Introduction
Breast cancer (BC) is a global health problem and
one of the principal causes of female morbidity and mortality
(1,2). It is a major public health concern in
both developed and developing countries (3). Approximately 10–17% of breast cancers
are defined as triple-negative (TN), i.e, the absence of estrogen
and progesterone receptor, and of overexpression and/or
amplification of the HER-2 (4,5).
Although advances have made in clinical and experimental oncology
studies, the prognosis of TN breast cancer remains extremely poor
(6). The clinical impact of
molecular-targeted therapy in the TNBC population remains unclear
(7). Mounting evidence of the
signal transduction pathways for transcription factors suggests
that these pathways could offer crucial targets for cancer
therapy.
The transcription factor hypoxia-inducible factor-1
(HIF-1) is a heterodimeric protein composed of an oxygen-regulated
HIF-1α and a constitutively-expressed HIF-1β subunit. Under
normoxic conditions, HIF-1α is hydroxylated on proline residue 402
and/or 564, which is required for binding of the von Hippel-Lindau
protein, the recognition subunit of an E3 ubiquitin ligase that
targets HIF-1α for proteasomal degradation (8). However, hydroxylation decreases under
hypoxic conditions, enabling HIF-1α to accumulate and dimerize with
HIF-1β. The functional transcription factor then binds at the core
hypoxia response element, 5′-RCGTG-3′, to induce genes involved in
angiogenesis, glycolysis, de-differentiation, invasion and
metastasis. Overexpression of HIF-1α is associated with increased
mortality in many cancer types (9–11).
Numerous cancer chemotherapeutic agents target
angiogenesis in tumors to reduce primary tumor growth. Response to
anti-vascular endothelial growth factor (VEGF) therapy has,
however, been poor, as intratumoral hypoxia arising from impaired
angiogenesis causes HIF-1-dependent metastasis and expansion of
cancer stem cell pools (12).
HIF-1α is the main transcription factor mediating the hypoxic
response, which promotes the transcription of angiogenic factors,
such as VEGF and leads to increased glycolysis via the inhibition
of mitochondrial oxidative phosphorylation (13). The critical role of the hypoxia
response network and HIF-1α has resulted in it being viewed as an
ideal target for small molecule intervention. Small molecule
inhibitors of HIF-1α are widely studied and are considered
important due to their central role in tumorigenesis.
Combretastatins are natural cis-stilbenes that are
isolated from the bark of the African willow tree Combretum
caffrum. Combretastatin A4 is the most prominent representative
of this group of compounds, which exerts high anti-mitotic and
anti-angiogenic activities (14).
Previous studies (15) showed that
a new CA4 analogue, (Z)-3,4′,5-trimethoxylstilbene-3′-O-phosphate
disodium (M410) was a potent inhibitor of bovine brain tubulin
polymerization in vitro. In experiments on nude mice in
vivo, M410 inhibited the growth of human colon carcinoma
xenografts and reduced microvessel density in tumor tissues. M410
exhibited a prominent cytotoxic effect, which downregulated HIF-1α
expression, reduced nuclear HIF-1α and subsequently downregulated
VEGF mRNA. In the present study, we assessed the activity of M410
on TNBC cell lines and confirmed the mechanism of the inhibitory
effects of M410 on a MDA-MB-231 breast cancer cell line.
Materials and methods
Cell lines and culture conditions
The Human BT549, MDA-MB-453, MDA-MB-231 and SK-BR-3
BC cell lines were maintained in Dulbecco’s modified Eagle’s medium
(DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS;
Hyclone, Logan, uT, uSA), 100 u/ml penicillin and 100 µg/ml
streptomycin. The cells were cultured in a humidified atmosphere
containing 5% CO2 at 37°C. For the treatment of hypoxia,
the cells were incubated in an MIC-101 chamber (Billups Rothenberg,
Inc., Del Mar, CA, uSA) containing 5% CO2 balanced with
N2 (<0.1% O2) or exposed to
CoCl2 (200 µM) for 4–6 h.
Reagents and antibodies
M410 was synthesized by the Guangzhou Institute of
Chemistry, Chinese Academy of Sciences (15). M410 was dissolved in distilled water
to yield a 10-mM stock solution. Cycloheximide (CHX) was dissolved
in distilled water to yield a 10-mg/ml stock. MG132 (C2211) and
monastrol (M8515) (both from Sigma-Aldrich, St. Louis, MO, uSA)
were respectively dissolved in DMSO as stocks of 10 mM. The primary
antibodies used were: HIF-1α antibody (610959) and HIF-1β antibody
(611078) (both from BD Transduction Laboratories, Bedford, MA,
uSA), β-tubulin antibody (no. 2128; Cell Signaling Technology,
Danvers, MA, uSA), monoclonal anti-β-tubulin antibody (D00057;
Sigma-Aldrich), β-actin antibody (no. 3700; Cell Signaling
Technology), NF-κB-p65 antibody (SAB, no. 21012) and c-Fos antibody
(SAB, no. 21667).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells were plated in a 96-well plate and cultured in
medium with various concentrations of M410 added after 24 h. After
68-h incubation, MTT was added to each well (100 µg/well)
and incubated for an additional 4 h. The insoluble formazan
produced was dissolved with 200 µl DMSO, and optical density
was measured using an ELISA reader (Thermo Labsystems, Espoo,
Finland) at wavelengths of 570 and 630 nm. Experiments were
performed in triplicate. From these results, the percentages of
live cells in each well were estimated and plotted against the drug
concentrations as dose-response curves, from which the 50% growth
inhibition (GI50) was derived.
Immunofluorescence and confocal
microscopy
Exponentially growing cells were placed on 15-mm
coverslips in 6-well plates and the cells were allowed to attach
overnight. The following day, the cells were treated with the
indicated agents for 24 h and subjected to hypoxia or remained
normoxic for an additional 6 h. The cells were fixed with
pre-warmed 4% para-formaldehyde (PFA) at 37°C for 30 min and washed
with PBS for four times. After being permeabilized with PBS
containing 0.5% Triton X-100 (vol/vol) for 15 min at room
temperature and washed with PBS, the cells were blocked with 5%
bovine serum albumin (BSA) for 1 h and then incubated with primary
antibody at 4°C overnight. Subsequently, the cells were washed with
PBS and re-incubated with DyLight™ 549 or 488-labeled antibody in a
dark room for 1 h. The cells were then stained for the nuclei with
0.1 µg/ml DAPI in a dark room for 10 min. The coverlips were
fixed on the slides using an antifade reagent (Life Technologies,
Carlsbad, CA, USA) and then observed using an Olympus FV100
confocal microscope.
ELISA for VEGF
VEGF concentrations in media from treated and
untreated cells were determined using a quantitative sandwich
enzyme immunoassay (Human VEGF Quantikine ELISA kit; R&D
Systems, Inc., Minneapolis, MN, uSA) according to the
manufacturer’s instructions. The results were expressed as a
concentration of VEGF (pg/ml) per total protein amount from each
well.
Immunoblot analysis
Lysates were prepared from 4×105 cells by
dissolving cell pellets in 100 µl of lysis buffer (20 mM
Na2PO4, pH 7.4, 150 mM NaCl, 1% Triton X-100,
1% aprotinin, 1 mM phenymethysulfonyl fluoride, 10 mg/ml leupeptin,
100 mM NaF and 2 mM Na3VO4). The lysates were
centrifuged at 14,000 rpm for 20 min. The supernatant was
collected. Protein concentrations were determined using a BCA
protein assay kit (Thermo Scientific). The protein content was
determined using the Bio-Rad protein assay. Protein (10 µg)
was loaded in each well of 10–12% SDS-PAGE gels. Resolved proteins
were electrophoretically transferred to PVDF membranes and
incubated sequentially with primary antibody and HRP-conjugated
secondary antibody (Cell Signaling Technology). After washing, the
bound antibody complex was detected using LumiGLO reagent (no.
7003; Cell Signaling Technology) and XAR film (XBT-1; Kodak,
Rochester, NY, uSA) according to the manufacturer’s
instructions.
Isolation and analysis of RNA
Total RNA was isolated from MDA-MB-231 cells treated
and untreated with M410 using TRIzol reagent (Life Technologies).
Then, 1 µg of RNA was reverse transcribed in a 20-µl
reaction using a Transcriptor First Strand cDNA Synthesis kit
(Roche Applied Science, Mannheim, Germany) according to the
manufacturer’s instructions. PCR was performed using PCR Master Mix
(Promega, Madison, WI, uSA) and 2 µl of cDNA was used for
each reaction. The PCR conditions were: denaturation for 2 min at
94°C, 35 cycles of 94°C for 45 sec, annealing temperatures for 45
sec, and extension at 72°C for 60 sec. A 10-min extension at 72°C
was carried out to the end. PCR products were visualized with
GelRed on 1.5% agarose gels. The primers were designed using the
Primer-BLAST and shown in Table
I.
 | Table IPrimers used for RT-PCR. |
Table I
Primers used for RT-PCR.
| mRNA | Forward primer | Reverse primer | Product size
(bp) | Annealing temperature
(°C) |
|---|
| GLuT1 |
GTGCCCATGTATGTGGGTGA |
CTAGCGCGATGGTCATGAGT | 649 | 60 |
| HIF-1α |
CCCCAGATTCAGGATCAGACA |
CCATCATGTTCCATTTTTCGC | 704 | 59 |
| VEGFA |
TCACCAAGGCCAGCACATAG |
GAGGCTCCAGGGCATTAGAC | 202 | 62 |
| β-actin |
TCTACAATGAGCTGCGTGTG |
GGTGAGGATCTTCATGAGGT | 314 | 56 |
Statistical analysis
Experiments were repeated three times. The results
of multiple experiments are given as the mean ± SE. Statistical
analysis was performed using the statistical software package SPSS
17.0. P-values were calculated using a one-way ANOVA test or the
Student’s t-test. P<0.05 was considered to be statistically
significant.
Results
M410 reduces HIF-1α protein level in
MDA-MB-231 cells
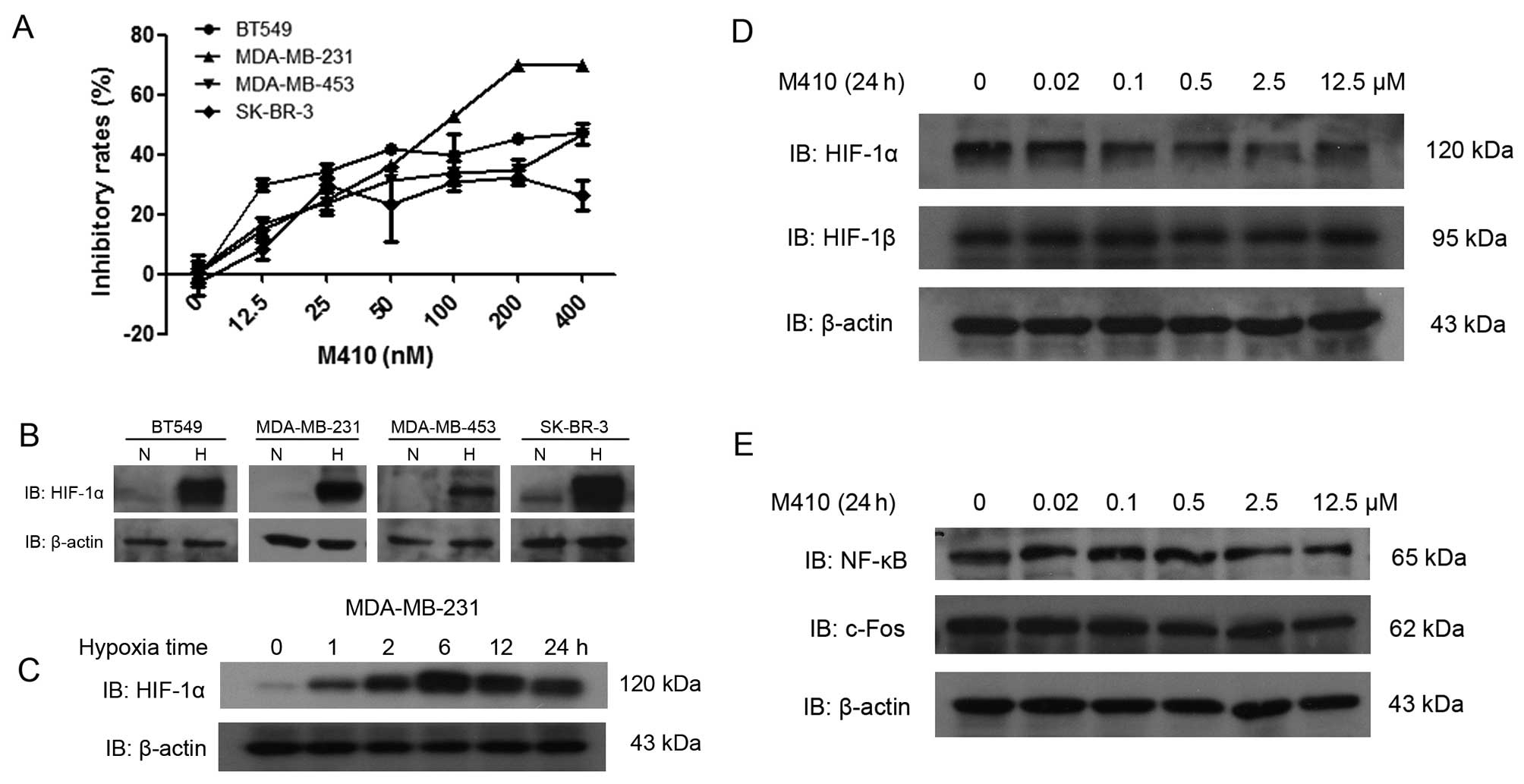
First, we examined the viability of the four BC cell
lines by MTT assay in the presence of M410 with different
concentrations (0–400 nM). MDA-MB-231 was the most sensitive, with
the concentration of GI50 of 111.4±2.2 nM at 72 h.
Western blot analyses showed that HIF-1α was weakly expressed in
the SK-BR-3 cells, but was not expressed in the remaining three BC
cells under normoxia. Following treatment with hypoxia for 6 h, the
expression of HIF-1α was highly induced in all the cell lines
(Fig. 1B). under the indicated time
of hypoxia, the levels of HIF-1α protein increased rapidly, peaked
at the 6-h time-point, and was then reduced gradually (Fig. 1C). HIF-1α expression was clearly
reduced after M410 treatment in a dose-dependent manner. To examine
whether these inhibitions were specific for HIF-1α, the regulated
subunit of HIF-1, we also assessed the effect of M410 on HIF-1β
expression. HIF-1β was not affected by M410 treatment. Similarly,
transcription factors such as NF-κB and c-Fos were not affected
(Fig. 1D and E).
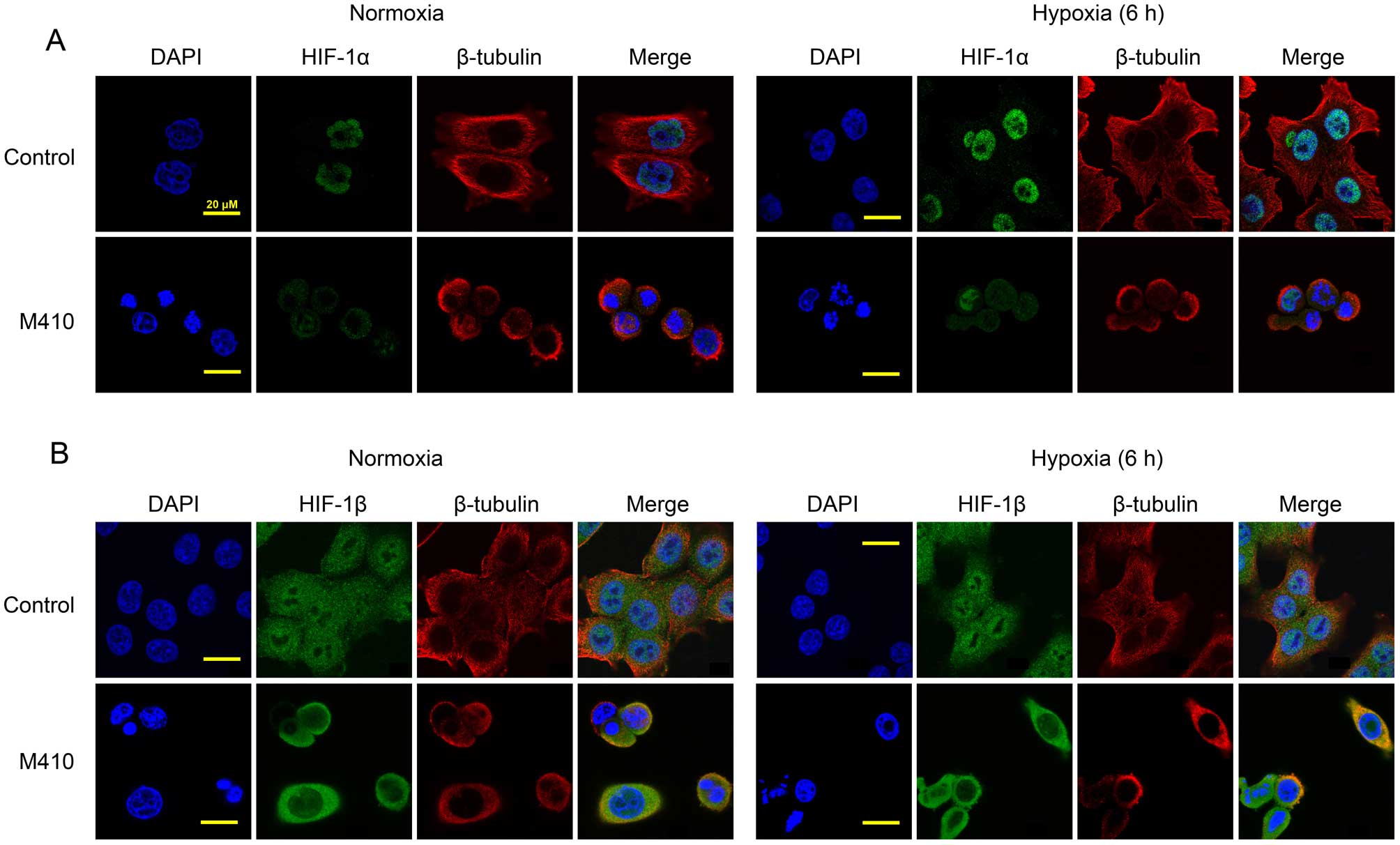
M410 depolymerizes microtubules and
inhibits the nuclear accumulation of HIF-1α
M410 has been shown to depolymerize microtubules in
human vesicular endothelial cells (HuVECs) as well as in tumor
cells resulting in G2/M arrest (15). We investigated the correlation
between the effects of M410 on microtubules and its effects on
HIF-1α (Fig. 2). using laser
scanning confocal microscopy, MDA-MB-231 cells treated with or
without M410 were observed by double-labeled antibodies against
β-tubulin and HIF-1α. In untreated control cells, we observed an
intricate and intact microtubule network while we observed the
depolymerization of microtubules in the M410-treated cells. No
significant changes in the microtubule network were observed in the
control cells after hypoxia. Under the normoxic conditions, HIF-1α
was barely detectable while it predominantly accumulated in the
nucleus after exposure to hypoxia. Nuclear localization of HIF-1α
was confirmed by staining with DAPI. The treatment of M410
significantly reduced the hypoxia-induced nuclear accumulation of
HIF-1α. Consistent with the results of western blotting, the
treatment of M410 had no effect on the expression of HIF-1β
(Fig. 2B). In the untreated cells,
HIF-1β was localized in the nucleus and the cytoplasm, especially
in the nucleus. Under hypoxic conditions, HIF-1β did not accumulate
in the nucleus. The treatment of M410 affected the subcellular
localization of HIF-1β.
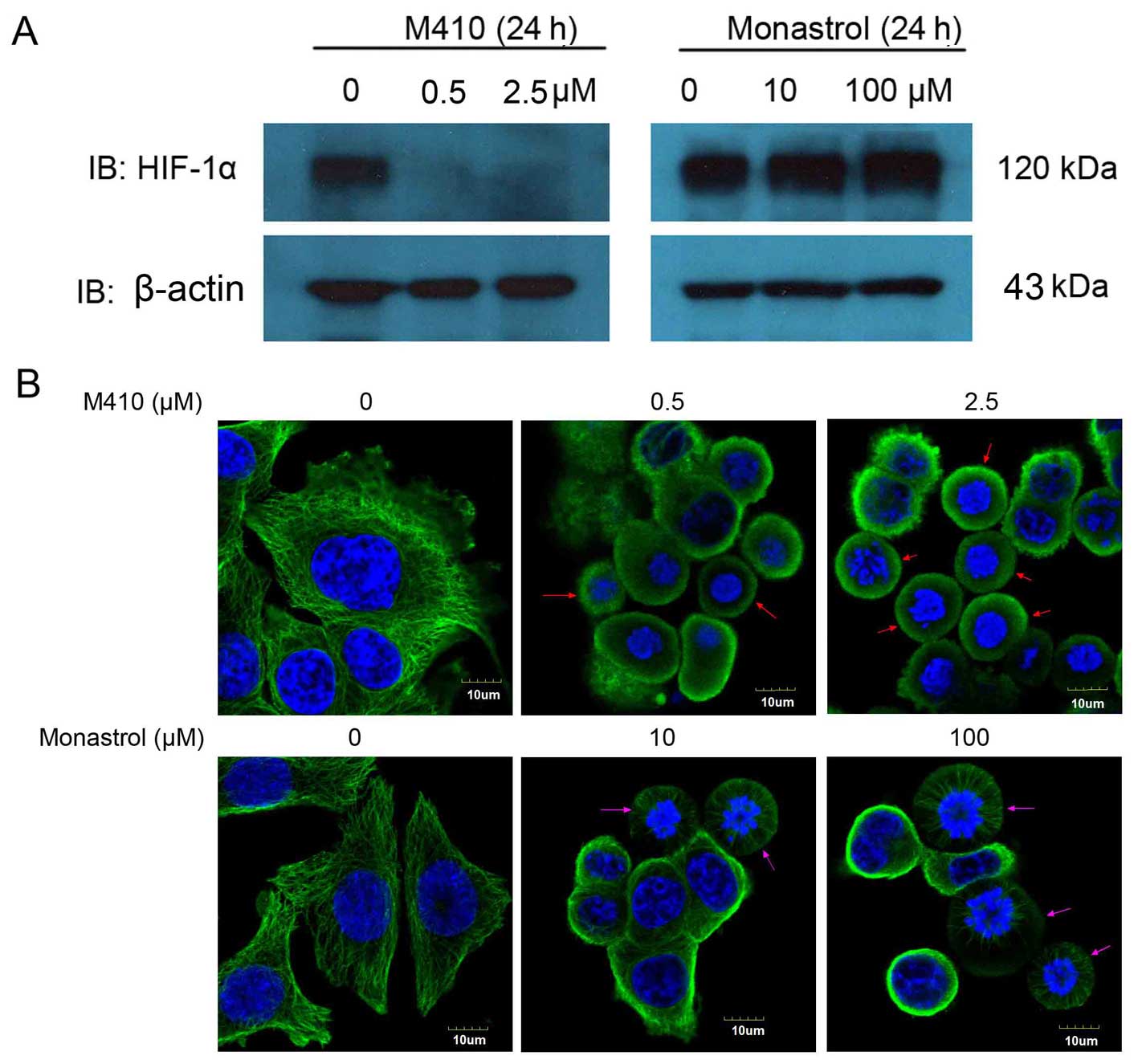
M410 inhibition of HIF-1α function is
independent of mitotic arrest
Since M410 has been shown to induce mitotic arrest
(15), we clarified the correlation
between the inhibition of HIF-1α and the mitotic arrest.
Subsequently, we treated MDA-MB-231 cells with monastrol, which is
not a microtubule-targeting compound and is known to induce mitotic
arrest by inhibiting the mitotic kinesin Eg5, utilizing M410 as a
positive control. As shown in Fig.
3A, monastrol had no effect on HIF-1α protein levels even at
concentrations that induced a significant mitotic arrest. Although
arrested in the interphase, the micro-tubule network and the
spindles (arrow) were intact in the monastrol-treated cells
compared with the depolymerization of microtubule in the
M410-treated cells. These results suggested that the inhibition of
HIF-1α function by M410 was independent of M410-induced mitotic
arrest (Fig. 3B).
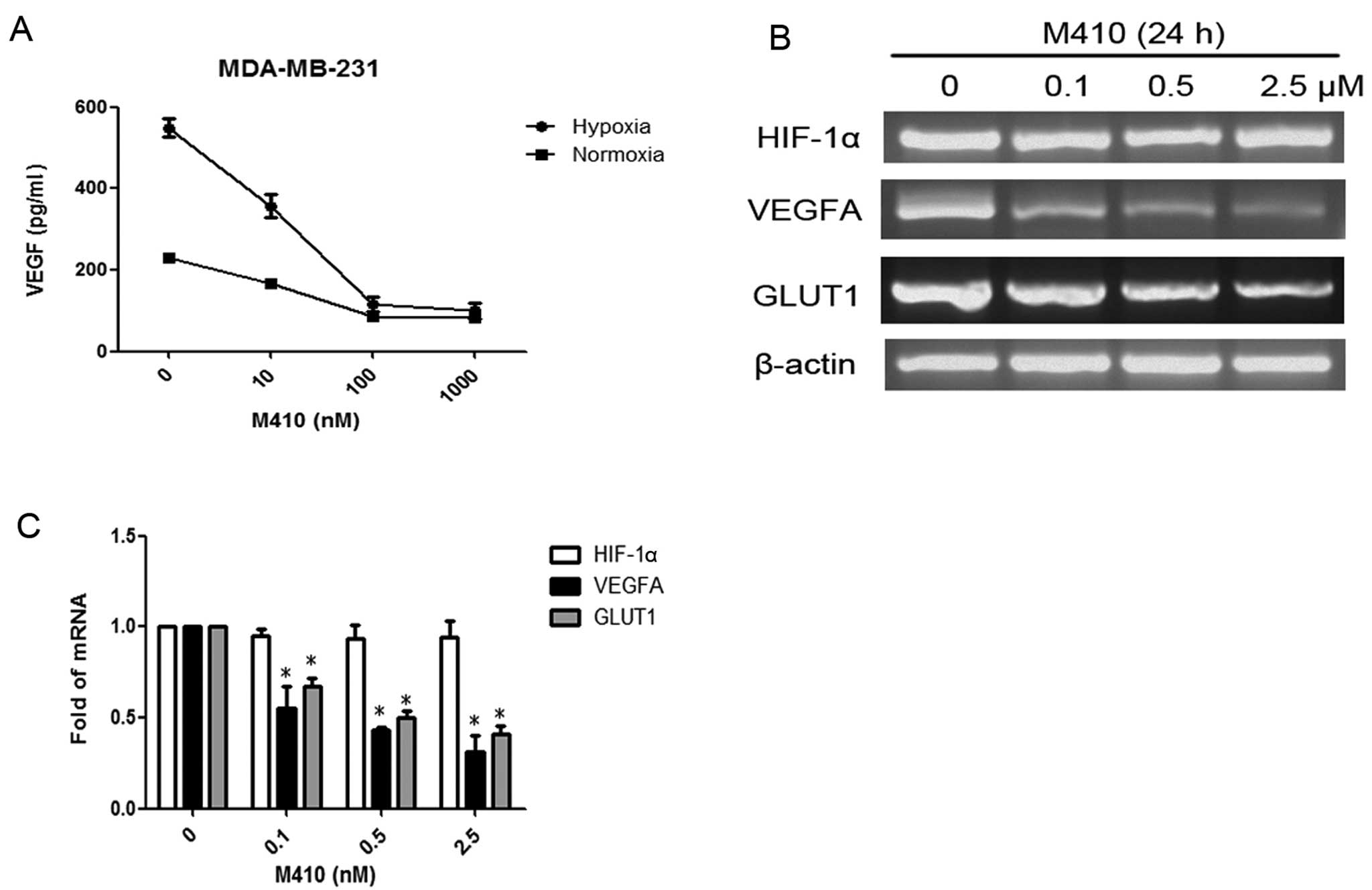
M410 inhibits HIF-1α transcriptional
activity
To determine the effects of M410 treatment on HIF-1
transcriptional activity, we examined the VEGF protein level in the
supernatant medium from M410-treated MDA-MB-231 cells. Consistently
with the reduced levels of HIF-1α protein levels by M410, VEGF
protein levels were also significantly decreased in a
dose-dependent manner under hypoxia (Fig. 4A). To determine whether the
downregulation of HIF-1α by M410 occurred at the transcriptional
level, we extracted total RNA from M410-treated MDA-MB-231 cells
and performed two-step RT-PCR. HIF-1α mRNA levels were not
significantly changed by M410 (Fig. 4B
and C). On the other hand, the mRNA levels of VEGF and another
HIF-1 target gene, i.e., GLUT1 glucose transporter were
significantly decreased by M410 treatment.
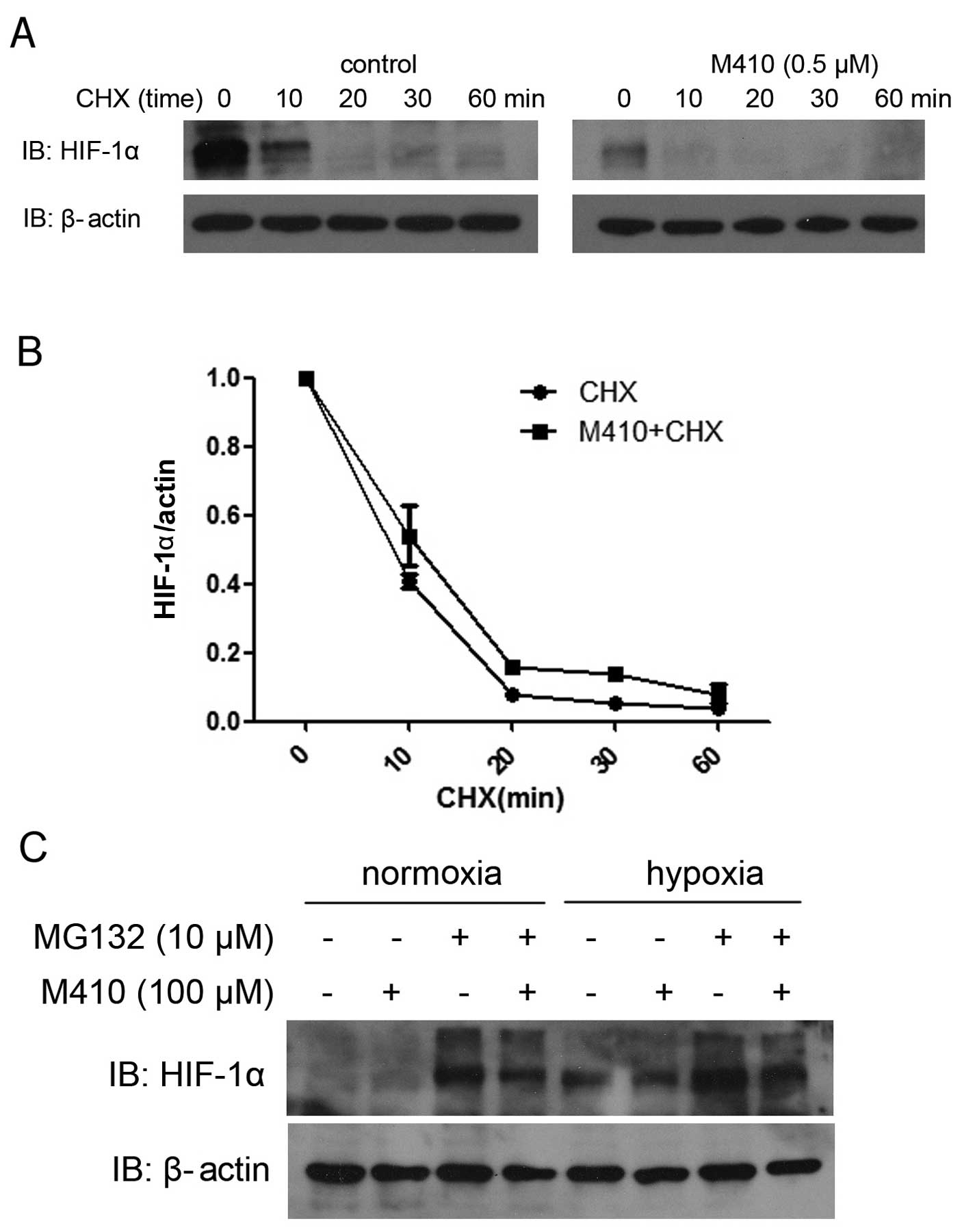
M410 downregulates HIF-1α expression in a
proteasome-independent manner
To examine the effect of M410 on the HIF-1
post-transcriptional process, we examined the M410 treatment on
HIF-1α protein stability by using the protein translation inhibitor
CHX. As CHX inhibits new protein synthesis, the HIF-1α level would
mostly reflect the degradation process of HIF-1α protein. untreated
or M410-treated cells were exposed to CHX from 0 to 60 min and
HIF-1α levels were analyzed by western blotting (Fig. 5A). We assessed whether M410 combined
with CHX treatment affected the half-life of HIF-1α compared with
CHX treatment alone. The results indicated no statistical
significance (Fig. 5B). To
eliminate the possibility that M410 affects HIF-1α ubiquitination
and degradation via the proteasome pathway, we treated MDA-MB-231
cells with the MG132 proteasome inhibitor. In the untreated cells,
MG132 led to enhanced HIF-1α protein levels while in the
M410-treated cells, MG132 did not restore the inhibition of M410 on
HIF-1α protein levels under hypoxia or normoxia (Fig. 5C).
Discussion
Hypoxia is a feature of most tumors that often
arises due to rapid cell division, aberrant tumor angiogenesis and
blood flow. Persistent hypoxia leads to a selection of genotypes
favoring survival and promoting tumor angiogenesis,
epithelial-tomesenchymal transition, invasiveness and metastasis,
as well as suppression of immune reactivity (16,17).
HIFs are optimally characterized markers mediating cell responses
to hypoxic stress. Of the HIF family members, HIF-1α is the most
well characterized (18). Increased
HIF-1α levels are associated with increased risk of mortality in
many human cancer types, including those of the breast, brain,
colon, bladder, esophageal, head/neck/oropharynx, liver,
pancreatic, lung, gastric, and uterus, skin, as well as in acute
lymphocytic and myeloid leukemias (19). It was shown that HIF-1α was
overexpressed in BRCA-1 germline mutation-related breast cancer
(20) and associated independently
with shortened survival in patients with lymph node-negative breast
carcinoma and in patients with lymph node-positive ones (21,22).
HIF-1α has been a prime target for anticancer therapies (23). PX-478 is the first inhibitor of
HIF-1α, which decreased the HIF-1α protein expression and had
potent antitumor activity (24).
Kong et al (25) reported
that echinomycin inhibited HIF-1 DNA binding to endogenous
promoters and resulted in cell apoptosis. Kim et al
(26) found that a potent
angiogenesis inhibitor G0811, targeted HIF-1α signal transduction
and suppressed HIF-1α stability in cancer cells and inhibited in
vitro and in vivo angiogenesis. In addition, G0811
effectively decreased the expression of VEGF, which is one of the
target genes of HIF-1α. In a previous study (15), six synthesized stilbene derivatives
were screened for their cytotoxic activity against human tumor
cells and of these compound M410 exhibited a most prominent
cytotoxic effect. M410 has been shown to compete with colchicine
for tubulin binding and to disrupt microtubules leading to mitotic
arrest in colon cancer cell lines. However, the exact mechanism
whereby M410 destabilizes microtubules and inhibits HIF-1α, as well
as the relationship between the two remains unclear.
In the present study, we first investigated the
antitumor effect of M410 in BC cell lines in a
concentration-dependent manner. We then confirmed that M410
depolymerizes microtubules and inhibits the nuclear accumulation of
HIF-1α expression in a proteasome-independent manner in hypoxia.
The effect was specific as other transcription factors such as
HIF-1β, NF-κB and c-Fos, were not affected. Since M410 has been
shown to induce mitotic arrest, we clarified the relationship
between the inhibition of HIF-1α and mitotic arrest. We used the
non-microtubule-targeting agent, monastrol, to exclude the
possibility that the inhibition of M410 on HIF-1α is the
consequence of mitotic arrest. The results suggest that the strong
correlation between disruption of the microtubule cytoskeleton and
inhibition of HIF-1α function is independent of mitotic arrest. We
also assessed the role of microtubule-targeting agents, such as
Vincristine (VCR) and Taxol, on the HIF-1α protein levels in breast
cancer cells (data not shown). VCR and Taxol had similar effects on
HIF-1α as M410, suggesting a strong correlation between the
disruption of microtubules and inhibition of HIF-1α. However, the
mechanisms regarding how the stability of microtubules affects the
translation of HIF-1α levels remain to be determined. Furthermore,
we examined whether the downregulation of HIF-1α by M410 occurred
at the transcriptional or translational level or other sides by
extracting total RNA from MDA-MB-231 cells and found HIF-1α mRNA
levels were not significantly changed by M410. Thus, we concluded
that M410 inhibited HIF-1α at the translation level. VEGF is the
most potent angiogenic growth factor in solid tumors. It also has a
range of other functions, including induction of vascular
permeability and supporting survival of endothelial cells. We
showed that the mRNA level of VEGF was significantly decreased by
M410 treatment, which was consistent with findings of a previous
study (25). Our results identified
that HIF-1 target gene, GLUT1 glucose transporter was also
significantly decreased by M410 treatment. However, future in
vivo investigation is required to confirm these results.
In conclusion, our results have shown that M410
depolymerizes microtubules and downregulates HIF-1α protein levels
in a proteasome-independent manner and reduces the mRNA of
HIF-1-targeted genes in the MDA-MB-231 breast cancer cell line.
Notably, we suggest a strong correlation between the inhibition of
HIF-1α and the disruption of microtubules in breast cancer
cells.
Acknowledgments
This study was supported by grants as follows: Young
Scientist Project of the National Natural Science Foundation of
China (no. 81201716), Core Technology Program for Strategy Emerging
Industries of Guangdong Province (no. 2011A081401002) and the
Science and Technology Program of Guangzhou City (no.
2014J4100224).
References
|
1
|
Althuis MD, Dozier JM, Anderson WF, Devesa
SS and Brinton LA: Global trends in breast cancer incidence and
mortality 1973–1997. Int J Epidemiol. 34:405–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forbes JF: The incidence of breast cancer:
the global burden, public health considerations. Semin Oncol.
24(Suppl 1): S1–20–S1–35. 1997.
|
|
4
|
Rakha EA, El-Sayed ME, Green AR, Lee AH,
Robertson JF and Ellis IO: Prognostic markers in triple-negative
breast cancer. Cancer. 109:25–32. 2007. View Article : Google Scholar
|
|
5
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hudis CA and Gianni L: Triple-negative
breast cancer: an unmet medical need. Oncologist. 16(Suppl 1):
1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: the central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
10
|
Sun HC, Qiu ZJ, Liu J, Sun J, Jiang T,
Huang KJ, Yao M and Huang C: Expression of hypoxia-inducible
factor-1α and associated proteins in pancreatic ductal
adenocarcinoma and their impact on prognosis. Int J Oncol.
30:1359–1367. 2007.PubMed/NCBI
|
|
11
|
Rasheed S, Harris AL, Tekkis PP, Turley H,
Silver A, McDonald PJ, Talbot IC, Glynne-Jones R, Northover JM and
Guenther T: Hypoxia-inducible factor-1alpha and -2alpha are
expressed in most rectal cancers but only hypoxia-inducible
factor-1alpha is associated with prognosis. Br J Cancer.
100:1666–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conley SJ, Gheordunescu E, Kakarala P,
Newman B, Korkaya H, Heath AN, Clouthier SG and Wicha MS:
Antiangiogenic agents increase breast cancer stem cells via the
generation of tumor hypoxia. Proc Natl Acad Sci uSA. 109:2784–2789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papandreou I, Cairns RA, Fontana L, Lim AL
and Denko NC: HIF-1 mediates adaptation to hypoxia by actively
downregulating mitochondrial oxygen consumption. Cell Metab.
3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mikstacka R, Stefański T and Różański J:
Tubulin-interactive stilbene derivatives as anticancer agents. Cell
Mol Biol Lett. 18:368–397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai YC, Zou Y, Ye YL, Sun HY, Su QG, Wang
ZX, Zeng ZL and Xian LJ: Anti-tumor activity and mechanisms of a
novel vascular disrupting agent,
(Z)-3,4′,5-trimethoxylstilbene-3′-O-phosphate disodium (M410).
Invest New Drugs. 29:300–311. 2011. View Article : Google Scholar
|
|
16
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaupel P, Mayer A and Höckel M: Tumor
hypoxia and malignant progression. Methods Enzymol. 381:335–354.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gong L, Zhang W, Zhou J, Lu J, Xiong H,
Shi X and Chen J: Prognostic value of HIFs expression in head and
neck cancer: a systematic review. PLoS One. 8:e750942013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar :
|
|
20
|
van der Groep P, Bouter A, Menko FH, van
der Wall E and van Diest PJ: High frequency of HIF-1alpha
overexpression in BRCA1 related breast cancer. Breast Cancer Res
Treat. 111:475–480. 2008. View Article : Google Scholar
|
|
21
|
Bos R, van der Groep P, Greijer AE,
Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible factor-1α independently
predict prognosis in patients with lymph node negative breast
carcinoma. Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schindl M, Schoppmann SF, Samonigg H,
Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P
and Oberhuber G; Austrian Breast and Colorectal Cancer Study Group:
Overexpression of hypoxia-inducible factor 1α is associated with an
unfavorable prognosis in lymph node-positive breast cancer. Clin
Cancer Res. 8:1831–1837. 2002.PubMed/NCBI
|
|
23
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Welsh S, Williams R, Kirkpatrick L,
Paine-Murrieta G and Powis G: Antitumor activity and
pharmacodynamic properties of PX-478, an inhibitor of
hypoxia-inducible factor-1alpha. Mol Cancer Ther. 3:233–244.
2004.PubMed/NCBI
|
|
25
|
Kong D, Park EJ, Stephen AG, Calvani M,
Cardellina JH, Monks A, Fisher RJ, Shoemaker RH and Melillo G:
Echi-nomycin, a small-molecule inhibitor of hypoxia-inducible
factor-1 DNA-binding activity. Cancer Res. 65:9047–9055. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim KH, Jung HJ and Kwon HJ: A new
anti-angiogenic small molecule, G0811, inhibits angiogenesis via
targeting hypoxia inducible factor (HIF)-1α signal transduction.
Biochem Biophys Res Commun. 441:399–404. 2013. View Article : Google Scholar : PubMed/NCBI
|