Introduction
Purinergic signaling acts as an important pathway in
the regulation of cell growth, differentiation and development
(1). Extracellular ATP, which is
widely distributed in the tumor microenvironment, has been reported
to be involved in the progression of cancer (2). ATP acts through P2 receptors, which
are further categorized as P2X and P2Y receptors. P2Y receptors
belong to the G-protein coupled receptors, while P2X receptors
belong to the ligand-gated ion channel receptors. To date, seven
P2X receptor subtypes (P2X1-7) and eight P2Y receptor subtypes
(P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) have been
cloned in human cells (3). As one
subtype of the P2X receptors, the P2X7 receptor has been found to
be highly expressed in many types of cancers, such as acute
myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL),
chronic myelogenous leukemia (CML) and thyroid papillary cancer
(4,5). However, the level of the P2X7 receptor
is low in tissues of complex hyperplasia with atypia or in
endometrial adenocarcinoma and uterine epithelial cancer (6,7).
Breast cancer is one of the most common cancers
among women worldwide. Advancements in treatment including surgery,
chemotherapy and radiotherapy have increased the overall survival
rate of breast cancer patients (8).
However, invasion and metastasis remain the major reasons for
breast cancer-related mortality (9). One study reported that P2X7 receptor
activation participated in the SK3 channel- and cystein
cathepsin-dependent cancer cell invasiveness in MDA-MB-435s breast
cancer cells (10), but the role of
the P2X7 receptor in breast cancer cell invasion and metastasis
requires further clarification.
In the present study, we identified that P2X7
receptor activation via extracellular ATP promoted the invasion and
migration of T47D breast cancer cells. We also elucidated the
function of the AKT pathway in the P2X7-mediated cell invasion and
migration.
Materials and methods
Reagents
ATP, BzATP, ADP, P2X inhibitor APPDS and AKT
inhibitor LY294002 were obtained from Sigma Chemical Co. (St.
Louis, MO, USA). Antibodies to E-cadherin and β-actin were obtained
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to
phospho-AKT and AKT were obtained from Cell Signaling Technology
(Danvers, MA, USA).
Cell culture
Human T47D breast cancer cells were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA) and
were maintained in RPMI-1640 medium, supplemented with 10% fetal
bovine serum (FBS), 100 mg/ml streptomycin, 100 U/ml penicillin and
2 mM L-glutamine. Cells were grown at 37°C in a humidified
atmosphere of 95% air and 5% CO2.
Transwell invasion assay
A 24-well cell culture Transwell chamber (Corning
Costar, San Diego, CA, USA) was used to analyze the cell invasion
capacity. The filters of the upper inserts were coated with
Matrigel before being used. The upper inserts were seeded with
1×105 cells/200 µl in RPMI-1640 medium and the
lower inserts were filled with RPMI-1640 medium, supplemented with
20% FBS. After stimulation with different nucleotides (ATP, BzATP
or ADP), the cells that invaded through the Matrigel and filters
were fixed with methanol, and the nuclei were labeled with
4′,6-diamidino-2-phenylindole (DAPI). Nuclei were counted using
immunofluorescence microscopy (Nikon, Tokyo, Japan) at ×200
magnification.
Transwell migration assay
Cell migration capacity was also determined using
24-well cell culture Transwell chambers (Corning Costar). Briefly,
the upper inserts were seeded with 5×105 cells/200
µl in RPMI-1640 medium, and the lower inserts were filled
with RPMI-1640 medium supplemented with 20% FBS as a
chemoattractant. The cells were stimulated with or without the
different nucleotides (ATP, BzATP or ADP). After an 18-h incubation
at 37°C, the migrated cells were fixed with methanol, and the
nuclei were labeled with DAPI. Finally, the nuclei were counted in
seven random fields using immunofluo-rescence microscopy (Nikon) at
×200 magnification.
RNA extraction, reverse transcription and
real-time PCR
Total RNA was isolated from the T47D cells by TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). Then 2 µg of RNA
was reverse-transcribed into cDNA using the cDNA synthesis kit
(Tiangen Biotech Co., Ltd., Beijing, China). Real-time PCR was
performed with a SYBR-Green PCR kit (Tiangen Biotech) and the
primers are listed in Table I. The
fold-change in the expression of each objective gene relative to
β-actin was calculated based on the 2−ΔΔCt method.
 | Table IReal-time PCR primers. |
Table I
Real-time PCR primers.
| Gene | Sequence (5′-3′) |
|---|
| P2X1 |
GGCTGACTACGTCTTCCCAG |
|
GCGCAGTAGCCTTGAGTCT |
| P2X2 |
AGCTGGGCTTTATCGTGGAGA |
|
TTGGGGTTGCACTCCGATG |
| P2X3 |
AGTCGGTGGTTGTGAAGAGC |
|
AGCCTTCTCGTGCAAGAAAAC |
| P2X4 |
CTACCAGGAAACTGACTCCGT |
|
GGTATCACATAATCCGCCACAT |
| P2X5 |
CTGTCGCTGTTCGACTACAAG |
|
CCCATACGACCAGGTACGC |
| P2X6 |
GAACCCCAGTTTTCCATCATCA |
|
GGCGTCACAAGGAAGTTGGT |
| P2X7 |
TATGAGACGAACAAAGTCACTCG |
|
GCAAAGCAAACGTAGGAAAAGAT |
| MMP-13 |
ACTGAGAGGCTCCGAGAAATG |
|
GAACCCCGCATCTTGGCTT |
| β-actin |
AGCGCGGCTACAGCTTCA |
|
CGTAGCACAGCTTCTCCTTAAT |
Small interfering RNA transfection
A P2X7 siRNA (siP2X7) was purchased from Shanghai
Genechem Co., Ltd. (Shanghai, China), with the sequence of
5′-CCGAGAAACAGGCGAUAAU-3′. A scramble sequence not targeting any
known gene was used as a control siRNA (siCtrl). T47D cells were
plated in 24-well plates at the density of 1×104
cells/ml. Six hours later, the cells were transfected with siP2X7
or siCtrl using Lipofectamine 2000 (Invitrogen). After 36 h of
transfection, real-time PCR was performed to assess the knockdown
efficiency.
Western blot analysis
The cells were stimulated with or without the
different nucleotides (ATP, BzATP or ADP) for various times, and
the inhibitors were applied 30 min before ATP stimulation when the
inhibitors were used. The cells were lysed in ice-cold RIPA buffer
containing protease and phosphatase inhibitors (Applygen
Technologies Inc., Beijing, China). Protein concentrations were
determined with the BCA protein assay kit (Applygen Technologies).
Then fifty micrograms of proteins were separated on 10% SDS gel
electrophoresis and transferred to a PVDF membrane. After blocking
with 5% BSA in buffered saline, the membrane was further probed
with primary antibodies overnight at 4°C, and then incubated with
the secondary antibodies for 1 h at room temperature. The
immunoreactive bands were detected using ECL reagents (Applygen
Technologies).
ELISA assay
After stimulation with or without ATP, the
supernatant was collected and centrifuged at 10,000 × g for 15 min
at 4°C. The MMP-13 ELISA kit was purchased from Invitrogen
(Carlsbad, CA, USA) and used to measure the protein level of
MMP-13, according to the manufacturer’s instructions.
Analysis of data
Experiments were repeated at least three times. Data
are expressed as the means ± SD, and were analyzed using the
Student’s t-test. Statistical significance is indicated when
P<0.05.
Results
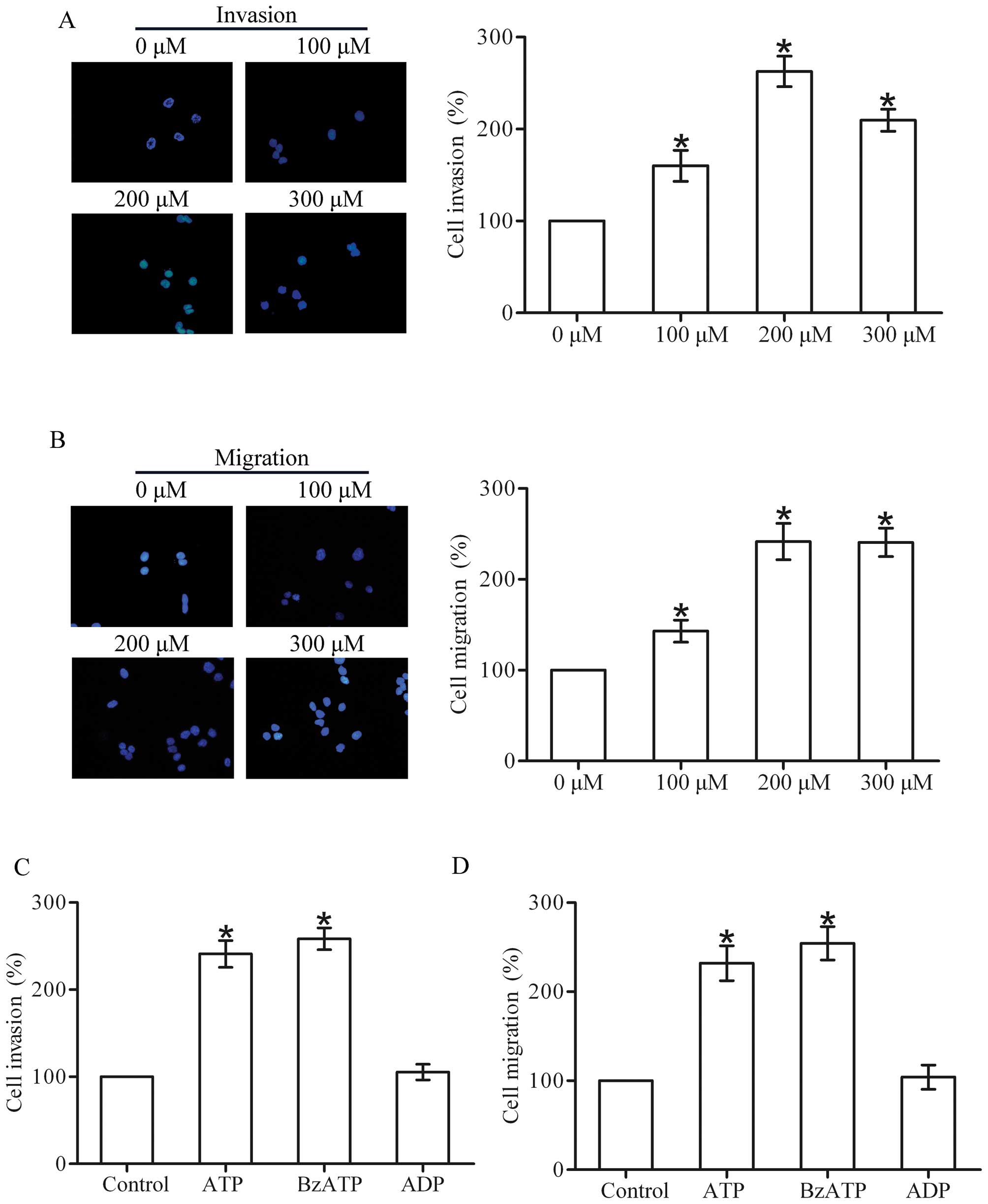
Extracellular ATP promotes the invasion
and migration of breast cancer cells
To determine the effect of ATP on the invasion and
migration of breast cancer cells, we performed Transwell invasion
and migration assays in the T47D cells. The results showed that ATP
produced a concentration-dependent (100–300 µM) increase in
the invasion and migration capacities of the T47D cells, with a
maximal effect occurring at 200 µM (Fig. 1A and B). Therefore, subsequent
experiments were carried out using 200 µM. Furthermore, the
ATP analogue BzATP also promoted the invasion and migration of the
T47D cells. However, ADP had little effect on the invasion and
migration (Fig. 1C and D).
Together, these data suggest that extracellular ATP promotes the
invasion and migration of breast cancer cells.
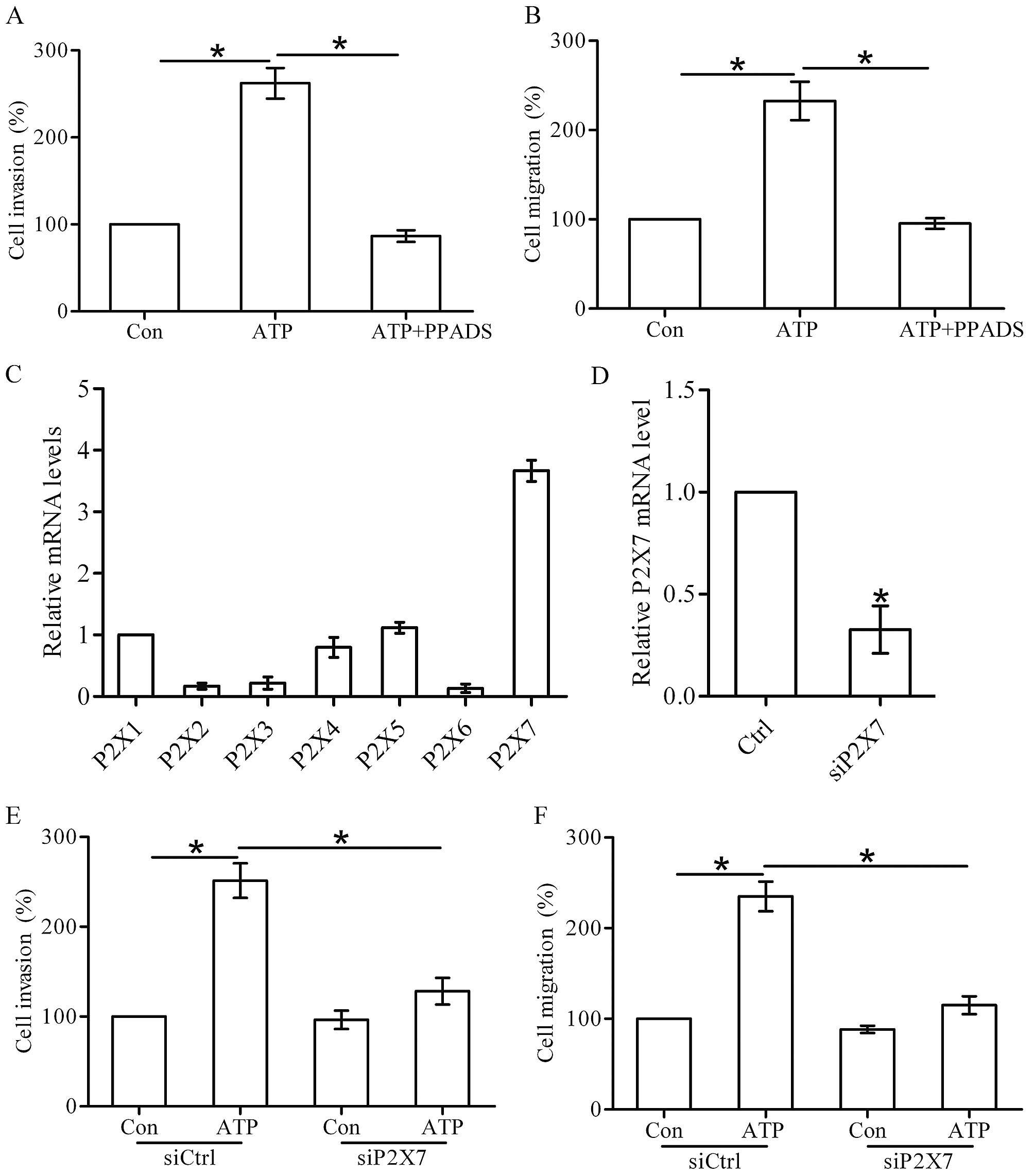
Effect of P2X7 receptor activation on the
invasion and migration of breast cancer cells
Next, we found that PPADS (100 µM), a
non-selective P2X receptor antagonist, attenuated the ATP-induced
invasion and migration of the T47D cells (Fig. 2A and B). Seven P2X receptor subtypes
(P2X1-7) have been cloned in human cells, and the P2X7 receptor has
been reported to play an important role in tumor progression
(11). Using real-time PCR, we
found that the P2X7 receptor was significantly expressed in the
T47D cells (Fig. 2C). Thus, we
silenced the expression of P2X7 receptor in the T47D cells by
transfection of siRNA (Fig. 2D),
and then investigated the effect of P2X7 knockdown on cell invasion
and migration. We found that knockdown of the P2X7 receptor
markedly inhibited the invasion and migration of the T47D cells
promoted by ATP stimulation (Fig. 2E
and F), indicating the involvement of the P2X7 receptor in
breast cancer cell invasion and migration.
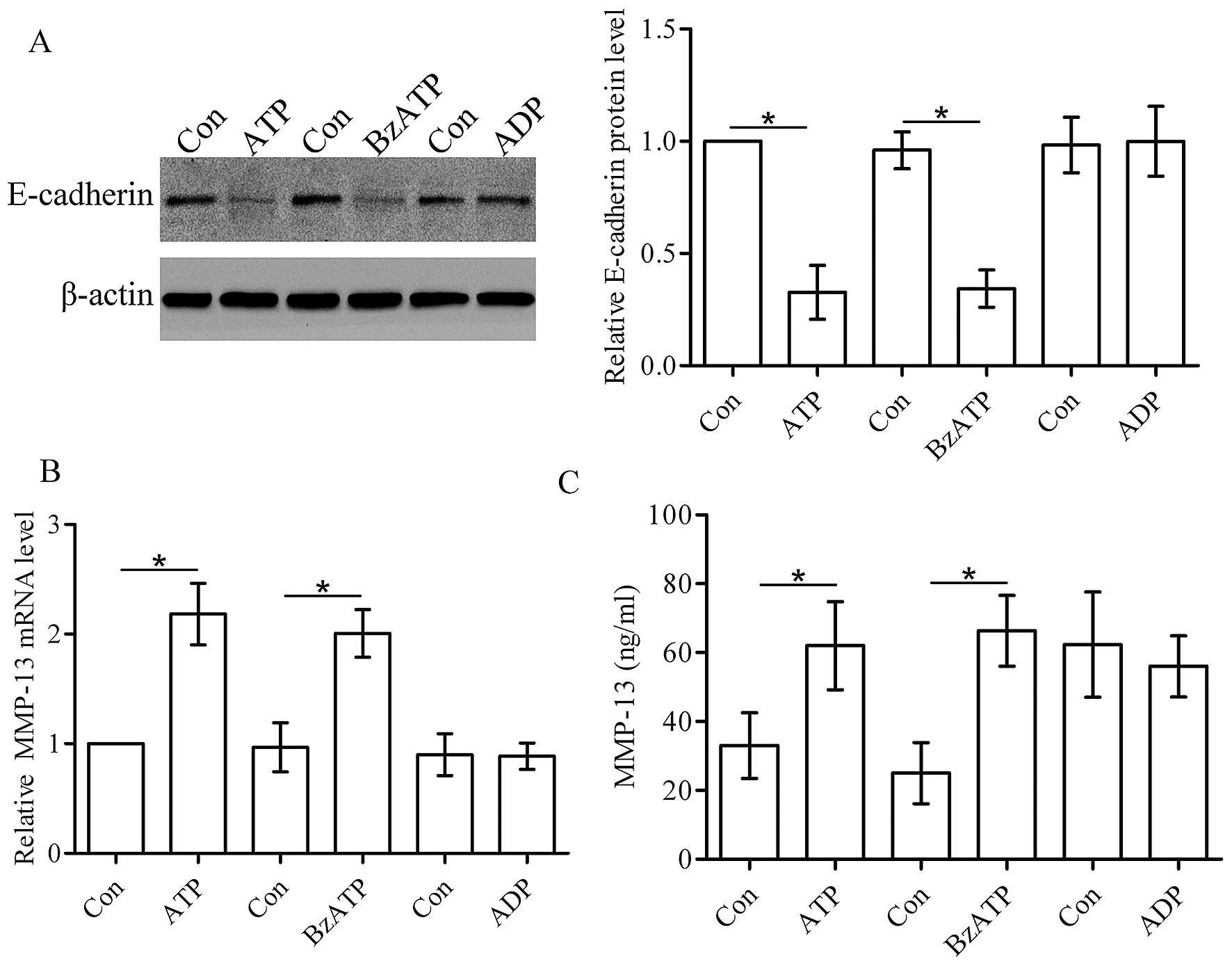
Activation of the P2X7 receptor affects
the levels of E-cadherin and MMP-13
E-cadherin is essential for tumor invasion and
metastasis (12). Using western
blot analysis, we found that ATP and its analogue BzATP
downregulated the protein level of E-cadherin in the T47D cells,
whereas ADP did not affect the protein level of E-cadherin
(Fig. 3A). MMP-13 plays an
important role in tumor invasion. In the present study, real-time
PCR and ELISA assay showed that ATP and its analogue BzATP
increased the expression and secretion of MMP-13 in the T47D cells.
However, ADP did not affect the production of MMP-13 (Fig. 3B and C).
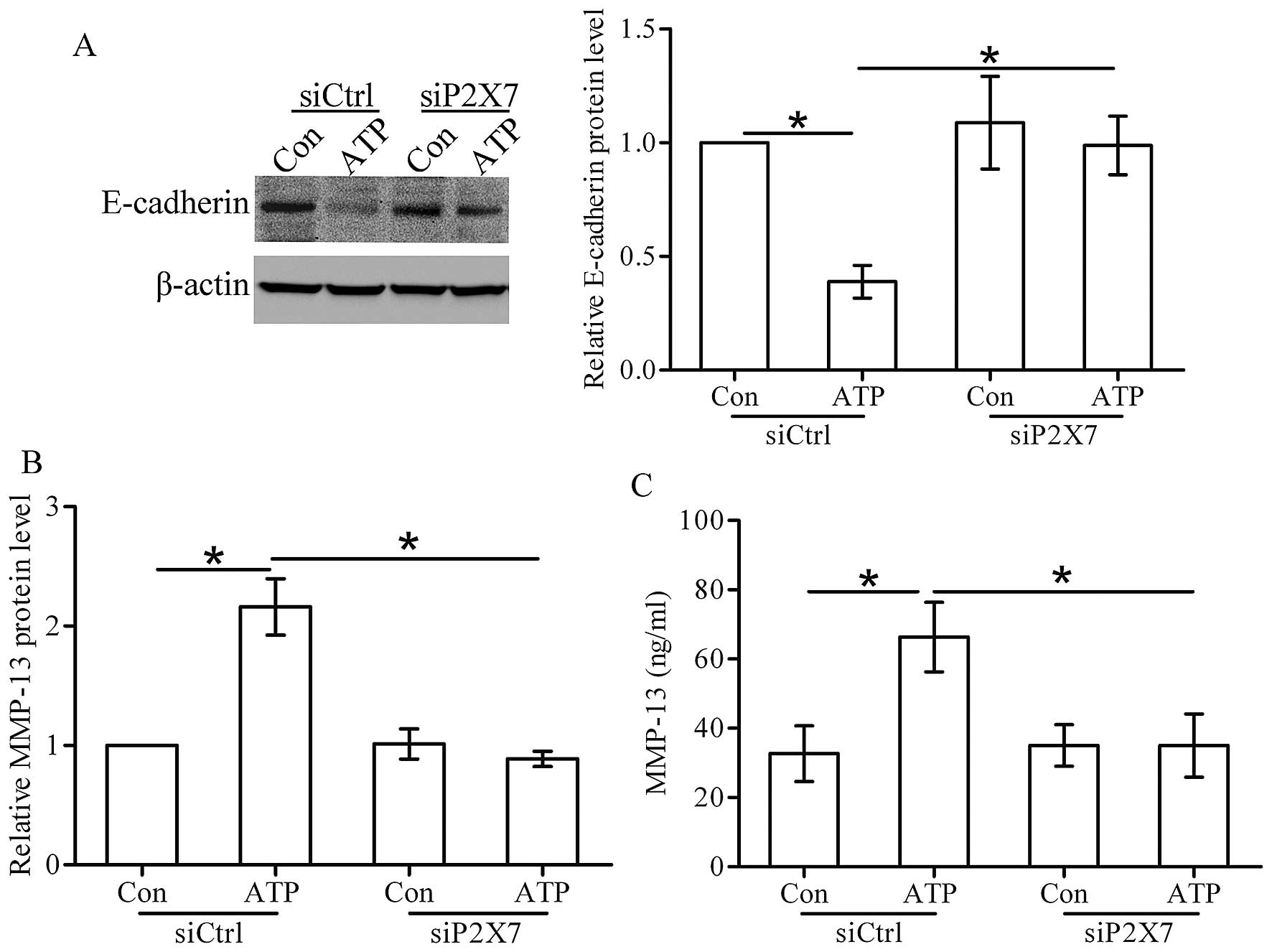
Extracellular ATP regulates the
expression of E-cadherin and MMP-13 via the P2X7 receptor
As shown in Fig. 4A,
after stimulation of ATP, the expression of E-cdherin was decreased
in the control siRNA (siCtrl) cells. However, the expression of
E-cadherin did not show any change in the P2X7 siRNA (siP2X7) cells
stimulated with ATP. Furthermore, ATP increased the expression and
secretion of MMP-13 in the siCtrl cells, whereas ATP stimulation
had little effect on MMP-13 production in the siP2X7 cells
(Fig. 4B and C). These findings
imply that activation of the P2X7 receptor by ATP decreases the
expression level of E-cadherin and MMP-13 in breast cancer
cells.
Activation of the P2X7 receptor by ATP
induces the AKT pathway in breast cancer cells
AKT, a pivotal kinase in the cell signaling pathway,
participates in tumor metastasis and development (13). Therefore, we examined whether ATP
could stimulate the activation of the AKT pathway in breast cancer
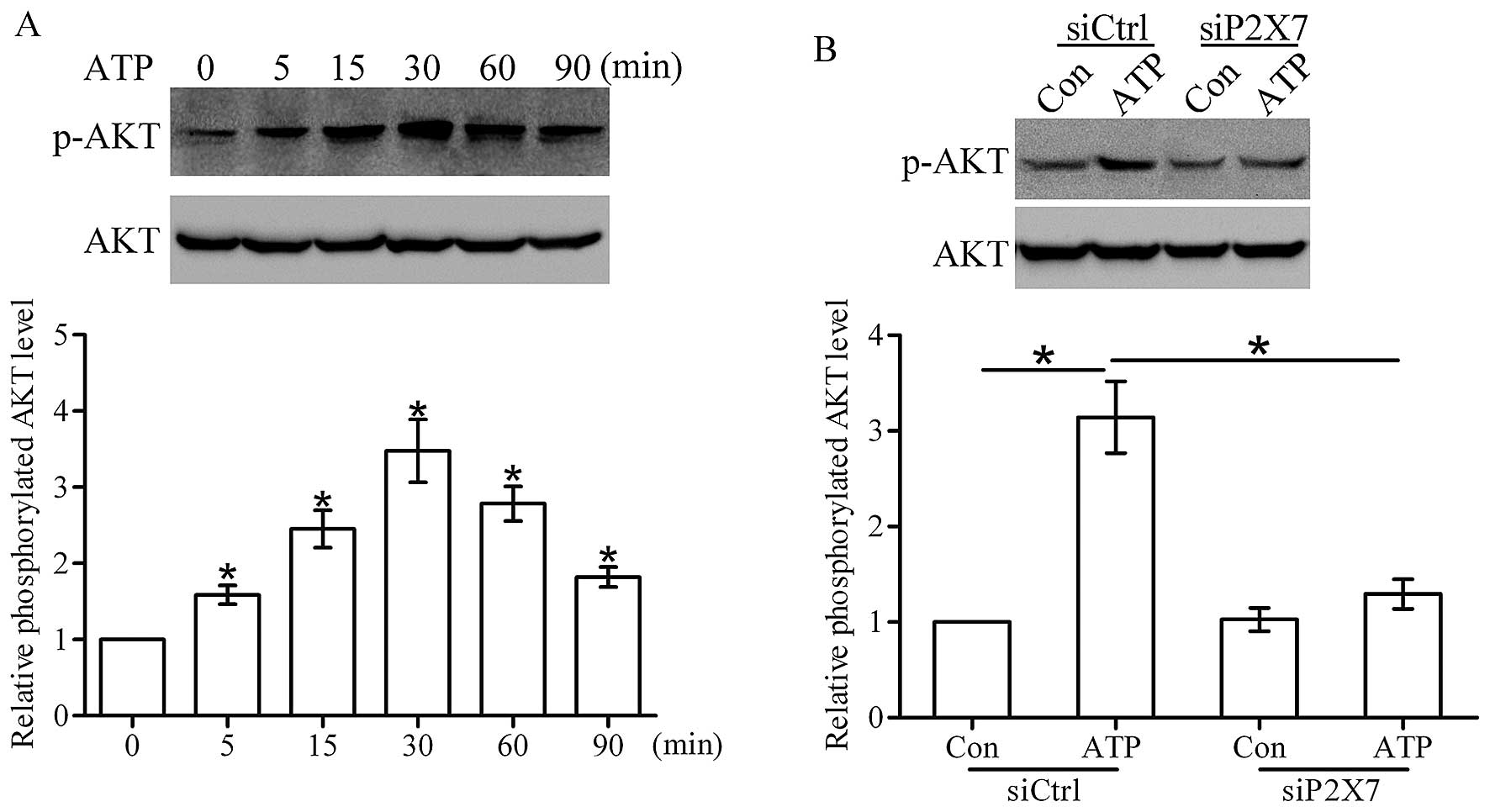
cells. As shown in Fig. 5A, ATP
(200 µM) time-dependently stimulated a marked increase in
the level of phosphorylated AKT in the T47D cells, and peak
activation occurred at 30 min. Moreover, western blot analysis
showed that the ATP-induced activation of AKT was greatly inhibited
after knockdown of the P2X7 receptor (Fig. 5B), suggesting that the P2X7 receptor
contributes to the ATP-induced activation of the AKT pathway in
breast cancer cells.
Effect of the AKT pathway on
P2X7-mediated invasion and migration
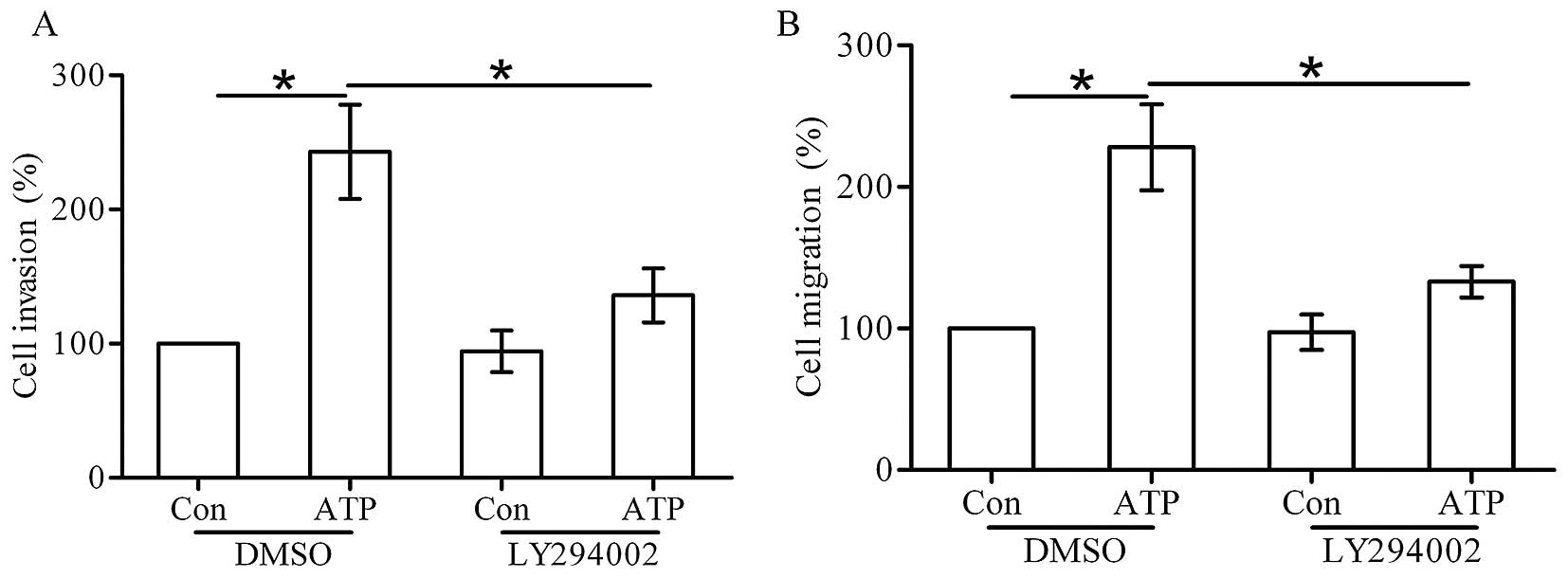
To determine whether the P2X7 receptor-stimulated
cell invasion and migration are mediated through the AKT pathway,
T47D cells were pretreated with 10 mM LY294002 for 30 min before
ATP stimulation. Transwell invasion and migration assays showed
that LY294002 inhibited the cell invasion and migration induced by
ATP (Fig. 6A and B). These data
confirm that the AKT pathway is required for the invasion and
migration induced by ATP.
Effect of the AKT pathway on
P2X7-mediated expression changes of E-cadherin and MMP-13
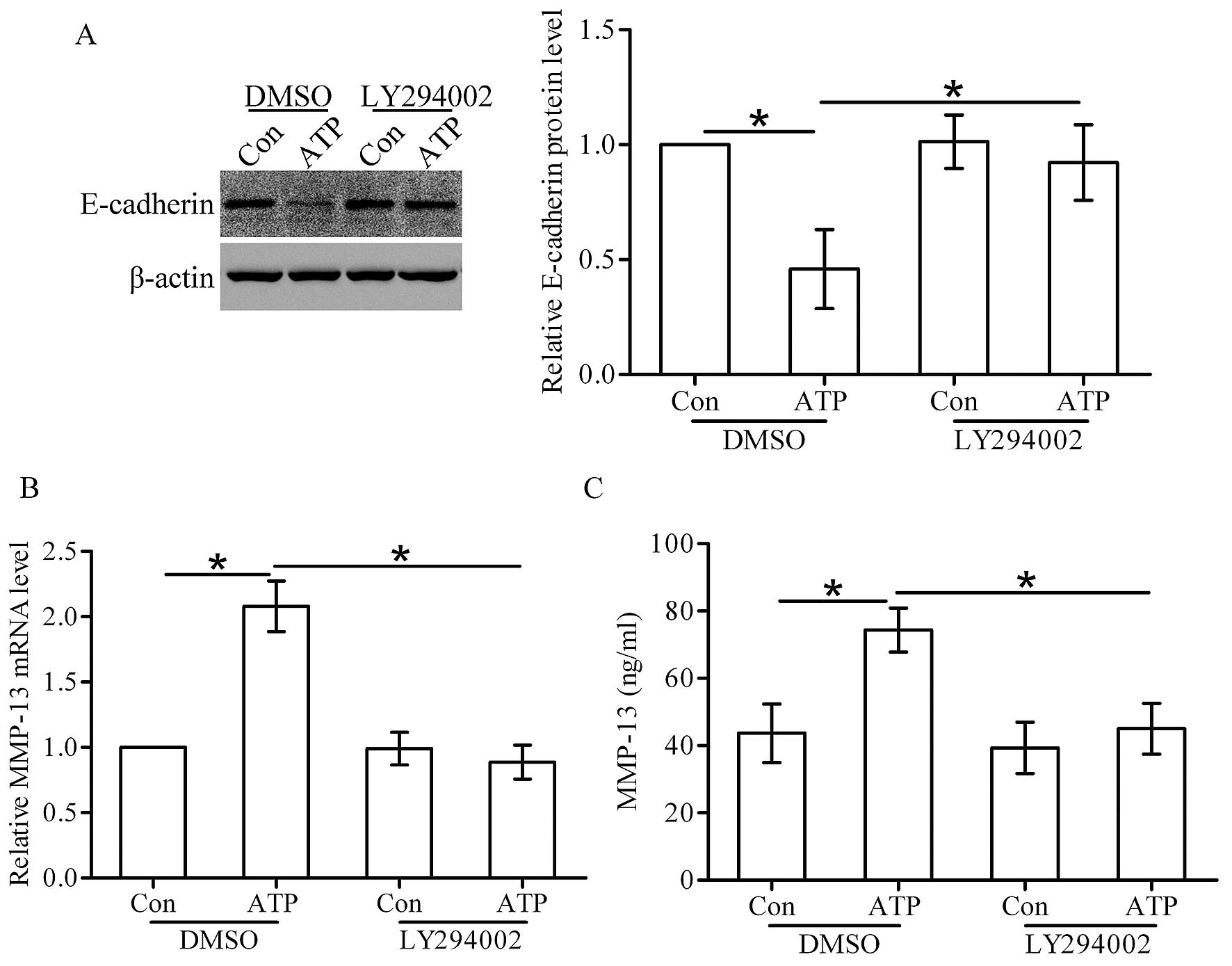
Western blot analysis showed that ATP stimulation
decreased the protein level of E-cadherin in the DMSO-treated
cells, whereas the effect of ATP on E-cadherin expression was
attenuated in the LY294002-treated cells (Fig. 7A). Furthermore, real-time PCR and
ELISA assay showed that ATP stimulation increased the expression
and secretion of MMP-13 in the DMSO-treated cells, while in the
LY294002-treated cells, this effect was significantly attenuated
(Fig. 7B and C).
Discussion
Many reports have proved the important functions of
purinergic signaling in tumor progression. In the present study, we
examined the capability of P2X7 receptor activation to induce
breast cancer cell invasion and migration. We found that ATP and
its analogue BzATP stimulated the invasion and migration of human
breast cancer T47D cells. P2X receptor inhibitor PPADS suppressed
the ATP-mediated cell invasion and migration. Furthermore,
knockdown of the P2X7 receptor inhibited the invasion and migration
stimulated by ATP. Altogether, our data suggest that activation of
the P2X7 receptor by ATP contributes to the invasion and migration
of breast cancer cells.
Previously, studies have found that extracellular
ATP stimulation leads to a decrease in the growth of tumor cells
(14). Further studies showed that
ATP participates in tumor motility, invasion and metastasis
(15,16). In the present study, we found that
ATP dose-dependently increased the invasion and migration of T47D
cells. We further found that BzATP also stimulated cell invasion
and migration, whereas ADP stimulation did not affect the invasion
and migration of T47D cells, confirming the involvement of ATP in
regulating breast cancer cell invasion and migration. ATP functions
via P2 receptors, which are divided into P2X and P2Y receptors. In
the present study, we found that the P2X receptor inhibitor PPADS
could greatly suppress the ATP-induced cell invasion and migration,
suggesting that P2X receptors may participate in the invasion and
migration of breast cancer cells. There are seven P2X receptor
subtypes (P2X1-7) that have been cloned in human cells. Using
real-time PCR, we found that the P2X7 receptor was significantly
expressed in the T47D cells. Studies have reported that expression
of the P2X7 receptor is elevated in thyroid papillary cancer, and
is closely associated with poor prognostic factors and lymph node
metastasis (4,17,18).
It was reported that activation of the P2X7 receptor increased the
growth of B16 melanoma cells in vitro and in vivo
(19,20) and influenced the motile activity of
lung cancer cells (21). Jelassi
et al (10) found that P2X7
receptor activation enhanced SK3 channel- and cystein
cathepsin-dependent cancer cell invasiveness of MDA-MB-435s breast
cancer cells. In the present study, we found that the knockdown of
the P2X7 receptor inhibited the ATP-mediated invasion and migration
of T47D cells, further confirming that P2X7 receptor activation
promotes breast cancer cell invasion and migration.
Epithelial-mesenchymal transition (EMT) is essential
for the invasion and metastasis of breast cancer cells (22). Davis et al (23) showed that ATP stimulated the
expression of vimentin in MDA-MB-468 breast cancer cells, and Li
et al (15) further found
that ATP increased the protein levels of E-cadherin and Snail in
prostate cancer cells, suggesting that ATP may affect the EMT
process in tumor cells. E-cadherin, which is an important
EMT-related marker in tumors, regulates cell-cell adhesion and is
often weakly expressed in tumor cells (24). In the present study, we found that
ATP and its analogue BzATP downregulated the expression of
E-cadherin, but ADP had little effect on the expression of
E-cadherin in breast cancer cells. Using siRNA technology, we
confirmed that ATP stimulation decreased the protein level of
E-cadherin via the P2X7 receptor.
A member of the metalloproteinases (MMPs), MMP-13
plays a key role in regulating tumor invasion and metastasis. Many
studies have reported that MMP-13 contributes to the bone
metastasis of breast cancer (25,26).
It was reported that extracellular ATP stimulated MMP-13 mRNA
expression in DU-145 prostate cancer cells (27). However, there is no report
concerning the effect of the P2X7 receptor on MMP-13 expression.
Here, we found that activation of the P2X7 receptor by ATP promoted
the expression and secretion of MMP-13 in breast cancer cells.
Many intracellular signaling pathways can be
activated by the P2X7 receptor. Studies have confirmed that
activation of the P2X7 receptor enhanced the proliferation of
ovarian carcinoma cells via the AKT and ERK1/2 pathways (28), and mediated tumor cell death via
PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways (29). In the present study, we found that
activation of the P2X7 receptor by ATP time-dependently induced the
AKT pathway in the T47D cells. It is well known that the AKT
pathway plays a vital role in tumor progression (30). The present study showed that
blocking of the AKT pathway attenuated the effect of ATP on the
invasion and migration in T47D cells. We further identified that
inhibition of the AKT pathway suppressed the changes in
P2X7-mediated E-cadherin and MMP-13 expression. These data indicate
that activation of the P2X7 receptor stimulates breast cancer cell
invasion and migration via the AKT pathway.
In conclusion, the present study demonstrated that
ATP stimulation promotes the invasion and migration of breast
cancer cells via activation of the P2X7 receptor. The function of
the P2X7 receptor may be triggered by activation of the AKT pathway
and subsequent regulation of E-cadherin and MMP-13 expression.
Thus, the P2X7 receptor could act as a new target for the
anticancer therapy of breast cancer.
Acknowledgments
The present study was supported by a grant from the
Luzhou Administration of Science and Technology, no.
2014-S-44(5/8).
References
|
1
|
Burnstock G: Purinergic signalling:
Pathophysiology and therapeutic potential. Keio J Med. 62:63–73.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braganhol E, Wink MR, Lenz G and
Battastini AM: Purinergic signaling in glioma progression. Adv Exp
Med Biol. 986:81–102. 2013. View Article : Google Scholar
|
|
3
|
Burnstock G: Introduction: P2 receptors.
Curr Top Med Chem. 4:793–803. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Solini A, Cuccato S, Ferrari D, Santini E,
Gulinelli S, Callegari MG, Dardano A, Faviana P, Madec S, Di
Virgilio F, et al: Increased P2X7 receptor expression and function
in thyroid papillary cancer: A new potential marker of the disease?
Endocrinology. 149:389–396. 2008. View Article : Google Scholar
|
|
5
|
Zhang XJ, Zheng GG, Ma XT, Yang YH, Li G,
Rao Q, Nie K and Wu KF: Expression of P2X7 in human hematopoietic
cell lines and leukemia patients. Leuk Res. 28:1313–1322. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Qi X, Zhou L, Catera D, Rote NS,
Potashkin J, Abdul-Karim FW and Gorodeski GI: Decreased expression
of P2X7 in endometrial epithelial pre-cancerous and cancer cells.
Gynecol Oncol. 106:233–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Zhou L, Feng YH, Abdul-Karim FW and
Gorodeski GI: The P2X7 receptor: A novel biomarker of uterine
epithelial cancers. Cancer Epidemiol Biomarkers Prev. 15:1906–1913.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar
|
|
9
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jelassi B, Chantôme A, Alcaraz-Pérez F,
Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A and Roger S:
P2X(7) receptor activation enhances SK3 channels- and cystein
cathepsin-dependent cancer cells invasiveness. Oncogene.
30:2108–2122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun SH: Roles of P2X7 receptor in glial
and neuroblastoma cells: The therapeutic potential of P2X7 receptor
antagonists. Mol Neurobiol. 41:351–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheng S, Qiao M and Pardee AB: Metastasis
and AKT activation. J Cell Physiol. 218:451–454. 2009. View Article : Google Scholar
|
|
14
|
Yang G, Zhang S, Zhang Y, Zhou Q, Peng S,
Zhang T, Yang C, Zhu Z and Zhang F: The inhibitory effects of
extracellular ATP on the growth of nasopharyngeal carcinoma cells
via P2Y2 receptor and osteopontin. J Exp Clin Cancer Res.
33:532014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li WH, Qiu Y, Zhang HQ, Liu Y, You JF,
Tian XX and Fang WG: P2Y2 receptor promotes cell invasion and
metastasis in prostate cancer cells. Br J Cancer. 109:1666–1675.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi K, Queiroz KC, Stap J, Richel DJ and
Spek CA: Protease-activated receptor-2 induces migration of
pancreatic cancer cells in an extracellular ATP-dependent manner. J
Thromb Haemost. 11:1892–1902. 2013.PubMed/NCBI
|
|
17
|
Gu LQ, Li FY, Zhao L, Liu Y, Chu Q, Zang
XX, Liu JM, Ning G and Zhao YJ: Association of XIAP and P2X7
receptor expression with lymph node metastasis in papillary thyroid
carcinoma. Endocrine. 38:276–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon JH, Nam ES, Shin HS, Cho SJ, Park HR
and Kwon MJ: P2X7 receptor expression in coexistence of papillary
thyroid carcinoma with Hashimoto’s thyroiditis. Korean J Pathol.
48:30–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adinolfi E, Raffaghello L, Giuliani AL,
Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V
and Di Virgilio F: Expression of P2X7 receptor increases in vivo
tumor growth. Cancer Res. 72:2957–2969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hattori F, Ohshima Y, Seki S, Tsukimoto M,
Sato M, Takenouchi T, Suzuki A, Takai E, Kitani H, Harada H, et al:
Feasibility study of B16 melanoma therapy using oxidized ATP to
target purinergic receptor P2X7. Eur J Pharmacol. 695:20–26. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takai E, Tsukimoto M, Harada H and Kojima
S: Autocrine signaling via release of ATP and activation of P2X7
receptor influences motile activity of human lung cancer cells.
Purinergic Signal. 10:487–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y and Zhou BP: Epithelial-mesenchymal
transition in breast cancer progression and metastasis. Chin J
Cancer. 30:603–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davis FM, Kenny PA, Soo ET, van Denderen
BJ, Thompson EW, Cabot PJ, Parat MO, Roberts-Thomson SJ and
Monteith GR: Remodeling of purinergic receptor-mediated
Ca2+ signaling as a consequence of EGF-induced
epithelial-mesenchymal transition in breast cancer cells. PLoS One.
6:e234642011. View Article : Google Scholar
|
|
24
|
Paredes J, Figueiredo J, Albergaria A,
Oliveira P, Carvalho J, Ribeiro AS, Caldeira J, Costa AM,
Simões-Correia J, Oliveira MJ, et al: Epithelial E- and
P-cadherins: Role and clinical significance in cancer. Biochim
Biophys Acta. 1826:297–311. 2012.PubMed/NCBI
|
|
25
|
Morrison C, Mancini S, Cipollone J,
Kappelhoff R, Roskelley C and Overall C: Microarray and proteomic
analysis of breast cancer cell and osteoblast co-cultures: Role of
osteoblast matrix metalloproteinase (MMP)-13 in bone metastasis. J
Biol Chem. 286:34271–34285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pivetta E, Scapolan M, Pecolo M,
Wassermann B, Abu-Rumeileh I, Balestreri L, Borsatti E, Tripodo C,
Colombatti A and Spessotto P: MMP-13 stimulates osteoclast
differentiation and activation in tumour breast bone metastases.
Breast Cancer Res. 13:R1052011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Gong LH, Zhang HQ, Du Q, You JF,
Tian XX and Fang WG: Extracellular ATP enhances in vitro invasion
of prostate cancer cells by activating Rho GTPase and upregulating
MMPs expression. Cancer Lett. 293:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vázquez-Cuevas FG, Martínez-Ramírez AS,
Robles-Martínez L, Garay E, García-Carrancá A, Pérez-Montiel D,
Castañeda-García C and Arellano RO: Paracrine stimulation of P2X7
receptor by ATP activates a proliferative pathway in ovarian
carcinoma cells. J Cell Biochem. 115:1955–1966. 2014.PubMed/NCBI
|
|
29
|
Bian S, Sun X, Bai A, Zhang C, Li L,
Enjyoji K, Junger WG, Robson SC and Wu Y: P2X7 integrates PI3K/AKT
and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell
death. PLoS One. 8:e601842013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lauring J, Park BH and Wolff AC: The
phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target
in breast cancer. J Natl Compr Canc Netw. 11:670–678.
2013.PubMed/NCBI
|