Introduction
Radiotherapy is widely employed in the clinic to
treat a variety of cancers. However, since ionizing radiation also
affects normal cells, side-effects are commonly observed in
patients receiving radiotherapy. An important adverse effect
induced by radiotherapy and chemotherapy is myelosuppression, with
a significant decline in the number of circulating neutrophils
(1,2). However, no effective treatment has
been developed to mitigate the radiation-induced injury.
Granulocyte colony stimulating factor (G-CSF), an
agent clinically approved for neutropenia treatment, is often used
to increase the number of neutrophils after radiation and
chemotherapy (3,4). Furthermore, the ability of G-CSF to
reverse myelosuppression following radiation exposure has been
demonstrated in animal models (5).
In addition to the hematopoietic functions, G-CSF has been
suggested to offer protective effects in other tissues, including
the liver (6), brain (7) and intestine (8). Moreover, a recent in vivo study
reported that G-CSF treatment administered before or after
irradiation attenuated focal irradiation-induced intestinal mucosal
damage (9,10). G-CSF also ameliorated
radiation-induced apoptosis in intestinal epithelial cells in
vitro (10). Although G-CSF may
have a radio-protective effect against gastrointestinal damage
after irradiation exposure, its exact influence on cancer cells
after irradiation remain poorly understood.
The induction of neutrophilia by G-CSF may have
antitumor effects in cancer patients (11). Additionally, in the experimental
animals, antitumor effects by G-CSF have been observed in a murine
xenograft model of human medulloblastoma (12). In contrast, G-CSF has been shown to
stimulate cancer angiogenesis and promote tumor growth (13). G-CSF treatment with chemotherapy has
also been suggested to facilitate revascularization and enhance
tumor growth (14). Thus, the
effects of G-CSF on tumor growth, especially under irradiation
therapy, are controversial and require further investigation.
Nevertheless, the precise involvement of G-CSF treatment in colon
cancer following radiotherapy remains unknown.
Therefore, the present study evaluated tumor growth
following radiotherapy and G-CSF administration in a murine
xenograft model of colon cancer. Changes in the expression levels
of myeloperoxidase (MPO), vascular endothelial growth factor
(VEGF), matrix metalloproteinase-9 (MMP-9) and CD31 were also
assessed in the mouse cancer induced by injection of colon cancer
cells.
Materials and methods
Cell culture
CT26 BALB/c mouse colon cancer cells (Korean Cell
Line Bank, Seoul, Korea) were cultured in the Roswell Park Memorial
Institute 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan)
containing 10% heat-inactivated fetal bovine serum, 100 units/ml
penicillin, 0.1 mg/ml streptomycin and 0.5 mM glutamine
(Invitrogen, Grand Island, NY, USA). Cells were incubated at 37°C
in a humidified atmosphere with 5% CO2.
Animal experiments
Female BALB/c mice (6-week-old) were purchased from
the Central Lab. Animal Inc. (Seoul, Korea) and used after one week
of quarantine and acclimatization. All animals were maintained in a
room at 23±2°C with a relative humidity of 50±5%, artificial
lighting from 08:00 to 20:00 daily and 13–18 air changes/hour. They
had ad libitum access to standard laboratory diet and water.
After acclimatization, mice were randomly divided into four groups
(n=6 mice/group): control, treatment with radiation, treatment with
G-CSF, and treatment with radiation and G-CSF. CT26 cells
(1×106 cells/animal in 100 µl PBS) were injected
subcutaneously in the right flank of BALB/c mice. Tumor volume was
measured at 7, 14, 17, and 21 days after implantation using
calipers. At the end of the study, the tumor of each animal was
quickly frozen and immediately stored for protein extraction
(n=4/group) or fixed in buffered formalin for immunohistochemistry
(n=2/group).
All experimental procedures were conducted according
to the National Institutes of Health Guidelines for the Care and
Use of Laboratory Animals and following a protocol approved by the
Institutional Animal Care and Use Committee of the Dongnam
Institute of Radiological and Medical Sciences. All animals were
cared for in accordance with the National Animal Welfare Law of
Korea.
Irradiation and G-CSF treatment
A schematic schedule for radiation and G-CSF
treatments is shown in Fig. 1A.
When tumors reached a mean volume of ~100 mm3 (day 7),
mice received sham or focal radiation. Anesthetized mice were
positioned on a tray with the intestine and cancer lesion in the
radiation field. Partial body irradiation was administered at a
daily dose of 2 Gy for 5 days using 6-MV high-energy photon rays
(Elekta, Stockholm, Sweden) at a dose rate of 3.8 Gy/min.
Sham-irradiated mice underwent similar procedures without
radiation.
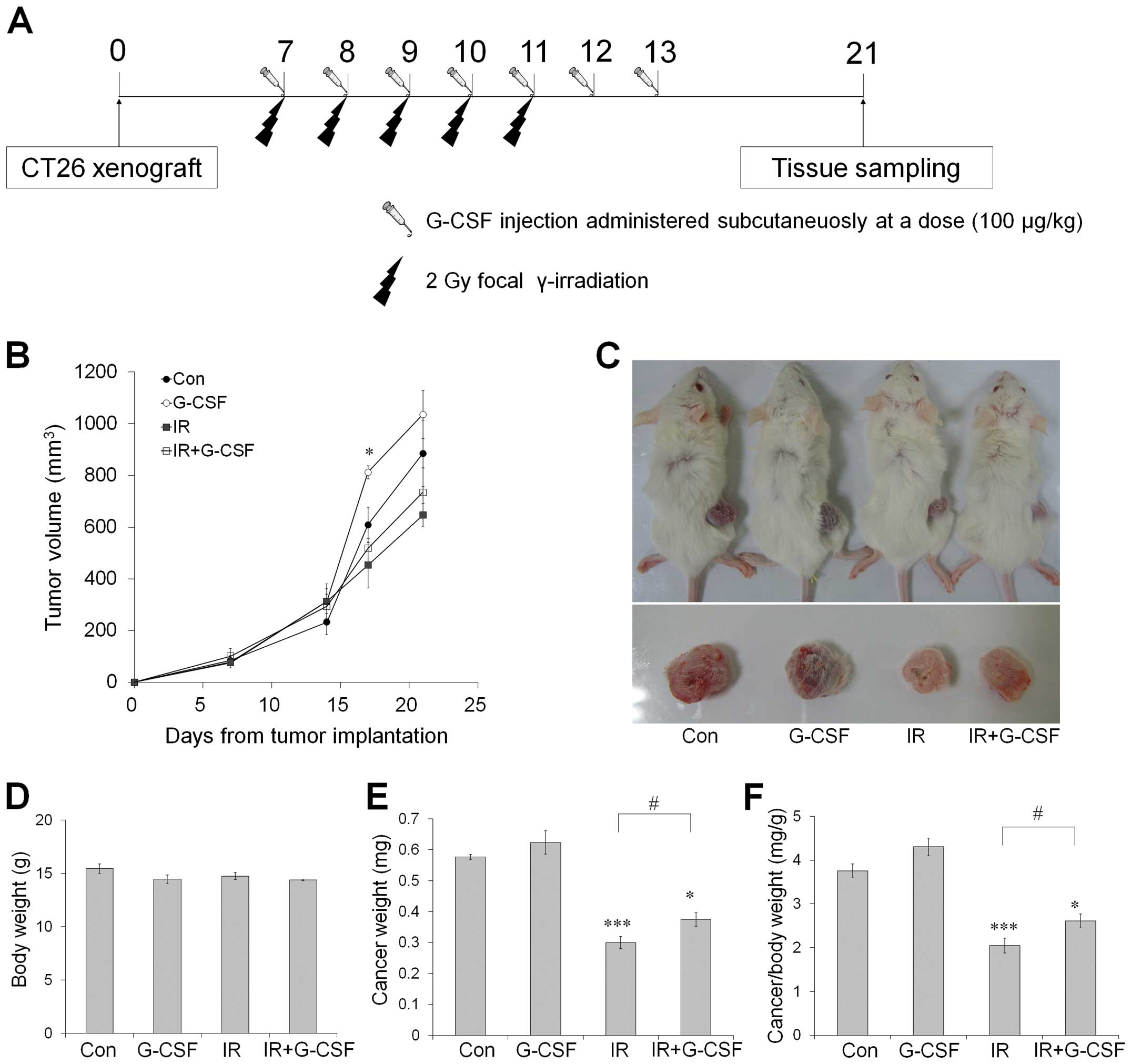 | Figure 1CT26 tumor growth in BALB/c mice
treated with radiation and G-CSF, alone or in combination. (A)
Schematic diagram of the experimental procedure, (B) Tumor volumes
measured by calipers at 7, 14, 17 and 21 days after implantation,
(C) Representative photographs of tumor-bearing mice treated with
radiation and G-CSF, alone or in combination, 21 days after tumor
implantation. Before tissue sampling, the body weight (D), tumor
weight (E) and relative tumor weight (F) were calculated. Data are
reported as means ± SE (n=6/group). *p<0.05,
**p<0.01, ***p<0.001 vs. sham controls;
#p<0.05 vs. sham-treated irradiated groups. G-CSF,
granulocyte-colony stimulating factor. |
Recombinant human G-CSF (Neutrogin, Choongwae Pharm.
Co., Seoul, Korea) was dissolved in 0.9% saline and injected
intraperitoneally at 100 µg/kg for 7 days from the day of
radiation treatment. Sham-treated mice received the same volume of
saline.
Blood collection
Blood samples were collected from the vena cava into
tubes containing ethylenediaminetetraacetic acid. Peripheral white
blood cells (WBC) and neutrophils were monitored by automatic
counting using a Hemavet (Drew Scientific Inc., UK).
Immunohistochemistry
Tissue samples were processed in paraffin, cut into
4-µm thick coronal sections and deparaffinized. The sections
were incubated in normal goat serum (Vector ABC Elite kit; Vector
Laboratories Inc., Burlingame, CA, USA) for 60 min to prevent
non-specific binding, followed by incubation with rabbit monoclonal
anti-MPO (1:200; Cell Signaling Technology, Beverly, MA, USA),
rabbit monoclonal anti-CD31 (1:1,000; Cell Signaling Technology),
rabbit monoclonal anti-MMP-9 (1:200; Acris Antibodies GmbH,
Herford, Germany) for 2 h at room temperature (RT). Then, the
sections were reacted with biotinylated goat anti-rabbit IgG
(Vector ABC Elite kit) for 60 min at RT. Immunoreactivity was
performed for 60 min at RT using the avidin-biotin peroxidase
complex (Vector ABC Elite kit) prepared according to the
manufacturer’s instructions and the peroxidase reaction was
developed using a diaminobenzidine substrate kit (SK-4100; Vector).
For controls, incubation with the primary antibodies was omitted.
All sections were counterstained with Harris’ hematoxylin prior to
being mounted.
Western blot analysis
Harvested tumor tissues were homogenized in lysis
buffer and the protein concentration was determined using a Bio-Rad
protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) with
bovine serum albumin set as the standard. A sodium dodecyl sulfate
sample buffer (4X) was added to each homogenized sample and the
samples were heated at 100°C for 10 min. The samples were then
separated by electrophoresis, transferred to a polyvinylidene
difluoride membrane, and blocked with 5% skim milk in
phosphate-buffered saline containing 0.1% Tween 20 (PBS-T) for 30
min at RT. The membranes were incubated with rabbit monoclonal
anti-MPO (1:200; Cell Signaling Technology), anti-VEGF (1:1,000;
Cell Signaling Technology), rabbit monoclonal anti-CD31 (1:1,000;
Cell Signaling Technology) and rabbit monoclonal anti-MMP-9 (1:200;
Acris Antibodies GmbH) in PBS-T overnight at 4°C. After extensive
washing and incubation with horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:20,000; Pierce, Rockford, IL, USA), the signals
were visualized using chemiluminescence method (SuperSignal West
Pico; Pierce). For normalization of expression, the membranes were
incubated with a mouse monoclonal antibody for β-actin (1:20,000;
Sigma-Aldrich, St. Louis, MO, USA) and processed as mentioned
above. Several exposure times were used to obtain signals in a
linear range. Band intensities were quantified using Scion Image
Beta 4.0.2 for Windows XP software (Scion Corp., Frederick, MD,
USA). Values shown represent the mean ± standard error (SE).
Cytotoxicity and cell viability
evaluation
Cytotoxicity was measured using a lactate
dehydrogenase (LDH) cytotoxicity assay kit (BioVision, Mountain
View, CA, USA) according to the manufacturer’s recommendations. The
assay was assessed quantitatively via the measurement of LDH, which
was released from damaged or destroyed cells in the extracellular
fluid. The LDH activity was quantified by the colorimetric
reduction of a tetrazolium salt (yellow) to formazan (red). After a
30-min incubation at RT, absorbance at 450 nm was measured to
determine LDH activity. Data shown are the mean ± standard error
(SE) of 4 cultures/condition. Blank LDH levels were subtracted from
the LDH values for each sample and the results were normalized to
100%.
Cell viability was evaluated using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay (Sigma Chemicals Co.). The assay was based on the reduction
of MTT by living cells to yield a soluble formazan product that
could be detected colorimetrically. MTT was added to cultured cells
at 0.6 mg/ml culture medium without phenol red. Cell plates were
incubated at 37°C for 4 h before the MTT solution was removed and
cells were dissolved in a solubilization solution (dimethyl
sulfoxide and ethanol, 1:1 ratio). The absorbance of solubilized
formazan product was quantified by a microplate reader at 560 nm.
Data shown are the mean ± SE of 4 replicates/condition. Blank MTT
levels were subtracted from the MTT values for each sample and the
results were normalized to 100%.
Statistical analysis
The data are reported as the mean ± SE and were
analyzed using one-way analysis of variance followed by the
Student-Newman-Keuls post-hoc test for multiple comparisons. A
p-value of <0.05 was considered significant.
Results
G-CSF induces tumor growth in BALB/c mice
bearing CT26 xenografts
The potential clinical implications of G-CSF
combined with radiation were assessed in vivo using a CT26
xenograft model. Tumor-bearing mice were sham-irradiated or
received a total dose of 10 Gy, with or without G-CSF at a dosage
of 100 µg/kg/day. All treatments were well tolerated, with
no signs of toxicity observed or gross pathologic abnormalities
noted on necropsy. Tumors exposed to fractionated radiation were
significantly smaller than those sham-irradiated (p<0.01 at 21
days after tumor implantation) (Fig.
1B). However, tumors treated with G-CSF alone significantly
increased in size at 17 days after implantation (Fig. 1B). Tumors exposed to radiation with
G-CSF had significantly increased absolute and relative tumor
weight on day 21 after implantation compared with those receiving
radiation only (Fig. 1E and F).
Thus, these data indicate that G-CSF may support tumor growth and
interfere with antitumor effect of radiotherapy.
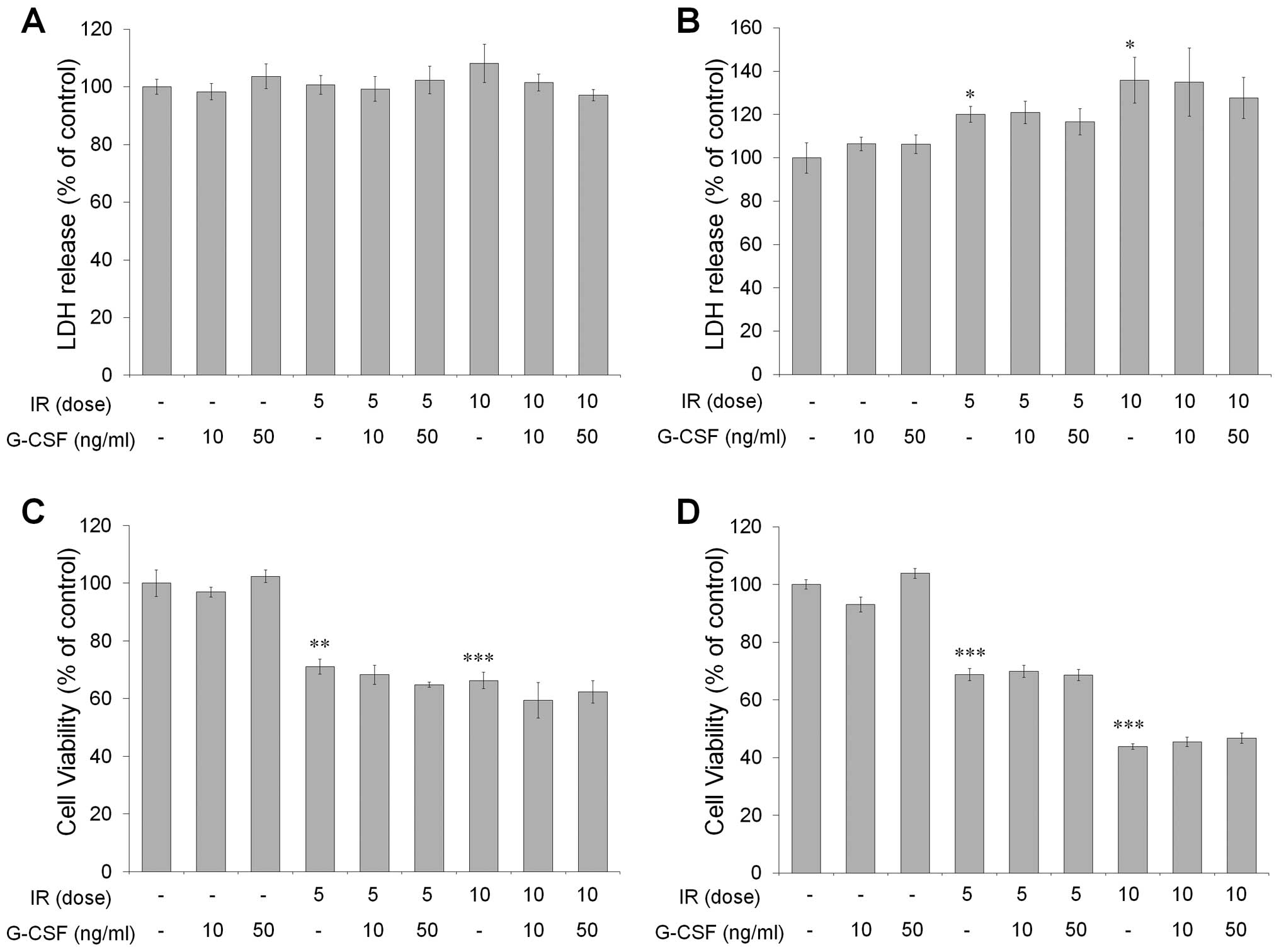
G-CSF does not affect radiation-induced
cytotoxicity and cell viability in vitro
To examine the effect of G-CSF on radiation-induced
cytotoxicity and cell viability, CT-26 cells were irradiated with 5
or 10 Gy and treated with or without 10–50 ng/ml of G-CSF. At 48 h
after irradiation, cytotoxicity assessed by the LDH assay showed no
changes by radiation or G-CSF treatment (Fig. 2A). However, at 72 h, significantly
increased cytotoxicity was observed in cells receiving radiation,
but G-CSF did not exert such an effect compared to sham-treated
controls (Fig. 2B). Although cell
viability, evaluated by the MTT assay, decreased significantly with
radiation, G-CSF treatment did not alter cell viability at 48 and
72 h after irradiation (Fig. 2C and
D). Thus, irradiation-induced cytotoxicity and cell viability
were not altered by G-CSF treatment.
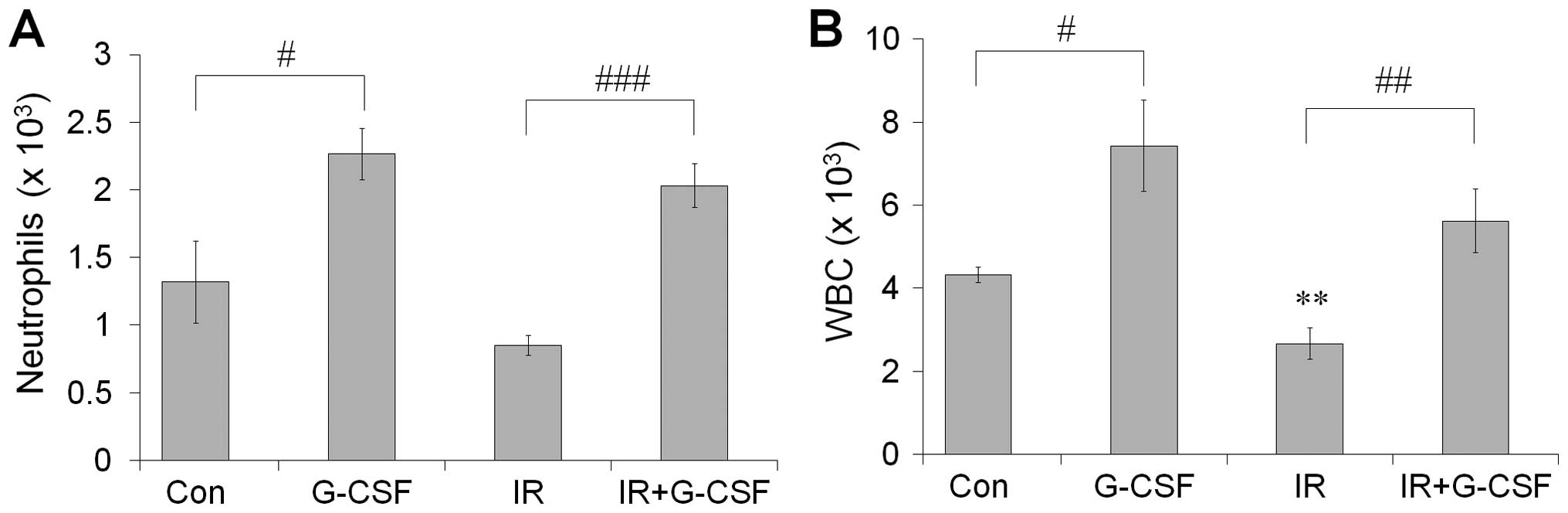
G-CSF increased the number of peripheral
WBCs and neutrophils
The number of circulating WBCs and neutrophils was
assessed 21 days after radiotherapy to evaluate the effect of
radiotherapy on blood counts (Fig.
3). We found, as expected, that WBCs and neutrophils were
decreased after irradiation. However, the number of WBCs and
neutrophils increased significantly following G-CSF treatment at 21
days after irradiation. Thus, G-CSF treatment induced the
upregulation of circulation WBCs and neutrophils in tumor-bearing
mice.
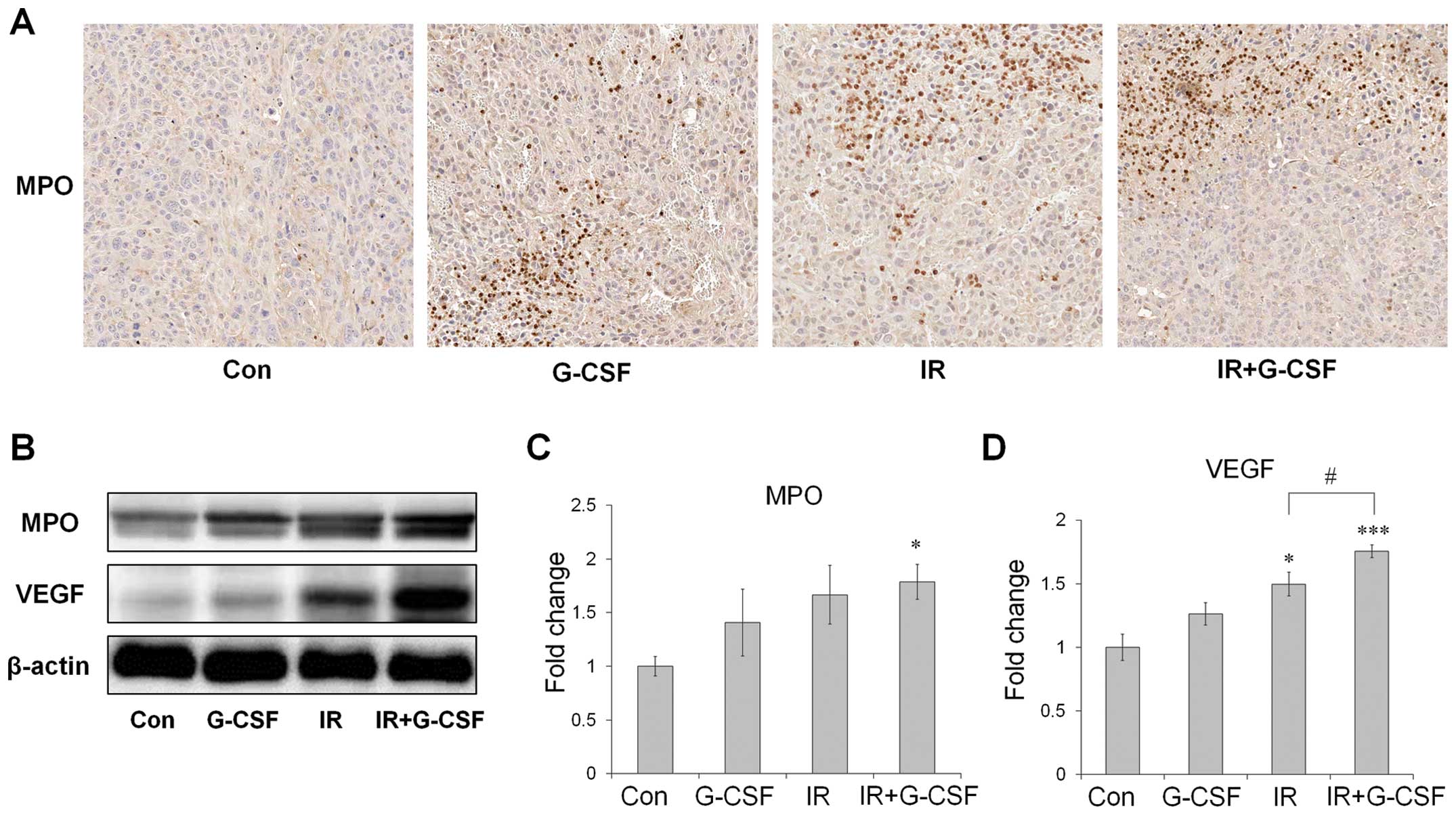
The expression levels of MPO and VEGF are
upregulated in tumors treated with radiation and G-CSF
Next, we assessed MPO, a marker for neutrophils, to
determine whether G-CSF induced the recruitment of neutrophils into
the tumor after irradiation (Fig.
4). MPO protein levels were shown to increase after radiation
by both immunohistochemistry and western blot analysis and G-CSF
supplementation further promoted such an increase (Fig. 4A-C). VEGF protein levels, determined
by western blot analysis, also increased after fractionated
irradiation, and change in the patterns of VEGF were similar to
those of MPO (Fig. 4D). These
results indicate that radiation-induced neutrohphil infiltration
accompanied by VEGF is aggravated by G-CSF treatment.
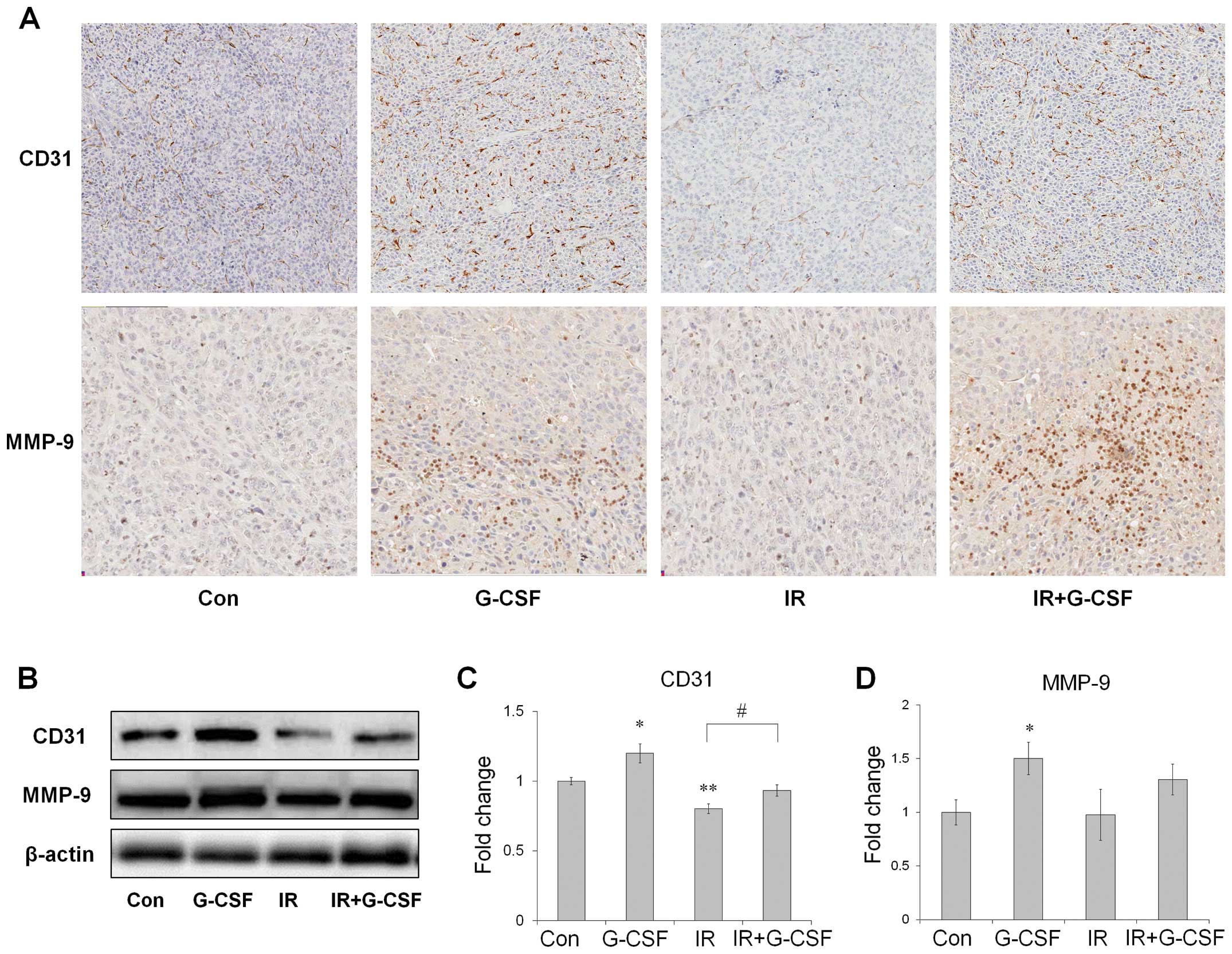
Irradiation-induced vascular damage is
mitigated by G-CSF administration
To investigate the possible mechanisms involved in
tumor growth after G-CSF treatment, CD31 and MMP-9 levels were
assessed (Fig. 5). CD31 expression
levels were reduced significantly after irradiation as determined
by western blot analysis and the number of CD31-positive cells also
decreased in tumor tissue as determined by immunohistochemistry
(Fig. 5A and C). After G-CSF
supplementation, CD31 levels increased significantly compared to
vehicle-treated controls with or without irradiation. MMP-9 levels
did not change after irradiation monotherapy. However, western blot
analysis data showed that G-CSF treatment significantly increased
MMP-9 levels (Fig. 5D) and
comparable result was also observed by immunohistochemistry in the
tumor tissue (Fig. 5A), although
the differences between radiation with G-CSF and radiation only
were not significant. These data suggest that G-CSF supplementation
may decrease radiation-induced vascular damages and increase MMP-9
expression levels in tumors.
Discussion
The present study demonstrates that G-CSF may
attenuate the antitumor effect of radiotherapy. Although G-CSF may
induce neutrophil upregulation via bone marrow stimulation, it
could also promote tumor vascularization, possibly via production
of pro-angiogenic factors.
Radiotherapy is a commonly used method to treat
tumors in cancer patients. Because G-CSF is known to increase
neutrophil production by mobilizing hematopoietic stem cells from
the bone marrow, it is often used clinically to treat neutropenia
induced by chemotherapy or radiotherapy (15,16).
In experimental animals, a previous study by us, and other showed
the protective effects of G-CSF against radiation-induced injury of
the normal intestine both in vivo and in vitro
(10,17). Additionally, G-CSF treatment after
irradiation has been shown to enhance survival and minimize the
effects of radiation on gastrointestinal injury in mice (9). Furthermore, there is evidence that
G-CSF may have a therapeutic effect induced by brain irradiation by
inhibiting the detrimental effects on hippocampal neurogenesis
(7).
The effects of tumor by irradiation can be different
between high single dose and small fractionated dose. Although high
single dose of irradiation is more effective in tumor treatment
(18), this schedule is not
equivalent to the clinical fractionated radiation protocol (~2
Gy/fraction). It was reported that the effects of G-CSF was
dose-dependent, however, the number of neutrophil counts in
irradiated mice treated with 300 and 600 µg/kg were higher
than that of neutrophil counts in sham-irradiated control (19). Although many previous studies have
used various G-CSF doses (13,14,20),
we used G-CSF at 100 µg/kg, which is consistent with our
previous studies to determine the protective effect of G-CSF
against radiation (7,9). In a previous study, tumors were
collected in the late tumor growth to examine the role of tumor
vasculature by G-CSF (20).
Further, tumors were excised when they reached a size of 10 to 12
mm, which is relevant to ~1,000 mm3 in size, to
investigate vascular response with fractionated radiation protocol
(13,21). Therefore, in the present study, we
have examined the side-effects and vascular changes in tumors in
the late tumor growth phase following fractionated irradiation with
G-CSF treatment.
Clinically, G-CSF, with or without chemotherapy, has
demonstrated anticancer effects (22,23).
However, it is important to comment that the administration of
G-CSF administration may also associate with cancer progression.
Previously, it was reported that G-CSF may have potential to
promote tumor growth in mice inoculated with colon cancer (13). Additionally, despite its known
clinical benefits, G-CSF may attenuate the antitumor activity of
chemotherapy (14). Therefore, the
exact effects of G-CSF administration on cancer cells remain
controversial. The mechanisms associated with tumor growth when
exogenous G-CSF is administered with radiotherapy are also unknown.
Thus, this preclinical study aimed to determine whether G-CSF
diminished the antitumor effects of radiation. We found that
compared to radiation alone, combinational G-CSF administration
attenuated the tumor suppression effects of radiotherapy,
suggesting that G-CSF may contribute to tumor growth after
irradiation.
The use of G-CSF supplementation in neutropenic
cancer patients treated with radiation or chemotherapy have been
suggested. Because it is well-known that G-CSF administration
increases the production of neutrophils, it was suggested that
cytotoxic mediators produced by neutrophils could kill the cancer
cells (24,25). In the present study, as expected, we
confirmed that G-CSF increased the number of circulating
neutrophils. Because it was previously reported that neutrophils
can be identified by the cytoplasmic marker MPO coupled with cell
morphology (26), we assessed MPO
levels in xenograft tumors treated with radiation and G-CSF.
Although irradiation alone led to increased expression levels of
MPO, G-CSF treatment upregulated the number of neutrophils in
tumors. G-CSF has been shown to promote angiogenesis by
upregulating VEGF, which is released by neutrophils, but not by
monocytes (27). In addition, it
has been reported that VEGF signaling is required for
vasculogenesis and angiogenesis (28), and tumor-produced VEGF not only
promotes local angiogenesis but also mobilizes hematopoietic
progenitors from the bone marrow (29,30).
In agreement with these previous findings, we showed that VEGF
levels increased in tumors treated with both radiation and G-CSF
compared to controls and those receiving radiation only. Moreover,
the pattern of VEGF changes was similar to that of MPO. Our
findings suggest that both radiation and G-CSF may associate with
VEGF upregulation, possibly via increased neutrophil infiltration
into tumors and radiotherapy combined with G-CSF may facilitate
vascularization.
Tumor can expand its vasculature by angiogenesis,
which results from nearby endothelial cell proliferation, or by
vasculogenesis, which occurs via the recruitment of circulating
endothelial and other cells from the bone marrow (31). Radiation has been reported to induce
angiogenesis blockage by abrogating endothelial cells (32), thereby forcing the tumor to rely on
vasculogenesis promoted by increased hypoxia (31). We demonstrated that CD31-positive
endothelial cells in the tumor were significantly decreased
following irradiation; G-CSF, however, reduced such
radiation-induced vascular damage. MMP-9 is involved in
extracellular matrix degradation and vascular remodeling (33) and may enhance local angiogenesis by
releasing angiogenic factors such as VEGF (34). Additionally, subcutaneously
implanted tumors could not grow in MMP-9 knockout mice, but tumor
growth was restored by transplantation of wild-type bone marrow,
implicating vasculogenesis involvement (35). We found that G-CSF, regardless of
radiation, elevated MMP-9 levels in tumors. Thus, despite
radiation-induced endothelial abrogation in tumor, G-CSF may
promote tumor growth by enhancing angiogenesis and vasculogenesis.
Further studies are needed to verify the specific mechanisms
underlying tumor growth after G-CSF treatment and associated
vasculogenesis following ionizing irradiation.
In conclusion, the present study demonstrates that
CT26 tumor growth in mice may be facilitated by G-CSF administered
with radiotherapy. Although G-CSF increased neutrophils levels, it
was accompanied by VEGF upregulation. Additionally, levels of
vascularization-related factors characterized by CD31 and MMP-9 in
tumors were correlated with increased tumor growth by G-CSF
treatment. Consequently, we suggested that G-CSF treatment may
enhance tumor growth after radiation, possibly via increasing
levels of vascularization in the tumor. More comprehensive studies
are needed to improve the outcomes of cancer patients receiving
radiotherapy.
Acknowledgments
The present study was supported by the Nuclear
R&D Program of the Ministry of Education, Science and
Technology, Korea (Grant no., 50496-2013).
References
|
1
|
Mauch P, Constine L, Greenberger J, Knospe
W, Sullivan J, Liesveld JL and Deeg HJ: Hematopoietic stem cell
compartment: Acute and late effects of radiation therapy and
chemotherapy. Int J Radiat Oncol Biol Phys. 31:1319–1339. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friberg LE, Henningsson A, Maas H, Nguyen
L and Karlsson MO: Model of chemotherapy-induced myelosuppression
with parameter consistency across drugs. J Clin Oncol.
20:4713–4721. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung L and Dror Y: Clinical applications
of granulocyte-colony stimulating factor. Front Biosci.
12:1988–2002. 2007. View
Article : Google Scholar
|
|
4
|
MacVittie TJ and Farese AM: Cytokine-based
treatment of radiation injury: Potential benefits after low-level
radiation exposure. Mil Med. 167(Suppl): 68–70. 2002.PubMed/NCBI
|
|
5
|
Uckun FM, Souza L, Waddick KG, Wick M and
Song CW: In vivo radioprotective effects of recombinant human
granulocyte colony-stimulating factor in lethally irradiated mice.
Blood. 75:638–645. 1990.PubMed/NCBI
|
|
6
|
Hou XW, Jiang Y, Wang LF, Xu HY, Lin HM,
He XY, He JJ and Zhang S: Protective role of granulocyte
colony-stimulating factor against adriamycin induced cardiac, renal
and hepatic toxicities. Toxicol Lett. 187:40–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JS, Yang M, Jang H, Oui H, Kim SH,
Shin T, Jang WS, Lee SS and Moon C: Granulocyte-colony stimulating
factor ameliorates irradiation-induced suppression of hippocampal
neurogenesis in adult mice. Neurosci Lett. 486:43–46. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudo T, Matsumoto T, Nakamichi I, Yada S,
Esaki M, Jo Y, Ohji Y, Yao T and Iida M: Recombinant human
granulocyte colony-stimulating factor reduces colonic epithelial
cell apoptosis and ameliorates murine dextran sulfate
sodium-induced colitis. Scand J Gastroenterol. 43:689–697. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JS, Ryoo SB, Heo K, Kim JG, Son TG,
Moon C and Yang K: Attenuating effects of granulocyte-colony
stimulating factor (G-CSF) in radiation induced intestinal injury
in mice. Food Chem Toxicol. 50:3174–3180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JS, Yang M, Lee CG, Kim SD, Kim JK and
Yang K: In vitro and in vivo protective effects of granulocyte
colony-stimulating factor against radiation-induced intestinal
injury. Arch Pharm Res. 36:1252–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lopez-Lazaro M: Granulocyte
colony-stimulating factor (G-CSF): A novel anticancer therapy based
on the ‘universal dynamics of tumor growth’? Exp Oncol. 28:249–251.
2006.PubMed/NCBI
|
|
12
|
Maeda H, Uozumi T, Kurisu K, Matsuoka T,
Kawamoto K, Kiya K, Ogasawara H, Sugiyama K, Mikami T, Monden S, et
al: Combined antitumor effects of TNF and G-CSF on a human
medulloblastoma xenograft line. J Neurooncol. 21:203–213. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Natori T, Sata M, Washida M, Hirata Y,
Nagai R and Makuuchi M: G-CSF stimulates angiogenesis and promotes
tumor growth: Potential contribution of bone marrow-derived
endothelial progenitor cells. Biochem Biophys Res Commun.
297:1058–1061. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Voloshin T, Gingis-Velitski S, Bril R,
Benayoun L, Munster M, Milsom C, Man S, Kerbel RS and Shaked Y:
G-CSF supplementation with chemotherapy can promote
revascularization and subsequent tumor regrowth: Prevention by a
CXCR4 antagonist. Blood. 118:3426–3435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roberts AW: G-CSF: A key regulator of
neutrophil production, but that’s not all! Growth Factors.
23:33–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hérodin F and Drouet M: Cytokine-based
treatment of accidentally irradiated victims and new approaches.
Exp Hematol. 33:1071–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh VK, Fatanmi OO, Singh PK and
Whitnall MH: Role of radiation-induced granulocyte
colony-stimulating factor in recovery from whole body
gamma-irradiation. Cytokine. 58:406–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia-Barros M, Paris F, Cordon-Cardo C,
Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z and Kolesnick R:
Tumor response to radiotherapy regulated by endothelial cell
apoptosis. Science. 300:1155–1159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romero-Weaver AL, Wan XS, Diffenderfer ES,
Lin L and Kennedy AR: Kinetics of neutrophils in mice exposed to
radiation and/or granulocyte colony-stimulating factor treatment.
Radiat Res. 180:177–188. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shojaei F, Wu X, Qu X, Kowanetz M, Yu L,
Tan M, Meng YG and Ferrara N: G-CSF-initiated myeloid cell
mobilization and angiogenesis mediate tumor refractoriness to
anti-VEGF therapy in mouse models. Proc Natl Acad Sci USA.
106:6742–6747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen FH, Fu SY, Yang YC, Wang CC, Chiang
CS and Hong JH: Combination of vessel-targeting agents and
fractionated radiation therapy: The role of the SDF-1/CXCR4
pathway. Int J Radiat Oncol Biol Phys. 86:777–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bottoni U, Bonaccorsi P, Devirgiliis V,
Panasiti V, Borroni RG, Trasimeni G, Clerico R and Calvieri S:
Complete remission of brain metastases in three patients with stage
IV melanoma treated with BOLD and G-CSF. Jpn J Clin Oncol.
35:507–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Löwenberg B, van Putten W, Theobald M,
Gmür J, Verdonck L, Sonneveld P, Fey M, Schouten H, de Greef G,
Ferrant A, et al: Effect of priming with granulocyte
colony-stimulating factor on the outcome of chemotherapy for acute
myeloid leukemia. N Engl J Med. 349:743–752. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Di Carlo E, Forni G, Lollini P, Colombo
MP, Modesti A and Musiani P: The intriguing role of
polymorphonuclear neutrophils in antitumor reactions. Blood.
97:339–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koga Y, Matsuzaki A, Suminoe A, Hattori H
and Hara T: Neutrophil-derived TNF-related apoptosis-inducing
ligand (TRAIL): A novel mechanism of antitumor effect by
neutrophils. Cancer Res. 64:1037–1043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu
Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, et al:
Granulocyte colony-stimulating factor promotes neovascularization
by releasing vascular endothelial growth factor from neutrophils.
FASEB J. 19:2005–2007. 2005.PubMed/NCBI
|
|
28
|
Roskoski R Jr: Vascular endothelial growth
factor (VEGF) signaling in tumor progression. Crit Rev Oncol
Hematol. 62:179–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heissig B, Hattori K, Dias S, Friedrich M,
Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et
al: Recruitment of stem and progenitor cells from the bone marrow
niche requires MMP-9 mediated release of kit-ligand. Cell.
109:625–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hattori K, Heissig B, Tashiro K, Honjo T,
Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S,
et al: Plasma elevation of stromal cell-derived factor-1 induces
mobilization of mature and immature hematopoietic progenitor and
stem cells. Blood. 97:3354–3360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kioi M, Vogel H, Schultz G, Hoffman RM,
Harsh GR and Brown JM: Inhibition of vasculogenesis, but not
angiogenesis, prevents the recurrence of glioblastoma after
irradiation in mice. J Clin Invest. 120:694–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prionas SD, Kowalski J, Fajardo LF, Kaplan
I, Kwan HH and Allison AC: Effects of X irradiation on
angiogenesis. Radiat Res. 124:43–49. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heissig B, Hattori K, Friedrich M, Rafii S
and Werb Z: Angiogenesis: Vascular remodeling of the extracellular
matrix involves metalloproteinases. Curr Opin Hematol. 10:136–141.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bergers G, Brekken R, McMahon G, Vu TH,
Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al:
Matrix metallopro-teinase-9 triggers the angiogenic switch during
carcinogenesis. Nat Cell Biol. 2:737–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahn GO and Brown JM: Matrix
metalloproteinase-9 is required for tumor vasculogenesis but not
for angiogenesis: Role of bone marrow-derived myelomonocytic cells.
Cancer Cell. 13:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|