Introduction
Breast cancer is a common malignancy in females
worldwide and is a heterogeneous disease that encompasses several
distinct entities with different biological characteristics and
clinical behaviors. Currently, breast cancer patients are managed
according to different treatment approaches based on various
clinical parameters in conjunction with the assessment of the
status of sex steroid receptor (estrogen and progesterone
receptors). Approximately 70 to 80% of primary breast cancers are
positive for estrogen receptor (ER) and/or progesterone receptor
(PR), such as MCF-7 breast cancer cells and ER+ breast
cancers typically have a better prognosis and are often responsive
to antiestrogen therapy; however, ER-independent breast cancer
cells, such as MDA-MB-231, are more aggressive, possess high
potential to metastasize and are unresponsive to antiestrogens
(1). Thus, it is important to
investigate the differences between high- and low-metastatic cancer
cells.
In a previous study, we showed that the highly
metastatic breast cancer cell line MDA-MB-231, released
significantly more ATP than the less metastatic breast cancer cell
line MCF-7 or normal epithelial or endothelial cells (ECs) under
both normoxic and hypoxic conditions (2,3). In
addition, MDA-MB-231 cells showed higher P2Y2 purinergic
receptor (P2Y2R) activity and increased invasion into
the extracellular matrix (ECM) compared with MCF-7 cells (2). P2Y2R is a G protein-coupled
purinergic receptor equally activated by both extracellular ATP and
UTP (4). Many studies have shown
that extracellular purines accumulate in the tumor
micro-environment and directly affect cancer progression through
purinergic receptors. The activation of P2Y2Rs also
supports the progression of each step of metastasis, including
angiogenesis, intravasation and invasion and tumor growth (5-7). Thus,
it is important to determine which P2Y2R-related
signaling pathway is involved in the invasion of breast cancer
cells.
Gq-coupled P2Y2R activates several
intracellular signal transduction pathways, resulting in
intracellular calcium mobilization and phospholipase C (PLC) and
protein kinase C (PKC) activation. Through the extracellularly
oriented RGD domain, P2Y2R interacts with
αVβ3/5 integrins to regulate the activities
of Rho and ROCK, which regulate cell movement. Src-homology-3
binding domains (PXXP) within the C terminus of P2Y2R
bind Src to enable ATP or UTP to transactivate growth factor
receptors and downstream mitogen-activated protein kinases (MAPKs).
Accordingly, in the present study, we investigated whether
P2Y2R activation mediates breast cancer cell invasion
through PKC and extracellular signal-regulated kinase (ERK)
signaling pathways.
Materials and methods
Materials
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from HyClone (South Logan, UT, USA). Antibiotics
(penicillin/streptomycin), glutamine and collagenase were purchased
from Gibco-BRL (Rockville, MD, USA). Anti-Snail, anti-N-cadherin,
anti-β-catenin and anti-E-cadherin antibodies were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Control siRNA or
P2Y2R siRNA was obtained from Bioneer (Daejeon, Korea).
Cell culture inserts (8 µm) and the Basement Membrane Matrix
(Matrigel) were obtained from BD Bioscience (San Jose, CA, USA).
Enhanced chemiluminescence (ECL) western blotting detection reagent
was purchased from Amersham (Buckinghamshire, UK). All other
chemicals, including adenosine triphosphate (ATP), uridine
5′-triphosphate (UTP) and anti-β-actin antibody, were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The human breast cancer cell lines MCF-7,
MDA-MB-231, SK-BR-3 and T47D were obtained from the Korea Cell Line
Bank (Seoul, Korea). The cells were grown in RPMI-1640 supplemented
with 10% FBS, 100 IU/ml penicillin and 10 µg/ml
streptomycin.
Gene silencing with small interfering RNA
(siRNA)
Gene silencing experiments were performed with three
independent P2Y2R siRNAs. The cells were transfected
with 100 nM control or P2Y2R siRNA in serum-containing
medium using Turbofect® (Thermo Scientific, Rockford,
IL, USA). The gene silencing efficiency was determined by reverse
transcription-polymerase chain reaction (RT-PCR) and western blot
analysis.
RT-PCR
RT-PCR was performed using the TOPscript One-step RT
PCR DryMix (Enzynomics, Daejeon, Korea) according to the
manufacturer’s instructions. The following primer sets were used:
hP2Y2R, 5′-GTG CTC TAC TTC CTG GCT-3′ and 5′-CTG AAG TGT
TCT GCT CCT AC-3′; hGAPDH, 5′-TCA ACA GCG ACA CCC ACT CC-3′ and
5′-TGA GGT CCA CCA CCC TGT TG-3′. Thirty cycles of amplification
were performed under the following conditions: melting at 95°C for
30 sec, annealing at 56°C for 30 sec and extension at 72°C for 30
sec.
Measurement of intracellular calcium ion
concentration ([Ca2+]i)
The [Ca2+]i concentration was
measured as previously described, with minor modifications
(2). Briefly, the cells were
stained with 5 µM fluo-3-AM and washed with physiological
solution (125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM
HEPES, 5 mM glucose and 1 mM CaCl2). Subsequently, the
cells were treated with ATP and the fluorescent images were scanned
using a confocal microscope (IX70 Fluoview, Olympus; excitation
wavelength: 488 nm, emission wavelength: 530 nm). The changes in
[Ca2+]i were calculated as
(Fmax−F0)/F0 (F, fluorescence
intensity; F0, basal fluorescence intensity before
treatment; Fmax, maximum level of fluorescence
intensity, which occurred after the addition of ATP).
Extracellular ATP release
measurements
The cells were incubated for 15 min at 37°C with
HEPES buffer (pH 7.4) containing AOPCP, a selective inhibitor of
ecto-5′-nucle-otidase. The cells were treated with or without TNF-α
for an additional 5 min. The supernatants were collected at the
indicated time-points and ATP release was measured using the
Enliten ATP Assay system (Promega, Madison, WI, USA). ATP levels
were calculated based on an ATP standard curve.
Western blot analysis
The cells were lysed using Pro-PREP protein
extraction solution (iNtRON Biotechnology, Seoul, Korea).
Subsequently, the lysate was centrifuged at 13,000 rpm for 15 min
at 4°C and the supernatants were collected for determination of the
protein concentration using the Bradford method. Aliquots of 40
µg of protein were subjected to 7.5–12.5% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis for 2 h at 100 V. The
separated proteins were transferred onto Hybond-P+
polyvinylidene difluoride membranes (Amersham). The membranes were
blocked with 5% nonfat milk in Tris-buffered saline containing
0.05% Tween-20 (TBS-T) for 2 h at room temperature, followed by
incubation with the indicated primary antibodies. The bound
antibodies were detected using horseradish peroxidase-conjugated
secondary antibodies and ECL western blotting detection reagent
(Bionote, Gyeonggi-do, Korea).
Matrigel invasion assay
The upper chambers of the inserts were coated with
100 µl of Matrigel (1 mg/ml, BD Bioscience). Control or
P2Y2R siRNA-transfected breast cancer cells
(2×105 cells/insert) were added to the upper chambers in
serum-free media and 500 µl of RPMI media with or without
apyrase was added to the lower chambers. The cells were incubated
for 16 h to facilitate invasion, and subsequently the cells on the
lower part of the insert membranes were stained with
4′,6-diamidino-2-phenylindole (DAPI) and counted in a 500×500
µm field under an Olympus microscope (CKX41) equipped with a
camera (Nikon, DS-U3). Three independent experiments were performed
in triplicate.
Data analysis
Image Master® VDS (Pharmacia Biotech
Inc., San Francisco, CA, USA) was used for scanning densitometry.
All results are representative of three independent experiments
performed in triplicate. Significant differences within experiments
were evaluated using one-way analysis of variance and the Scheffe
post-hoc test. P<0.05 was considered statistically
significant.
Results
Highly metastatic breast cancer cells
MDA-MB-231 and SK-BR-3 show much higher ATP release and
P2Y2R activity than less metastatic breast cancer cells
MCF-7 and T47D
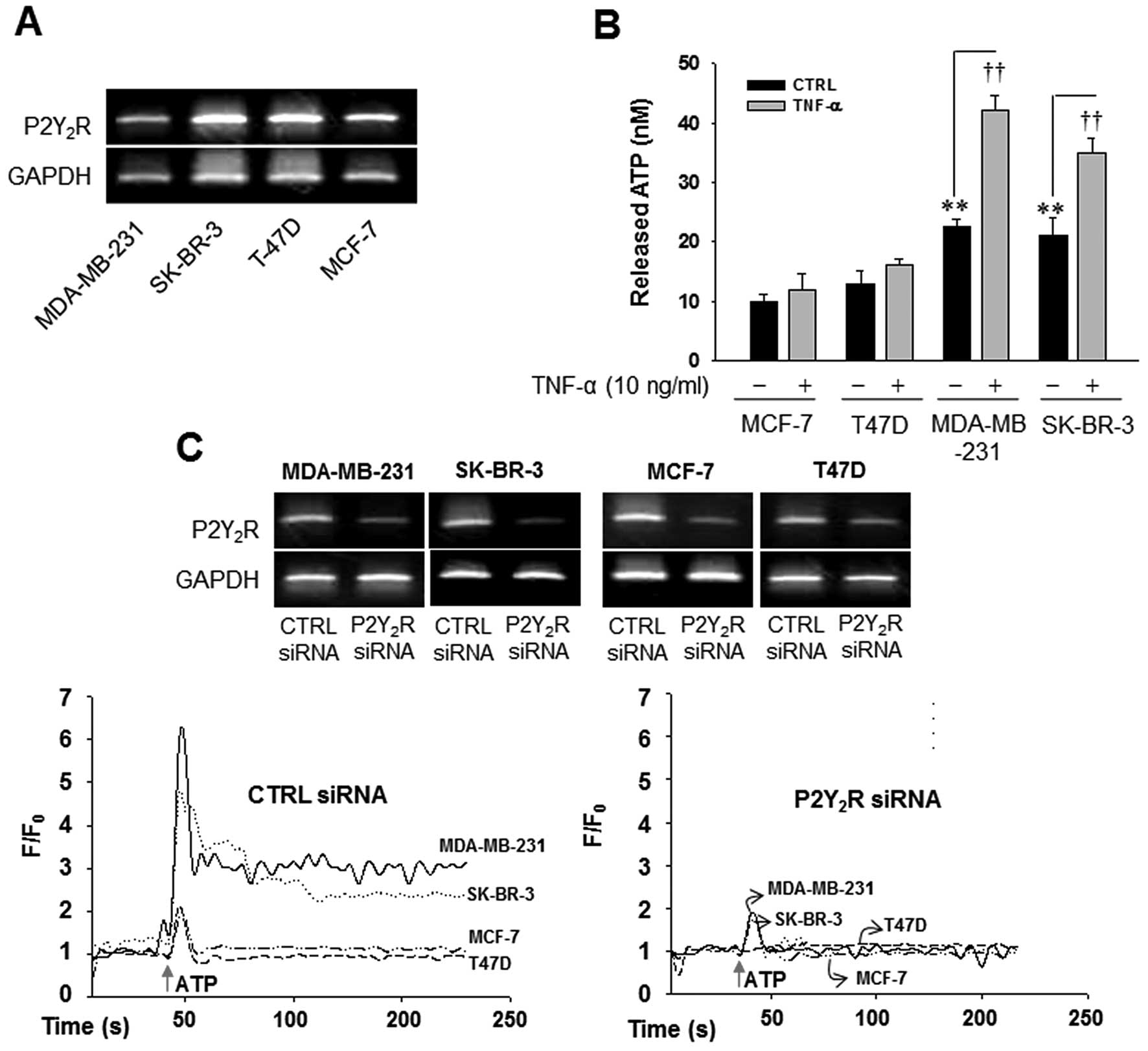
In a previous study, the highly metastatic breast
cancer cell line MDA-MB-231 released ATP at a much higher level
than the less metastatic breast cancer cell line MCF-7, although
P2Y2R expression was not different between the two cell
types (2). Thus, we confirmed the
levels of ATP released into the extracellular medium in several
breast cancer cells with different metastatic properties. Fig. 1 showed that there were no
significant differences between P2Y2R mRNA expression in
highly metastatic breast cancer cells (MDA-MB-231 and SK-BR-3) and
low metastatic breast cancer cells (MCF-7 and T47D) (Fig. 1A); however, the highly metastatic
breast cancer cells MDA-MB-231 and SK-BR-3 released markedly more
ATP in comparison to the low metastatic breast cancer cells MCF-7
and T47D, In addition, TNF-α, an essential factor in tumor
progression and metastasis (8,9),
significantly enhanced the release of ATP in MDA-MB-231 and SK-BR-3
(Fig. 1B). Next, we compared
P2Y2R activity between the highly metastatic breast
cancer cells MDA-MB-231 and SK-BR-3 and the low metastatic breast
cancer cells MCF-7 and T47D. As shown in Fig. 1C, ATP (10 µM) a
P2Y2R agonist elicited immediate and rapid augmentation
in [Ca2+]i in MDA-MB-231 and SK-BR-3, which
were significantly reduced in P2Y2R knocked down
MDA-MB-231 and SK-BR-3. As expected, the transient elevation of
[Ca2+]i levels in MCF-7 and T47D were much
lower than MDA-MB-231 and SK-BR-3, suggesting higher
P2Y2R activity in response to nucleotides in MDA-MB-231
and SK-BR-3.
P2Y2R activation by ATP
released from highly metastatic breast cancer cells increases
invasion of breast cancer cells
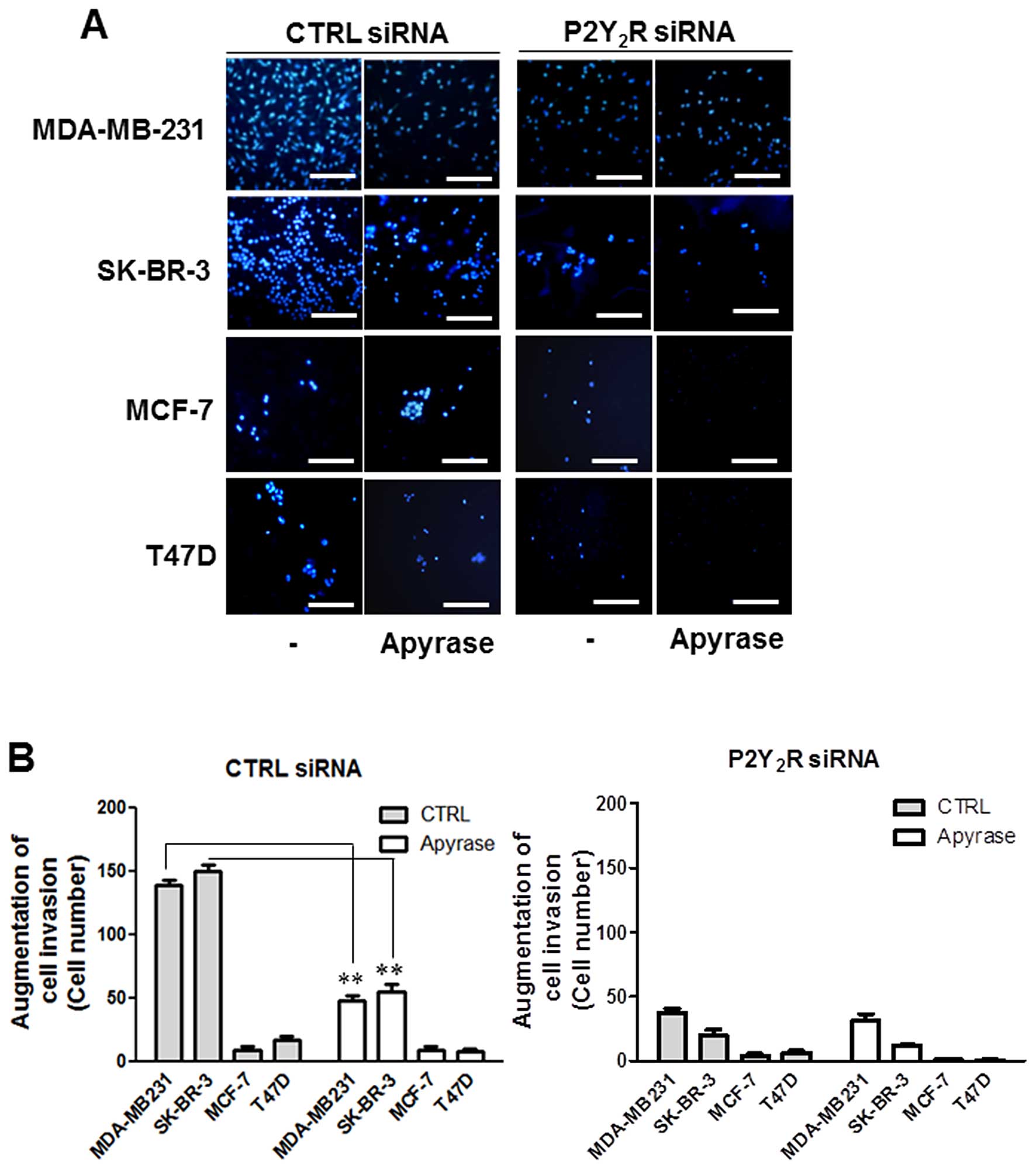
Next, to investigate whether nucleotides released
from highly metastatic breast cancer cells could increase the
invasion of these cells, we performed Matrigel invasion assays.
Control siRNA- or P2Y2R siRNA-transfected breast cancer
cells were seeded on the Matrigel-coated insert wells in serum-free
media and RPMI media with or without apyrase was added to the lower
chambers. After 16 h-incubation, MDA-MB-231 and SK-BR-3 showed a
higher invasion than MCF-7 and T47D in basal level, which was
abolished in the presence of apyrase. In addition, the induced
invasion of MDA-MB-231 and SK-BR-3 was significantly reduced in
P2Y2R siRNA-transfected MDA-MB-231 and SK-BR-3. These
results suggest that ATP released from highly metastatic breast
cancer cells increases invasion of breast cancer cells through
P2Y2R activation (Fig.
2).
ATP released from highly metastatic
breast cancer cells induces the expression of mesenchymal markers,
Snail, Vimentin and N-cadherin, but not the epithelial marker
E-cadherin, through P2Y2R activation in MDA-MB-231
cells
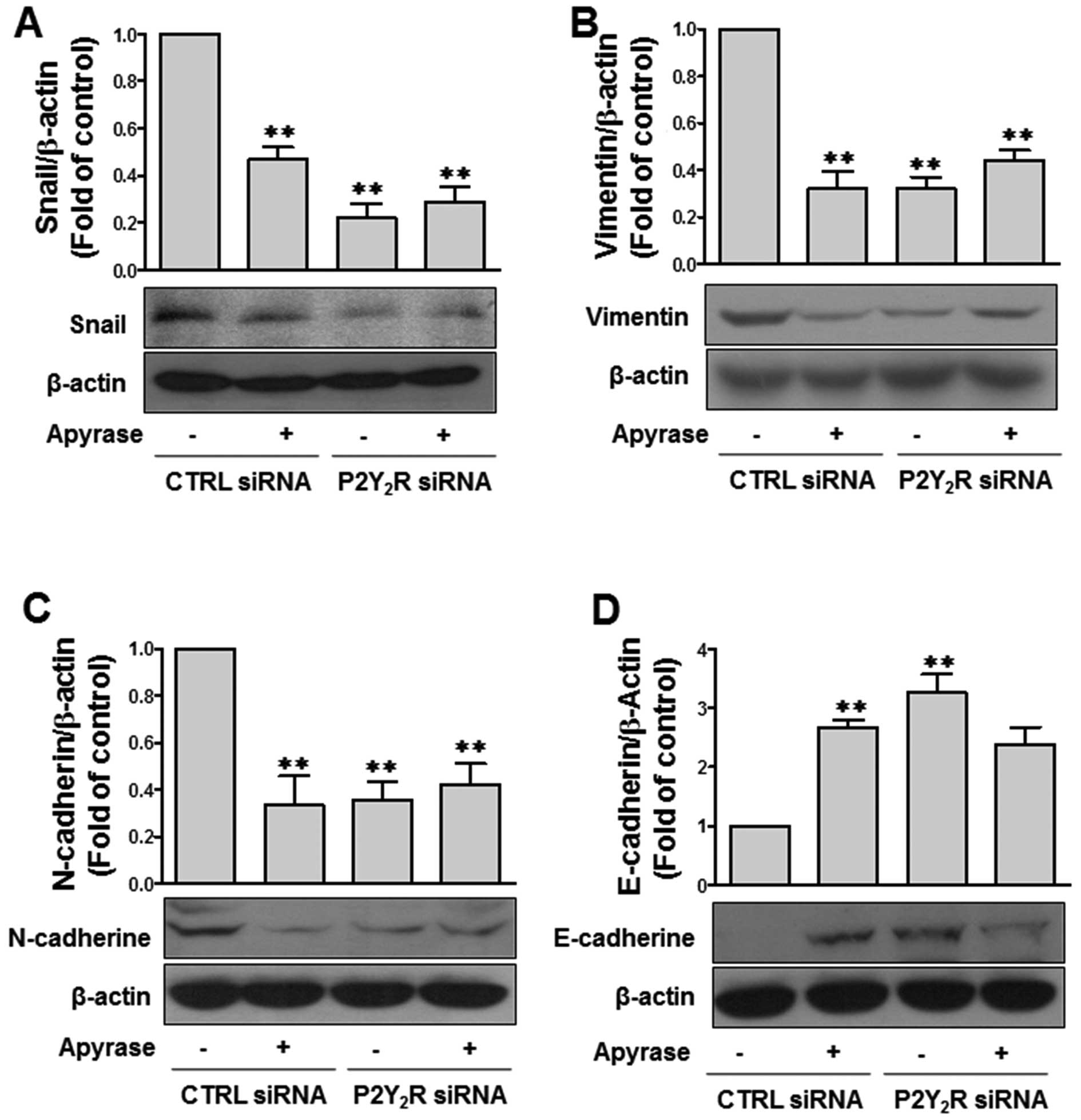
Next, we assessed whether P2Y2R
activation by ATP released from the highly metastatic breast cancer
cells affects the expression of epithelial-mesenchymal transition
(EMT)-related proteins, including the mesenchymal markers Snail,
Vimentin and N-cadherin and the epithelial marker E-cadherin. The
levels of the mesenchymal markers Snail, Vimentin and N-cadherin
were highly induced at a basal level in MDA-MB-231 cells, and this
effect was significantly reduced in the presence of apyrase or
after P2Y2R knockdown. In contrast, the epithelial
marker E-cadherin was not detected at the basal level and ATP
degradation using apyrase or siRNA-mediated P2Y2R
knockdown induced E-cadherin levels (Fig. 3), thereby implicating the release of
ATP from MDA-MB-231 cells in EMT through P2Y2R
activation.
ERK and PKC pathways are over-activated
in highly metastatic breast cancer cells
As shown in Fig. 2
and 3, ATP-mediated
P2Y2R activation increased invasion and EMT-related
protein expression in highly metastatic breast cancer cells. Thus,
we examined which P2Y2R-related signaling pathway is
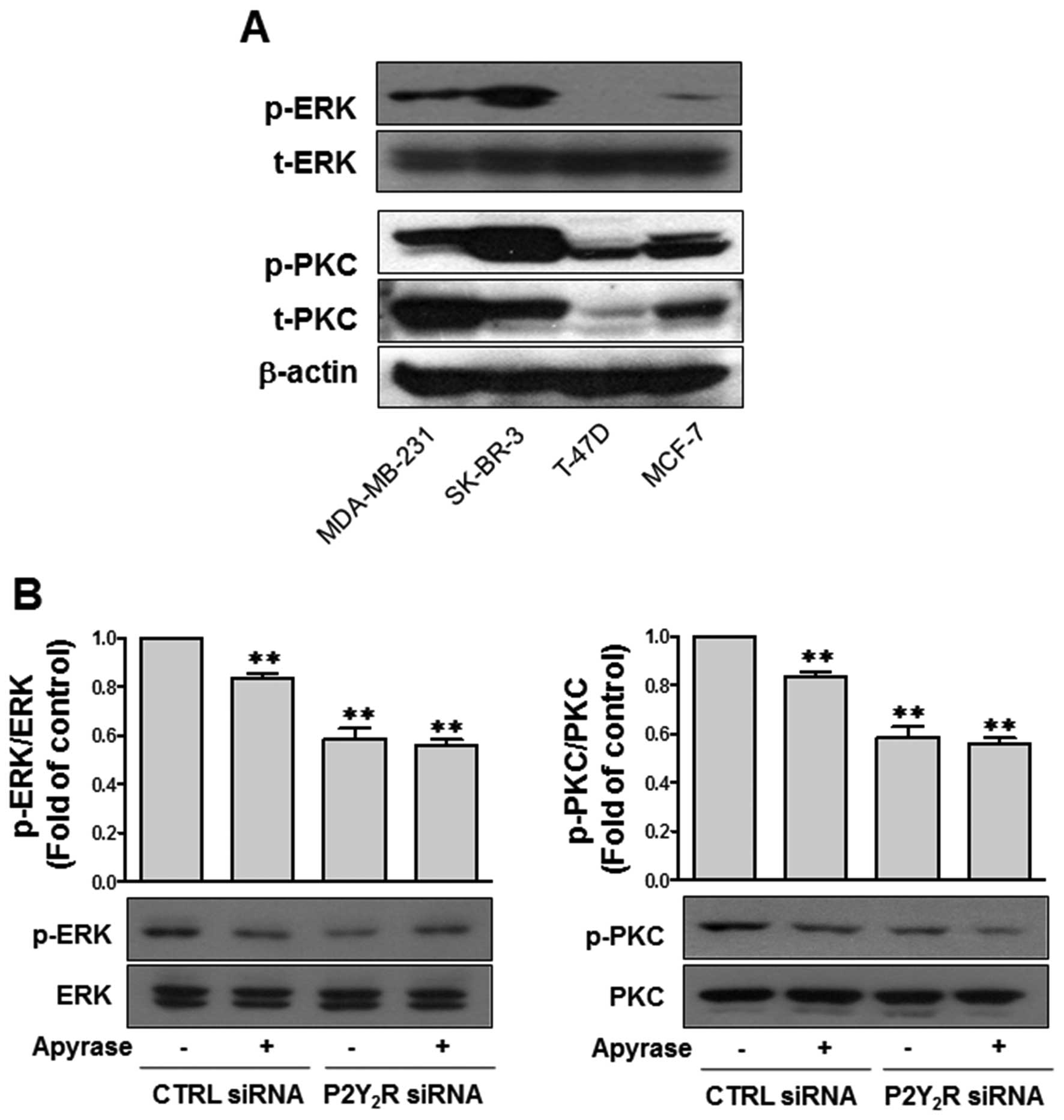
involved in these responses. Preliminary data suggested that
ERK/MAPK and PKC pathways were activated in MDA-MB-231 cells
compared with MCF-7 cells (data not shown). Thus, we further
examined the levels of phospho-ERK and phospho-PKC in SK-BR-3 and
T47D cells. The results shown in Fig.
4A indicated that SK-BR-3 and MDA-MB231 cells exhibited
upregulated ERK and PKC phosphorylation levels at the basal level;
however, phospho-ERK and phospho-PKC were not detectable or
significantly low in T47D and MCF-7 cells. As shown in Fig. 4B, the upregulated ERK and PKC
phosphorylation levels in MDA-MB-231 cells were significantly
downregulated after treatment with apyrase or transfection with
P2Y2R siRNA. These data suggest that over-activated ERK
and PKC pathways are associated with ATP-mediated P2Y2R
activation in highly metastatic breast cancer cells, such as
MDA-MB-231.
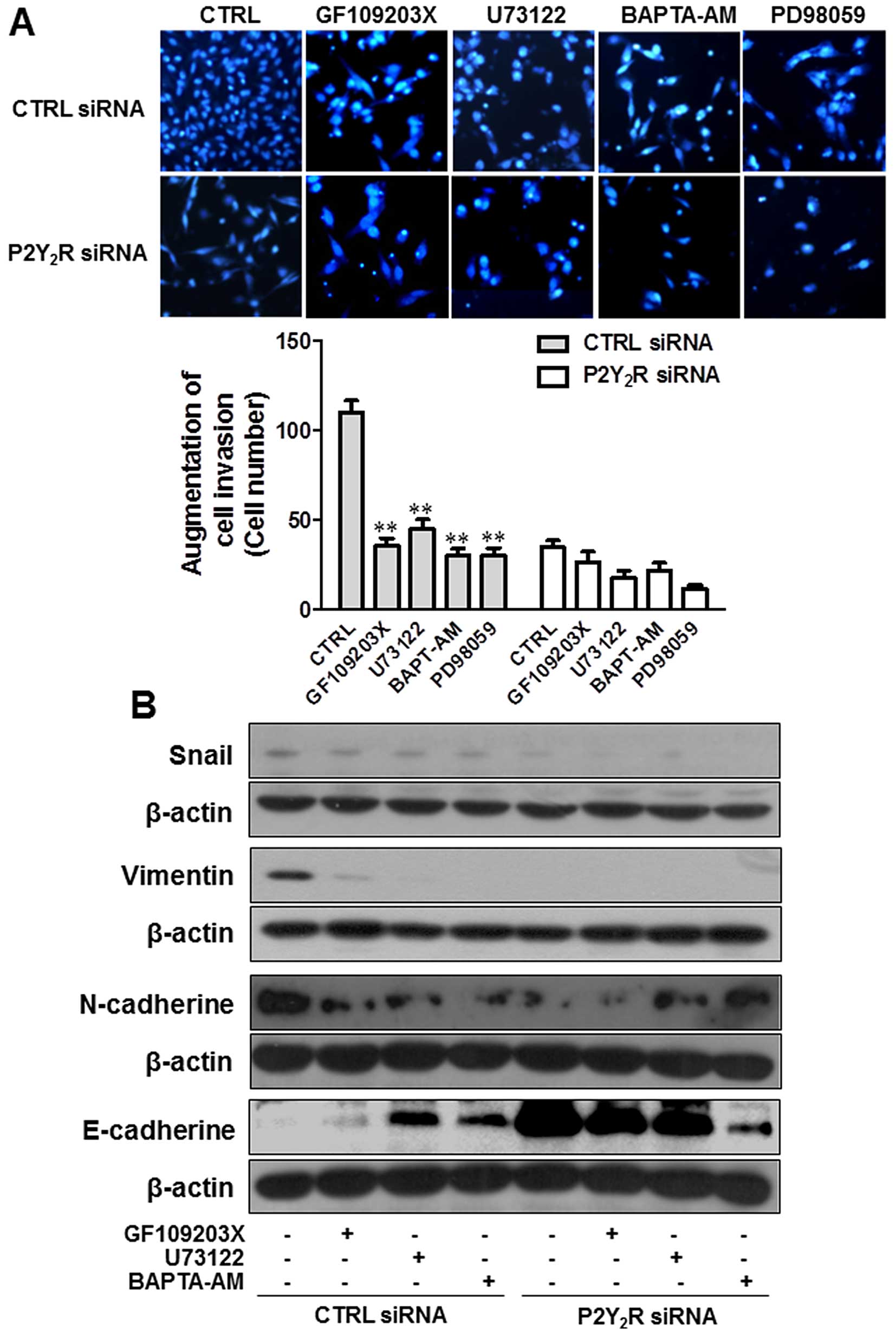
ERK and PKC pathways are involved in the
P2Y2R-mediated breast cancer cell invasion and
EMT-related protein expression
To confirm the involvement of ERK and PKC pathways
in the enhancement of P2Y2R-mediated invasion and
EMT-related protein expression, MDA-MB-231 cells were treated with
specific inhibitors of ERK, PKC and PLC. As expected, treatment
with specific inhibitors (PD98059, an ERK inhibitor; GF109203X, a
PKC inhibitor; U73122, a PLC inhibitor) markedly reduced the
invasion of MDA-MB-231. The intracellular Ca2+ chelator
BAPTA-AM also inhibited the invasion of MDA-MB-231 cells (Fig. 5A). Induced levels of Snail, Vimentin
and N-cadherin expression at the basal level were reduced by
treatment with GF109203X, U73122 and BAPTA-AM, but E-cadherin
expression was induced by these inhibitors and these responses were
a P2Y2R-dependent (Fig.
5B). These results suggest that ATP released from highly
metastatic breast cancer cells activates the P2Y2R
pathway, which mediates ERK and PKC-PLC activation, resulting in
the invasion and EMT of highly metastatic breast cancer cells.
Discussion
In metastasis, cancer cells spread from the site of
origin to adjacent sites and this process is responsible for the
majority of cancer-related mortalities, including breast cancer
(10–12). Therefore, many studies have focused
on elucidating the molecular mechanisms of metastasis. It has been
suggested that the tumor microenvironment affects tumor progression
and the formation of metastases. Recent studies have highlighted
the role and accumulation of nucleotides, such as ATP, within the
tumor microenvironment, which directly affects cancer progression
through purinergic receptors. Among the receptors engaged by
extracellular ATP (P2 receptors), the P2Y2R is most
consistently expressed (or overexpressed) on tumor cells. The
activation of P2Y2Rs supports the progression of each
step of metastasis, including angiogenesis, intravasation and
invasion and tumor growth (5–7). In a
previous study, we reported that MDA-MB-231, a highly metastatic
breast cancer cell line, released higher levels of ATP and showed
higher P2Y2R activity compared with the low-metastatic
breast cancer cell line MCF-7 and the ATP-mediated activation of
P2Y2R plays an important role in cancer metastasis
through the modulation of crosstalk between cancer cells and ECs
(2). In addition, we showed that
P2Y2R activation by ATP released from highly metastatic
breast cancer cells induces HIF-1α expression, lysyl oxidase
secretion and collagen crosslinking, generating a receptive
microenvironment for pre-metastatic niche formation (3). In the present study, we also confirmed
that the highly metastatic breast cancer cell lines SK-BR-3 and
MDA-MB-231 released markedly higher levels of ATP and showed higher
P2Y2R activity compared with the low metastatic breast
cancer cell lines MCF-7 and T47D. Furthermore, we observed that ERK
and PKC pathways are activated in highly metastatic breast cancer
cells and ATP-mediated P2Y2R activation induces EMT
invasion through ERK and PKC pathways.
Breast cancer is a heterogeneous disease, classified
into luminal A (ER+ or PR+,
HER2−), luminal B (ER+ or PR+,
HER2+), HER2-positve (ER− and PR−,
HER2+) and triple-negative (ER− and
PR−, HER2−) subtypes. Generally,
triple-negative breast cancers (TNBCs) are known to be an
aggressive group of breast cancers with higher rates of relapse
compared with ER/PR− and HER2-positive breast cancers,
despite optimal locoregional and systemic therapies. However,
molecular analyses, including microarray, DNA copy-number variation
and DNA sequencing, have shown significant biological diversity
within this subgroup (13–15). Moreover, it has been reported that
ER-independent (ER−) breast cancers are more aggressive,
possess high metastatic potential and are unresponsive to
antiestrogens (1,16). Indeed, MDA-MB-231 cells present as
TNBCs with highly metastatic characteristics. Although SK-BR-3
cells are not TNBCs, these metastatic breast cancer cells are
ER-negative cells (ER−/PR−/HER2+)
and both SK-BR-3 and MDA-MB-231 cells exhibit increased
invasiveness.
Tumor metastasis is responsible for most cancer
deaths. Signal transduction in the microenvironment around the
primary tumor locus may trigger tumor metastasis, particularly at
the migration stage. Sustained MAPK signaling involved in
uncontrolled tumor cell migration relies on crosstalk between
integrin, receptor tyrosine kinase and PKC. In a previous study, we
reported that the conditions of the tumor microenvironment,
specifically the high level of ATP released from cancer cells,
induced tumor metastasis through P2Y2R activation
(2). Gq-coupled P2Y2R
activation by ATP or UTP results in intracellular calcium
mobilization and PKC and phosphatidylinositol 3-kinase (PI3K)
activation. In addition, src-homology-3 binding domains (PXXP)
within the C-terminus of P2Y2R bind Src to enable ATP or
UTP to transactivate growth factor receptors and downstream MAP
kinases (17), suggesting that
P2Y2R-mediated MAPK and PKC pathways are involved in the
induction of breast cancer cell invasion. Accordingly, we
determined the levels of phospho-MAPK and phospho-PKC in the highly
metastatic breast cancer cell line MDA-MB-231. SK-BR-3 and
MDA-MB-231 showed activated ERK and PKC levels, and upregulated ERK
and PKC phosphorylation levels in MDA-MB-231 cells were
significantly downregulated by treatment with apyrase or through
P2Y2R knockdown. Therefore, we suggest that
P2Y2R activation by ATP released from highly metastatic
breast cancer cells increases metastasis through ERK and PKC
pathways. These results are consistent with reports that ERK
regulates migration in several cell types (18,19)
and MAPK and PKC signaling pathways are involved in the regulation
of MMP-9 transcription, closely associated with the MMP-9 activity
in numerous cancer cells (20,21).
During EMT, epithelial tumor cells lose polarized
organization and cell-cell junctions. Thus, the cells undergo
changes in shape and cytoskeletal organization and acquire
mesenchymal characteristics important for metastasis (22,23).
The loss of E-cadherin expression in epithelial tumors has been
associated with a more invasive phenotype and metastasis (24). N-cadherin promotes cell motility and
migration, effects opposite to that of E-cadherin (24). PI3K/Akt activation results in the
phosphorylation of GSK-3β (inactivation of GSK-3β), which in turn
increases Snail and β-catenin protein levels, ultimately resulting
in the suppression of E-cadherin transcription and induction of
N-cadherin expression. In the present study, we observed that the
P2Y2R-mediated activation of ERK and PKC pathways
induced invasion and metastasis through the modulation of the EMT
process. According to Martiáñez et al (25), P2Y2R activation by UTP
induced N-cadherin expression via downstream pathways, such as
ROCK, PLC, Ca2+ and PKC and MAPKs, including ERK in
Schwann cells, a type of peripheral myelinating glial cell.
N-cadherin expression through P2Y2R activation could
also involve JNK, P38 and ERK pathways in MDA-MB-231 cells.
However, in the present study, we focused on identifying the
different signaling pathways involved in high and low-metastatic
cancer cells, and it was shown that ERK/MAPK and PKC are
over-activated in the MDA-MB-231 cells compared with MCF-7 cells.
Thus, it is proposed that ATP released from highly metastatic
breast cancer cells and the subsequent P2Y2R activation
by ATP mediate ERK and PKC-PLC activation, resulting in invasion
and EMT of highly metastatic breast cancer cells.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(2012R1A1A3003268).
Abbreviations:
|
ATP
|
adenosine triphosphate
|
|
CTRL
|
control
|
|
DAPI
|
4′,6-diamino-2-phenylindole
|
|
EC
|
endothelial cell
|
|
ECM
|
extracellular matrix
|
|
ECL
|
enhanced chemiluminescence
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ER
|
estrogen receptor
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
FBS
|
fetal bovine serum
|
|
HER2
|
human epidermal growth factor
receptor-2
|
|
MAPK
|
mitogen activated protein kinase
|
|
MTT
|
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
|
|
PBS
|
phosphate-buffered saline
|
|
PKC
|
protein kinase C
|
|
PLC
|
phospholipase C
|
|
PR
|
progesterone receptor
|
|
P2Y2R
|
P2Y2 receptor
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
siRNA
|
small interfering RNA
|
|
TBS-T
|
Tris-buffered saline/Tween-20
|
|
TNBC
|
triple-negative breast cancers
|
References
|
1
|
Anandappa SY, Sibson R, Platt-Higgins A,
Winstanley JH, Rudland PS and Barraclough R: Variant estrogen
receptor α mRNAs in human breast cancer specimens. Int J Cancer.
88:209–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin H, Lee JS, Park SW, Lee JH, Chang KC
and Kim HJ: P2Y2R activation by nucleotides released
from highly metastatic breast cancer cells increases tumor growth
and invasion via crosstalk with endothelial cells. Breast Cancer
Res. 16:R772014. View
Article : Google Scholar
|
|
3
|
Joo YN, Jin H, Eun SY, Park SW, Chang KC
and Kim HJ: P2Y2R activation by nucleotides released
from the highly metastatic breast cancer cell MDA-MB-231
contributes to pre-metastatic niche formation by mediating lysyl
oxidase secretion, collagen crosslinking, and monocyte recruitment.
Oncotarget. 5:9322–9334. 2014.PubMed/NCBI
|
|
4
|
Burnstock G: Purine and pyrimidine
receptors. Cell Mol Life Sci. 654:1471–1483. 2007. View Article : Google Scholar
|
|
5
|
Bilbao PS, Santillan G and Boland R: ATP
stimulates the proliferation of MCF-7 cells through the PI3K/Akt
signaling pathway. Arch Biochem Biophys. 499:40–48. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schafer R, Sedehizade F, Welte T and
Reiser G: ATP- and UTP-activated P2Y receptors differently regulate
proliferation of human lung epithelial tumor cells. Am J Physiol
Lung Cell Mol Physiol. 285:L376–L385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schumacher D, Strilic B, Sivaraj KK,
Wettschureck N and Offermanns S: Platelet-derived nucleotides
promote tumor-cell transendothelial migration and metastasis via
P2Y2 receptor. Cancer cell. 24:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suganuma M, Okabe S, Marino MW, Sakai A,
Sueoka E and Fujiki H: Essential role of tumor necrosis factor
alpha (TNF-alpha) in tumor promotion as revealed by
TNF-alpha-deficient mice. Cancer Res. 59:4516–4518. 1999.PubMed/NCBI
|
|
9
|
Egberts JH, Cloosters V, Noack A,
Schniewind B, Thon L, Klose S, Kettler B, von Forstner C, Kneitz C,
Tepel J, et al: Anti-tumor necrosis factor therapy inhibits
pancreatic tumor growth and metastasis. Cancer Res. 68:1443–1450.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steeg PS: Cancer: Micromanagement of
metastasis. Nature. 449:671–673. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eccles SA and Welch DR: Metastasis: Recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prat A, Adamo B, Cheang MC, Anders CK,
Carey LA and Perou CM: Molecular characterization of basal-like and
non-basal-like triple-negative breast cancer. Oncologist.
18:123–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keen JC and Davidson NE: The biology of
breast carcinoma. Cancer. 97:825–833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weisman GA, Ajit D, Garrad R, Peterson TS,
Woods LT, Thebeau C, Camden JM and Erb L: Neuroprotective roles of
the P2Y(2) receptor. Purinergic Signal. 8:559–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frogne T, Benjaminsen RV, Sonne-2 Hansen
K, Sorensen BS, Nexo E, Laenkholm AV, Rasmussen LM, Riese DJ II, de
Cremoux P, Stenvang J, et al: Activation of ErbB3, EGFR and Erk is
essential for growth of human breast cancer cell lines with
acquired resistance to fulvestrant. Breast Cancer Res Treat.
114:263–275. 2009. View Article : Google Scholar :
|
|
19
|
Zhang SS, Liu MG, Kano A, Zhang C, Fu XY
and Barnstable CJ: STAT3 activation in response to growth factors
or cytokines participates in retina precursor proliferation. Exp
Eye Res. 81:103–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin Y, Yoon SH, Choe EY, Cho SH, Woo CH,
Rho JY and Kim JH: PMA-induced up-regulation of MMP-9 is regulated
by a PKCalpha-NF-kappaB cascade in human lung epithelial cells. Exp
Mol Med. 39:97–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fini ME, Cook JR, Mohan R and Brinckerhoff
EC: Regulation of matrix metalloproteinase gene expression. Parks
WC and Mecham RP: Matrix metalloproteinases. Academic Press; New
York, NY: pp. 2991998, View Article : Google Scholar
|
|
22
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cavallaro U and Christofori G: Cell
adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev
Cancer. 4:118–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martiáñez T, Lamarca A, Casals N and Gella
A: N-cadherin expression is regulated by UTP in schwannoma cells.
Purinergic Signal. 9:259–270. 2013. View Article : Google Scholar :
|