSF6,
SH3BGRL2, SIVA1, SLC20A2, SLC30A4, SS18L1, SSTR1, TDRD6, TGDS,
TSPAN12, USP15, VAPA, ZNF131, ZNF236 | Downregulation |
| hsa-miR-99a | Downregulation | KBTBD8, CD93,
RASA3, ST6GALNAC4 | Upregulation |
| hsa-miR-409 | Upregulation | EML5, ACVR1C,
CYP3A4, KLF12, ME1, MPDZ, PTCD3, SALL3, SLC38A2, TUBE1, ASPA, EGFR,
GREM2, ACADSB, ALDH5A1, BCL2L15, C7orf58, CACNA2D4, CAPN5, CXCL2,
DBT, EFHA2, FAM123B, GALC, GHR, GRIA4, ITGB8, KCNB1, MBP, MCART6,
MMAB, MMP19, PIK3R1, POU4F1, PPARGC1A, PTPN2, RASSF6, RNF128,
SH2D4A, SH3RF2, SIRPB1, SLC30A4, SLC7A2, SNX29, TBX3, TDRD6, VAPA,
WNK3, ZDHHC23, ZNF236, ZNF425 | Downregulation |
| hsa-miR-542 | Upregulation | IL28RA, USP15, DES,
IER5L, DAK, FOSB, GIPR, GPR146, GUCA2B, HAPLN4, KLF12, MASP1,
MORN1, MTTP, NF1, PKLR, RGS16, SH2D4A, SH3RF2, SLC16A2, SLC1A2,
SMPD3, SNX29, SOX8, SRD5A2, SYT15, TP53INP2, VWA1 | Downregulation |
| hsa-miR-652 | Upregulation | KPNA1, LRRC1,
BCL2L15, IL28RA, KCNB1, PPIL2, SNX29, TNFRSF10D | Downregulation |
| hsa-miR-551 | Upregulation | ANKS1A, CACNA2D4,
IYD, KBTBD11, UPB1 | Downregulation |
| hsa-miR-532 | Upregulation | CACNA2D4, CMBL,
ETNK2, IL6R, KIAA1161, NF1, PRRG4, SH3RF2, SLC7A2, SMOC1, ZNF425,
ABCA8, ACACB, ACADSB, ACO1, AKR1D1, ANKS1A, APOA5, AQP9, AZGP1,
BDH2, C1orf116, C1orf187, C22orf36, CAPN6, CBLN3, CDC14B, CXCL12,
CYP2A13, CYP3A4, DBT, DES, DIRAS3, FAM123B, FNDC5, FOSB, FOXO1,
GCHFR, GLYAT, GPD1, GREM2, HAPLN4, HCN3, HLF, IGSF3, IYD, KANK4,
KATNAL2, KCNK5, LPIN3, LRRC27, MBP, MMAB, MMP19, MSRA, NBPF3,
NCAM1, OASL, PI16, PIK3R1, POU4F1, PPARGC1A, PPP1R3F, PRKCG, PSD3,
PTCD3, RCAN1, RGS16, SART1, SCAMP1, SEC14L2, SH3BGRL2, SHMT1,
SLC20A2, SMPD3, SNX29, SOX8, SPTB, SS18L1, SYT7, TBCD, TCAP, TDRD6,
TMEM105, TNFRSF10D, TNFRSF14, TRH, TUB, WFDC5, ZBTB7C, ZDHHC11,
ZNF577 | Downregulation |
| hsa-miR-503 | Upregulation | C7orf58, GADD45G,
CAPN6, PCDH9, SLC20A2, WNK3, ACADL, ALDH1A3, ANKS1A, C14orf180,
CCDC25, IL28RA, ITGB8, PTCD3, SNX29, ACADSB, AIM1, BDH2, CACNA2D4,
CBFA2T3, CDADC1, CDC14B, CIDEB, CLEC4G, EML5, EPB41L4B, FAM123B,
GABRP, GALC, GHR, GPD1, GREM2, HAPLN4, IYD, KCNK5, LHPP, MBP,
MCART6, MMAB, MPDZ, NF1, PIK3R1, PRKCG, PTPRD, RCL1, RNF43, RHBG,
SALL3, SH3BGRL2 SLC7A2, SMPD3, TCF7, TUB, USH2A, ZDHHC23 | Downregulation |